Epigenetic Properties of Compounds Contained in Functional Foods Against Cancer
Abstract
1. Introduction
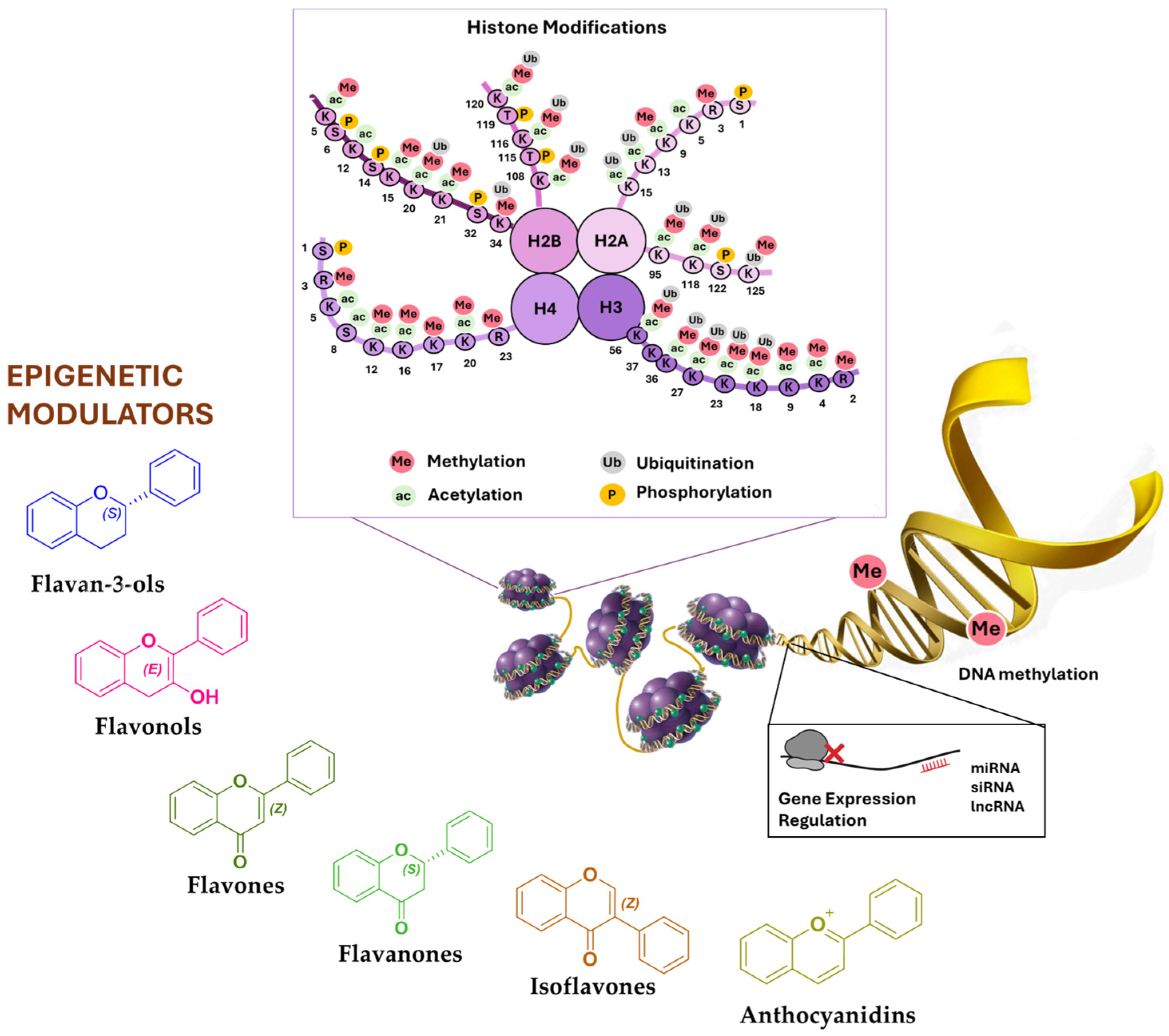
2. Epigenetic Mechanisms
2.1. DNA Methylation
2.2. Histone Modifications
2.3. Non-Coding RNAs
3. Phytochemicals Targeting Enzymes Involved in Epigenetic Regulation
3.1. Flavan-3-ols
| Compounds FLAVAN-3-OLS | Epigenetic Effects | High Content Food Quantity mg/100 g [81] | Role in Pathological Conditions |
|---|---|---|---|
| (−)-Epigallocatechin 3-gallate (EGCG) SubClass: Flavan-3-ols Class: Flavonoids Family: Polyphenols | Inhibition of DNMT activity (mainly DNMT1) in breast, colon, prostate, and esophageal cancer cells [61] Inhibition of hTERT promoter methylation in breast, lung, oral cavity, liver, and thyroid tumor cells [61] Demethylation of SCUBE2 promoter, inhibiting the epithelial-mesenchymal transition [80] Modulation of HAT activity [60] Regulation of miRNA expression in hepatocellular carcinoma and gastric cancer in vivo, targeting c-Kit, Bcl2, E2F, and RAS [62] | Carob Flour (109.40), Green tea (70.20), White tea (42.45), Oolong tea (34.48), Black tea (9.36), Nuts pecans (2.30), Fuji Apple with skin (1.93), Hazelnut (1.06), Cranberry (0.97), Blackberry (0.68), Raspberry (0.54), Plum (0.48), Pistachio nuts (0.40), Peach (Prunus persica) (0.30), Granny Smith Apple, with skin (0.24), Golden Delicious Apple, with skin (0.19), Pear (0.19), Avocado (0.15), Red Delicious Apple, with skin (0.13), Gala Apple, with skin (0.11), Strawberry (0.11) | Reduction in oxidative stress, angiogenesis, and inflammation in tumors [82] Cancer prevention [83,84] Induction of apoptosis by activating caspase 9, 8, and 3 and upregulating Bax protein in cancer cells [85] Induction of cell cycle arrest by enhancing p21 and p27 expression in tumor cells [85] |
| (−)-Epicatechin (EC), Epicatechin-3-gallate (ECG) Epigallocatechin (EGC) SubClass: Flavan-3-ols Class: Flavonoids Family: Polyphenols | Inhibition of DNMT activity [64] Regulation of HAT activity [20] Modulation of miRNA expression in cancer [63] | (−)-Epicatechin: Cocoa powder (158.3) Baking chocolate (141.83), Cacao seed (99.18), Grape seed (93.31), Dark chocolate (84.80), Soybean, mature seed (37.41), Broad beans, immature seed (Vicia faba) (28.96), Apple, skin only (28.73), Blueberry (25.66), Green tea, Quingmao (20.80), Sour cherry juice (12.97), Milk chocolate (10.88), Alcoholic beverage, red wine (10.66–3.79), Apple (9.83–4.09), Grape, black (Vitis vinifera) (8.68), Cherry (Prunus avium) (5.00), Apricot (Prunus armeniaca) (4.74), Apple juice (4.70), Blackberry (4.66), Cranberry (4.37), Peache, white, (4.09), Pear, raw (Pyrus communis) (3.76), Raspberry (Rubus spp.) (3.52), Plum (3.20), Nectarine (Prunus persica) (3.06–2.34), Buckwheat flour, whole-groat (3.02), Cranberry bush berries (Viburnum opulus) (2.69), Oolong tea (2.54), Vinegar, wine, red (2.20), Black tea (2.13), Arctic bramble berries (1.80), Grape, white (1.70), Strawberry tree fruit (Arbutus unedo) (1.56), Pistachio nuts (Pistacia vera) (0.83), Nut pecans (Carya illinoinensis) (0.82), Vinegar, cider (0.82), Cloudberry (0.80), Kiwifruit, gold (Actinidia chinensis) (0.64), Nut almonds (Prunus dulcis) (0.60), Alcoholic beverage, white wine (0.55), Medlar (Mespilus germanica) (0.53), Rhubarb (Rheum rhabarbarum) (0.51), Fig (Ficus carica) (0.50), Currants, European black (Ribes nigrum) (0.47) Epicatechin-3-gallate and Epigallocatechin: Cocoa powder (196.43), Cacao Bean (99.18), Carob Flour (30.06), Green tea (8.33), White tea (8.35), Oolong tea (2.54), Black tea (2.11), Nut almond (0.60), Nut pecan (0.82) | Antioxidative, anti-inflammatory, and anticancer effects [20] |
| (+)-Catechin, (+)-Gallocatechin SubClass: Flavan-3-ols Class: Flavonoids Family: Polyphenols | Inhibition of DNMT activity [64] Regulation of HAT activity [20] Modulation of miRNA expression in cancer [63] | (+)-Catechin: Blueberry (98.47), Cacao Bean (88.45), Grape seed (74.63), Tea green, Quingmao (67.60), Cacao powder (64.82), Carob Fluor (50.75), Blackberry (37.06), Cranberry (29.04), Chocolate, dark (24.20), Cocoa powder (21.51), Plum Black Diamond(17.22), Broad bean (Vicia faba) (14.29), Peache white (12.25), Grape Black (10.14), Nectarine (Prunus persica) (7.58), Apple skin only (7.40), Nut Pecan (7.24), Alcoholic beverage, wine red (from 6.21 to 7.70) Strawberry (6.70), Chard, red leaf (Beta vulgaris) (6.70), Apple (6.67–0.75) Banana (6.10), Blueberry (5.29), Bean, pinto (Phaseolus vulgaris) (5.07). Cider (4.85), Green tea (4.47), Cherry (4.36), Milk chocolate (4.16), Grape, white (3.73), Apricot (3.67), Vinegar, wine (3.60), Pistachio nuts, (3.57), Jujube (Ziziphus jujuba) (3.21), Strawberry (3.11), Juice sour cherry (3.18), Plum (2.89), Barley, hulled (Hordeum vulgare L.) (2.39), Arctic bramble berry (2.30), Rhubarb (Rheum rhabarbarum) (2.17), Mango (1.72), Gooseberry (1.67), Raspberry (1.31), Currants, red (1.27), Apple juice (1.25), Black tea (1.51), Nut, hazelnut or peanut (Corylus spp.) (1.19), Quinces (0.75) (+)-Gallocatechin: Cacao Bean (8262.00), Broad bean, immature seeds (Vicia faba) (4.15), Strawberry tree fruit (Arbutus) (1.60), Green Tea (1.54), Currants, red (1.28), Black tea (1.25), Pomegranate, raw (0.17), Persimmon (0.17), Star apple (0.53) | Antioxidative, anti-inflammatory, and anticancer effects [20] |
| Thearubigins SubClass: Flavan-3-ols Class: Flavonoids Family: Polyphenols | Inhibition of DNMT activity [86] | Black tea (81.30) | Antioxidative, anti-inflammatory, and anticancer effects [87,88] |
3.2. Flavonols
| Compounds FLAVONOLS | Epigenetic Effects | High Content Food Quantity mg/100 g | Role in PATHOLOGICAL Conditions |
|---|---|---|---|
| Kaempferol SubClass: Flavonols Class: Flavonoids Family: Polyphenols | Inhibitory activity towards HDAC enzymes in human hepatoma and colon cancer cell lines [65] Promotion of H3 histone hyperacetylation in hepatoma and colon tumor cells [65] Binding to DNMT1 in colorectal cancer cell lines, thus downregulating DACT2 methylation and inhibiting Wnt/β-catenin pathway [90] Regulation of miR-340 expression in lung tumor [7,66] | Caper (259.19), Caper canned (131.34), Kale (46.80), Arugula (34.89), Mustard green (38.30), Ginger (33.60), Watercress (23.03), Radish (21.85), Chia seed (12.30), Chives (10.0), Chard (9.20), Collards (8.74), Broccoli (7.84), Lovage (Levisticum officinale) (7.0), Fennel leaves (6.50), Dried Goji berries (6.20), Cherry powder (5.14), Thistle (3.80), Chicory green (2.45), Corn poppy (Papaver rhoeas) (2.30), Blueberry (1.66), Black Tea (1.41), Asparagus (1.39), Bee Pollen granules (1.12), Acerola (1.05), Green Tea (1.00), Ribes Nigrum (0.71), Red onion (0.70), Elderberry (0.51), Strawberry (0.49), Carob Flour (0.44), Grapefruit (0.40), Lingonberry (0.38), Blackberry (0.27), Apple (0.14), Cranberry (0.12) | Inhibition of fat formation, nervous system protection, and heart protection [87] Antioxidant, antiallergic and anticancer effects [87] Promotion of autophagic cell death in gastric tumor cells; inhibition of cancer cell proliferation and induction of cell apoptosis in lung tumor [7,66] |
| Quercetin SubClass: Flavonols Class: Flavonoids Family: Polyphenols Current dosage: 500 mg quercetin/day, 12 weeks Mean intake by fruits or vegetables: 5–500 mg/day IC50: 1.6 µmol/L | Reduction in the activity of DNMTs, HMTs, and HDACs, and activation of HATs in cervical tumor cells [66] Regulation of different miRNAs (like miR-145 and miR-146) and regulatory axes (such as miR-22/WNT1/β-catenin and p53/miR-34a/SIRT1) in several tumor cell lines [7] | Caper (233.84), Caper canned (172.55), Levisticum (170.0), Juice concentrate Elderberry (108.16), Radish (70.37), Arugula (66.19), Corianders leaves (52.90), Peppers yellow wax (50.63), Fennel leaves (48.80), Juniper berries ripe (46.16), Red onion ((39.21), Watercress (29.99), Elderberries (26.77), Corn poppy (Papaver rhoeas) (26.30), Carob flour (38.78), Onion cooked boiled drained (24.36), Kale (22.58), Been pollen (20.95), Apple skin (19.36), Chia seed (18.20), Cherries powder (17.44), Thistle (16.50), Arugula (15.16), Asparagu cook boiled-drained (15.16), Cranberries (14.84), Asparagus (13.98), Goji berry dried (13.60), Lingonberries (13.30), Plums black Diamond (12.45), Lovage Mustard green (8.80), Blueberries (7.67), Chard (7.50), Chicory green (6.49), Chives (4.77), Acerola (4.74), Ribes Nigrum (4.45), Apple (4.01), Blackberries (3.58), Broccoli (3.26), Collards (2.57) | Antioxidant, anticancer, anti-inflammatory, antiviral, antibacterial, neuroprotective and hypolipidemic impact [66] Inhibition of cell proliferation by promoting let-7c, Numb1, and Notch, and induction of cell apoptosis in pancreatic tumor cells [91] Regulation of β-catenin signal and promotion of BRCA1 expression in triple-negative breast cancer [92] |
| Fisetin SubClass: Flavonols Class: Flavonoids Family: Polyphenols IC50: 3.5 µmol/L | Inhibition of DNMT activity [95] Activation of SIRTs [97] | Strawberry (16.0), Apple (2.69), Persimmons (1.05), Onion (0.48), Grapes (0.39), Kiwi (0.2) | Interference with cancer cell growth, cell cycle progression, and promotion of cell apoptosis [95] Regulation of the Bcl-2 family protein expression and inhibition of signaling pathways in which p38 MAPK, ERK 1/2, or NF-kB are involved [96] |
| Myricetin SubClass: Flavonols Class: Flavonoids Family: Polyphenols IC50: 1.2 µmol/L | Inhibition of DNMT activity in a concentration-dependent manner [20] Indirect modulation of deacetylation to induce HIF-1α expression and suppress cMyc and β-catenin expression [98] | Juice concentrate black currant (20.85), Fennel leaves (19.80), Goji berry dried (11.40), Arugula (7.92), Carob flour (6.73), Cranberries (6.63), Thistle (3.60), Ribes Nigrum (6.18), Been pollen (3.34), Swiss Chard (2.20), Red onion (2.16), Blueberries (1.30), Corn poppy (Papaver rhoeas) (1.10), Tea green (1.00), Blackberry (0.67), Black tea (0.45), Strawberry (0.35), Broccoli (0.06), Apple (0.01), Plums Black Diamond (0.01) | Prevention of cardiovascular disorders, anticancer ability, and reduction in blood lipid levels, blood pressure, and both diabetes and bacteriostasis complications [4] |
3.3. Flavones and Flavanones
| Compounds FLAVONES and FLAVANONES | Epigenetic Effects | High Content Food Quantity mg/100 g | Role in Pathological Conditions |
|---|---|---|---|
| Apigenin SubClass: Flavones Class: Flavonoids Family: Polyphenols Dosage: 500–1000 mg | Inhibition of class I HDAC activity in prostate tumor cells [20,68] Acetylation of H3 histone in breast tumor cells [101] Inhibition of the activity of 5-cytosine DNMTs and silencing of NRF2 in skin epidermal cells [67] Induction of the expression of miR-16 and miR215-5p in glioma and colon cancer [7] | Parsley dried (4503.50), Celery seed (78.65), Vine Spinach (62.20), Kumquat (Citrus japonica) (21.87), Celery heart green (19.1), Red onion (0.24) | Inhibition of cell growth and promotion of cell cycle arrest and apoptosis in human prostate cancer cells [108] Blocking of the proliferation and growth of breast tumor cells through p21 transcription [101] Antioxidant and anticancer effects [67] |
| Luteolin SubClass: Flavones Class: Flavonoids Family: Polyphenols | Inhibition of DNMT1 and HDAC enzymes in a dose-dependent manner in colorectal tumor cells [69] Modification of the acetylation pattern of the gene promoter histone in prostate cancer cells [103] Regulation of the expression of different miRNAs in several tumor cell lines [4] | Juniper berries ripe (Juniperus communis L.) (69.05), Parsley dried (19.75), Red onion (0.16) | Use against diabetes and Alzheimer’s disorder [4] Inhibition of proliferation and metastasis in breast tumors by regulating MMP9 expression and reversal of the epithelial-to-mesenchymal transition through the regulation of β-catenin expression [102] Promotion of apoptosis in colorectal tumor cells [69] |
| Hesperetin SubClass: Flavanones Class: Flavonoids Family: Polyphenols | Reduction in methylation of the histone H3K79 in gastric cancer cells [105] Inhibition of DNMT activity [70] | Grapefruit (1.50), Citrus paradise (1.50) | Anticancer effects by reducing metastasis recurrence [20] |
| Naringenin SubClass: Flavanones Class: Flavonoids Family: Polyphenols Dose: 500–1000 mg/day | Inhibition of DNMT and HDAC activity [70] Reduction in the expression of several miRNAs involved in anti-inflammatory and antioxidant mechanisms (including miR-17-3p and miR-25-5p) in human colon adenocarcinoma to upregulate GPX and SOD expression [107]) | Grapefruit (53.0), Citrus paradise (53.0) | Antidiabetic, liver and heart-protective, antiviral, antioxidant, and antitumor effects; use in sepsis treatment [106] Promotion of apoptosis in neuroblastoma cancer cells [70] |
3.4. Isoflavones and Anthocyanidins
| Compounds ISOFLAVONES and ANTHOCYANIDINS | Epigenetic Effects | High Content Food Quantity mg/100 g | Role in Pathological Conditions |
|---|---|---|---|
| Genistein SubClass: Isoflavones Class: Flavonoids Family: Polyphenols (phytoestrogen group) | Activation of HAT activity and histone demethylation [73,74] Inhibition of DNMT enzymes [72] Modulation of several miRNAs in cancer [119] | Soy flour (89.42), Instant beverage Soy powder (62.18), Soy protein drink (42.91), Soy milk (42.85), Soybean (39.57), Natto (37.66), Tempeh (36.15), Miso (23.24), Green soybeans (22.57), Soy fiber (21.68) Soybean mature seeds (18.77), Tofu (16.01) Soy Yogurt (16.59) | Antitumor and antiproliferative effects [114] Suppressor of oncogene antiestrogenic activity [121] Induction of ROS-mediated apoptosis and reduction in epithelial-mesenchymal transition in both head and neck tumors, thus inhibiting tumor growth and proliferation [119] |
| Daidzein SubClass: Isoflavones Class: Flavonoids Family: Polyphenol (phytoestrogen group) | Activation of HAT activity [122] Inhibition of DNMT enzymes [115] | Soy flour (67.69), Soy Milk, dried (40.85), Instant beverage soy powder (40.07), Natto (33.22), Soy protein drink (27.98), Tempeh (22.66), Soybeans (21.75), Green soybeans (20.34), Soy fiber (18.80), Miso (16.43), Tofu (15.59), Soy Yogurt (13.77), Soybean mature seeds (12.86) | Antiestrogenic activity [123] |
| Delphinidin Subclass: Anthocyanidins Class: Flavonoids Family: Polyphenols | Inhibition of DNMT enzymes, which causes the activation of the Nrf2-ARE pathway [76] Increase in HDAC (in particular class I HDACs) activity [75] | Bilberry (97.59), Black currants (89.62), Eggplant (85.69), Blueberries (35.43), Black beans (18.50), Jambul (17.73), Red currants (9.32), Cranberries (7.67), Bananas (7.39), Pecans nuts (7.28), Jostaberry (6.61), Red onion (4.28), Red grapes (2.27) | Anticancer, antioxidative, and vision-protective functions; use in the prevention and treatment of type-2 diabetes and obesity [120] Upregulation of different pro-apoptotic genes and downregulation of anti-apoptotic ones [75] |
3.5. Other Compounds
| OTHER PHYTOCHEMICALS | Epigenetic Effects | High Content Food | Role in Pathological Conditions |
|---|---|---|---|
| Curcumin Dosage: Curcuma longa powered 500 mg/day. Dosage Indian cook: 1–2 g/day | Regulation of both HAT and HDAC activities [126] Inhibition of DNMT activity [154] Inhibition of oncogenic miRNAs and promotion of tumor suppressor miRNAs [127] | Curcuma longa powder contains 2% curcumin; 1 g Curcuma contains 20 mg curcumin; 1 g Curry contains 2.9 mg curcumin | Modulation of intracellular pathways implicated in inflammation, proliferation, invasion, survival, and apoptosis in cancer [125] Cancer prevention and suppressor [127,129] Toxic effects at high doses in normal cells like human dermal fibroblasts, resulting in a pronounced arrest of cell cycle progression and higher levels of cell death [155] |
| Folic Acid Family: Folate The recommended daily intake of folate for adults is about 400 µg. | A key element in the methyl-metabolism pathway [156] Regulation of the hepatic DNA methylation status [157] | Breakfast cereals Fortified (100–400 µg per 100 g), Peanuts (240 µg per 100 g), Black-eyed Peas (210 µg per 100 g), Fortified pasta (100–200 µg per 100 g), Spinach (194 µg per 100 g), Lentils (181 µg per 100 g), Chickpeas (Cicer arietinum) (172 µg per 100 g), Asparagus (149 µg per 100 g) Brussels Sprouts (140 µg per 100 g), Beetroot (Beta vulgaris subsp. vulgaris) (109 µg per 100 g), Lettuce (136 µg per 100 g), Broccoli (108 µg per 100 g), Almonds (50 µg per 100 g), Grains (20–40 µg per 100 g), Oranges (30 µg per 100 g), Eggs (22–47 µg per 100 g.), Bananas (20 µg per 100 g), Pasta (not fortified) (10–20 µg per 100 g) | Deficiency can modify hepatic DNA methylation patterns and induce liver (but also breast, lung, brain, and colorectal) cancer [158]. Antioxidant and pro-oxidant effects [159] |
| Indole-3-carbinol (I3C) and diindolylmethane (DIM) | Proteasomal degradation of class I HDAC enzymes [133] Modulation of the expression of several miRNAs in cancer [160] | Cruciferae family (Brassicaceae) as Broccoli (15–25 mg/100 g), Cabbage (10–15 mg/100 g), Cauliflower (20–40 mg/100 g), Brussels Sprouts (40–50 mg/100 g), Mustard (10–15 mg/100 g), radish (all of Brassica genus) (1–4 mg/100 g) | Attenuation of symptoms of cigarette smoke [161] Anticancer effects, including promotion of apoptotic mechanisms in several tumor cell lines [132] |
| Phenethyl isothiocyanate (PEITC) Family: Isothiocyanates | Inhibition of HDAC and DNMT activity [136] Induction of targeted histone acetylation and methylation in human prostate tumor cells [135] Regulation of the expression of several miRNAs in cancer [137] | Cruciferae family as Watercress, Cauliflower, Cabbage, Cress, Bok Choy (Brassica rapa subsp. Chinensis) (Chinese cabbage) (10–150 mg per 100 g); Radishes (Raphanus sativus) (1–4 mg per 100 g); Arugula (1–2 mg per 100 g). | Inhibition of carcinogenic processes and regulation of stress response and inflammation [134] |
| Lycopene Family: Tetraterpenoid | Inhibition of DNMTs [139] | Tomato (2.5–4.5 mg per 100 g) Watermelon (4–7 mg per 100 g) Pink Grapefruit (1–3 mg per 100 g), Pink Guava (5–6 mg per 100 g), Papaya (2–3 mg per 100 g) | Antioxidant capacity, DNA protection from oxidative damage, reduction in tumor growth and proliferation in breast, prostate, liver and lung cancer [138] Protective agent against ultraviolet-related tumorigenesis [140] |
| Resveratrol Family: Polyphenols | Promotion of HDAC activity [162] Resveratrol targets: SIRT1, -2 and -3 [162] H3 acetylation in breast cancer, enhancing the expression of BRCA1 [144] FOXO deacetylation in prostate cancer [163] Reduction in the expression of different oncogenic miRNAs in colon cancer cells [144] | Red grape skin (0.5–1.5 mg per 100 g), Red wine (0.2–5.8 mg per 100 mL), Blueberry, Mulberry, Cranberry (0.02–0.06 mg per 100 g), and Peanut (0.1–0.3 mg per 100 g) | Anti-inflammatory, anti-aging, and anticancer properties, which influence tumor cell proliferation, growth, invasion, and apoptosis [141] Promotion of apoptotic mechanisms in colon, breast, prostate tumor cells and leukemia [124] Anti-aging potential and suppression of mouse skin cancer in combination with tea polyphenols [162,164] |
| Sulforaphane Family: Isothiocyanate | Inhibition of HDAC activity, regulation of histone methylation, and reduction in H1 histone phosphorylation [146] Inhibition of the activity of DNMTs, mainly DNMT1, -3a, and -3b, in cervical, prostate, and breast tumor cell lines [149,150] Regulation of the expression of several miRNAs in different human tumors [137,147] | Broccoli Sprouts (around 30–100 mg per 100 g), Cruciferae as Cabbage, Brussels Sprouts, Broccoli, Kale (Brassica oleracea var. Sabellica) (0.1–2.5 mg per 100 g) | Reduction in oxidative stress levels, inhibition of cell proliferation, and induction of cell apoptosis in cervical, pancreatic, liver, lung, and ovarian cancers [145] Promotion of the expression of several cytoprotective genes (such as catalase, glutathione S-transferase, and superoxide dismutase) through the epigenetic reactivation of the Nrf2 pathway [147] Cell protection from ultraviolet-associated tumorigenesis [165] |
4. Bioavailability of Bioactive Molecules
5. Phytochemicals and Traditional Anticancer Therapies: A Comparative Analysis Including Clinical Studies
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hughes, V. Epigenetics: The sins of the father. Nature 2014, 507, 22–24. [Google Scholar] [CrossRef]
- Shannar, A.; Sarwar, M.S.; Kong, A.T. A New Frontier in Studying Dietary Phytochemicals in Cancer and in Health: Metabolic and Epigenetic Reprogramming. Prev. Nutr. Food Sci. 2022, 27, 335–346. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in Health and Disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar] [PubMed]
- Jiang, W.; Xia, T.; Liu, C.; Li, J.; Zhang, W.; Sun, C. Remodeling the Epigenetic Landscape of Cancer-Application Potential of Flavonoids in the Prevention and Treatment of Cancer. Front. Oncol. 2021, 11, 705903. [Google Scholar] [CrossRef]
- Russo, G.L.; Vastolo, V.; Ciccarelli, M.; Albano, L.; Macchia, P.E.; Ungaro, P. Dietary polyphenols and chromatin remodeling. Crit. Rev. Food Sci. Nutr. 2017, 57, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.A.; Aagaard-Tillery, K.M. Environmental influences on epigenetic profiles. Semin. Reprod. Med. 2009, 27, 380–390. [Google Scholar] [CrossRef]
- Fatima, N.; Baqri, S.S.R.; Bhattacharya, A.; Koney, N.K.; Husain, K.; Abbas, A.; Ansari, R.A. Role of Flavonoids as Epigenetic Modulators in Cancer Prevention and Therapy. Front. Genet. 2021, 12, 758733. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Kanwal, R.; Candamo, M.; Gupta, S. Dietary phytochemicals as epigenetic modifiers in cancer: Promise and challenges. Semin. Cancer Biol. 2016, 40–41, 82–99. [Google Scholar] [CrossRef]
- Choudhuri, S. From Waddington’s epigenetic landscape to small noncoding RNA: Some important milestones in the history of epigenetics research. Toxicol. Mech. Methods 2011, 21, 252–274. [Google Scholar] [CrossRef]
- Shoaib, S.; Ansari, M.A.; Ghazwani, M.; Hani, U.; Jamous, Y.F.; Alali, Z.; Wahab, S.; Ahmad, W.; Weir, S.A.; Alomary, M.N.; et al. Prospective Epigenetic Actions of Organo-Sulfur Compounds against Cancer: Perspectives and Molecular Mechanisms. Cancers 2023, 15, 697. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes. Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Shyh-Chang, N.; Locasale, J.W.; Lyssiotis, C.A.; Zheng, Y.; Teo, R.Y.; Ratanasirintrawoot, S.; Zhang, J.; Onder, T.; Unternaehrer, J.J.; Zhu, H.; et al. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 2013, 339, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Denis, H.; Ndlovu, M.N.; Fuks, F. Regulation of mammalian DNA methyltransferases: A route to new mechanisms. EMBO Rep. 2011, 12, 647–656. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. A decade of exploring the cancer epigenome—Biological and translational implications. Nat. Rev. Cancer 2011, 11, 726–734. [Google Scholar] [CrossRef]
- Cheung, H.H.; Lee, T.L.; Rennert, O.M.; Chan, W.Y. DNA methylation of cancer genome. Birth Defects Res. Part C Embryo Today 2009, 87, 335–350. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The epigenomics of cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.P.; Kantarjian, H.M. Targeting DNA methylation. Clin. Cancer Res. 2009, 15, 3938–3946. [Google Scholar] [CrossRef]
- Derissen, E.J.; Beijnen, J.H.; Schellens, J.H. Concise drug review: Azacitidine and decitabine. Oncologist 2013, 18, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Busch, C.; Burkard, M.; Leischner, C.; Lauer, U.M.; Frank, J.; Venturelli, S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenet. 2015, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of our epigenomes. Pharmacol. Res. 2012, 65, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zang, C.; Cui, K.; Schones, D.E.; Barski, A.; Peng, W.; Zhao, K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 2009, 138, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Struhl, K. Histone acetylation and transcriptional regulatory mechanisms. Genes. Dev. 1998, 12, 599–606. [Google Scholar] [CrossRef]
- Kuo, M.H.; Allis, C.D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 1998, 20, 615–626. [Google Scholar] [CrossRef]
- Di Cerbo, V.; Schneider, R. Cancers with wrong HATs: The impact of acetylation. Brief Funct. Genom. 2013, 12, 231–243. [Google Scholar] [CrossRef]
- Shi, M.Q.; Xu, Y.; Fu, X.; Pan, D.S.; Lu, X.P.; Xiao, Y.; Jiang, Y.Z. Advances in targeting histone deacetylase for treatment of solid tumors. J. Hematol. Oncol. 2024, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Delcuve, G.P.; Khan, D.H.; Davie, J.R. Roles of histone deacetylases in epigenetic regulation: Emerging paradigms from studies with inhibitors. Clin. Epigenet. 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Varier, R.A.; Timmers, H.T. Histone lysine methylation and demethylation pathways in cancer. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2011, 1815, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, D.; Avvakumov, N.; Cote, J. Histone phosphorylation: A chromatin modification involved in diverse nuclear events. Epigenetics 2012, 7, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Xu, Y.; Long, D.; Sun, H.; Li, H.; Xin, R.; Zhang, Z.; Li, Z.; Yang, Z.; Kang, Y. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677-3p/Sox9 axis. Stem Cell Res. Ther. 2021, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Rhim, J.; Baek, W.; Seo, Y.; Kim, J.H. From Molecular Mechanisms to Therapeutics: Understanding MicroRNA-21 in Cancer. Cells 2022, 11, 2791. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Ali, M.M.; Reischl, S.; Mahale, S.; Kosalai, S.T.; Huarte, M.; Kanduri, C. The DNA damage inducible lncRNA SCAT7 regulates genomic integrity and topoisomerase 1 turnover in lung adenocarcinoma. NAR Cancer 2021, 3, zcab002. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Marin-Bejar, O.; Marchese, F.P.; Athie, A.; Sanchez, Y.; Gonzalez, J.; Segura, V.; Huang, L.; Moreno, I.; Navarro, A.; Monzo, M.; et al. Pint lincRNA connects the p53 pathway with epigenetic silencing by the Polycomb repressive complex 2. Genome Biol. 2013, 14, R104. [Google Scholar] [CrossRef]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Nejadi Orang, F.; Abdoli Shadbad, M. CircRNA and lncRNA-associated competing endogenous RNA networks in medulloblastoma: A scoping review. Cancer Cell Int. 2024, 24, 248. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Wang, J.; Dong, Z.; Li, X. Cancer-related circular RNA: Diverse biological functions. Cancer Cell Int. 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Weiss, G.J. MicroRNAs and cancer: Past, present, and potential future. Mol. Cancer Ther. 2008, 7, 3655–3660. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Lou, U.K.; Fung, F.K.; Tong, J.H.M.; Zhang, C.H.; To, K.F.; Chan, S.L.; Chen, Y. CircRTN4 promotes pancreatic cancer progression through a novel CircRNA-miRNA-lncRNA pathway and stabilizing epithelial-mesenchymal transition protein. Mol. Cancer 2022, 21, 10. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Morresi, C.; Cianfruglia, L.; Armeni, T.; Mancini, F.; Tenore, G.C.; D’Urso, E.; Micheletti, A.; Ferretti, G.; Bacchetti, T. Polyphenolic compounds and nutraceutical properties of old and new apple cultivars. J. Food Biochem. 2018, 42, e12641. [Google Scholar] [CrossRef]
- Khan, H.; Belwal, T.; Efferth, T.; Farooqi, A.A.; Sanches-Silva, A.; Vacca, R.A.; Nabavi, S.F.; Khan, F.; Prasad Devkota, H.; Barreca, D.; et al. Targeting epigenetics in cancer: Therapeutic potential of flavonoids. Crit. Rev. Food Sci. Nutr. 2021, 61, 1616–1639. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Galeazzi, R.; Laudadio, E.; Amici, A.; Rusciano, D.; Armeni, T.; Cantarini, M.; Stipa, P.; Mobbili, G. Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation. Antioxidants 2020, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Circular RNAs: Novel Players in Cancer Mechanisms and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 10121. [Google Scholar] [CrossRef]
- Harper, K.L.; Mottram, T.J.; Whitehouse, A. Insights into the Evolving Roles of Circular RNAs in Cancer. Cancers 2021, 13, 4180. [Google Scholar] [CrossRef]
- Gerhauser, C. Cancer chemoprevention and nutriepigenetics: State of the art and future challenges. Nat. Prod. Cancer Prev. Ther. 2013, 329, 73–132. [Google Scholar]
- Minnelli, C.; Laudadio, E.; Sorci, L.; Sabbatini, G.; Galeazzi, R.; Amici, A.; Semrau, M.S.; Storici, P.; Rinaldi, S.; Stipa, P.; et al. Identification of a novel nitroflavone-based scaffold for designing mutant-selective EGFR tyrosine kinase inhibitors targeting T790M and C797S resistance in advanced NSCLC. Bioorg. Chem. 2022, 129, 106219. [Google Scholar] [CrossRef] [PubMed]
- Mobbili, G.; Romaldi, B.; Sabbatini, G.; Amici, A.; Marcaccio, M.; Galeazzi, R.; Laudadio, E.; Armeni, T.; Minnelli, C. Identification of Flavone Derivative Displaying a 4′-Aminophenoxy Moiety as Potential Selective Anticancer Agent in NSCLC Tumor Cells. Molecules 2023, 28, 3239. [Google Scholar] [CrossRef]
- Somsakeesit, L.O.; Senawong, T.; Senawong, G.; Kumboonma, P.; Samankul, A.; Namwan, N.; Yenjai, C.; Phaosiri, C. Evaluation and molecular docking study of two flavonoids from Oroxylum indicum (L.) Kurz and their semi-synthetic derivatives as histone deacetylase inhibitors. J. Nat. Med. 2024, 78, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Pechalrieu, D.; Dauzonne, D.; Arimondo, P.B.; Lopez, M. Synthesis of novel 3-halo-3-nitroflavanones and their activities as DNA methyltransferase inhibitors in cancer cells. Eur. J. Med. Chem. 2020, 186, 111829. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Choi, K.C.; Jung, M.G.; Lee, Y.H.; Yoon, J.C.; Kwon, S.H.; Kang, H.B.; Kim, M.J.; Cha, J.H.; Kim, Y.J.; Jun, W.J.; et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009, 69, 583–592. [Google Scholar] [CrossRef]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar] [PubMed]
- Kang, Q.; Tong, Y.; Gowd, V.; Wang, M.; Chen, F.; Cheng, K.W. Oral administration of EGCG solution equivalent to daily achievable dosages of regular tea drinkers effectively suppresses miR483-3p induced metastasis of hepatocellular carcinoma cells in mice. Food Funct. 2021, 12, 3381–3392. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, F.; Albertini, M.C.; Coletti, D.; Vilmercati, A.; Campanella, L.; Darzynkiewicz, Z.; Teodori, L. How Diet Intervention via Modulation of DNA Damage Response through MicroRNAs May Have an Effect on Cancer Prevention and Aging, an in Silico Study. Int. J. Mol. Sci. 2016, 17, 752. [Google Scholar] [CrossRef] [PubMed]
- Rajavelu, A.; Tulyasheva, Z.; Jaiswal, R.; Jeltsch, A.; Kuhnert, N. The inhibition of the mammalian DNA methyltransferase 3a (Dnmt3a) by dietary black tea and coffee polyphenols. BMC Biochem. 2011, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Venturelli, S.; Kallnischkies, M.; Bocker, A.; Busch, C.; Weiland, T.; Noor, S.; Leischner, C.; Weiss, T.S.; Lauer, U.M.; et al. Kaempferol, a new nutrition-derived pan-inhibitor of human histone deacetylases. J. Nutr. Biochem. 2013, 24, 977–985. [Google Scholar] [CrossRef]
- Kedhari Sundaram, M.; Hussain, A.; Haque, S.; Raina, R.; Afroze, N. Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell. Biochem. 2019, 120, 18357–18369. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Chen, D.; Yang, C.S. Dietary polyphenols may affect DNA methylation. J. Nutr. 2007, 137, 223S–228S. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Kaur, P.; Shukla, S.; Abbas, A.; Fu, P.; Gupta, S. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: In vitro and in vivo study. Mol. Carcinog. 2012, 51, 952–962. [Google Scholar] [CrossRef]
- Krifa, M.; Leloup, L.; Ghedira, K.; Mousli, M.; Chekir-Ghedira, L. Luteolin induces apoptosis in BE colorectal cancer cells by downregulating calpain, UHRF1, and DNMT1 expressions. Nutr. Cancer 2014, 66, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Marshall, G.M.; Liu, P.Y.; Xu, N.; Nelson, C.A.; Iismaa, S.E.; Liu, T. Enhancing the anticancer effect of the histone deacetylase inhibitor by activating transglutaminase. Eur. J. Cancer 2012, 48, 3278–3287. [Google Scholar] [CrossRef]
- Caba, L.; Florea, L.; Gug, C.; Dimitriu, D.C.; Gorduza, E.V. Circular RNA-Is the Circle Perfect? Biomolecules 2021, 11, 1755. [Google Scholar] [CrossRef]
- Fang, M.Z.; Chen, D.; Sun, Y.; Jin, Z.; Christman, J.K.; Yang, C.S. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin. Cancer Res. 2005, 11, 7033–7041. [Google Scholar] [CrossRef]
- Vahid, F.; Zand, H.; Nosrat-Mirshekarlou, E.; Najafi, R.; Hekmatdoost, A. The role dietary of bioactive compounds on the regulation of histone acetylases and deacetylases: A review. Gene 2015, 562, 8–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Chen, H. DNA methylation and histone modifications of Wnt genes by genistein during colon cancer development. Carcinogenesis 2013, 34, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.H.; Ko, H.; Jeon, H.; Sung, G.J.; Park, S.Y.; Jun, W.J.; Lee, Y.H.; Lee, J.; Lee, S.W.; Yoon, H.G.; et al. Delphinidin induces apoptosis via cleaved HDAC3-mediated p53 acetylation and oligomerization in prostate cancer cells. Oncotarget 2016, 7, 56767–56780. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.D.; Wu, R.; Li, S.; Yang, A.Y.; Kong, A.N. Anthocyanin Delphinidin Prevents Neoplastic Transformation of Mouse Skin JB6 P+ Cells: Epigenetic Re-activation of Nrf2-ARE Pathway. AAPS J. 2019, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Mobbili, G.; Galeazzi, R.; Armeni, T. Effect of Epigallocatechin-3-Gallate on EGFR Signaling and Migration in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 11833. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Patel, S.N.; Chan, T.H.; Tollefsbol, T.O. A novel prodrug of epigallocatechin-3-gallate: Differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev. Res. 2011, 4, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Shi, W.; Guo, H.; Long, W.; Wang, Y.; Qi, J.; Liu, J.; Xu, Y. The Inhibitory Effect of (-)-Epigallocatechin-3-Gallate on Breast Cancer Progression via Reducing SCUBE2 Methylation and DNMT Activity. Molecules 2019, 24, 2899. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Wasswa-Kintu, S.I.; Holden, J.M. USDA Develops a Database for Flavonoids to Assess Dietary Intakes. Procedia Food Sci. 2013, 2, 81–86. [Google Scholar] [CrossRef]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Liu, D. Flavonoids influence epigenetic-modifying enzyme activity: Structure-function relationships and the therapeutic potential for cancer. Curr. Med. Chem. 2010, 17, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Volate, S.R.; Muga, S.J.; Issa, A.Y.; Nitcheva, D.; Smith, T.; Wargovich, M.J. Epigenetic modulation of the retinoid X receptor alpha by green tea in the azoxymethane-Apc Min/+ mouse model of intestinal cancer. Mol. Carcinog. 2009, 48, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Feyes, D.K.; Nieminen, A.L.; Agarwal, R.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Chatterjee, R.; Mandal, A.K.A.; Mukhopadhyay, A.; Basu, S.; Giri, A.K.; Chatterji, U.; Bhattacharjee, P. Theaflavin-Containing Black Tea Extract: A Potential DNA Methyltransferase Inhibitor in Human Colon Cancer Cells and Ehrlich Ascites Carcinoma-Induced Solid Tumors in Mice. Nutr. Cancer 2021, 73, 2447–2459. [Google Scholar] [CrossRef]
- Imran, A.; Butt, M.S.; Xiao, H.; Imran, M.; Rauf, A.; Mubarak, M.S.; Ramadan, M.F. Inhibitory effect of black tea (Camellia sinensis) theaflavins and thearubigins against HCT 116 colon cancer cells and HT 460 lung cancer cells. J. Food Biochem. 2019, 43, e12822. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Ukil, A.; Karmakar, S.; Datta, N.; Chaudhuri, T.; Vedasiromoni, J.R.; Ganguly, D.K.; Das, P.K. Thearubigin, the major polyphenol of black tea, ameliorates mucosal injury in trinitrobenzene sulfonic acid-induced colitis. Eur. J. Pharmacol. 2003, 470, 103–112. [Google Scholar] [CrossRef]
- Russo, G.L.; Ungaro, P. Chapter 9—Epigenetic Mechanisms of Quercetin and Other Flavonoids in Cancer Therapy and Prevention. Transl. Epigenet. Epigenet. Cancer Prev. Acad. Press 2019, 8, 187–202. [Google Scholar]
- Lu, L.; Wang, Y.; Ou, R.; Feng, Q.; Ji, L.; Zheng, H.; Guo, Y.; Qi, X.; Kong, A.N.; Liu, Z. DACT2 Epigenetic Stimulator Exerts Dual Efficacy for Colorectal Cancer Prevention and Treatment. Pharmacol. Res. 2018, 129, 318–328. [Google Scholar] [CrossRef]
- Zheng, N.G.; Wang, J.L.; Yang, S.L.; Wu, J.L. Aberrant epigenetic alteration in Eca9706 cells modulated by nanoliposomal quercetin combined with butyrate mediated via epigenetic-NF-kappaB signaling. Asian Pac. J. Cancer Prev. 2014, 15, 4539–4543. [Google Scholar] [CrossRef]
- Srinivasan, A.; Thangavel, C.; Liu, Y.; Shoyele, S.; Den, R.B.; Selvakumar, P.; Lakshmikuttyamma, A. Quercetin regulates beta-catenin signaling and reduces the migration of triple negative breast cancer. Mol. Carcinog. 2016, 55, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Struewing, J.P.; Hartge, P.; Wacholder, S.; Baker, S.M.; Berlin, M.; McAdams, M.; Timmerman, M.M.; Brody, L.C.; Tucker, M.A. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N. Engl. J. Med. 1997, 336, 1401–1408. [Google Scholar] [CrossRef]
- Tao, S.F.; He, H.F.; Chen, Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015, 402, 93–100. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Gilani, S.A.; Shariati, M.A.; Imran, A.; Afzaal, M.; Atif, M.; Tufail, T.; Anjum, F.M. Fisetin: An anticancer perspective. Food Sci. Nutr. 2021, 9, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Pal, H.C.; Pearlman, R.L.; Afaq, F. Fisetin and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 213–244. [Google Scholar] [PubMed]
- Dashwood, R.H. Frontiers in polyphenols and cancer prevention. J. Nutr. 2007, 137, 267S–269S. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.S.; Park, J.I.; Kim, M.J.; Kim, H.B.; Lee, J.W.; Dao, T.T.; Oh, W.K.; Kang, C.D.; Kim, S.H. Involvement of SIRT1 in hypoxic down-regulation of c-Myc and beta-catenin and hypoxic preconditioning effect of polyphenols. Toxicol. Appl. Pharmacol. 2012, 259, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Matboli, M.; Ibrahim, D.; Hasanin, A.H.; Hassan, M.K.; Habib, E.K.; Bekhet, M.M.; Afifi, A.M.; Eissa, S. Epigenetic modulation of autophagy genes linked to diabetic nephropathy by administration of isorhamnetin in Type 2 diabetes mellitus rats. Epigenomics 2021, 13, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants 2020, 9, 288. [Google Scholar] [CrossRef]
- Tseng, T.H.; Chien, M.H.; Lin, W.L.; Wen, Y.C.; Chow, J.M.; Chen, C.K.; Kuo, T.C.; Lee, W.J. Inhibition of MDA-MB-231 breast cancer cell proliferation and tumor growth by apigenin through induction of G2/M arrest and histone H3 acetylation-mediated p21(WAF1/CIP1) expression. Environ. Toxicol. 2017, 32, 434–444. [Google Scholar] [CrossRef]
- Wu, H.T.; Lin, J.; Liu, Y.E.; Chen, H.F.; Hsu, K.W.; Lin, S.H.; Peng, K.Y.; Lin, K.J.; Hsieh, C.C.; Chen, D.R. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021, 81, 153437. [Google Scholar] [CrossRef] [PubMed]
- Shoulars, K.; Rodriguez, M.A.; Thompson, T.; Markaverich, B.M. Regulation of cell cycle and RNA transcription genes identified by microarray analysis of PC-3 human prostate cancer cells treated with luteolin. J. Steroid Biochem. Mol. Biol. 2010, 118, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Zill-E-Huma; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Wang, S.W.; Sheng, H.; Zheng, F.; Zhang, F. Hesperetin promotes DOT1L degradation and reduces histone H3K79 methylation to inhibit gastric cancer metastasis. Phytomedicine 2021, 84, 153499. [Google Scholar] [CrossRef]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Curti, V.; Di Lorenzo, A.; Rossi, D.; Martino, E.; Capelli, E.; Collina, S.; Daglia, M. Enantioselective Modulatory Effects of Naringenin Enantiomers on the Expression Levels of miR-17-3p Involved in Endogenous Antioxidant Defenses. Nutrients 2017, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Gupta, S.C.; Nabavizadeh, A.; Aggarwal, B.B. Regulation of cell signaling pathways by dietary agents for cancer prevention and treatment. Semin. Cancer Biol. 2017, 46, 158–181. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Tham, D.M.; Gardner, C.D.; Haskell, W.L. Clinical review 97: Potential health benefits of dietary phytoestrogens: A review of the clinical, epidemiological, and mechanistic evidence. J. Clin. Endocrinol. Metab. 1998, 83, 2223–2235. [Google Scholar]
- Keinan-Boker, L.; van Der Schouw, Y.T.; Grobbee, D.E.; Peeters, P.H. Dietary phytoestrogens and breast cancer risk. Am. J. Clin. Nutr. 2004, 79, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Chen, M.L.; Qin, Y.; Zhang, Q.Y.; Xu, H.X.; Zhou, Y.; Mi, M.T.; Zhu, J.D. Isoflavone consumption and risk of breast cancer: A dose-response meta-analysis of observational studies. Asia Pac. J. Clin. Nutr. 2013, 22, 118–127. [Google Scholar] [PubMed]
- Huser, S.; Guth, S.; Joost, H.G.; Soukup, S.T.; Kohrle, J.; Kreienbrock, L.; Diel, P.; Lachenmeier, D.W.; Eisenbrand, G.; Vollmer, G.; et al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Arch. Toxicol. 2018, 92, 2703–2748. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Adjakly, M.; Bosviel, R.; Rabiau, N.; Boiteux, J.P.; Bignon, Y.J.; Guy, L.; Bernard-Gallon, D. DNA methylation and soy phytoestrogens: Quantitative study in DU-145 and PC-3 human prostate cancer cell lines. Epigenomics 2011, 3, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H. Genistein, an epigenome modifier during cancer prevention. Epigenetics 2011, 6, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meeran, S.M.; Patel, S.N.; Chen, H.; Hardy, T.M.; Tollefsbol, T.O. Epigenetic reactivation of estrogen receptor-alpha (ERalpha) by genistein enhances hormonal therapy sensitivity in ERalpha-negative breast cancer. Mol. Cancer 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Bosviel, R.; Dumollard, E.; Dechelotte, P.; Bignon, Y.J.; Bernard-Gallon, D. Can soy phytoestrogens decrease DNA methylation in BRCA1 and BRCA2 oncosuppressor genes in breast cancer? OMICS 2012, 16, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.L.; Liao, Y.W.; Hsieh, C.W.; Chen, P.N.; Yu, C.C. Soy Isoflavone Genistein Impedes Cancer Stemness and Mesenchymal Transition in Head and Neck Cancer through Activating miR-34a/RTCB Axis. Nutrients 2020, 12, 1924. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Meza, E.; Bergel, M.; Maier, C. Estrogenic, Antiestrogenic and Antiproliferative Activities of Euphorbia bicolor (Euphorbiaceae) Latex Extracts and Its Phytochemicals. Nutrients 2019, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Nakagawa, T.; Pan, W.; Kim, M.Y.; Kraus, W.L.; Ikehara, T.; Yasui, K.; Aihara, H.; Takebe, M.; Muramatsu, M.; et al. Isoflavones stimulate estrogen receptor-mediated core histone acetylation. Biochem. Biophys. Res. Commun. 2004, 317, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, W.J.; Li, Z.Y.; Li, L.; Wang, Y.; Zhao, Y.; Wang, C.; Yu, L.R.; Li, L.Z.; Zhang, Y.L. Daidzein increases OPG/RANKL ratio and suppresses IL-6 in MG-63 osteoblast cells. Int. Immunopharmacol. 2016, 40, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Kumar, D.; Srivastava, R.K. Epigenetic modifications by dietary phytochemicals: Implications for personalized nutrition. Pharmacol. Ther. 2013, 138, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Eifes, S.; Dicato, M.; Diederich, M. Curcumin-the paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins 2010, 2, 128–162. [Google Scholar] [CrossRef] [PubMed]
- Marcu, M.G.; Jung, Y.J.; Lee, S.; Chung, E.J.; Lee, M.J.; Trepel, J.; Neckers, L. Curcumin is an inhibitor of p300 histone acetylatransferase. Med. Chem. 2006, 2, 169–174. [Google Scholar] [PubMed]
- Sun, M.; Estrov, Z.; Ji, Y.; Coombes, K.R.; Harris, D.H.; Kurzrock, R. Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Mol. Cancer Ther. 2008, 7, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, Y.; Sun, J.; Zhang, Y. Curcumin reduces the expression of Bcl-2 by upregulating miR-15a and miR-16 in MCF-7 cells. Med. Oncol. 2010, 27, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef]
- Banerjee, S.; Kong, D.; Wang, Z.; Bao, B.; Hillman, G.G.; Sarkar, F.H. Attenuation of multi-targeted proliferation-linked signaling by 3,3′-diindolylmethane (DIM): From bench to clinic. Mutat. Res. Mol. Mech. Mutagen. 2011, 728, 47–66. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Guo, B. Chemopreventive agent 3,3′-diindolylmethane selectively induces proteasomal degradation of class I histone deacetylases. Cancer Res. 2010, 70, 646–654. [Google Scholar] [CrossRef]
- Cheung, K.L.; Kong, A.N. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010, 12, 87–97. [Google Scholar] [CrossRef]
- Wang, L.G.; Beklemisheva, A.; Liu, X.M.; Ferrari, A.C.; Feng, J.; Chiao, J.W. Dual action on promoter demethylation and chromatin by an isothiocyanate restored GSTP1 silenced in prostate cancer. Mol. Carcinog. 2007, 46, 24–31. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Hao, M. Phenethyl isothiocyanate reduces breast cancer stem cell-like properties by epigenetic reactivation of CDH1. Oncol. Rep. 2021, 45, 337–348. [Google Scholar] [CrossRef]
- Izzotti, A.; Larghero, P.; Cartiglia, C.; Longobardi, M.; Pfeffer, U.; Steele, V.E.; De Flora, S. Modulation of microRNA expression by budesonide, phenethyl isothiocyanate and cigarette smoke in mouse liver and lung. Carcinogenesis 2010, 31, 894–901. [Google Scholar] [CrossRef]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef] [PubMed]
- King-Batoon, A.; Leszczynska, J.M.; Klein, C.B. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environ. Mol. Mutagen. 2008, 49, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, Z.; Gao, D.; Saladi, R.N.; Lu, Y.; Lebwohl, M.; Wei, H. Protective effects of lycopene against ultraviolet B-induced photodamage. Nutr. Cancer 2003, 47, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Unterman, T.G.; Shankar, S. FOXO transcription factors and VEGF neutralizing antibody enhance antiangiogenic effects of resveratrol. Mol. Cell. Biochem. 2010, 337, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Pyo, I.S.; Yun, S.; Yoon, Y.E.; Choi, J.W.; Lee, S.J. Mechanisms of Aging and the Preventive Effects of Resveratrol on Age-Related Diseases. Molecules 2020, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Torres, E.; Hernandez-Oliveras, A.; Meneses-Morales, I.; Rodriguez, G.; Fuentes-Garcia, G.; Zarain-Herzberg, A. Resveratrol up-regulates ATP2A3 gene expression in breast cancer cell lines through epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2019, 113, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J.; Alder, H.; Volinia, S.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFbeta signaling pathway in SW480 cells. Biochem. Pharmacol. 2010, 80, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Patel, S.N.; Tollefsbol, T.O. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PLoS ONE 2010, 5, e11457. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer Activity of Sulforaphane: The Epigenetic Mechanisms and the Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef]
- Shankar, S.; Ganapathy, S.; Srivastava, R.K. Sulforaphane enhances the therapeutic potential of TRAIL in prostate cancer orthotopic model through regulation of apoptosis, metastasis, and angiogenesis. Clin. Cancer Res. 2008, 14, 6855–6866. [Google Scholar] [CrossRef]
- Ali Khan, M.; Kedhari Sundaram, M.; Hamza, A.; Quraishi, U.; Gunasekera, D.; Ramesh, L.; Goala, P.; Al Alami, U.; Ansari, M.Z.; Rizvi, T.A.; et al. Sulforaphane Reverses the Expression of Various Tumor Suppressor Genes by Targeting DNMT3B and HDAC1 in Human Cervical Cancer Cells. Evid. Based Complement Altern. Med. 2015, 2015, 412149. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.; Wong, C.P.; Yu, Z.; Williams, D.E.; Dashwood, R.H.; Ho, E. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clin. Epigenet. 2011, 3, 3. [Google Scholar] [CrossRef]
- Li, Y.; Buckhaults, P.; Cui, X.; Tollefsbol, T.O. Combinatorial epigenetic mechanisms and efficacy of early breast cancer inhibition by nutritive botanicals. Epigenomics 2016, 8, 1019–1037. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meeran, S.M.; Tollefsbol, T.O. Combinatorial bioactive botanicals re-sensitize tamoxifen treatment in ER-negative breast cancer via epigenetic reactivation of ERalpha expression. Sci. Rep. 2017, 7, 9345. [Google Scholar]
- Paul, B.; Li, Y.; Tollefsbol, T.O. The Effects of Combinatorial Genistein and Sulforaphane in Breast Tumor Inhibition: Role in Epigenetic Regulation. Int. J. Mol. Sci. 2018, 19, 1754. [Google Scholar] [CrossRef]
- Liu, Q.; Loo, W.T.; Sze, S.C.; Tong, Y. Curcumin inhibits cell proliferation of MDA-MB-231 and BT-483 breast cancer cells mediated by down-regulation of NFkappaB, cyclinD and MMP-1 transcription. Phytomedicine 2009, 16, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Cianfruglia, L.; Minnelli, C.; Laudadio, E.; Scire, A.; Armeni, T. Side Effects of Curcumin: Epigenetic and Antiproliferative Implications for Normal Dermal Fibroblast and Breast Cancer Cells. Antioxidants 2019, 8, 382. [Google Scholar] [CrossRef]
- Luan, Y.; Leclerc, D.; Cosin-Tomas, M.; Malysheva, O.V.; Wasek, B.; Bottiglieri, T.; Caudill, M.A.; Rozen, R. Moderate Folic Acid Supplementation in Pregnant Mice Results in Altered Methyl Metabolism and in Sex-Specific Placental Transcription Changes. Mol. Nutr. Food Res. 2021, 65, e2100197. [Google Scholar] [CrossRef] [PubMed]
- Jing-Bo, L.; Ying, Y.; Bing, Y.; Xiang-Bing, M.; Zhi-Qing, H.; Guo-Quan, H.; Hong, C.; Dai-Wen, C. Folic acid supplementation prevents the changes in hepatic promoter methylation status and gene expression in intrauterine growth-retarded piglets during early weaning period. J. Anim. Physiol. Anim. Nutr. 2013, 97, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J. Epigenetic modifications and human pathologies: Cancer and CVD. Proc. Nutr. Soc. 2011, 70, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, S.; Orlando, P.; Armeni, T.; Padella, L.; Bruge, F.; Seddaiu, G.; Littarru, G.P.; Tiano, L. Coenzyme Q10 and alpha-lipoic acid: Antioxidant and pro-oxidant effects in plasma and peripheral blood lymphocytes of supplemented subjects. J. Clin. Biochem. Nutr. 2015, 57, 21–26. [Google Scholar] [CrossRef]
- Li, Y.; VandenBoom, T.G., 2nd; Kong, D.; Wang, Z.; Ali, S.; Philip, P.A.; Sarkar, F.H. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009, 69, 6704–6712. [Google Scholar] [CrossRef]
- Izzotti, A.; Calin, G.A.; Steele, V.E.; Cartiglia, C.; Longobardi, M.; Croce, C.M.; De Flora, S. Chemoprevention of cigarette smoke-induced alterations of MicroRNA expression in rat lungs. Cancer Prev. Res. 2010, 3, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Chen, Q.; Fu, J.; Shankar, S.; Srivastava, R.K. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS ONE 2011, 6, e25166. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ganapathy, S.; Singh, K.P.; Shankar, S.; Srivastava, R.K. Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS ONE 2010, 5, e15288. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Y.; Sargsyan, D.; Wu, R.; Yin, R.; Kuo, H.D.; Yang, I.; Wang, L.; Cheng, D.; Ramirez, C.N.; et al. Epigenome, Transcriptome, and Protection by Sulforaphane at Different Stages of UVB-Induced Skin Carcinogenesis. Cancer Prev. Res. 2020, 13, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19, 954–994. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Molteni, C.; La Motta, C.; Valoppi, F. Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review. Antioxidants 2022, 11, 1931. [Google Scholar] [CrossRef]
- Aatif, M. Current Understanding of Polyphenols to Enhance Bioavailability for Better Therapies. Biomedicines 2023, 11, 2078. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef]
- Berdasco, M.; Esteller, M. Clinical epigenetics: Seizing opportunities for translation. Nat. Rev. Genet. 2019, 20, 109–127. [Google Scholar] [CrossRef]
- Herman, J.G.; Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003, 349, 2042–2054. [Google Scholar] [CrossRef] [PubMed]
- Kronfol, M.M.; Dozmorov, M.G.; Huang, R.; Slattum, P.W.; McClay, J.L. The role of epigenomics in personalized medicine. Expert Rev. Precis. Med. Drug Dev. 2017, 2, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Gu, J.J.; Zhang, Q.; Mavis, C.; Hernandez-Ilizaliturri, F.J.; Czuczman, M.S.; Guo, Y. Vorinostat, a histone deacetylase (HDAC) inhibitor, promotes cell cycle arrest and re-sensitizes rituximab- and chemo-resistant lymphoma cells to chemotherapy agents. J. Cancer Res. Clin. Oncol. 2016, 142, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef] [PubMed]
- Mahalmani, V.; Sinha, S.; Prakash, A.; Medhi, B. Translational research: Bridging the gap between preclinical and clinical research. Indian J. Pharmacol. 2022, 54, 393–396. [Google Scholar] [CrossRef] [PubMed]
- el Bahhaj, F.; Dekker, F.J.; Martinet, N.; Bertrand, P. Delivery of epidrugs. Drug Discov. Today 2014, 19, 1337–1352. [Google Scholar] [CrossRef]
- NCT01606124. Randomized Phase II Trial of Polyphenon E vs. Placebo in Patients at High Risk of Recurrent Colonic Neoplasia. 2012. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT01606124/ (accessed on 4 November 2024).
- NCT00917735. Phase II, Randomized, Double-blind, Placebo-controlled, Study of the Efficacy of Green Tea Extract on Biomarkers of Breast Cancer Risk in High Risk Women with Differing Catechol-O-methyl Transferase (COMT) Genotypes. 2009. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT00917735/ (accessed on 4 November 2024).
- NCT00666562. A Phase II Randomized, Placebo-Controlled Trial of Polyphenon E to Evaluate Bladder Tissue Levels of EGCG. 2008. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT00666562/ (accessed on 4 November 2024).
- NCT00573885. Phase II Trial of Polyphenon E in Former Smokers with Abnormal Sputa. 2007. Available online: http://cdek.pharmacy.purdue.edu/trial/NCT00573885/ (accessed on 4 November 2024).
- NCT00303823. A Phase II Trial of Polyphenon E for Cervical Cancer Prevention. 2006. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT00303823/ (accessed on 4 November 2024).
- NCT01032031. The Effect of Dietary Bioactive Compounds on Skin Health in Humans In Vivo. 2009. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT01032031/ (accessed on 4 November 2024).
- NCT02580279. Phase II Study of Topical Epigallocatechin-3-gallate (EGCG) in Patients with Breast Cancer Receiving Adjuvant Radiotherapy. 2015. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT02580279/ (accessed on 4 November 2024).
- NCT02577393. Epigallocatechin-3-gallate (EGCG) for Esophagus Protection in Patients with Lung Cancer Receiving Radial Radiotherapy. 2015. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT02577393/ (accessed on 4 November 2024).
- NCT02891538. A Pilot Study to Evaluate the Chemopreventive Effects of Epigallocatechin Gallate (EGCG) in Colorectal Cancer (CRC) Patients with Curative Resections. 2016. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT02891538/ (accessed on 4 November 2024).
- Pintova, S.; Dharmupari, S.; Moshier, E.; Zubizarreta, N.; Ang, C.; Holcombe, R.F. Genistein combined with FOLFOX or FOLFOX-Bevacizumab for the treatment of metastatic colorectal cancer: Phase I/II pilot study. Cancer Chemother. Pharmacol. 2019, 84, 591–598. [Google Scholar] [CrossRef]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef]
- NCT03288298. Therapeutic Effect of Luteolin Natural Extract Versus Its Nanoparticles on Tongue Squamous Cell Carcinoma Cell Line: In Vitro Study. 2017. Available online: https://cdek.pharmacy.purdue.edu/trial/NCT03288298/ (accessed on 4 November 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casari, G.; Romaldi, B.; Scirè, A.; Minnelli, C.; Marzioni, D.; Ferretti, G.; Armeni, T. Epigenetic Properties of Compounds Contained in Functional Foods Against Cancer. Biomolecules 2025, 15, 15. https://doi.org/10.3390/biom15010015
Casari G, Romaldi B, Scirè A, Minnelli C, Marzioni D, Ferretti G, Armeni T. Epigenetic Properties of Compounds Contained in Functional Foods Against Cancer. Biomolecules. 2025; 15(1):15. https://doi.org/10.3390/biom15010015
Chicago/Turabian StyleCasari, Giulia, Brenda Romaldi, Andrea Scirè, Cristina Minnelli, Daniela Marzioni, Gianna Ferretti, and Tatiana Armeni. 2025. "Epigenetic Properties of Compounds Contained in Functional Foods Against Cancer" Biomolecules 15, no. 1: 15. https://doi.org/10.3390/biom15010015
APA StyleCasari, G., Romaldi, B., Scirè, A., Minnelli, C., Marzioni, D., Ferretti, G., & Armeni, T. (2025). Epigenetic Properties of Compounds Contained in Functional Foods Against Cancer. Biomolecules, 15(1), 15. https://doi.org/10.3390/biom15010015












