Fine Wrinkle Improvement through Bioactive Materials That Modulate EDAR and BNC2 Gene Expression
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Transfection of Keratinocytes and Fibroblasts with siRNA Duplexes for RNAi Experiments
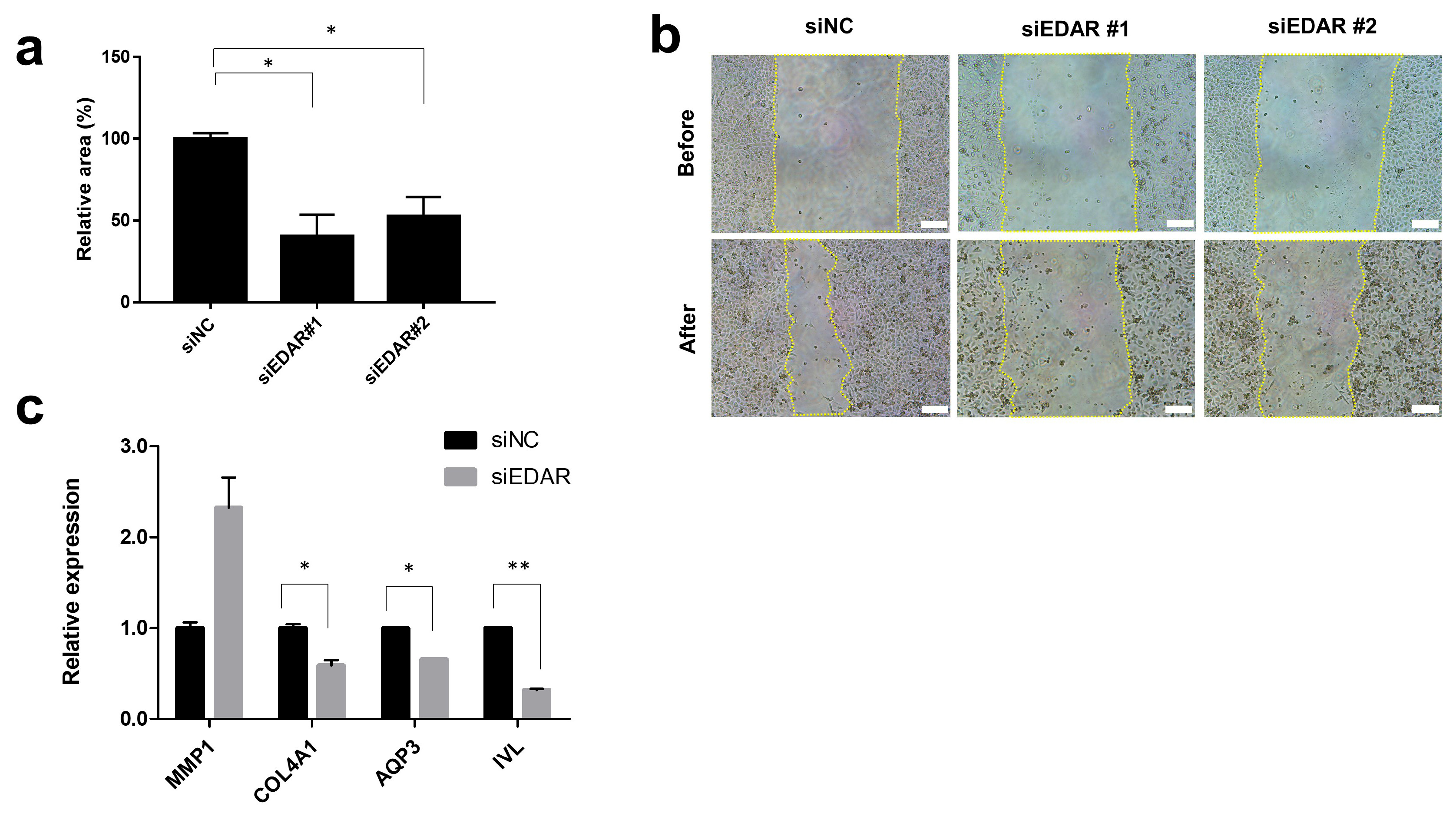
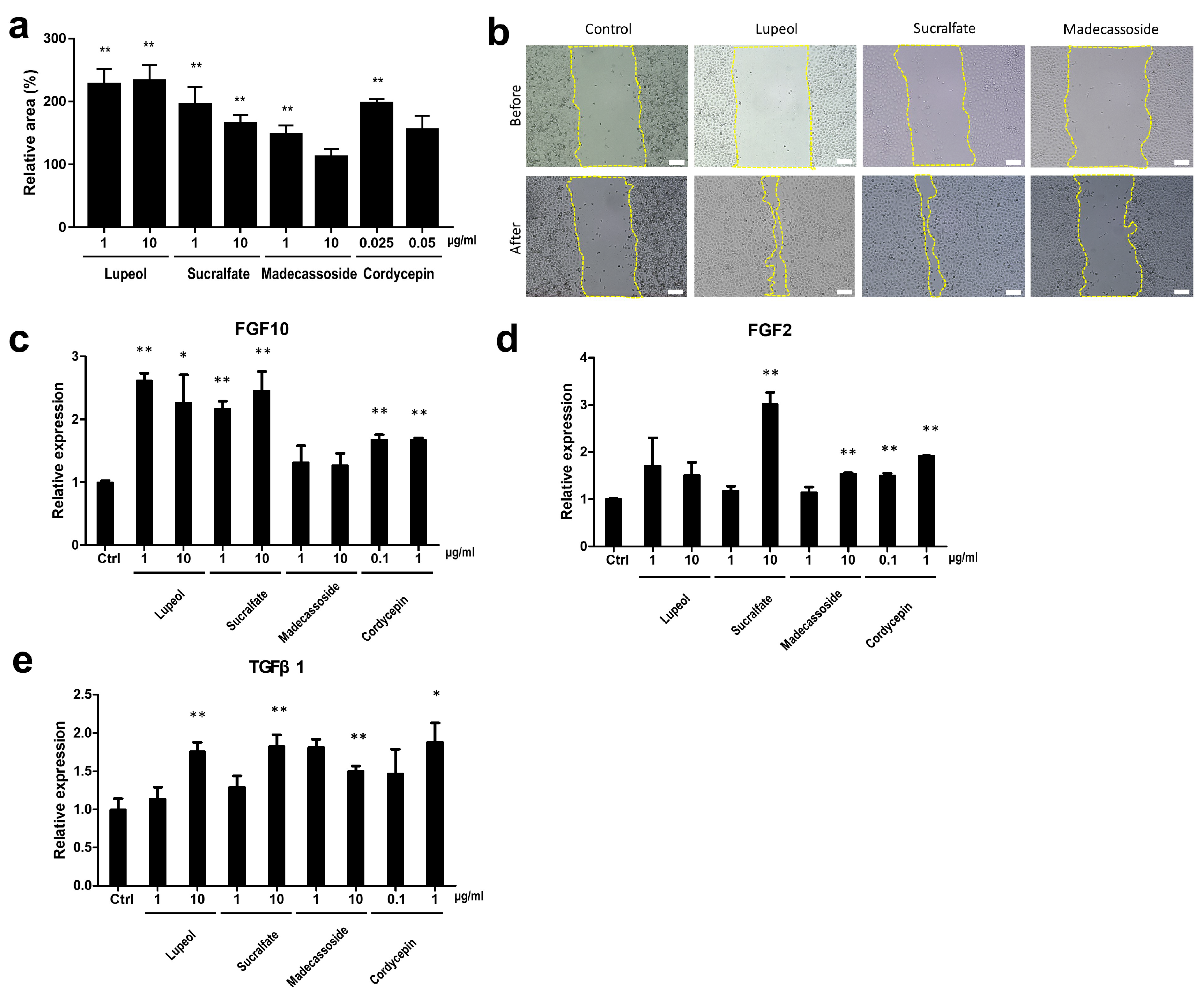
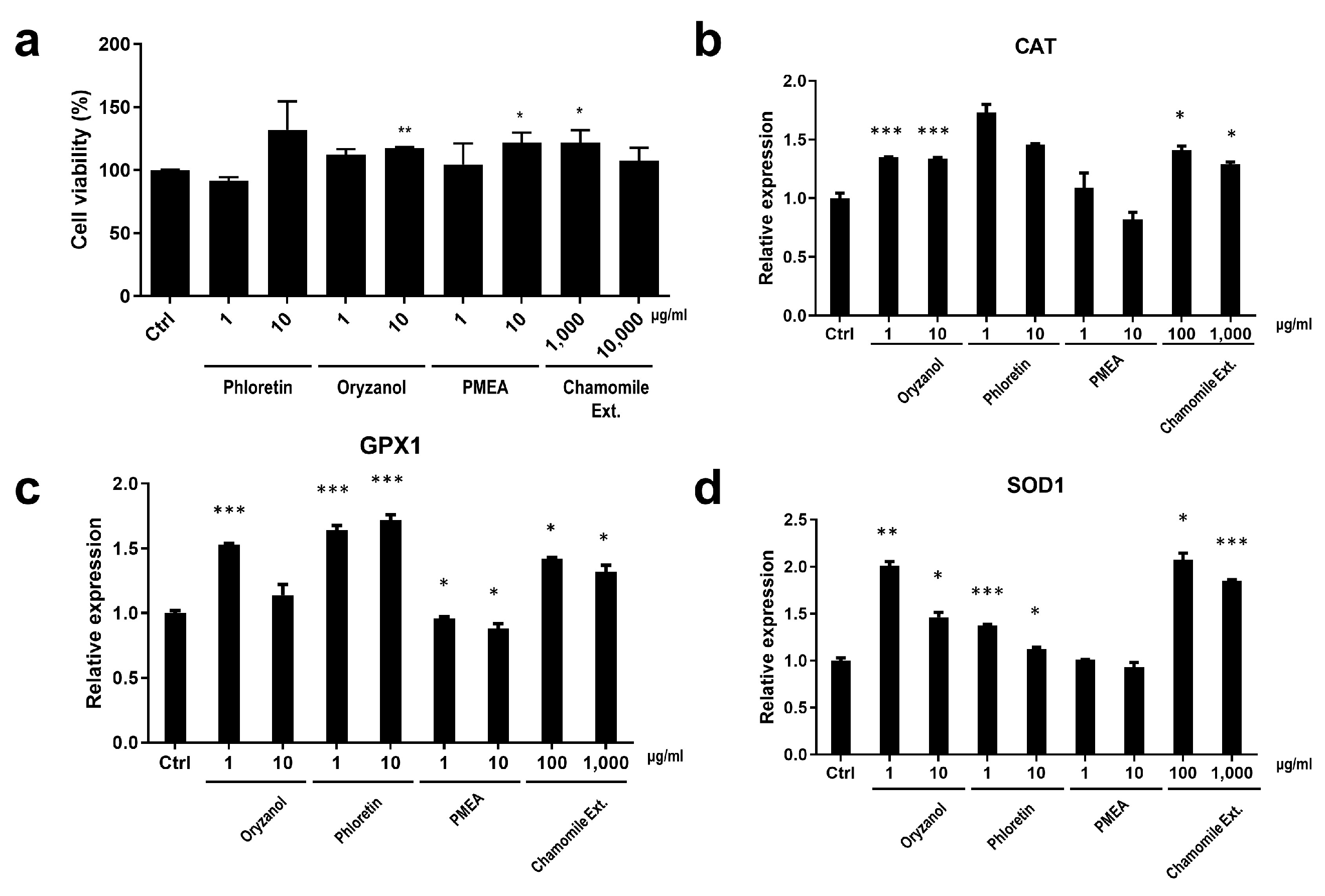
2.3. RT-qPCR
2.4. Cell Migration (Scratch Assay)
2.5. Cell Viability (CCK-8 Assay)
2.6. 3D Skin Experiment
2.7. Human Clinical Trial
2.8. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Functional Study on Periorbital Skin Wrinkle-Related Genes, EDAR and BNC2
3.1.1. Prevention of HaCaT Cell Migration and Wound Healing-Related Gene Expression by EDAR Knockdown
3.1.2. Increase in Cell Survival and Skin Extracellular Matrix-Related Gene Expression by BNC2 Knockdown
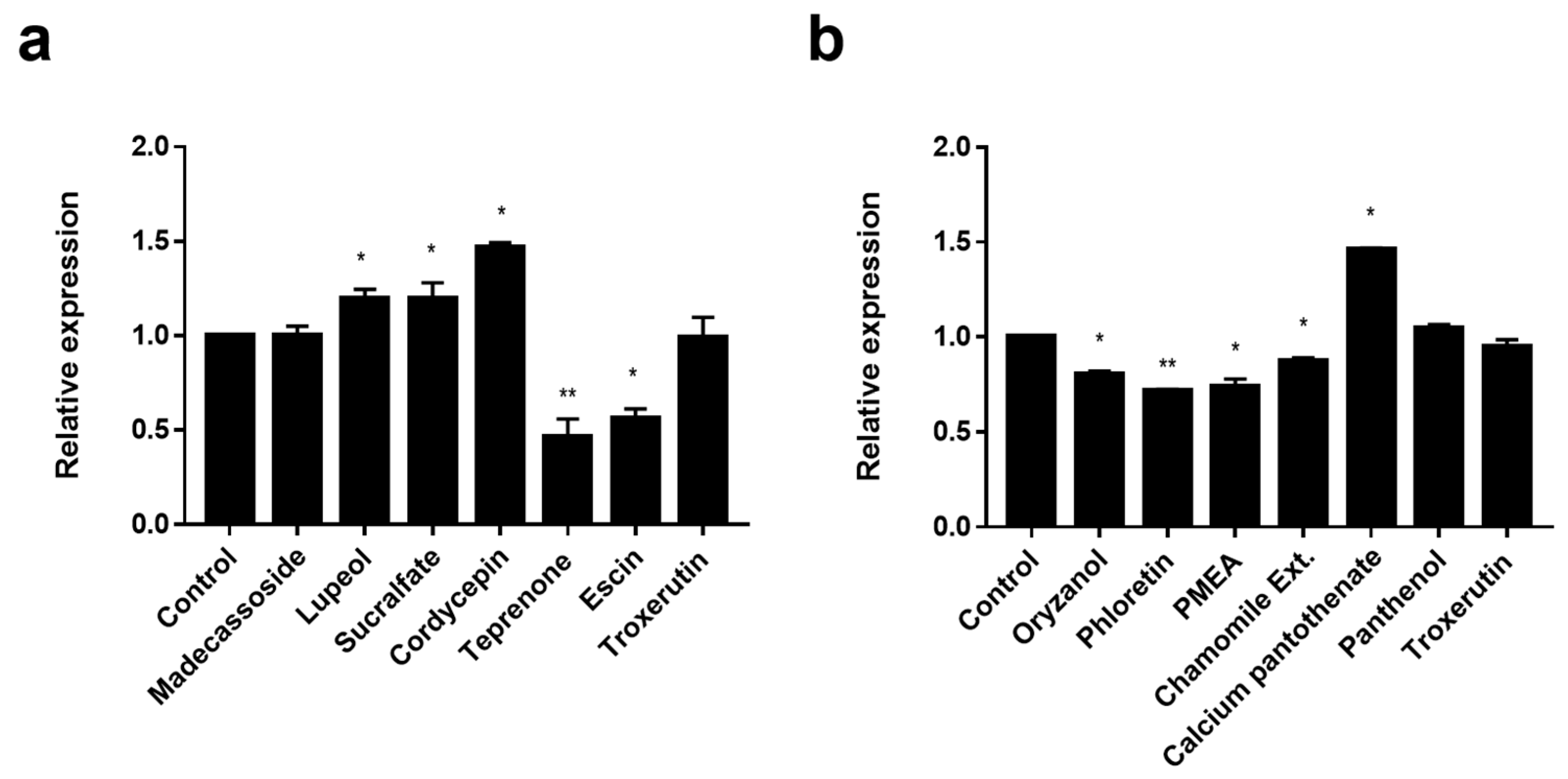
3.2. Screening of EDAR and BNC2 Expression-Regulating Materials
3.3. Wound-Healing and Anti-Oxidant Effect of EDAR and BNC2 Expression-Regulating Materials
3.3.1. Wound-Healing Effect and Enhanced Expression of Wound Healing-Related Genes by EDAR-Upregulating Materials
3.3.2. Cell Protection Effect and the Enhanced Expression of Anti-Oxidant System-Related Genes by BNC2-Downregulating Materials
3.4. Collagen Enhancement and Fine Wrinkle Improvement by an LG Formula Containing EDAR and BNC2 Expression-Regulating Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the Aging Skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef]
- Tupker, R.A.; Pinnagoda, J.; Nater, J.P. The Transient and Cumulative Effect of Sodium Lauryl Sulphate on the Epidermal Barrier Assessed by Transepidermal Water Loss: Inter-Individual Variation. Acta Derm. Venereol. 1990, 70, 1–5. [Google Scholar] [CrossRef]
- El-Domyati, M.; Attia, S.; Saleh, F.; Brown, D.; Birk, D.E.; Gasparro, F.; Ahmad, H.; Uitto, J. Intrinsic Aging vs. Photoaging: A Comparative Histopathological, Immunohistochemical, and Ultrastructural Study of Skin. Exp. Dermatol. 2002, 11, 398–405. [Google Scholar] [CrossRef]
- Sgonc, R.; Gruber, J. Age-Related Aspects of Cutaneous Wound Healing: A Mini-Review. Gerontology 2013, 59, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Landau, M. Exogenous Factors in Skin Aging. In Current Problems in Dermatology; Tur, E., Ed.; S. Karger AG: Basel, Switzerland, 2007; Volume 35, pp. 1–13. ISBN 978-3-8055-8313-8. [Google Scholar]
- Yaar, M.; Eller, M.S.; Gilchrest, B.A. Fifty Years of Skin Aging. J. Investig. Dermatol. Symp. Proc. 2002, 7, 51–58. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and Extrinsic Factors in Skin Ageing: A Review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wurm, E.M.T.; Longo, C.; Curchin, C.; Soyer, H.P.; Prow, T.W.; Pellacani, G. In Vivo Assessment of Chronological Ageing and Photoageing in Forearm Skin Using Reflectance Confocal Microscopy: Assessment of Chronological Ageing and Photoageing Using RCM. Br. J. Dermatol. 2012, 167, 270–279. [Google Scholar] [CrossRef]
- Orioli, D.; Dellambra, E. Epigenetic Regulation of Skin Cells in Natural Aging and Premature Aging Diseases. Cells 2018, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, W.; Koellmann, C.; Le Clerc, S.; Hüls, A.; Li, B.; Peng, Q.; Wu, S.; Ding, A.; Yang, Y.; et al. Genome-Wide Scan Identified Genetic Variants Associated with Skin Aging in a Chinese Female Population. J. Dermatol. Sci. 2019, 96, 42–49. [Google Scholar] [CrossRef]
- Chen, Y.; André, M.; Adhikari, K.; Blin, M.; Bonfante, B.; Mendoza-Revilla, J.; Fuentes-Guajardo, M.; Palmal, S.; Chacón-Duque, J.C.; Hurtado, M.; et al. A Genome-wide Association Study Identifies Novel Gene Associations with Facial Skin Wrinkling and Mole Count in Latin Americans. Br. J. Dermatol. 2021, 185, 988–998. [Google Scholar] [CrossRef]
- Liu, F.; Hamer, M.A.; Deelen, J.; Lall, J.S.; Jacobs, L.; van Heemst, D.; Murray, P.G.; Wollstein, A.; de Craen, A.J.M.; Uh, H.-W.; et al. The MC1R Gene and Youthful Looks. Curr. Biol. 2016, 26, 1213–1220. [Google Scholar] [CrossRef]
- Oh Kim, J.; Park, B.; Yoon Choi, J.; Ra Lee, S.O.; Jin Yu, S.O.; Goh, M.; Lee, H.; Park, W.-S.; Soo Suh, I.N.; Koh, D.-S.; et al. Identifi Cation of the Underlying Genetic Factors of Skin Aging in a Korean Population Study. J. Cosmet. Sci. 2021, 72, 63–80. [Google Scholar]
- Colvan, L.; Fleck, T.; Vega, V.L. Global Periorbital Skin Rejuvenation by a Topical Eye Cream Containing Low Molecular Weight Heparan Sulfate (LMW-HS) and a Blend of Naturally Derived Extracts. J. Cosmet. Dermatol. 2019, 18, 530–538. [Google Scholar] [CrossRef]
- Takema, Y.; Tsukahara, K.; Fujimura, T.; Hattori, M. Age-Related Changes in the Three-Dimensional Morphological Structure of Human Facial Skin. Ski. Res. Technol. 1997, 3, 95–100. [Google Scholar] [CrossRef]
- Alexis, A.F.; Grimes, P.; Boyd, C.; Downie, J.; Drinkwater, A.; Garcia, J.K.; Gallagher, C.J. Racial and Ethnic Differences in Self-Assessed Facial Aging in Women: Results From a Multinational Study. Dermatol. Surg. 2019, 45, 1635–1648. [Google Scholar] [CrossRef]
- Merinville, E.; Messaraa, C.; O’Connor, C.; Grennan, G.; Mavon, A. What Makes Indian Women Look Older—An Exploratory Study on Facial Skin Features. Cosmetics 2018, 5, 3. [Google Scholar] [CrossRef]
- Rossi, A.M.; Eviatar, J.; Green, J.B.; Anolik, R.; Eidelman, M.; Keaney, T.C.; Narurkar, V.; Jones, D.; Kolodziejczyk, J.; Drinkwater, A.; et al. Signs of Facial Aging in Men in a Diverse, Multinational Study: Timing and Preventive Behaviors. Dermatol. Surg. 2017, 43, S210–S220. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.A.C.; Cox, S.E.; Jones, D.; Lei, X.; Gallagher, C.J. Heterogeneity of Crow’s Feet Line Patterns in Clinical Trial Subjects. Dermatol. Surg. 2015, 41, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Shin, J.-G.; Kim, Y.; Leem, S.; Park, S.G.; Won, H.-H.; Kang, N.G. Identification of Genetic Loci Associated with Facial Wrinkles in a Large Korean Population. J. Investig. Dermatol. 2022, 142, 2824–2827. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.; Wu, P.; Zhang, W.; Zheng, Y.; Li, Q.; Jin, R.; Chen, H.; You, C.; Guo, S.; Han, C.; et al. Regeneration of Skin Appendages and Nerves: Current Status and Further Challenges. J. Transl. Med. 2020, 18, 53. [Google Scholar] [CrossRef] [PubMed]
- Ambrozova, N.; Ulrichova, J.; Galandakova, A. Models for the Study of Skin Wound Healing. The Role of Nrf2 and NF-κB. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2017, 161, 1–13. [Google Scholar] [CrossRef]
- Garcin, C.L.; Huttner, K.M.; Kirby, N.; Schneider, P.; Hardman, M.J. Ectodysplasin A Pathway Contributes to Human and Murine Skin Repair. J. Investig. Dermatol. 2016, 136, 1022–1030. [Google Scholar] [CrossRef]
- Kim, M.J.; Won, K.J.; Kim, D.Y.; Won, Y.R.; Kim, N.Y.; Lee, D.K.; Hong, B.S.; Lee, H.M. Skin Wound Healing and Anti-Wrinkle-Promoting In Vitro Biological Activities of Caragana sinica Flower Absolute and Its Chemical Composition. Pharmaceuticals 2023, 16, 235. [Google Scholar] [CrossRef]
- Mahendra, C.K.; Tan, L.T.H.; Mahendra, C.K.; Ser, H.-L.; Pusparajah, P.; Htar, T.T.; Chuah, L.-H.; Yap, W.H.; Tang, S.Y.; Ming, L.C.; et al. The Potential of Sky Fruit as an Anti-Aging and Wound Healing Cosmeceutical Agent. Cosmetics 2021, 8, 79. [Google Scholar] [CrossRef]
- Romano, R.-A.; Li, H.; Tummala, R.; Maul, R.; Sinha, S. Identification of Basonuclin2, a DNA-Binding Zinc-Finger Protein Expressed in Germ Tissues and Skin Keratinocytes. Genomics 2004, 83, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Liu, Y.; Lu, F.; Chen, X. Decreased Expression of BNC1 and BNC2 Is Associated with Genetic or Epigenetic Regulation in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2016, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 26, 757–772. [Google Scholar] [CrossRef]
- JSander, C.S.; Chang, H.; Salzmann, S.; Muller, C.S.; Ekanayake-Mudiyanselage, S.; Elsner, P.; Thiele, J.J. Photoaging is associated with protein oxidation in human skin in vivo. J. Investig. Dermatol. 2002, 118, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Palstra, R.-J.; Kayser, M. Human Skin Color Is Influenced by an Intergenic DNA Polymorphism Regulating Transcription of the Nearby BNC2 Pigmentation Gene. Hum. Mol. Genet. 2014, 23, 5750–5762. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Functional and Physiological Characteristics of the Aging Skin. Aging Clin. Exp. Res. 2008, 20, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT Cells as a Reliable In Vitro Differentiation Model to Dissect the Inflammatory/Repair Response of Human Keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Matsuura-Hachiya, Y.; Arai, K.Y.; Muraguchi, T.; Sasaki, T.; Nishiyama, T. Type IV Collagen Aggregates Promote Keratinocyte Proliferation and Formation of Epidermal Layer in Human Skin Equivalents. Exp. Dermatol. 2018, 27, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B.; Aitkens, L.; White, J.; Hyndman, K.A. Aquaporin-3 in the Epidermis: More than Skin Deep. Am. J. Physiol.-Cell Physiol. 2020, 318, C1144–C1153. [Google Scholar] [CrossRef]
- Boury-Jamot, M.; Daraspe, J.; Bonte, F.; Perrier, E.; Schnebert, S.; Dumas, M.; Verbavatz, J.M. Skin aquaporins: Function in hydration, wound healing, and skin epidermis homeostasis. Handb. Exp. Pharmacol. 2009, 190, 205–217. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, Z.; Li, D.; Wang, L.; Chen, Y.; Liang, Y.; Jiao, W.; Niu, H. Development of a CAFs-Related Gene Signature to Predict Survival and Drug Response in Bladder Cancer. Hum. Cell 2022, 35, 649–664. [Google Scholar] [CrossRef]
- Van Deursen, J.M. The Role of Senescent Cells in Ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from Plants Protect against Skin Photoaging. Oxidative Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging Hallmarks and the Role of Oxidative Stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of Skin Collagen Metabolism in Aged and Photoaged Human Skin In Vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef]
- Namazi, M.R.; Fallahzadeh, M.K.; Schwartz, R.A. Strategies for Prevention of Scars: What Can We Learn from Fetal Skin? Strategies for Prevention of Scars. Int. J. Dermatol. 2011, 50, 85–93. [Google Scholar] [CrossRef]
- Malaisse, J.; Bourguignon, V.; De Vuyst, E.; Lambert De Rouvroit, C.; Nikkels, A.F.; Flamion, B.; Poumay, Y. Hyaluronan Metabolism in Human Keratinocytes and Atopic Dermatitis Skin Is Driven by a Balance of Hyaluronan Synthases 1 and 3. J. Investig. Dermatol. 2014, 134, 2174–2182. [Google Scholar] [CrossRef]
- Tsutsui, K.; Machida, H.; Nakagawa, A.; Ahn, K.; Morita, R.; Sekiguchi, K.; Miner, J.H.; Fujiwara, H. Mapping the Molecular and Structural Specialization of the Skin Basement Membrane for Inter-Tissue Interactions. Nat. Commun. 2021, 12, 2577. [Google Scholar] [CrossRef]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Brzezińska, M. Centella Asiatica in Cosmetology. Postępy Dermatol. I Alergol. 2013, 1, 46–49. [Google Scholar] [CrossRef]
- Lee, J.Y.; Min, D.-J.; Kim, W.; Bin, B.-H.; Kim, K.; Cho, E.-G. Non Pharmacological High-Intensity Ultrasound Treatment of Human Dermal Fibroblasts to Accelerate Wound Healing. Sci. Rep. 2021, 11, 2465. [Google Scholar] [CrossRef]
- Fisher, G.; Rittié, L. Restoration of the Basement Membrane after Wounding: A Hallmark of Young Human Skin Altered with Aging. J. Cell Commun. Signal 2018, 12, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yamanishi, K.; Mori, O.; Kamikawa, M.; Andersen, B.; Kato, S.; Toyoda, T.; Yamada, G. Defective Terminal Differentiation and Hypoplasia of the Epidermis in Mice Lacking the Fgf10 Gene. FEBS Lett. 2000, 481, 53–56. [Google Scholar] [CrossRef]
- Liu, M.; Dai, Y.; Li, Y.; Luo, Y.; Huang, F.; Gong, Z.; Meng, Q. Madecassoside Isolated from Centella Asiatica Herbs Facilitates Burn Wound Healing in Mice. Planta Med. 2008, 74, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Bian, D.; Liu, M.; Li, Y.; Xia, Y.; Gong, Z.; Dai, Y. Madecassoside, a Triterpenoid Saponin Isolated from Centella Asiatica Herbs, Protects Endothelial Cells against Oxidative Stress. J. Biochem. Mol. Toxicol. 2012, 26, 399–406. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant Responses and Cellular Adjustments to Oxidative Stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione Peroxidases. Biochim. Et. Biophys. Acta (BBA)–Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Mödinger, Y.; Schön, C.; Vogel, K.; Brandt, M.; Bielfeldt, S.; Wilhelm, K.P. Evaluation of a Food Supplement with Collagen Hydrolysate and Micronutrients on Skin Appearance and Beauty Effects: A Randomized, Double-Blind, PlaceboControlled Clinical Study with Healthy Subjects. J. Clin. Cosmet. Dermatol. 2021, 5, 1–5. [Google Scholar] [CrossRef]


| Active Ingredients | Concentration |
|---|---|
| Oryzanol | 10 μg/mL |
| Phloretin | 10 μg/mL |
| Cordycepin | 0.1 μg/mL |
| Lupeol | 10 μg/mL |
| Teprenone | 10 μg/mL |
| Escin | 10 μg/mL |
| Sucralfate | 10 μg/mL |
| Madecassoside | 10 μg/mL |
| Palmitamide MEA | 10 μg/mL |
| Chamomile extract | 1000 μg/mL |
| Calcium pantothenate | 0.01 μg/mL |
| Panthenol | 1 μg/mL |
| Troxerutin | 1 μg/mL |
| Retinol | 10 μM |
| Matrixyl-3000 | 1000 μg/mL |
| Peptilium | 0.1 μg/mL |
| Polydatin | 0.5 μg/mL |
| Peptilium | 0.1 μg/mL |
| Bakuchiol | 20 μM |
| Panax ginseng root protoplasts | 100 μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Ye, S.; Kim, M.; Lee, H.; Jun, S.-H.; Kang, N.-G. Fine Wrinkle Improvement through Bioactive Materials That Modulate EDAR and BNC2 Gene Expression. Biomolecules 2024, 14, 279. https://doi.org/10.3390/biom14030279
Lee S, Ye S, Kim M, Lee H, Jun S-H, Kang N-G. Fine Wrinkle Improvement through Bioactive Materials That Modulate EDAR and BNC2 Gene Expression. Biomolecules. 2024; 14(3):279. https://doi.org/10.3390/biom14030279
Chicago/Turabian StyleLee, Seonju, Sanghyun Ye, Mina Kim, Hyejin Lee, Seung-Hyun Jun, and Nae-Gyu Kang. 2024. "Fine Wrinkle Improvement through Bioactive Materials That Modulate EDAR and BNC2 Gene Expression" Biomolecules 14, no. 3: 279. https://doi.org/10.3390/biom14030279
APA StyleLee, S., Ye, S., Kim, M., Lee, H., Jun, S.-H., & Kang, N.-G. (2024). Fine Wrinkle Improvement through Bioactive Materials That Modulate EDAR and BNC2 Gene Expression. Biomolecules, 14(3), 279. https://doi.org/10.3390/biom14030279





