Biomarkers of Oxidative and Radical Stress
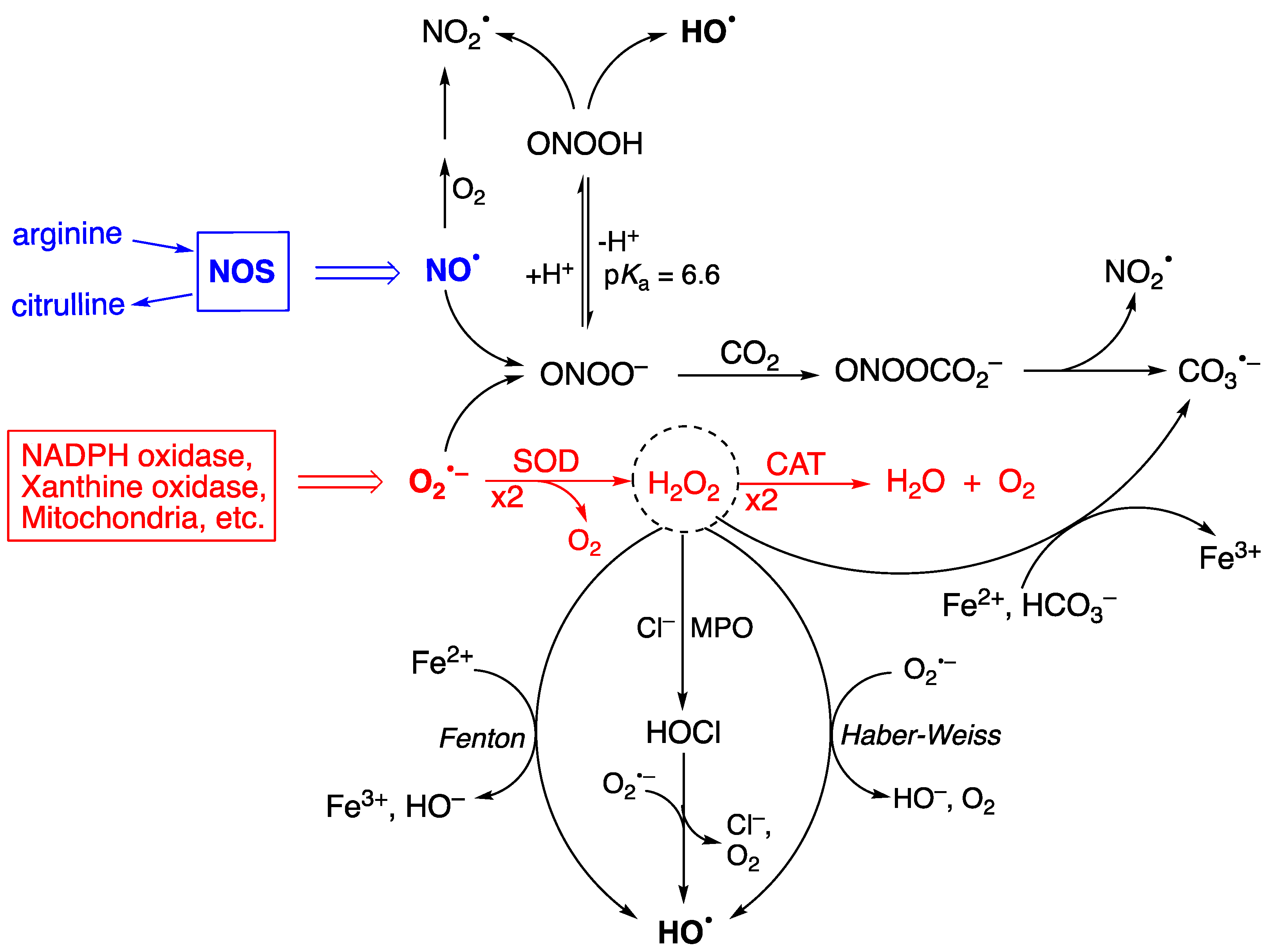
1. Reactive Species, Oxidative Damage, and Biomarkers
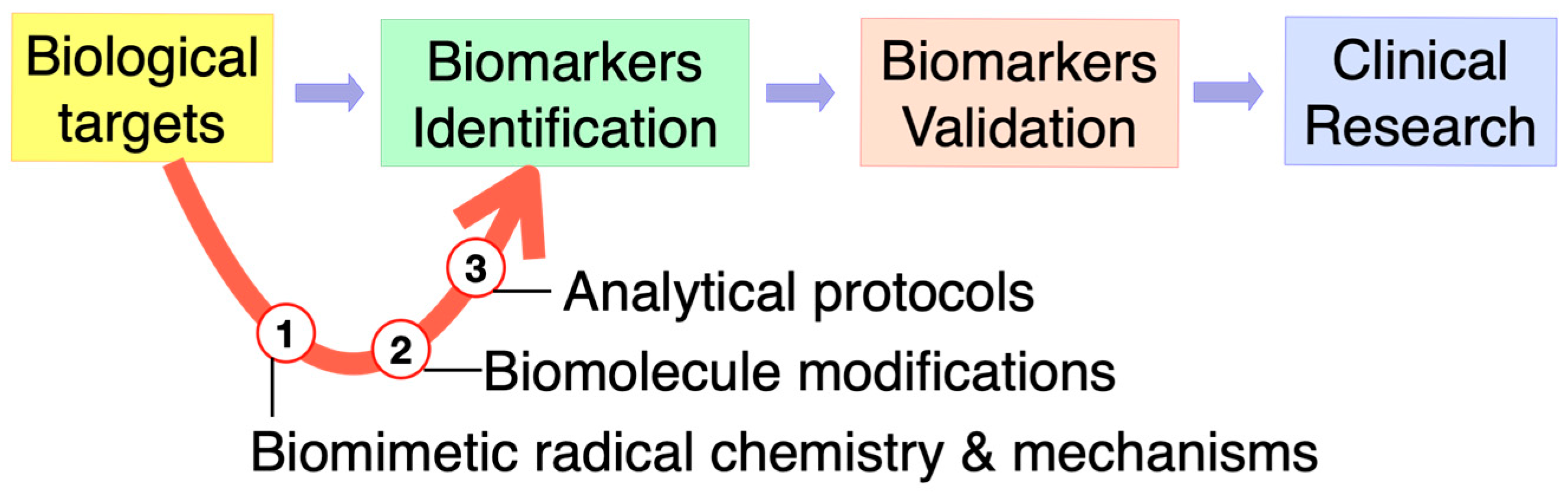
2. Brief Overview of the Special Issue
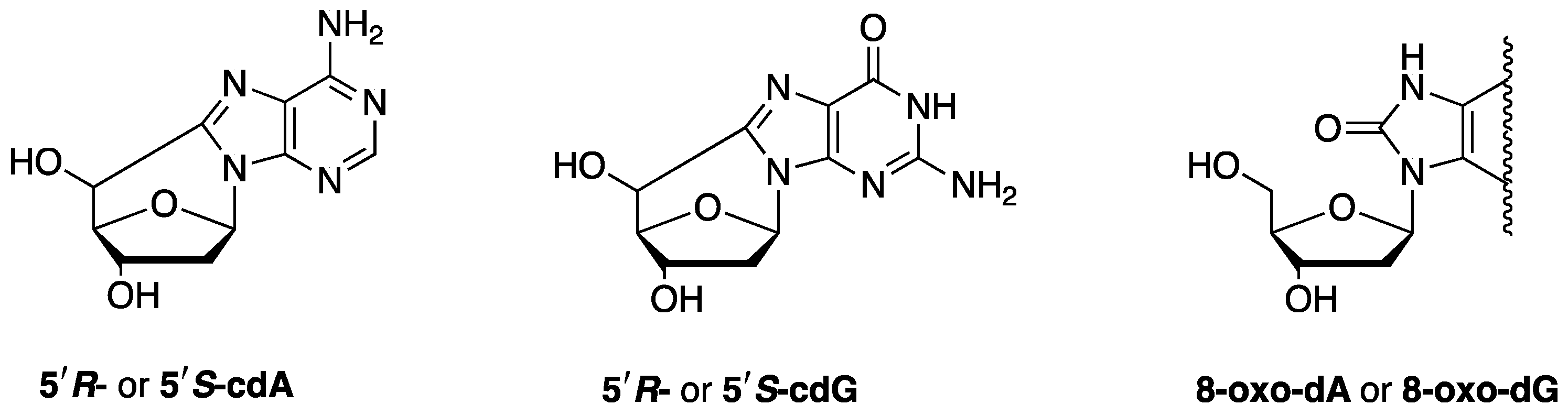
2.1. Biomarkers of DNA Damage
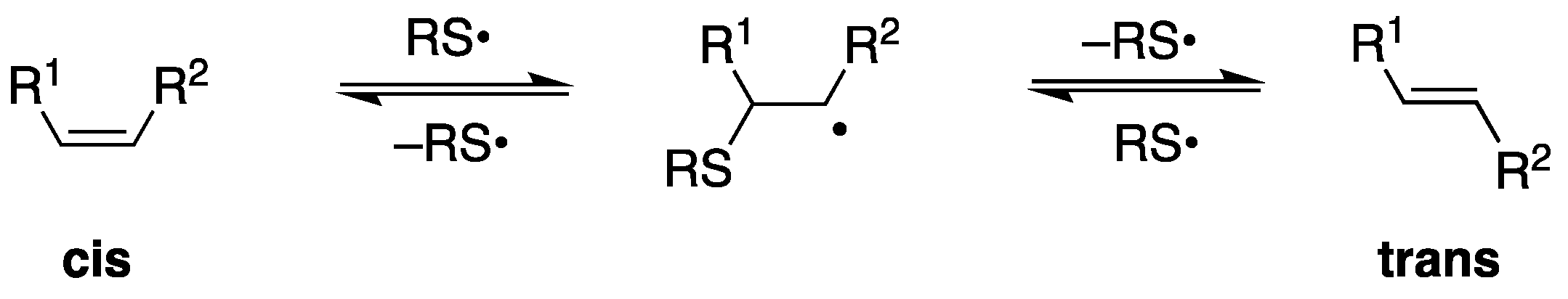
2.2. Biomarkers of Lipid Damage
2.3. Antioxidant Protection
2.4. Oxidative and Radical Stress: Measurements and Protein Studies
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- D’Autreáux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Geacintov, N.E.; Shafirovich, V. Reactions of small reactive species with DNA. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: Chichester, UK, 2012; Volume 3, pp. 1284–1317. [Google Scholar]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Ferreri, C.; Krokidis, M.G.; Masi, A.; Terzidis, M.A. On the relevance of hydroxyl radical to purine DNA damage. Free Radic. Res. 2021, 55, 384–404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z. Hydroxyl radical generation from the physiologically relevant Fenton-like reactions. Free Radic. Biol. Med. 2023, 208, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Sansone, A.; Torreggiani, A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014, 114, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, C.; Krokidis, M.G.; Masi, A.; Barata-Vallejo, S.; Ferreri, C.; Pascucci, B.; D’Errico, M. Assessing the Formation of Purine Lesions in Mitochondrial DNA of Cockayne Syndrome Cells. Biomolecules 2022, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- Krokidis, M.G.; Prasinou, P.; Efthimiadou, E.K.; Boari, A.; Ferreri, C.; Chatgilialoglu, C. Effects of Aging and Disease Conditions in Brain of Tumor-Bearing Mice: Evaluation of Purine DNA Damages and Fatty Acid Pool Changes. Biomolecules 2022, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Foret, M.K.; Lincoln, R.; Do Cormo, S.; Cuello, A.C.; Cosa, G. Connecting the “Dots”: From Free Radical Lipid Autoxidation to Cell Pathology and Disease. Chem. Rev. 2020, 120, 12757–12787. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef]
- Perkovic, M.N.; Jaganjac, M.; Milkovic, L.; Horvat, T.; Rojo, D.; Zarkovic, K.; Ćorić, M.; Hudolin, T.; Waeg, G.; Orehovec, B.; et al. Relationship between 4-Hydroxynonenal (4-HNE) as Systemic Biomarker of Lipid Peroxidation and Metabolomic Profiling of Patients with Prostate Cancer. Biomolecules 2023, 13, 145. [Google Scholar] [CrossRef]
- Ferreri, C.; Ferocino, A.; Batani, G.; Chatgilialoglu, C.; Randi, V.; Riontino, M.V.; Vetica, F.; Sansone, A. Plasmalogens: Free Radical Reactivity and Identification of Trans Isomers Relevant to Biological Membranes. Biomolecules 2023, 13, 730. [Google Scholar] [CrossRef]
- Hirata, Y.; Ferreri, C.; Yamada, Y.; Inoue, A.; Sansone, A.; Vetica, F.; Suzuki, W.; Saya Takano, S.; Noguchi, T.; Matsuzawa, A.; et al. Geometrical isomerization of arachidonic acid during lipid peroxidation Interferes with ferroptosis. Free Radic. Biol. Med. 2023, 204, 374–384. [Google Scholar] [CrossRef]
- Orrico, F.; Laurance, S.; Lopez, A.C.; Lefevre, S.D.; Thomson, L.; Möller, M.N.; Ostuni, M.A. Oxidative Stress in Healthy and Pathological Red Blood Cells. Biomolecules 2023, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A.; Bukowiecka-Matusiak, M. Impact of Polyphenols on Inflammatory and Oxidative Stress Factors in Diabetes Mellitus: Nutritional Antioxidants and Their Application in Improving Antidiabetic Therapy. Biomolecules 2023, 13, 1402. [Google Scholar] [CrossRef] [PubMed]
- Migni, A.; Mancuso, F.; Baroni, T.; Di Sante, G.; Rende, M.; Galli, F.; Bartolini, D. Melatonin as a Repairing Agent in Cadmium- and Free Fatty Acid-Induced Lipotoxicity. Biomolecules 2023, 13, 1758. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Factors Important in the Use of Fluorescent or Luminescent Probes and Other Chemical Reagents to Measure Oxidative and Radical Stress. Biomolecules 2023, 13, 1041. [Google Scholar] [CrossRef] [PubMed]
- Stanca, M.; Gaidau, C.; Zaharescu, T.; Balan, G.-A.; Matei, I.; Precupas, A.; Leonties, A.R.; Ionita, G. Physico-Chemical Changes Induced by Gamma Irradiation on Some Structural Protein Extracts. Biomolecules 2023, 13, 774. [Google Scholar] [CrossRef]
- Schöneich, C. Primary Processes of Free Radical Formation in Pharmaceutical Formulations of Therapeutic Proteins. Biomolecules 2023, 13, 1142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatgilialoglu, C. Biomarkers of Oxidative and Radical Stress. Biomolecules 2024, 14, 194. https://doi.org/10.3390/biom14020194
Chatgilialoglu C. Biomarkers of Oxidative and Radical Stress. Biomolecules. 2024; 14(2):194. https://doi.org/10.3390/biom14020194
Chicago/Turabian StyleChatgilialoglu, Chryssostomos. 2024. "Biomarkers of Oxidative and Radical Stress" Biomolecules 14, no. 2: 194. https://doi.org/10.3390/biom14020194
APA StyleChatgilialoglu, C. (2024). Biomarkers of Oxidative and Radical Stress. Biomolecules, 14(2), 194. https://doi.org/10.3390/biom14020194




