Comparative Analysis of Dehydrins from Woody Plant Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Dehydrin Sequences and Motif Search
2.2. Calculation of Biochemical Properties
2.3. Phylogenetic Tree Construction
2.4. Obtaining of Promoter Sequences and Analyses of Cis Regulatory Sequences
3. Results
3.1. Presence of K, Y, S, and F Segments in Dehydrins
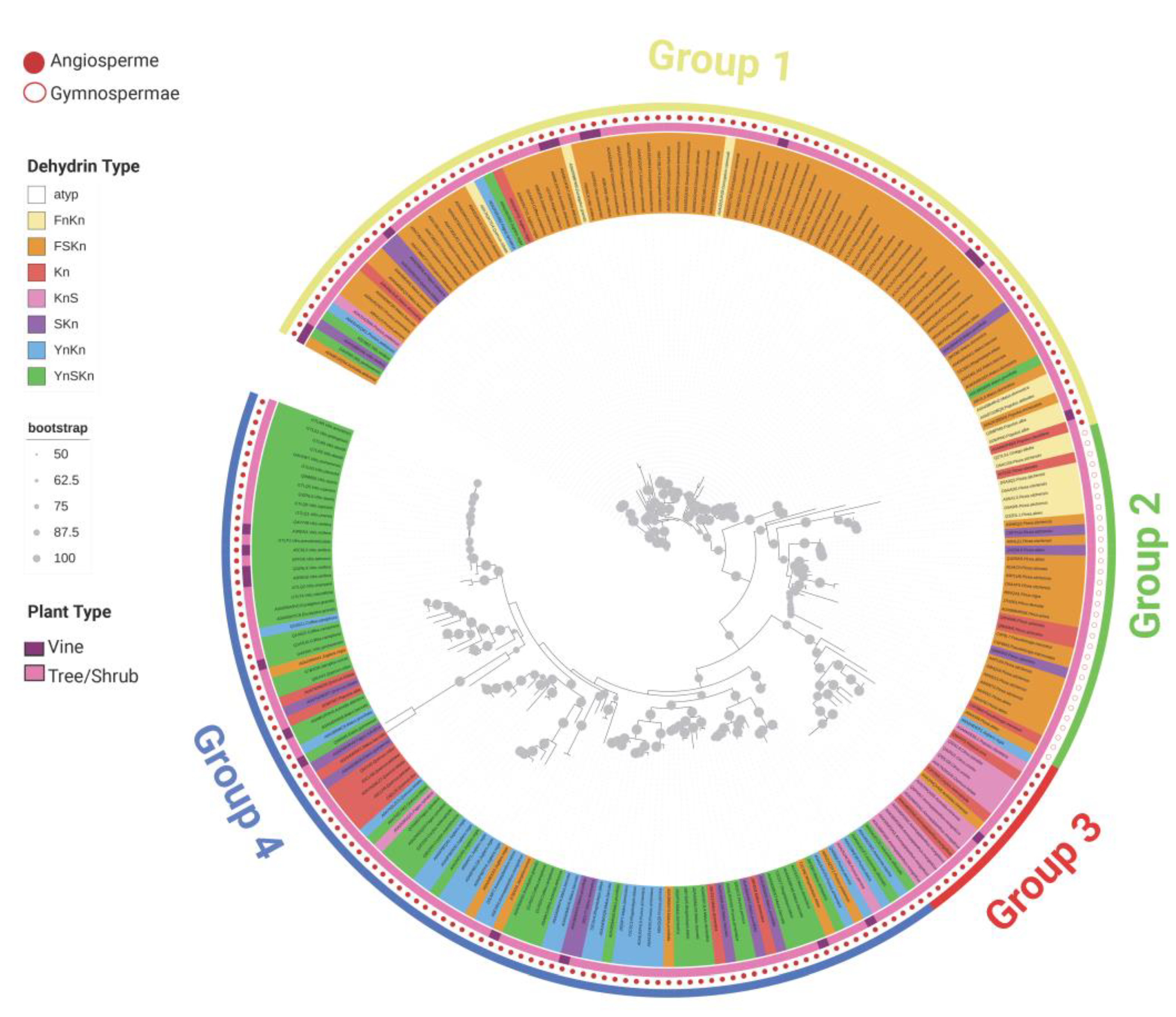
3.2. Analysis of Architecture, Biochemical Characteristics, and Protective Properties of Dehydrins in Woody Plants
3.3. Regulation of Dehydrins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amara, I.; Zaidi, I.; Masmoudi, K.; Ludevid, M.D.; Pages, M.; Goday, A.; Brini, F. Insights into Late Embryogenesis Abundant (LEA) Proteins in Plants: From Structure to the Functions. Am. J. Plant Sci. 2014, 5, 3440–3455. [Google Scholar] [CrossRef]
- Hernandez-Sanchez, I.E.; Lopez, I.M.; Martinez-Martinez, C.; Janis, B.; Jimenez-Bremont, J.F.; Covarrubias, A.A.; Menze, M.A.; Graether, S.P.; Thalhammer, A. LEAfing through literature: Late embryogenesis abundant proteins coming of age-achievements and perspectives. J. Exp. Bot. 2022, 73, 6525–6546. [Google Scholar] [CrossRef]
- Sun, Z.P.; Li, S.Y.; Chen, W.Y.; Zhang, J.Q.; Zhang, L.X.; Sun, W.; Wang, Z.L. Plant Dehydrins: Expression, Regulatory Networks, and Protective Roles in Plants Challenged by Abiotic Stress. Int. J. Mol. Sci. 2021, 22, 12619. [Google Scholar] [CrossRef]
- Graether, S.P.; Boddington, K.F. Disorder and function: A review of the dehydrin protein family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef]
- Smith, M.A.; Graether, S.P. The Disordered Dehydrin and Its Role in Plant Protection: A Biochemical Perspective. Biomolecules 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.A.; Veltri, M.; Boddington, K.F.; Singh, K.K.; Graether, S.P. Genome Analysis of Conserved Dehydrin Motifs in Vascular Plants. Front. Plant Sci. 2017, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Close, T.J. Dehydrins: Emergence of a biochemical role of a family of plant dehydration proteins. Physiol. Plant. 1996, 97, 795–803. [Google Scholar] [CrossRef]
- Hara, M. The multifunctionality of dehydrins: An overview. Plant Signal. Behav. 2010, 5, 503–508. [Google Scholar] [CrossRef]
- Alsheikh, M.K.; Heyen, B.J.; Randall, S.K. Ion binding properties of the dehydrin ERD14 are dependent upon phosphorylation. J. Biol. Chem. 2003, 278, 40882–40889. [Google Scholar] [CrossRef] [PubMed]
- Goday, A.; Jensen, A.B.; Culianezmacia, F.A.; Alba, M.M.; Figueras, M.; Serratosa, J.; Torrent, M.; Pages, M. The maize abscisic acid-responsive protein Rab17 is located in the nucleus and interacts with nuclear-localization signals. Plant Cell 1994, 6, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Strimbeck, G.R. Hiding in plain sight: The F segment and other conserved features of seed plant SKn dehydrins. Planta 2017, 245, 1061–1066. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: A commonality in the response of plants to dehydration and low temperature. Physiol. Plant. 1997, 100, 291–296. [Google Scholar] [CrossRef]
- Kosova, K.; Vitamvas, P.; Prasil, I.T. The role of dehydrins in plant response to cold. Biol. Plant. 2007, 51, 601–617. [Google Scholar] [CrossRef]
- Berry, Z.C.; Avila-Lovera, E.; De Guzman, M.E.; O’Keefe, K.; Emery, N.C. Beneath the Bark: Assessing Woody Stem Water and Carbon Fluxes and Its Prevalence Across Climates and the Woody Plant Phylogeny. Front. For. Glob. Chang. 2021, 4, 675299. [Google Scholar] [CrossRef]
- Azarkovich, M.I. Dehydrins in Orthodox and Recalcitrant Seeds. Russ. J. Plant Physiol. 2020, 67, 221–230. [Google Scholar] [CrossRef]
- Schneider, T.D.; Stephens, R.M. Sequence logos—A new way to display consensus sequences. Nucleic Acids Res. 1990, 18, 6097–6100. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. QuanTest2: Benchmarking multiple sequence alignments using secondary structure prediction. Bioinformatics 2020, 36, 90–95. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Svensson, J.; Ismail, A.M.; Palva, E.T.; Close, T.J. Dehydrins. In Cell and Molecular Response to Stress; Storey, K.B., Storey, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 3, pp. 155–171. [Google Scholar] [CrossRef]
- Xiao, H.G.; Nassuth, A. Stress- and development-induced expression of spliced and unspliced transcripts from two highly similar dehydrin 1 genes in V-riparia and V-vinifera. Plant Cell Rep. 2006, 25, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.W.; Wang, Y.; Yu, T.Q.; Chen, S.L.; Chen, Y.Z.; Lu, C.F. Heterologous Expression of Three Ammopiptanthus mongolicus Dehydrin Genes Confers Abiotic Stress Tolerance in Arabidopsis thaliana. Plants 2020, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.K.; Lee, H.; Lee, J.S.; Noh, E.W. Differential expression of a poplar SK2-type dehydrin gene in response to various stresses. BMB Rep. 2009, 42, 439–443. [Google Scholar] [CrossRef][Green Version]
- Zhang, D.; Yang, T.C.; Ren, L. Y2SK2- and SK3-type dehydrins from Agapanthus praecox act as protectants to improve plant cell viability during cryopreservation. Plant Cell Tissue Organ Cult. 2021, 144, 271–279. [Google Scholar] [CrossRef]
- Hara, M.; Endo, T.; Kamiya, K.; Kameyama, A. The role of hydrophobic amino acids of K-segments in the cryoprotection of lactate dehydrogenase by dehydrins. J. Plant Physiol. 2017, 210, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Ohkubo, T.; Kamiya, K.; Hara, M. Cryoprotective activity of Arabidopsis KS-type dehydrin depends on the hydrophobic amino acids of two active segments. Arch. Biochem. Biophys. 2020, 691, 108510. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.R.; Graether, S.P. Physiological, Structural, and Functional Insights Into the Cryoprotection of Membranes by the Dehydrins. Front. Plant Sci. 2022, 13, 886525. [Google Scholar] [CrossRef]
- Smith, M.A.; Graether, S.P. The Effect of Positive Charge Distribution on the Cryoprotective Activity of Dehydrins. Biomolecules 2022, 12, 1510. [Google Scholar] [CrossRef]
- Eriksson, S.K.; Kutzer, M.; Procek, J.; Grobner, G.; Harryson, P. Tunable Membrane Binding of the Intrinsically Disordered Dehydrin Lti30, a Cold-Induced Plant Stress Protein. Plant Cell 2011, 23, 2391–2404. [Google Scholar] [CrossRef]
- Koag, M.C.; Wilkens, S.; Fenton, R.D.; Resnik, J.; Vo, E.; Close, T.J. The K-Segment of Maize DHN1 Mediates Binding to Anionic Phospholipid Vesicles and Concomitant Structural Changes. Plant Physiol. 2009, 150, 1503–1514. [Google Scholar] [CrossRef]
- Maszkowska, J.; Debski, J.; Kulik, A.; Kistowski, M.; Bucholc, M.; Lichocka, M.; Klimecka, M.; Sztatelman, O.; Szymanska, K.P.; Dadlez, M.; et al. Phosphoproteomic analysis reveals that dehydrins ERD10 and ERD14 are phosphorylated by SNF1-related protein kinase 2.10 in response to osmotic stress. Plant Cell Environ. 2019, 42, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Shinoda, Y.; Tanaka, Y.; Kuboi, T. DNA binding of citrus dehydrin promoted by zinc ion. Plant Cell Environ. 2009, 32, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Boddington, K.F.; Graether, S.P. Binding of a Vitis riparia dehydrin to DNA. Plant Sci. 2019, 287, 110172. [Google Scholar] [CrossRef]
- Hara, M.; Terashima, S.; Fukaya, T.; Kuboi, T. Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 2003, 217, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Fujinaga, M.; Kuboi, T. Radical scavenging activity and oxidative modification of citrus dehydrin. Plant Physiol. Biochem. 2004, 42, 657–662. [Google Scholar] [CrossRef]
- Realini, C.; Rogers, S.W.; Rechsteiner, M. KEKE motifs: Proposed roles in protein-protein association and presentation of peptides by MHC Class-I receptors. FEBS Lett. 1994, 348, 109–113. [Google Scholar] [CrossRef]
- Ohkubo, T.; Kameyama, A.; Kamiya, K.; Kondo, M.; Hara, M. F-segments of Arabidopsis dehydrins show cryoprotective activities for lactate dehydrogenase depending on the hydrophobic residues. Phytochemistry 2020, 173, 112300. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Radwan, A.; Hara, M.; Selmar, D. Dehydrin expression in seeds: An issue of maturation drying. Front. Plant Sci. 2014, 5, 402. [Google Scholar] [CrossRef][Green Version]
- Sunderlikova, V.; Salaj, J.; Kopecky, D.; Salaj, T.; Wilhem, E.; Matusikova, I. Dehydrin genes and their expression in recalcitrant oak (Quercus robur) embryos. Plant Cell Rep. 2009, 28, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Pacini, E.; Dolferus, R. Pollen developmental arrest: Maintaining pollen fertility in a world with a changing climate. Front. Plant Sci. 2019, 10, 679. [Google Scholar] [CrossRef]
- Firon, N.; Nepi, M.; Pacini, E. Water status and associated processes mark critical stages in pollen development and functioning. Ann. Bot. 2012, 109, 1201–1214. [Google Scholar] [CrossRef]
- Liang, D.; Xia, H.; Wu, S.; Ma, F.W. Genome-wide identification and expression profiling of dehydrin gene family in Malus domestica. Mol. Biol. Rep. 2012, 39, 10759–10768. [Google Scholar] [CrossRef]
- Zolotarov, Y.; Stromvik, M. De Novo Regulatory Motif Discovery Identifies Significant Motifs in Promoters of Five Classes of Plant Dehydrin Genes. PLoS ONE 2015, 10, e0129016. [Google Scholar] [CrossRef]
- Jyothi-Prakash, P.A.; Mohanty, B.; Wijaya, E.; Lim, T.M.; Lin, Q.S.; Loh, C.S.; Kumar, P.P. Identification of salt gland-associated genes and characterization of a dehydrin from the salt secretor mangrove Avicennia officinalis. BMC Plant Biol. 2014, 14, 291. [Google Scholar] [CrossRef]
- Sadder, M.T.; Al-Doss, A.A. Characterization of dehydrin AhDHN from Mediterranean saltbush (Atriplex halimus). Turk. J. Biol. 2014, 38, 469–477. [Google Scholar] [CrossRef]
- Porat, R.; Pasentsis, K.; Rozentzvieg, D.; Gerasopoulos, D.; Falara, V.; Samach, A.; Lurie, S.; Kanellis, A.K. Isolation of a dehydrin cDNA from orange and grapefruit citrus fruit that is specifically induced by the combination of heat followed by chilling temperatures. Physiol. Plant. 2004, 120, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Porat, R.; Pavoncello, D.; Lurie, S.; McCollum, T.G. Identification of a grapefruit cDNA belonging to a unique class of citrus dehydrins and characterization of its expression patterns under temperature stress conditions. Physiol. Plant. 2002, 115, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, E.M.; Pukacka, S. Changes in late embryogenesis abundant proteins and a small heat shock protein during storage of beech (Fagus sylvatica L.) seeds. Environ. Exp. Bot. 2008, 63, 274–280. [Google Scholar] [CrossRef]
- Deng, Z.X.; Wang, Y.D.; Jiang, K.J.; Liu, X.F.; Wu, W.S.; Gao, S.; Lin, J.; Sun, X.F.; Tang, K.X. Molecular cloning and characterization of a novel dehydrin gene from Ginkgo biloba. Biosci. Rep. 2006, 26, 203–215. [Google Scholar] [CrossRef]
- Omar, S.A.; Elsheery, N.I.; Kalaji, H.M.; Ebrahim, M.K.H.; Pietkiewicz, S.; Lee, C.H.; Allakhverdiev, S.I.; Xu, Z.F. Identification and differential expression of two dehydrin cDNAs during maturation of Jatropha curcas seeds. Biochem. Mosc. 2013, 78, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Kjellsen, T.D.; Yakovlev, I.A.; Fossdal, C.G.; Strimbeck, G.R. Dehydrin accumulation and extreme low-temperature tolerance in Siberian spruce (Picea obovata). Tree Physiol. 2013, 33, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Yang, Y.; Xie, L.; Li, X.Y.; Feng, C.; Chen, J.W.; Xu, C.J. Involvement of Multiple Types of Dehydrins in the Freezing Response in Loquat (Eriobotrya japonica). PLoS ONE 2014, 9, e87575. [Google Scholar] [CrossRef] [PubMed]
- Vornam, B.; Gailing, O.; Derory, J.; Plomion, C.; Kremer, A.; Finkeldey, R. Characterisation and natural variation of a dehydrin gene in Quercus petraea (Matt.) Liebl. Plant Biol. 2011, 13, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Caballero, C.; Rosales, R.; Romero, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Unraveling the roles of CBF1, CBF4 and dehydrin 1 genes in the response of table grapes to high CO2 levels and low temperature. J. Plant Physiol. 2012, 169, 744–748. [Google Scholar] [CrossRef]
- Yang, Y.Z.; He, M.Y.; Zhu, Z.G.; Li, S.X.; Xu, Y.; Zhang, C.H.; Singer, S.D.; Wang, Y.J. Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biol. 2012, 12, 140. [Google Scholar] [CrossRef]
- Wisniewski, M.; Webb, R.; Balsamo, R.; Close, T.J.; Yu, X.-M.; Criffith, M. Purification. Immunolocalization, cryoprotective, and antifreeze activity of PCA60: A dehydrin from peach (Prunus persica). Physiol. Plant. 1999, 105, 600–608. [Google Scholar] [CrossRef]
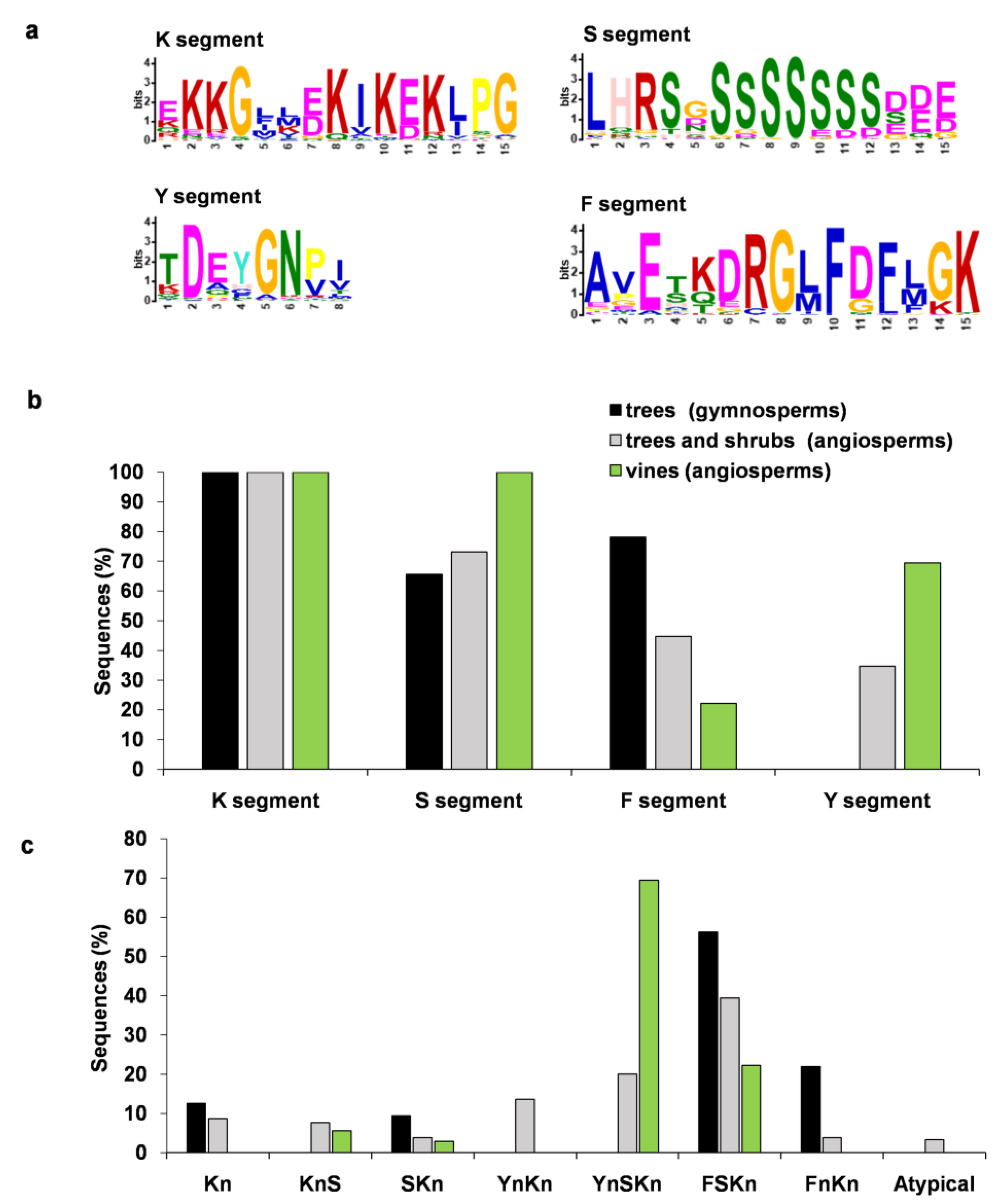


| Kn | KnS | SKn | YnKn | YnSKn | FnKn | FSKn | Atypical | |
|---|---|---|---|---|---|---|---|---|
| Motif | Ang(T,S/V) //Gym(T) [%] | Ang(T,S/V) //Gym(T) [%] | Ang(T,S/V) //Gym(T) [%] | Ang(T,S/V) //Gym(T) [%] | Ang(T,S/V) //Gym(T) [%] | Ang(T,S/V) //Gym(T) [%] | Ang(T,S/V) //Gym(T) [%] | Ang(T,S/V) //Gym(T) [%] |
| HH-K- seg-HH | - | - | - | 8.0/0//0 | 1.6/3.3//0 | - | - | - |
| Poly K motif | - | 31.3/0//0 | - | - | - | - | 36.0/0//0 | - |
| His-rich motifs: | ||||||||
| H-H a | - | 18.0/0//0 | 36.4/0//0 | 12.0/0//0 | 3.3/0//0 | - | 9.0/1.0//0 | 1.7/0//0 |
| HH-X3-HH b | - | 1.7/0//0 | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karas, M.; Vešelényiová, D.; Boszorádová, E.; Nemeček, P.; Gerši, Z.; Moravčíková, J. Comparative Analysis of Dehydrins from Woody Plant Species. Biomolecules 2024, 14, 250. https://doi.org/10.3390/biom14030250
Karas M, Vešelényiová D, Boszorádová E, Nemeček P, Gerši Z, Moravčíková J. Comparative Analysis of Dehydrins from Woody Plant Species. Biomolecules. 2024; 14(3):250. https://doi.org/10.3390/biom14030250
Chicago/Turabian StyleKaras, Milan, Dominika Vešelényiová, Eva Boszorádová, Peter Nemeček, Zuzana Gerši, and Jana Moravčíková. 2024. "Comparative Analysis of Dehydrins from Woody Plant Species" Biomolecules 14, no. 3: 250. https://doi.org/10.3390/biom14030250
APA StyleKaras, M., Vešelényiová, D., Boszorádová, E., Nemeček, P., Gerši, Z., & Moravčíková, J. (2024). Comparative Analysis of Dehydrins from Woody Plant Species. Biomolecules, 14(3), 250. https://doi.org/10.3390/biom14030250


_Kwok.png)



