Abstract
Concerns exist regarding the effects of 5-alpha reductase inhibitors (5-ARIs) on multipa-rametric magnetic resonance imaging (mpMRI) and clinically significant prostate cancer (csPCa) detection. Our objective is to analyze the effect of 5-ARI on the prostate imaging–reporting and data system (PI-RADS) distribution and csPCa and insignificant PCa (iPCa) detection. Among 2212 men with serum prostate-specific antigen levels of >3.0 ng/mL and/or suspicious digital rectal examinations who underwent mpMRI and targeted and/or systematic biopsies, 120 individuals exposed to 5-ARI treatment for over a year were identified. CsPCa was defined when the grade group (GG) was >2. The overall csPCa and iPCa detection rates were 44.6% and 18.8%, respectively. Since logistic regression revealed independent predictors of PCa, a randomized matched group of 236 individuals was selected for analysis. The PI-RADS distribution was comparable with 5-ARI exposure (p 0.685). The CsPCa detection rates in 5-ARI-naïve men and 5-ARI-exposed men were 52.6% and 47.4%, respectively (p 0.596). IPCa was detected in 37.6 and 62.5%, respectively (p 0.089). The tumor GG distribution based on 5-ARI exposure was similar (p 0.149) to the rates of csPCa and iPCa across the PI-RADS categories. We conclude that exposure to 5-ARI in suspected PCa men did not change the PI-RADS distribution and the csPCa and iPCa detection rates.
1. Introduction
The early detection of prostate cancer (PCa) has evolved toward clinically significant PCa (csPCa) [1]. This paradigm shift has resulted from the evidence generated by the European Randomized Study of Screening for Prostate Cancer (ERSPC), which showed a 30% decrease in PCa-specific mortality in the screened population after seven years of follow-up [2]. This decrease has been maintained for 22 years of follow-up in the Göteborg section of the ERSPC [3]. This change was made possible through the introduction and widespread adoption of multiparametric magnetic resonance imaging (mpMRI) for the selection of candidates for prostate biopsy among men suspected of having PCa due to elevated serum prostate-specific antigen (PSA) levels and/or suspicious digital rectal examinations (DREs) [4]. MpMRI can identify lesions with csPCa and classify their risk using the prostate imaging–reporting and data system (PI-RADS) [5]. Today, the role of mpMRI in improving the early detection of csPCa is well accepted [6], although non-negligible insignificant PCa (iPCa) over-detection remains in PI-RADS 3 lesions and systematic biopsies [7].
Benign prostatic hyperplasia (BPH) is a common condition, with over half of men developing symptomatic disease between the ages of 50 and 70 [8], which is the recommended age for the early detection of csPCa [9]. Consequently, many men suspected of having PCa undergo medical treatment for symptomatic BPH. 5-alpha reductase inhibitors (5-ARIs) are one of the primary treatment options, reducing the prostate volume by approximately 20%, alleviating obstructive symptoms, and slowing disease progression. Finasteride and dutasteride (5-ARIs) reduce serum PSA levels by approximately 50% and decrease the risk of developing PCa [10,11,12]. The Prostate Cancer Prevention Trial (PCPT) [11] and the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial [12] have shown a reduction in PCa incidence in men receiving finasteride for seven years and dutasteride for four years, respectively, compared with those receiving a placebo, although there was a concerning increase in high-grade Gleason tumors. This initial concern was challenged by the long-term follow-up of the PCPT [13]. The relative increase in high-grade Gleason tumors seems to result from the effective prevention and treatment of low-grade PCa [14]. The role of 5-ARIs in preventing the progression of low-grade PCa under active surveillance is currently under discussion [15].
The MRI in Primary Prostate Cancer after Exposure to Dutasteride (MAPPED) study was designed to provide the radiological effects of six-month exposure to dutasteride on low-grade PCa volume [16]. Preliminary data suggested that dutasteride was associated with an increase in the tumor apparent diffusion coefficient (ADC) and reduced tumor visibility in diffusion-weighted imaging (DWI) without effects on T2 sequences in mpMRI [17,18,19]. Starobinets et al. suggested improved discrimination in mpMRI between the areas with tumors and those with benign tissue in the peripheral zone [20]. However, the effect of 5-ARI exposure on the PI-RADS category and its corresponding detection of csPCa and iPCa in men suspected of having PCa has been poorly analyzed [21,22,23].
Our main goal was to analyze the effect of 5-ARI exposure on the PI-RADS distribution in men suspected of having PCa. Additionally, we aimed to compare the csPCa and insignificant PCa (iPCa) detection rates based on 5-ARI exposure.
2. Materials and Methods
2.1. Design, Setting, and Participants
This was a retrospective case-control study conducted among 2212 men suspected of having PCa due to serum PSA levels of >3.0 ng/mL and/or suspicious digital rectal examinations (DREs), in whom mpMRI and targeted and/or systematic biopsies were performed at 10 participant centers of the csPCa early detection program in Catalonia during 2022. Catalonia is a Spanish region with 7.9 million inhabitants. A subset of 120 (5.4%) participants were identified as having received 5-ARI treatment for over a year, while 12 individuals were previously excluded from this study due to 5-ARI exposure of less than a year.
2.2. MpMRI and Prostate Biopsy Characteristics
MpMRI exams were conducted at each participant center using a pelvic phased-array surface coil and reported with the PI-RADS v.2.1 by experienced radiologists. A magnetic strength field of 1.5 Tesla was utilized in four centers and 3.0 Tesla in six centers. The acquisition protocol followed in all participant centers included T2-weighted imaging (T2W), diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) imaging, according to the guidelines of the European Society of Urogenital Radiology. MRI-TRUS image fusion was performed for all prostate biopsies using a cognitive technique in five centers and a software technique in five centers. TRUS-assisted prostate biopsies were carried out via the transrectal route in four centers and the transperineal route in six centers. Targeted biopsies, ranging from 2 to 6 cores, were obtained for each suspected lesion (PI-RADS > 3) in addition to a 12-core TRUS systematic prostate biopsy. All included men with negative MRI results (PI-RADS < 3) only underwent a 12-core TRUS systematic biopsy [24]. The prostate biopsies were performed by experienced urologists at each parti-cipant center. The biopsy materials were analyzed in the pathology department of each participant center by experienced uropathologists who used the International Society of Urologic Pathology grade groups (GGs) to classify detected PCa as csPCa when the GG was 2 or higher [25].
2.3. Outcome Variables of This Study
The outcome variables of this study were the distribution of PI-RADS categories and the detection rates of csPCa and iPCa.
2.4. Statistical Analysis
Statistical analysis was conducted following the harmonization of anonymized datasets. Quantitative variables were defined as medians and interquartile ranges (IQR: 25–75 percentile), while qualitative variables were defined as numbers and percentages. Pearson’s chi-square test was employed to compare the distributions of qualitative variables based on 5-ARI exposure. After univariate analysis, logistic regression was used to identify independent predictive variables for csPCa and iPCa detection. If independent predictive variables for PCa detection were found, a randomized 1:1 matching group was selected based on the binary variable indicating the use of 5-ARI treatment to normalize its effect. Significant differences were considered when the p-values were below 0.05. p-values between 0.05 and 0.1 were considered as non-significant increasing or decreasing trends. The statistical analysis was conducted using IBM SPSS v.29.0.
3. Results
3.1. Characteristics of Study Population and Comparison According to 5-ARI Exposure
Table 1 summarizes the overall characteristics of the entire population of men suspected of having PCa and their comparison based on 5-ARI exposure. Notably, 5-ARI u-sers exhibited a significantly higher median age than 5-ARI-naïve individuals (72 vs. 68, respectively; p 0.001). The median serum PSA level was similar in both groups (8.0 vs. 7.3 ng/mL; p 0.107). A non-significant trend for a higher percentage of suspicious DREs was observed in 5-ARI users (35% vs. 26.8%; p 0.057), along with a similar percentage of men with previous negative prostate biopsies (36.7% vs. 31.4%; p 0.228) and a family history of PCa (4.2% vs. 8.1%; p 0.160). There was a higher median prostate volume in 5-ARI users than in 5-ARI-naïve men (69 vs. 53 cc; p < 0.001), accompanied by a lower PSA density (0.12 vs. 0.14; p < 0.001).

Table 1.
Characteristics of included population according to the 5-ARI exposure.
The distributions of PI-RADS categories according to 5-ARI exposure were similar (p 0.238). A non-significant decreasing trend in overall PCa detection was observed in 5-ARI users (55% vs. 63.9%; p 0.052), along with similar rates of csPCa detection (42.5% vs. 44.7%; p 0.706) and a non-significant decreasing trend in iPCa detection among 5-ARI users (12.5% vs. 19.2%; p 0.072).
3.2. Search for Independent Predictive Variables of csPCa and iPCa
Considering the significant differences observed in the characteristics between 5-ARI users and 5-ARI-naïve men, we investigated the existence of independent predictive va-riables for csPCa and iPCa. The logistic regression analyses revealed that 5-ARI exposure and a family history of PCa were not independent predictors for overall csPCa and iPCa. Age emerged as an independent predictor for csPCa detection, while serum PSA level was identified as an independent predictive variable for csPCa. Additionally, DRE results, prostate biopsy type (initial vs. repeated), prostate volume, and PI-RADS category were independent predictors of csPCa and iPCa (Table 2).

Table 2.
Multivariate analysis to identify independent predictive variables for csPCa and iPCa detection.
It was considered essential to normalize the effect of 5-ARI exposure according to the identified independent predictive variables influencing the detection of csPCa and iPCa. A randomized group of 1:1 matched pairs of 5-ARI users and 5-ARI-naïve individuals was selected.
3.3. Characteristics of the Randomized Matched Group According to 5-ARI Exposure
Table 3 presents the characteristics of the 118 pairs of men suspected of having PCa, constituting the randomized matched group. Notably, all characteristics were comparable between 5-ARI users and 5-ARI-naïve men, including serum PSA levels (8.0 and 7.5 ng/mL, respectively; p 0.304) and the distribution of PI-RADS categories (p 0.685), which were not included in the normalization process. The csPCa detection rate was 52.6% in 5-ARI users and 47.4% in 5-ARI-naïve men (p 0.596). A non-significant increasing trend in iPCa detection rates was observed in 5-ARI-naïve men compared with 5-ARI users (62.5% vs. 37.5%, respectively; p 0.089).

Table 3.
Characteristics of randomized matched group analyzed based on exposure to 5-ARI.
3.4. Distribution of csPCa and iPCa in 5-ARI Users and 5-ARI-Naïve Men According to PI-RADS Category
Table 4 illustrates that no significant differences were observed in the detection rates of csPCa and iPCa across the PI-RADS categories. In the subset of men with PI-RADS 4, a non-significant increasing trend in csPCa was found in 5-ARI users compared with 5-ARI-naïve men (46.4% vs. 28.6%; p 0.078). Additionally, a non-significant decreasing trend in iPCa detection was noted in men with PI-RADS 5 in 5-ARI users compared with 5-ARI-naïve men (3.7% vs. 16.7%; p 0.080).

Table 4.
Distribution of csPCa and iPCa according to the 5-ARI exposure and the PI-RADS score.
3.5. Distribution of Grade Groups in Tumors Detected in 5-ARI Users and 5-ARI-Naïve Men
The distributions of the GGs of tumors detected were comparable between 5-ARI u-sers and 5-ARI-naïve men (p 0.149), as illustrated in Figure 1. High-grade PCa (GG > 3) was observed in 57% of tumors detected in 5-ARI users compared with 38.6% in 5-ARI-naïve men (p 0.146).
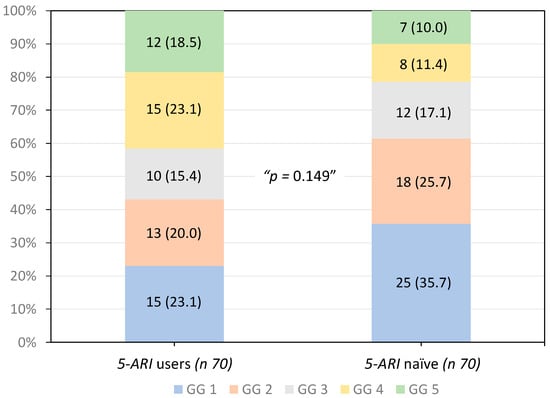
Figure 1.
Distribution of the ISUP grade groups of tumors detected according to the 5-ARI exposure.
4. Discussion
The present study reported unexpected comparable serum PSA levels in 5-ARI users and 5-ARI-naïve men suspected of having PCa. We note that reported serum PSA levels in 5-ARI users corresponded to the real measurements without any adjustment. This observation was true for the entire population and the selected matched group for analysis. Similar findings have been reported by Kim et al. [21] and Wang et al. [23]. This contrasts with the expected lower serum PSA levels in 5-ARI users compared with those observed in 5-ARI-naïve men because of the effect of 5-ARI exposure on serum PSA levels. This observation may be due to not following the recommendation to closely monitor serum PSA levels after reaching their nadir and to establish suspicion of PCa based on a confirmed increase in levels higher than 0.3 ng/mL [26]. Chang et al. recently underlined the necessity to closely monitor serum PSA levels in patients exposed to 5-ARI treatment to prevent delays in diagnosing high-grade PCa [27].
This is the first study analyzing the effect of 5-ARI exposure in a randomized matched group to normalize the influence of confusing independent variables for csPCa and iPCa. The present study suggests that 5-ARI exposure does not modify the distribution of PI-RADS categories. Furthermore, we observed comparable detection rates of csPCa and iPCa according to 5-ARI exposure. However, a non-significant decreasing trend in iPCa detection was noted in men exposed to 5-ARI. This non-significant decreased trend was observed in both the overall population and the randomized matched group. Kim et al. first reported the effects of 5-ARI exposure on the PI-RADS distribution and the detection of csPCa in 2019 [21]. Among 706 men with suspected PCa undergoing targeted and systematic biopsies, 80 (11.3%) were identified as receiving 5-ARI treatment for over a year. The authors found a similar distribution of PI-RADS categories between 5-ARI users and 5-ARI-naïve men. Additionally, comparable rates of csPCa and iPCa were observed. In 2023, Wang et al. reported a study conducted on 351 individuals suspected of having PCa who underwent saturation biopsies and targeted biopsies. They identified 54 (15.3%) individuals undergoing 5-ARI treatment for over a year. The authors observed a comparable distribution of PI-RADS categories between 5-ARI users and 5-ARI-naïve men. However, they found a significant reduction in overall PCa detection in 5-ARI users compared with 5-ARI-naïve men (68.0% vs. 46.3%, respectively), with a similar rate of csPCa. Although data on iPCa were not reported, a decreased rate of iPCa in 5-ARI users can be inferred in this series. The findings from this study align with those of previously mentioned studies, even though these analyses were not conducted in randomized matched groups.
The recently reported Prostate MRI Outcome Database (PROMOD) study included 705 men receiving 5-ARI treatment for more than three months and 6913 5-ARI-naïve men. The study involved mpMRI and targeted and/or systematic biopsies performed at 36 centers between 2020 and 2022. The authors concluded that 5-ARI exposure did not affect the PI-RADS distribution and its association with csPCa detection. The rate of csPCa was comparable in both groups, although a higher rate of high-grade PCa (GG > 3) was observed in 5-ARI users with PI-RADS 5. The median serum PSA levels reported in this study were 6.0 ng/mL in 5-ARI users and 6.5 ng/mL in 5-ARI-naïve men. Although a significant difference was reported between both serum PSA levels, those in 5-ARI users were notably higher than those expected [28]. In 2021, Forte et al. evaluated the PI-RADS v.2.0 in 75 men suspected of having PCa who underwent 5-ARI treatment. They concluded that the PI-RADS v.2.0 exhibited good accuracy in predicting csPCa [22]. In 2021, Artiles et al. concluded that 5-ARI exposure was an independent predictor of csPCa after analyzing 34 men suspected of having PCa with negative mpMRI results who underwent saturation biopsies [29].
The limitations of the present study are its retrospective design and the small size of the case group. The non-significant trends observed may be significant differences with an appropriate size previously calculated. Another limitation is that the length of 5-ARI exposure was not controlled beyond one year, possibly not allowing adequate time to observe changes in the PI-RADS distribution. Notably, the PCPT had a follow-up period of seven years, while the REDUCE trial spanned four years [11,13]. Additionally, the csPCa definition used in prostate biopsies may not fully represent the true pathology in entire prostate specimens.
In summary, few studies have analyzed the effect of 5-ARI exposure on the PI-RADS distribution and the corresponding detection rates of csPCa and iPCa. There is no evidence of changes in PI-RADS categories secondary to 5-ARI exposure. While there is a recognized delay in prostate biopsies for men undergoing 5-ARI based on serum PSA levels, our findings indicate no significant changes in the PI-RADS distribution and no significant differences in csPCa and iPCa detection rates related to 5-ARI exposure.
5. Conclusions
The PI-RADS score distribution in 5-ARI users was similar to that in 5-ARI-naïve men. The detection rates for csPCa and iPCa were also similar in both groups of men suspected of having PCa.
Author Contributions
Conceptualization, J.M.; methodology, J.M.; formal analysis, J.M. and B.M.; resources, J.M.; data curation, N.P. (Natàlia Picola), N.P. (Nahuel Paesano), J.M.-R., X.R.-P., M.V.M.-R., A.C., G.G.-d.M., P.S. and J.M.A.; writing—original draft preparation, J.M.; writing—review and editing, P.S. and J.M.A.; supervision, J.M.; project administration, J.M.; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto de Salut Carlos III (SP) and the European Union (PI20/01666).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. It was approved by the Institutional Ethics Committee of the Vall d’Hebron Research Institute (PRAG-02/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Van Poppel, H.; Albreht, T.; Basu, P.; Hogenhout, R.; Collen, S.; Roobol, M. Serum PSA-based early detection of prostate cancer in Europe and globally: Past, present and future. Nat. Rev. Urol. 2022, 19, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Schroder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef]
- Frånlund, M.; Månsson, M.; Godtman, R.A.; Aus, G.; Holmberg, E.; Kollberg, K.S.; Lodding, P.; Pihl, C.G.; Stranne, J.; Lilja, H.; et al. Results from 22 years of Followup in the Göteborg Randomized Population-Based Prostate Cancer Screening Trial. J. Urol. 2022, 208, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Padhani, A.R.; Rouvière, O.; Barentsz, J.O.; Richenberg, J. Analysis of Magnetic Resonance Imaging-directed Biopsy Strategies for Changing the Paradigm of Prostate Cancer Diagnosis. Eur. Urol. Oncol. 2020, 3, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2018, 20, 100–109. [Google Scholar] [CrossRef]
- Hagens, M.J.; Noordzij, M.A.; Mazel, J.W.; Jager, A.; Boellaard, T.N.; Tielbeek, J.A.W.; Henebiens, M.; Schoots, I.G.; van Leeuwen, P.J.; van der Poel, H.G.; et al. An Magnetic Resonance Imaging-directed Targeted-plus-perilesional Biopsy Approach for Prostate Cancer Diagnosis: “Less Is More”. Eur. Urol. Open Sci. 2022, 43, 68–73. [Google Scholar] [CrossRef]
- Jacobsen, S.J.; Girman, C.J.; Lieber, M.M. Natural history of benign prostatic hyperplasia. Urology 2001, 58, 5–16. [Google Scholar] [CrossRef]
- Van Poppel, H.; Hogenhout, R.; Albers, P.; van den Bergh, R.C.N.; Barentsz, J.O.; Roobol, M.J. A European Model for an Orga-nised Risk-stratified Early Detection Programme for Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 731–739. [Google Scholar] [CrossRef]
- Edwards, J.E.; Moore, R.A. Finasteride in the treatment of clinical benign prostatic hyperplasia: A systematic review of rando-mised trials. BMC Urol. 2002, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Lucia, M.S.; Miller, G.J.; Ford, L.G.; Lieber, M.M.; Cespedes, R.D.; Atkins, J.N.; Lippman, S.M.; et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003, 349, 215–224. [Google Scholar] [CrossRef]
- Andriole, G.L.; Bostwick, D.G.; Brawley, O.W.; Gomella, L.G.; Marberger, M.; Montorsi, F.; Pettaway, C.A.; Tammela, T.L.; Teloken, C.; Tindall, D.J.; et al. Effect of dutasteride on the risk of prostate cancer. N. Engl. J. Med. 2010, 362, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Goodman, P.J.; Tangen, C.M.; Parnes, H.L.; Minasian, L.M.; Godley, P.A.; Lucia, M.S.; Ford, L.G. Long-term survival of participants in the prostate cancer prevention trial. N. Engl. J. Med. 2013, 369, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Redman, M.W.; Tangen, C.M.; Goodman, P.J.; Lucia, M.S.; Coltman, C.A., Jr.; Thompson, I.M. Finasteride does not increase the risk of high-grade prostate cancer: A bias-adjusted modeling approach. Cancer Prev. Res. 2008, 1, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, H.; Klaassen, Z.; Chandrasekar, T.; Fleshner, N. Preventing clinical progression and need for treatment in patients on active surveillance for prostate cancer. Curr. Opin. Urol. 2018, 28, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.L.; Moore, C.M.; Ambler, G.; Bott, S.R.; Freeman, A.; Gambarota, G.; Jameson, C.; Mitra, A.V.; Whitcher, B.; Winkler, M.; et al. MAPPED study design: A 6 month randomised controlled study to evaluate the effect of dutasteride on prostate cancer volume using magnetic resonance imaging. Contemp. Clin. Trials 2013, 34, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Robertson, N.L.; Jichi, F.; Damola, A.; Ambler, G.; Giganti, F.; Ridout, A.J.; Bott, S.R.; Winkler, M.; Ahmed, H.U.; et al. The Effect of Dutasteride on Magnetic Resonance Imaging Defined Prostate Cancer: MAPPED-A Randomized, Placebo Controlled, Double-Blind Clinical Trial. J. Urol. 2017, 197, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Giganti, F.; Moore, C.M.; Robertson, N.L.; McCartan, N.; Jameson, C.; Bott, S.R.J.; Winkler, M.; Gambarota, G.; Whitcher, B.; Castro, R.; et al. MRI findings in men on active surveillance for prostate cancer: Does dutasteride make MRI visible lesions less conspicuous? Results from a placebo-controlled, randomised clinical trial. Eur. Radiol. 2017, 27, 4767–4774. [Google Scholar] [CrossRef]
- Ginsburg, S.B.; Algohary, A.; Pahwa, S.; Gulani, V.; Ponsky, L.; Aronen, H.J.; Boström, P.J.; Böhm, M.; Haynes, A.M.; Brenner, P.; et al. Radiomic features for prostate cancer detection on MRI differ between the transition and peripheral zones: Preliminary findings from a multi-institutional study. J. Magn. Reason. Imaging 2017, 46, 184–193. [Google Scholar] [CrossRef]
- Starobinets, O.; Kurhanewicz, J.; Noworolski, S.M. Improved multiparametric MRI discrimination between low-risk prostate cancer and benign tissues in a small cohort of 5α-reductase inhibitor treated individuals as compared with an untreated cohort. NMR Biomed. 2017, 30, e3696. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, H.J.; Hwang, S.I.; Choe, G.; Kim, H.J.; Hong, S.K. The effect of 5 alpha-reductase inhibitor therapy on prostate cancer detection in the era of multi-parametric magnetic resonance imaging. Sci. Rep. 2019, 9, 17862. [Google Scholar] [CrossRef] [PubMed]
- Forte, V.; Cavallo, A.U.; Bertolo, R.; de Soccio, V.; Sperandio, M.; Bove, P.; Ciccariello, M. PI-RADS score v.2 in predicting malignancy in patients undergoing 5α-reductase inhibitor therapy. Prostate Cancer Prostatic. Dis. 2021, 24, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, K.; Ong, H.Y.; Tsang, W.C.; Wu, Q.H.; Chiong, E. 5-alpha reductase inhibitors and MRI prostates: Actively reducing prostate sizes and ambiguity. BMC Urol. 2023, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Confort, P.; van den Bergh, R.C.N.; Briers, E.; Eberli, D.; De Meerleer, G.; De Santis, M.; Gillessen, S.; Grummet, J.; Henry, A.M.; et al. EAU–EANM–ESTRO–ESUR–ISUP–SIOG Guidelines on Prostate Cancer. 2023. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 1 November 2023).
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Marberger, M.; Freedland, S.J.; Andriole, G.L.; Emberton, M.; Pettaway, C.; Montorsi, F.; Teloken, C.; Rittmaster, R.S.; Somerville, M.C.; Castro, R. Usefulness of prostate-specific antigen (PSA) rise as a marker of prostate cancer in men treated with dutasteride: Lessons from the REDUCE study. BJU Int. 2012, 109, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Wang, S.S.; Yang, C.K.; Lu, K.; Chen, C.S.; Cheng, C.L.; Hung, S.C.; Chiu, K.Y.; Hsu, C.Y.; Li, J.R. Risk Analysis of Prostate Cancer Development Following Five-alpha Reductase Inhibitor Treatment for Benign Prostate Hyperplasia. Anticancer Res. 2023, 43, 485–491. [Google Scholar] [CrossRef]
- Falagario, U.G.; Lantz, A.; Jambor, I.; Busetto, G.M.; Bettocchi, C.; Finati, M.; Ricapito, A.; Luzzago, S.; Ferro, M.; Musi, G.; et al. Diagnosis of prostate cancer with magnetic resonance imaging in men treated with 5-alpha-reductase inhibitors. World J. Urol. 2023, 41, 2967–2974. [Google Scholar] [CrossRef]
- Artiles Medina, A.; Rodríguez-Patrón Rodríguez, R.; Ruiz Hernández, M.; Mata Alcaraz, M.; García Barreras, S.; Fernández Conejo, G.; Fraile Poblador, A.; Sanz Mayayo, E.; Burgos Revilla, F.J. Identifying Risk Factors for MRI-Invisible Prostate Cancer in Patients Undergoing Transperineal Saturation Biopsy. Res. Rep. Urol. 2021, 13, 723–731. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).