Preparing a Phytosome for Promoting Delivery Efficiency and Biological Activities of Methyl Jasmonate-Treated Dendropanax morbifera Adventitious Root Extract (DMARE)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of Phytosome
2.2.1. Preparation of MeJA-DMARE
2.2.2. Phytosome Synthesis by Co-Solvency Methods
2.2.3. Phytosome Synthesis by Thin-Layer Film Method
2.3. Characterization of Phytosome
2.3.1. UV-Vis
2.3.2. Entrapment Efficiency (EE) and Loading Capacity (LC)
2.3.3. Tyndall Effect
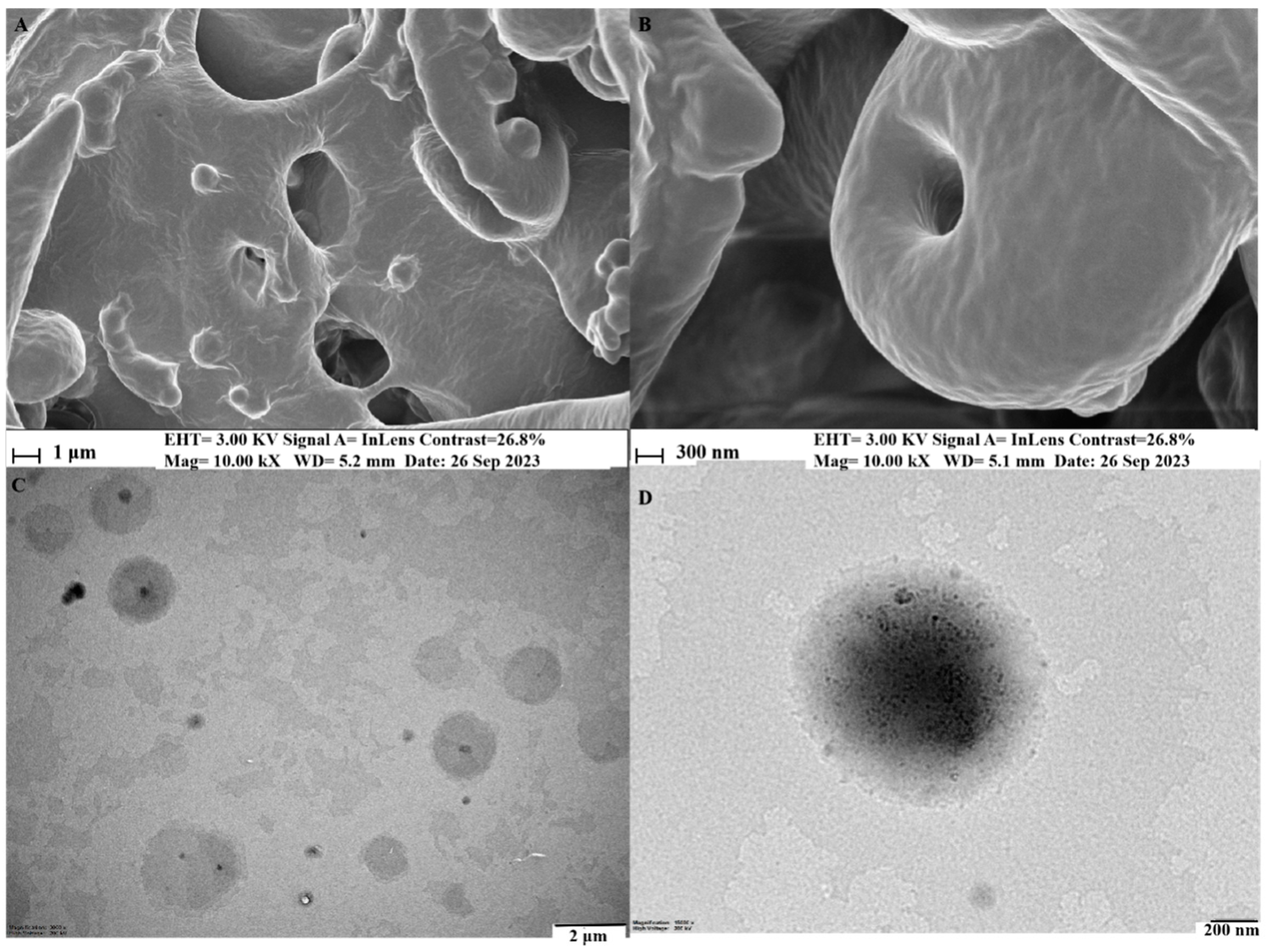
2.3.4. Morphology Study
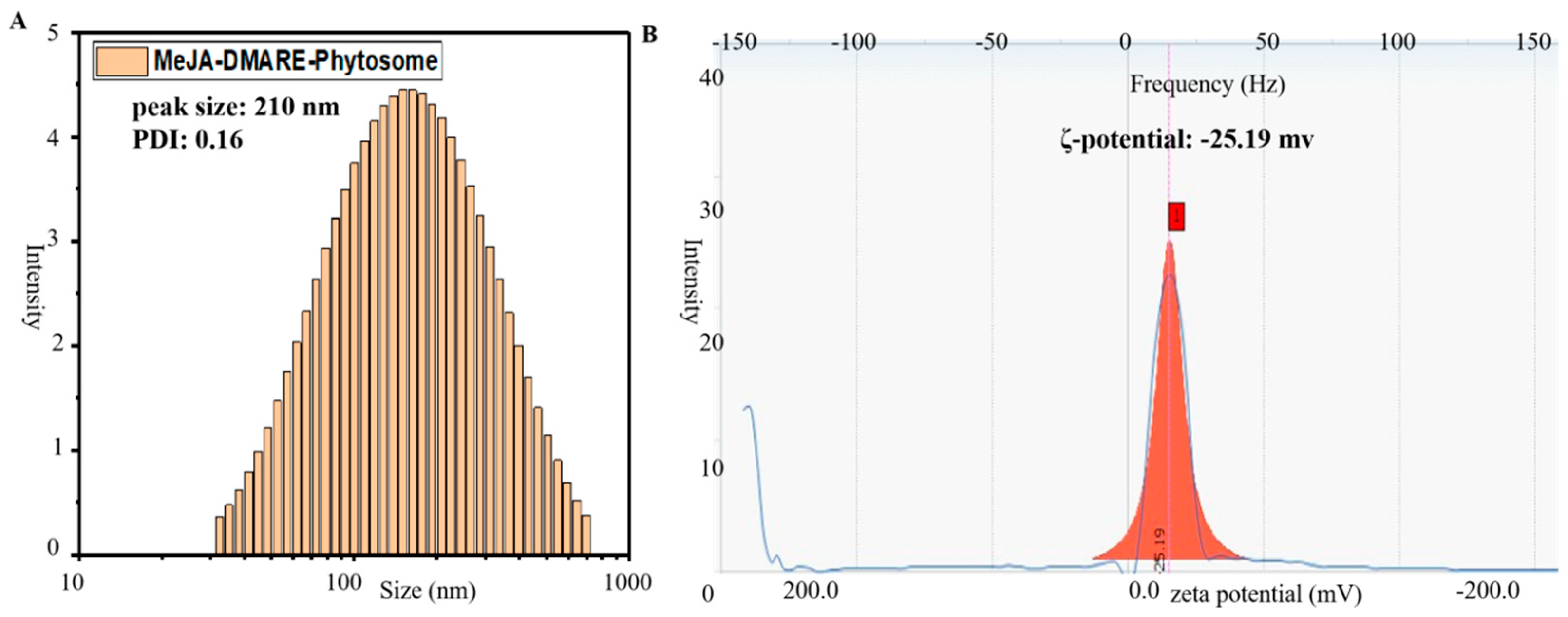
2.3.5. Particle Size, Polydispersity Index, and Zeta Potential
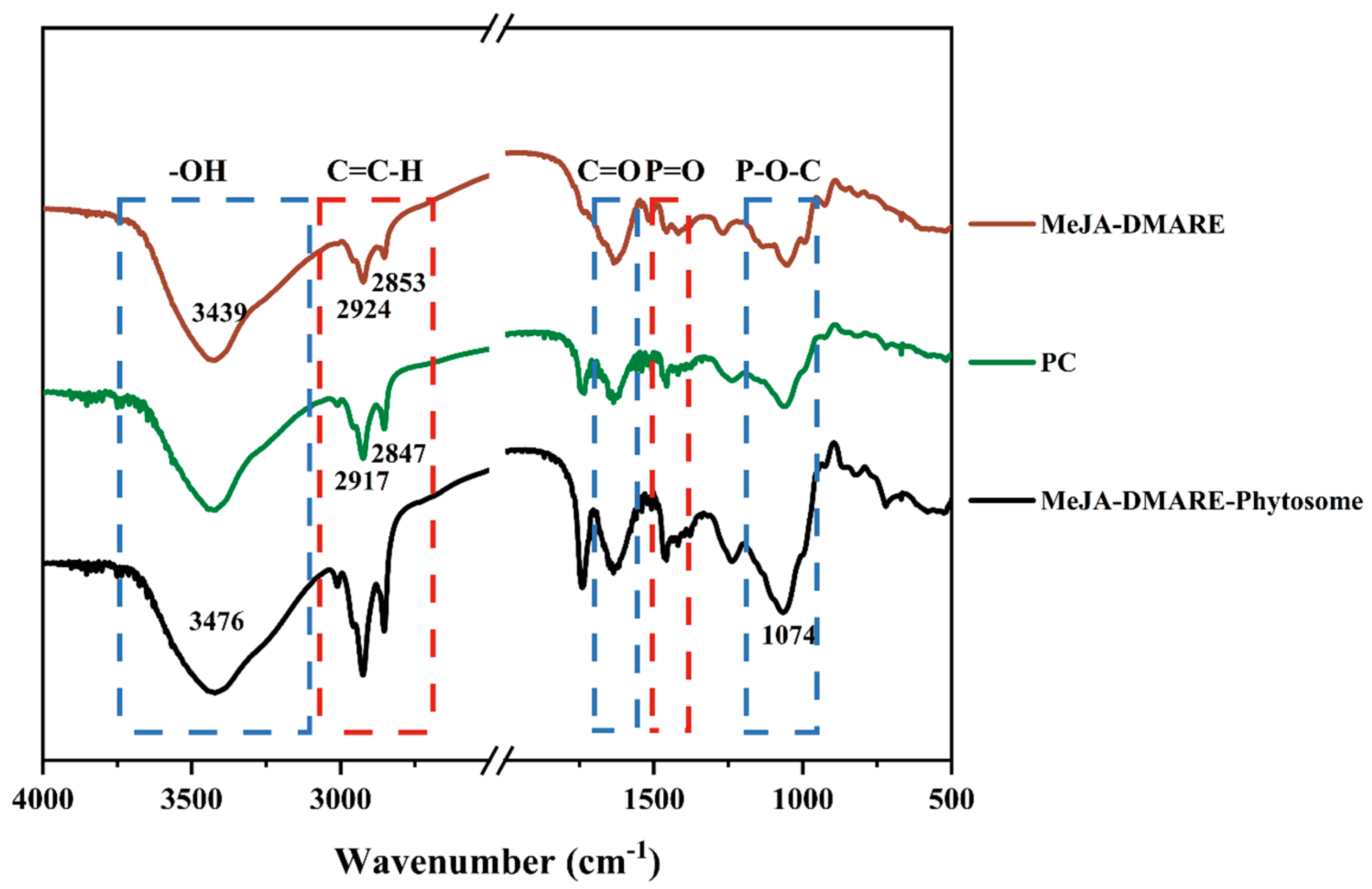
2.3.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.3.7. In Vitro Stability Studies
2.4. Evaluation of Biological Activities
2.4.1. Cell Cultures
2.4.2. Cytotoxicity Assay
2.4.3. Inhibition of Nitric Oxide (NO) Production
2.4.4. Reactive Oxygen Species (ROS) Generation Assay
2.4.5. The Analysis of Inflammation and Cancer-Related Gene Expression Levels
2.4.6. Wound-Healing Assay
2.5. Statistical Analysis
3. Results and Discussions
3.1. Formulation of MeJA-DMARE Phytosome Complex
3.2. Characterization of Phytosome
3.2.1. Consistency Analysis of Bioactive Components
3.2.2. Entrapment Efficiency (EE) and Loading Capacity (LC)
3.2.3. Molecular Characterization of Phytosomes
3.2.4. Potential Interactions between MeJA-DMARE and Phospholipids
3.2.5. In Vitro Stability
3.3. Cytotoxicity
3.4. Enhanced Anti-Inflammatory Activities
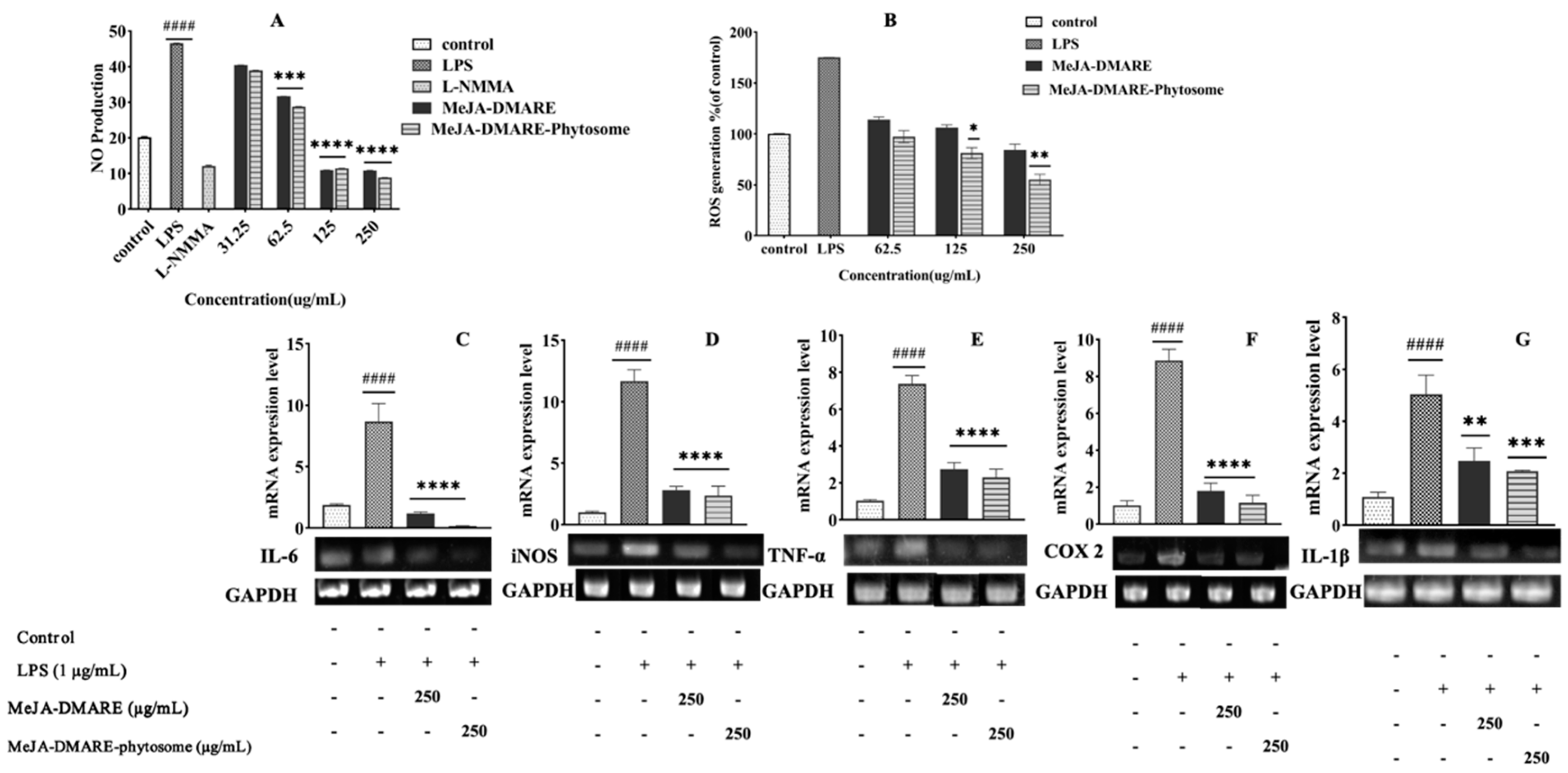
3.4.1. Inhibited NO and ROS Generation in RAW 264.7 Cells
3.4.2. Inhibition of Inflammation-Related Cytokines
3.5. Enhanced Anti-Lung Cancer Activities
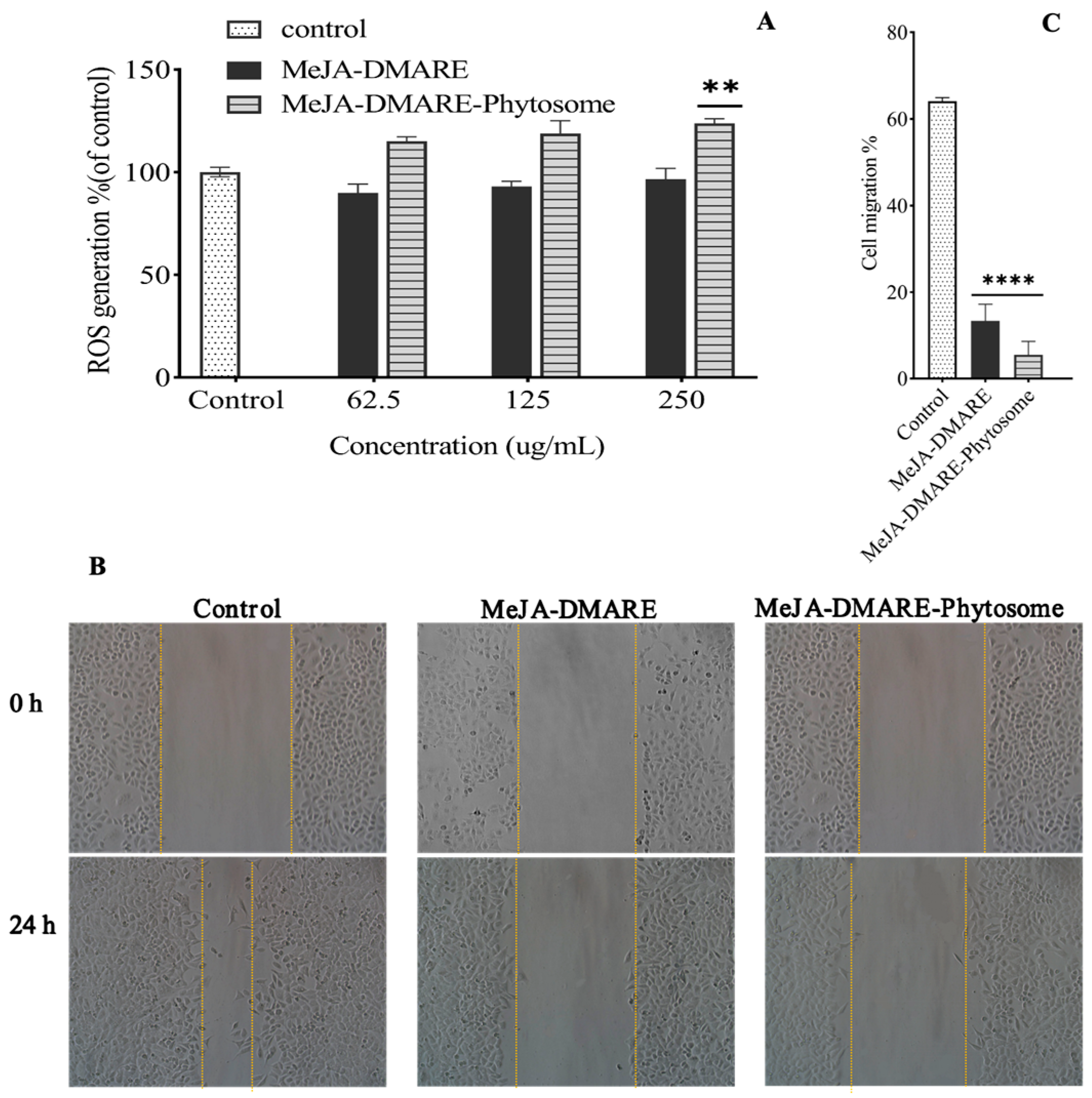
3.5.1. Increased ROS Generation in A549 Cells
3.5.2. Increased Inhibition of Lung Cancer Cell Migration
3.5.3. Apoptotic Gene Expression in Lung Cancer Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Song, J.-H.; Kang, H.-B.; Kim, J.H.; Kwak, S.; Sung, G.-J.; Park, S.-H.; Jeong, J.-H.; Kim, H.; Lee, J.; Jun, W. Antiobesity and cholesterol-lowering effects of Dendropanax morbifera water extracts in mouse 3T3-L1 Cells. J. Med. Food 2018, 21, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-J.; Kim, H.; Heldenbrand, B.; Jeon, J.; Lee, S. Ethnopharmacological survey of medicinal plants in Jeju Island, Korea. J. Ethnobiol. Ethnomedicine 2013, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.-J.; Park, S.; Lee, J.-W.; Park, H.-J.; Baek, S.-H.; Kim, E.-K.; Yu, S.-W. Extracts from Dendropanax morbifera Leaves Have Modulatory Effects on Neuroinflammation in Microglia. Am. J. Chin. Med. 2016, 44, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.-W.; Lee, S.-Y.; Kim, S.-G.; Heo, Y.-R.; Son, M.-K. Antimicrobial, antioxidant and cytotoxic activities of Dendropanax morbifera Léveille extract for mouthwash and denture cleaning solution. J. Adv. Prosthodont. 2016, 8, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Cho, D.-Y.; Su-Kim, I.; Choi, D.-K. Dendropanax morbiferus and other species from the genus dendropanax: Therapeutic potential of its traditional uses, phytochemistry, and pharmacology. Antioxidants 2020, 9, 962. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Kim, K.S.; Park, J.H.; Lee, S.H.; Kim, H.R.; Lee, S.H.; Choi, H.B.; Cao, S.; Kumar, V.; Kwak, J.H.; et al. Protective effects of dendropanoxide isolated from Dendropanax morbifera against cisplatin-induced acute kidney injury via the AMPK/mTOR signaling pathway. Food Chem. Toxicol. 2020, 145, 111605. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, C. Polyacetylenes in herbal medicine: A comprehensive review of its occurrence, pharmacology, toxicology, and pharmacokinetics (2014–2021). Phytochemistry 2022, 201, 113288. [Google Scholar] [CrossRef]
- Kim, M.-O.; Kang, M.-J.; Lee, S.-U.; Kim, D.-Y.; Jang, H.-J.; An, J.H.; Lee, H.-S.; Ryu, H.W.; Oh, S.-R. Polyacetylene (9Z, 16S)-16-hydroxy-9, 17-octadecadiene-12, 14-diynoic acid in Dendropanax morbifera leaves. Food Biosci. 2021, 40, 100878. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kim, M.-O.; Lee, H.; Kim, Y.; Kim, E.; Kim, J.-S. Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera Léveille. Food Chem. 2013, 141, 1947–1955. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, S.K.; Guo, T.J.; Kang, J.Y.; Ha, J.S.; Lee, U.; Heo, H.J. Anti-amnesic effect of Dendropanax morbifera via JNK signaling pathway on cognitive dysfunction in high-fat diet-induced diabetic mice. Behav. Brain Res. 2016, 312, 39–54. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, D.-W.; Park, S.-E.; Lee, H.-J.; Kim, K.-M.; Kim, K.-J.; Kim, M.-K.; Kim, S.-J.; Kim, S. Anti-thrombotic effect of rutin isolated from Dendropanax morbifera Leveille. J. Biosci. Bioeng. 2015, 120, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Kim, M.Y.; Park, W.-H.; Moon, H.-I. Antiatherogenic activity of Dendropanax morbifera essential oil in rats. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 547–549. [Google Scholar]
- Park, B.-Y.; Min, B.-S.; Oh, S.-R.; Kim, J.-H.; Kim, T.-J.; Kim, D.-H.; Bae, K.-H.; Lee, H.-K. Isolation and anticomplement activity of compounds from Dendropanax morbifera. J. Ethnopharmacol. 2004, 90, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Yoo, D.Y.; Jung, H.Y.; Kim, D.W.; Hwang, I.K.; Lee, J.Y.; Moon, S.M. Effects of Dendropanax morbifera Leveille extracts on cadmium and mercury secretion as well as oxidative capacity: A randomized, double-blind, placebo-controlled trial. Biomed. Rep. 2016, 4, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Valappil, A.K.; Zheng, S.; Zheng, B.; Yang, D.; Wang, Q. 3,5-DCQA as a Major Molecule in MeJA-Treated Dendropanax morbifera Adventitious Root to Promote Anti-Lung Cancer and Anti-Inflammatory Activities. Biomolecules 2024, 14, 705. [Google Scholar] [CrossRef]
- Pawar, H.A.; Bhangale, B.D. Phytosome as a novel biomedicine: A microencapsulated drug delivery system. J. Bioanal. Biomed. 2015, 7, 006–012. [Google Scholar]
- Anjana, R.; Kumar, S.; Sharma, H.; Khar, R. Phytosome drug delivery of natural products: A promising technique for enhancing bioavailability. Int. J. Drug Deliv. Technol. 2017, 7, 157–165. [Google Scholar] [CrossRef]
- Jain, S.; Dhanotiya, C.; Malviya, N. Physicochemical characterization and determination of free radical scavenging activity of rutin-phospholipid complex. Int. J. Pharm. Sci. Res. 2012, 3, 909. [Google Scholar]
- Dhase, A.S.; Saboo, S.S. Preparation and evaluation of phytosomes containing methanolic extract of leaves of Aegle marmelos (bael). Int. J. PharmTech Res. 2015, 8, 231–240. [Google Scholar]
- More, M.S.; Shende, M.A.; Kolhe, D.B.; Jaiswal, N.M. Herbosomes: Herbo-phospholipid complex an approach for absorption enhancement. Int. J. Bio. Pharm. Res. 2012, 3, 946–955. [Google Scholar]
- Youn, J.S.; Kim, Y.-J.; Na, H.J.; Jung, H.R.; Song, C.K.; Kang, S.Y.; Kim, J.Y. Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collecting season and extraction conditions. Food Sci. Biotechnol. 2019, 28, 201–207. [Google Scholar] [CrossRef] [PubMed]
- El-Menshawe, S.F.; Ali, A.A.; Rabeh, M.A.; Khalil, N.M. Nanosized soy phytosome-based thermogel as topical anti-obesity formulation: An approach for acceptable level of evidence of an effective novel herbal weight loss product. Int. J. Nanomed. 2018, 13, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A. Preparation of transfersomes encapsulating sildenafil aimed for transdermal drug delivery: Plackett–Burman design and characterization. J. Liposome Res. 2015, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Yang, D.C. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1150–1157. [Google Scholar]
- Leonard, K.; Ahmmad, B.; Okamura, H.; Kurawaki, J. In situ green synthesis of biocompatible ginseng capped gold nanoparticles with remarkable stability. Colloids Surf. B Biointerfaces 2011, 82, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 60. [Google Scholar] [CrossRef]
- Sasongko, R.E.; Surini, S.; Saputri, F.C. Formulation and characterization of bitter melon extract (momordica charantia) loaded phytosomes. Pharmacogn. J. 2019, 11, 1235–1241. [Google Scholar] [CrossRef]
- Das, M.K.; Kalita, B. Design and evaluation of phyto-phospholipid complexes (phytosomes) of rutin for transdermal application. J. Appl. Pharm. Sci. 2014, 4, 051–057. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, K.; Mo, X.; Chen, X.; Deng, Y.; Liu, C.; Yuan, Y.; Nie, J.; Zhang, Y. Tyndall-Effect-inspired assay with gold nanoparticles for the colorimetric discrimination and quantification of mercury ions and glutathione. Talanta 2022, 238, 122999. [Google Scholar] [CrossRef]
- Pan, F.; Hua, F.; Yan, Y.; Huang, X.; Yuan, L.; Tang, Y.; Yuan, Y.; Nie, J.; Zhang, Y. Sensitive, specific, smartphone-based quantitative sensing of glyphosate by integrating analyte-triggered anti-aggregation/anti-autocatalysis of metal nanoparticles with Tyndall-effect colorimetric signaling. Microchem. J. 2023, 190, 108707. [Google Scholar] [CrossRef]
- Wanjiru, J.; Gathirwa, J.; Sauli, E.; Swai, H.S. Formulation, optimization, and evaluation of Moringa oleifera leaf polyphenol-loaded phytosome delivery system against breast cancer cell lines. Molecules 2022, 27, 4430. [Google Scholar] [CrossRef] [PubMed]
- Leach, W.T.; Simpson, D.T.; Val, T.N.; Yu, Z.; Lim, K.T.; Park, E.J.; Williams, R.O.; Johnston, K.P. Encapsulation of protein nanoparticles into uniform-sized microspheres formed in a spinning oil film. AAPS PharmSciTech 2005, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Vandervoort, J.; Ludwig, A. Biocompatible stabilizers in the preparation of PLGA nanoparticles: A factorial design study. Int. J. Pharm. 2002, 238, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Nagai, M.; Omi, S. Preparation of uniform poly(lactide) microspheres by employing the Shirasu Porous Glass (SPG) emulsification technique. Colloids Surf. A Physicochem. Eng. Asp. 1999, 153, 383–394. [Google Scholar] [CrossRef]
- Bahari, L.A.S.; Hamishehkar, H. The impact of variables on particle size of solid lipid nanoparticles and nanostructured lipid carriers; a comparative literature review. Adv. Pharm. Bull. 2016, 6, 143. [Google Scholar] [CrossRef]
- Hou, Z.; Li, Y.; Huang, Y.; Zhou, C.; Lin, J.; Wang, Y.; Cui, F.; Zhou, S.; Jia, M.; Ye, S. Phytosomes loaded with mitomycin C–soybean phosphatidylcholine complex developed for drug delivery. Mol. Pharm. 2013, 10, 90–101. [Google Scholar] [CrossRef]
- Xie, S.; Pan, B.; Wang, M.; Zhu, L.; Wang, F.; Dong, Z.; Wang, X.; Zhou, W. Formulation, characterization and pharmacokinetics of praziquantel-loaded hydrogenated castor oil solid lipid nanoparticles. Nanomedicine 2010, 5, 693–701. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, Y.; Smith, E. Experimental design for the optimization of lipid nanoparticles. J. Pharm. Sci. 2009, 98, 1813–1819. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.-P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. Aaps Pharmscitech 2011, 12, 62–76. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Santra, K.; Pattnaik, G.; Ghosh, S. Preparation, characterization and in-vitro evaluation of sustained release protein-loaded nanoparticles based on biodegradable polymers. Int. J. Nanomed. 2008, 3, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Park, J.W.; Yoon, I.-S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): Recent advances in drug delivery. J. Pharm. Investig. 2013, 43, 353–362. [Google Scholar] [CrossRef]
- Molaveisi, M.; Noghabi, M.S.; Parastouei, K.; Taheri, R.A. Fate of nano-phytosomes containing bioactive compounds of Echinacea extract in an acidic food beverage. Food Struct. 2021, 27, 100177. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; Sterk, P.J.; Gaston, B.; Folkerts, G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004, 84, 731–765. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef]
- Akter, R.; Chan Ahn, J.; Nahar, J.; Awais, M.; Ramadhania, Z.M.; Oh, S.-W.; Oh, J.-H.; Kong, B.M.; Rupa, E.J.; Lee, D.W.; et al. Pomegranate juice fermented by tannin acyl hydrolase and Lactobacillus vespulae DCY75 enhance estrogen receptor expression and anti-inflammatory effect. Front. Pharmacol. 2022, 13, 1010103. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Hwang, J.-H.; Ko, H.-C.; Park, J.-G.; Kim, S.-J. Nobiletin from citrus fruit peel inhibits the DNA-binding activity of NF-κB and ROS production in LPS-activated RAW 264.7 cells. J. Ethnopharmacol. 2007, 113, 149–155. [Google Scholar] [CrossRef]
- Kabe, Y.; Ando, K.; Hirao, S.; Yoshida, M.; Handa, H. Redox regulation of NF-κB activation: Distinct redox regulation between the cytoplasm and the nucleus. Antioxid. Redox Signal. 2005, 7, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Damle, M.; Mallya, R. Development and evaluation of a novel delivery system containing phytophospholipid complex for skin aging. AAPS PharmSciTech 2016, 17, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.J.; Sung, J.J.; Cheon, K.K.; Tae, H.J. Anti-inflammatory effect of Centella asiatica phytosome in a mouse model of phthalic anhydride-induced atopic dermatitis. Phytomedicine 2018, 43, 110–119. [Google Scholar]
- Deleanu, M.; Toma, L.; Sanda, G.M.; Barbălată, T.; Niculescu, L.Ş.; Sima, A.V.; Deleanu, C.; Săcărescu, L.; Suciu, A.; Alexandru, G. Formulation of Phytosomes with Extracts of Ginger Rhizomes and Rosehips with Improved Bioavailability, Antioxidant and Anti-Inflammatory Effects In Vivo. Pharmaceutics 2023, 15, 1066. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.; Heredia, J.; Ambriz-Perez, D.; Leyva-López, N. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Bradford, B.; Yuan, K.; Farney, J.; Mamedova, L.; Carpenter, A. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef]
- Shin, J.-S.; Park, Y.M.; Choi, J.-H.; Park, H.-J.; Shin, M.C.; Lee, Y.S.; Lee, K.-T. Sulfuretin isolated from heartwood of Rhus verniciflua inhibits LPS-induced inducible nitric oxide synthase, cyclooxygenase-2, and pro-inflammatory cytokines expression via the down-regulation of NF-κB in RAW 264.7 murine macrophage cells. Int. Immunopharmacol. 2010, 10, 943–950. [Google Scholar] [CrossRef]
- Yoon, W.-J.; Heo, S.-J.; Han, S.-C.; Lee, H.-J.; Kang, G.-J.; Kang, H.-K.; Hyun, J.-W.; Koh, Y.-S.; Yoo, E.-S. Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch. Pharmacal Res. 2012, 35, 1421–1430. [Google Scholar] [CrossRef]
- Shin, J.-H.; Ryu, J.H.; Kang, M.J.; Hwang, C.R.; Han, J.; Kang, D. Short-term heating reduces the anti-inflammatory effects of fresh raw garlic extracts on the LPS-induced production of NO and pro-inflammatory cytokines by downregulating allicin activity in RAW 264.7 macrophages. Food Chem. Toxicol. 2013, 58, 545–551. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Montero, A.J.; Jassem, J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs 2011, 71, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Kuwano, K.; Hagimoto, N.; Matsuba, T.; Kunitake, R.; Tanaka, T.; Maeyama, T.; Hara, N. Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor. Am. J. Pathol. 2000, 157, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Fahmy, U.A.; Alruwaili, N.K.; Awan, Z.A.; Caruso, G.; Alfaleh, M.A.; Alaofi, A.L.; Arif, F.O.; Ahmed, O.A. Thymoquinone-loaded soy-phospholipid-based phytosomes exhibit anticancer potential against human lung cancer cells. Pharmaceutics 2020, 12, 761. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2009, 10, 445–457. [Google Scholar] [CrossRef]
- Beham, A.; Marin, M.C.; Fernandez, A.; Herrmann, J.; Brisbay, S.; Tari, A.M.; Lopez-Berestein, G.; Lozano, G.; Sarkiss, M.; McDonnell, T.J. Bcl-2 inhibits p53 nuclear import following DNA damage. Oncogene 1997, 15, 2767–2772. [Google Scholar] [CrossRef]
- Nawaz, H.; Garcia, A.; Meade, A.D.; Lyng, F.M.; Byrne, H.J. Raman micro spectroscopy study of the interaction of vincristine with A549 cells supported by expression analysis of bcl-2 protein. Analyst 2013, 138, 6177–6184. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Heme oxygenase-1: Molecular mechanisms of gene expression in oxygen-related stress. Antioxid. Redox Signal. 2002, 4, 625–632. [Google Scholar] [CrossRef]
- Fang, J.; Akaike, T.; Maeda, H. Antiapoptotic role of heme oxygenase (HO) and the potential of HO as a target in anticancer treatment. Apoptosis 2004, 9, 27–35. [Google Scholar] [CrossRef]
- No, J.H.; Kim, Y.B.; Song, Y.S. Targeting nrf2 signaling to combat chemoresistance. J. Cancer Prev. 2014, 19, 111–117. [Google Scholar] [CrossRef]
- Kweon, M.-H.; Adhami, V.M.; Lee, J.-S.; Mukhtar, H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J. Biol. Chem. 2006, 281, 33761–33772. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Srivastav, S.; Singh, M.P.; Singh, R.; Kumar, P.; Kush, P. Recent advances in phytosomes for the safe management of cancer. Phytomedicine Plus 2024, 4, 100540. [Google Scholar] [CrossRef]
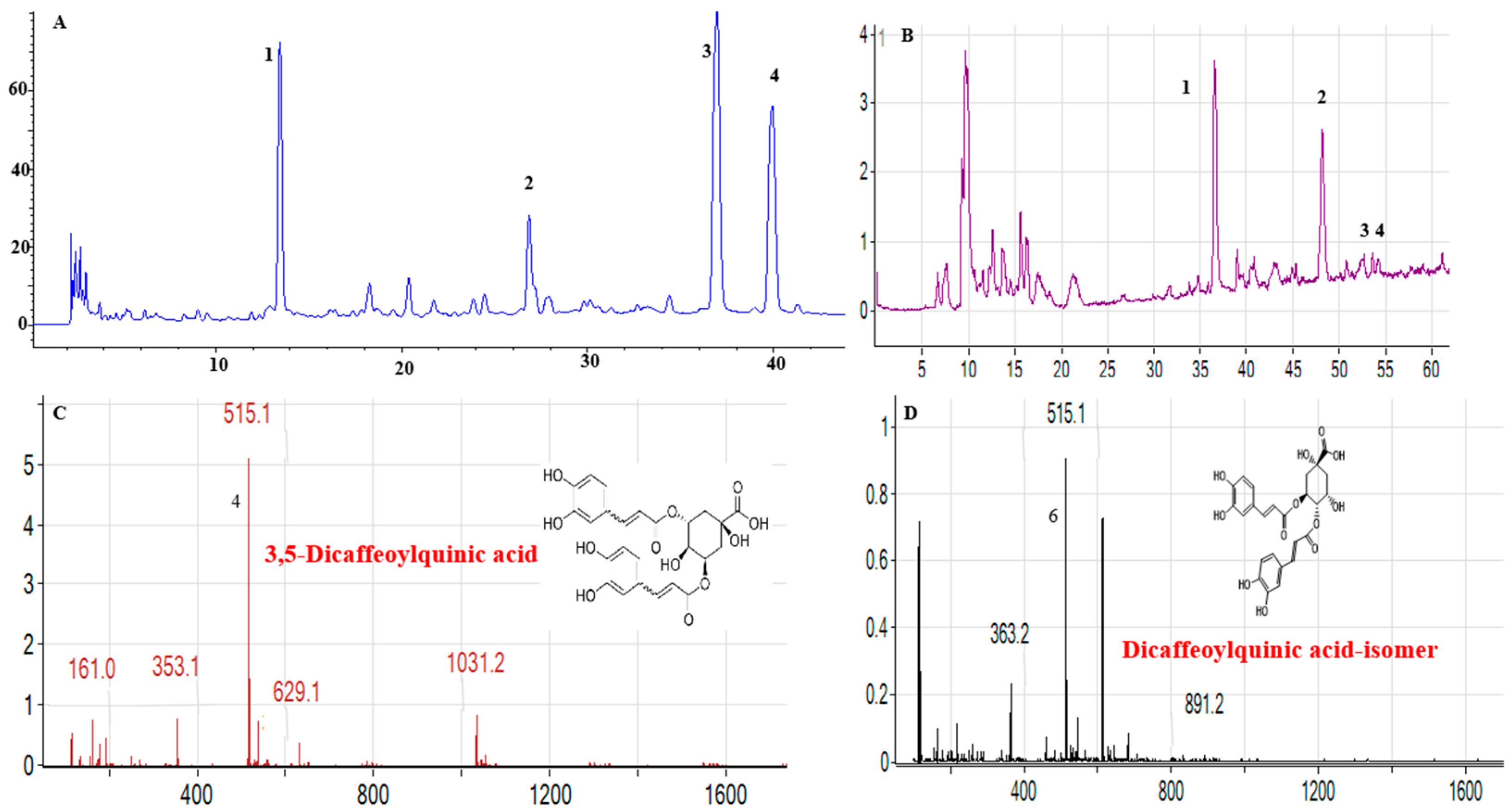
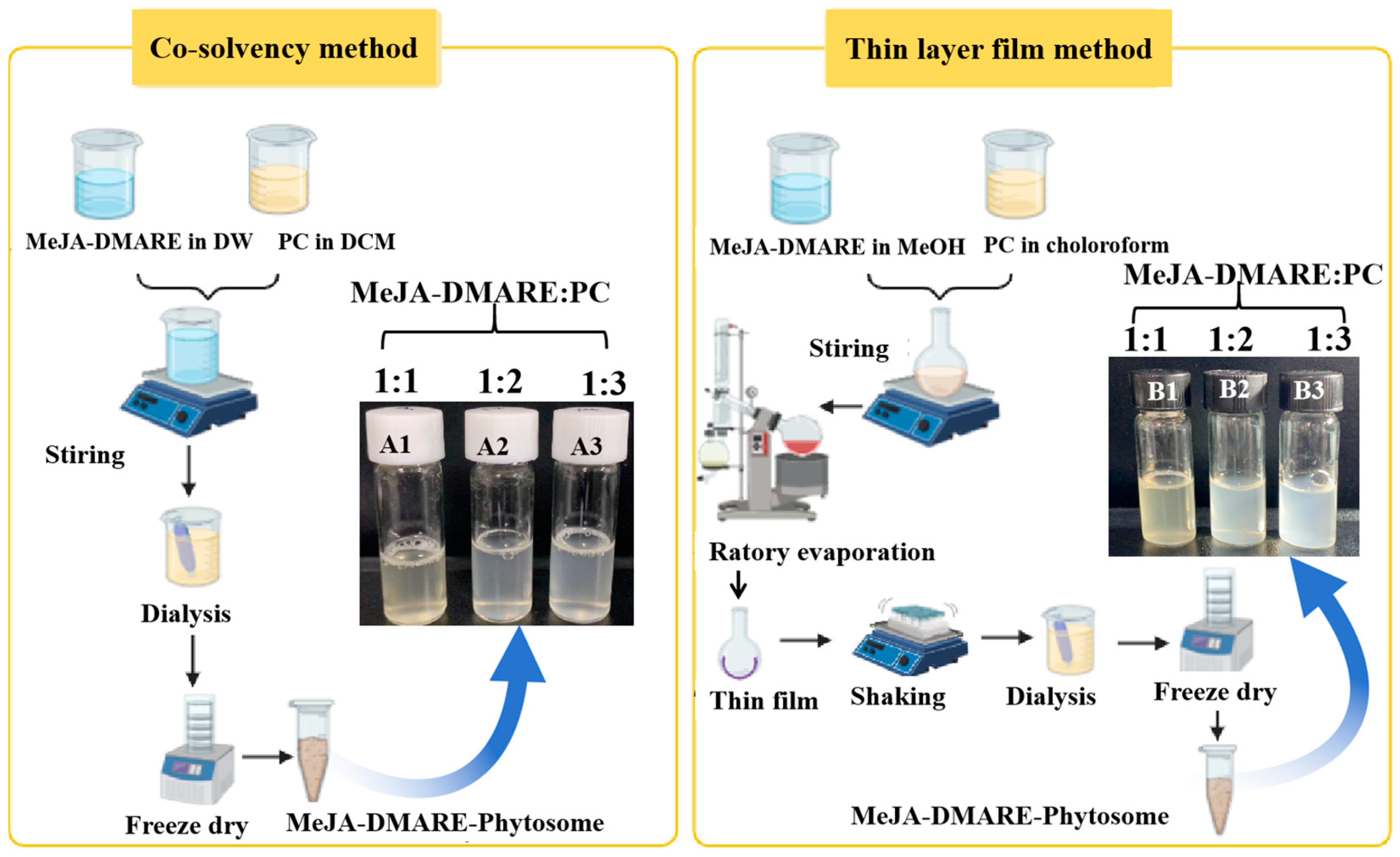
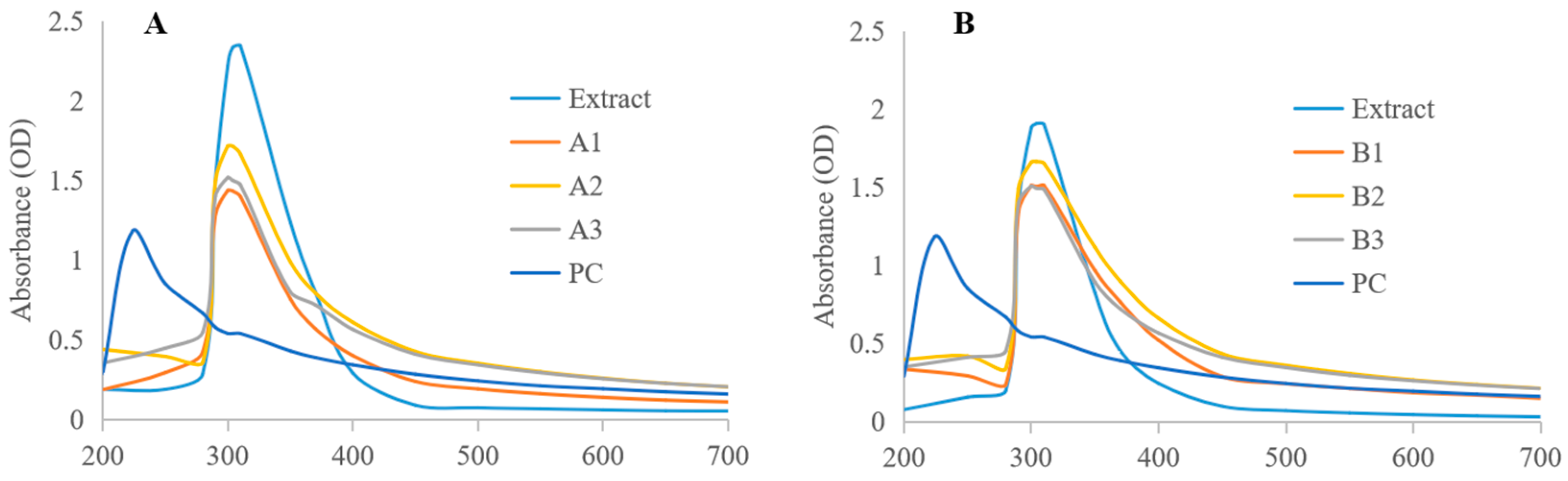
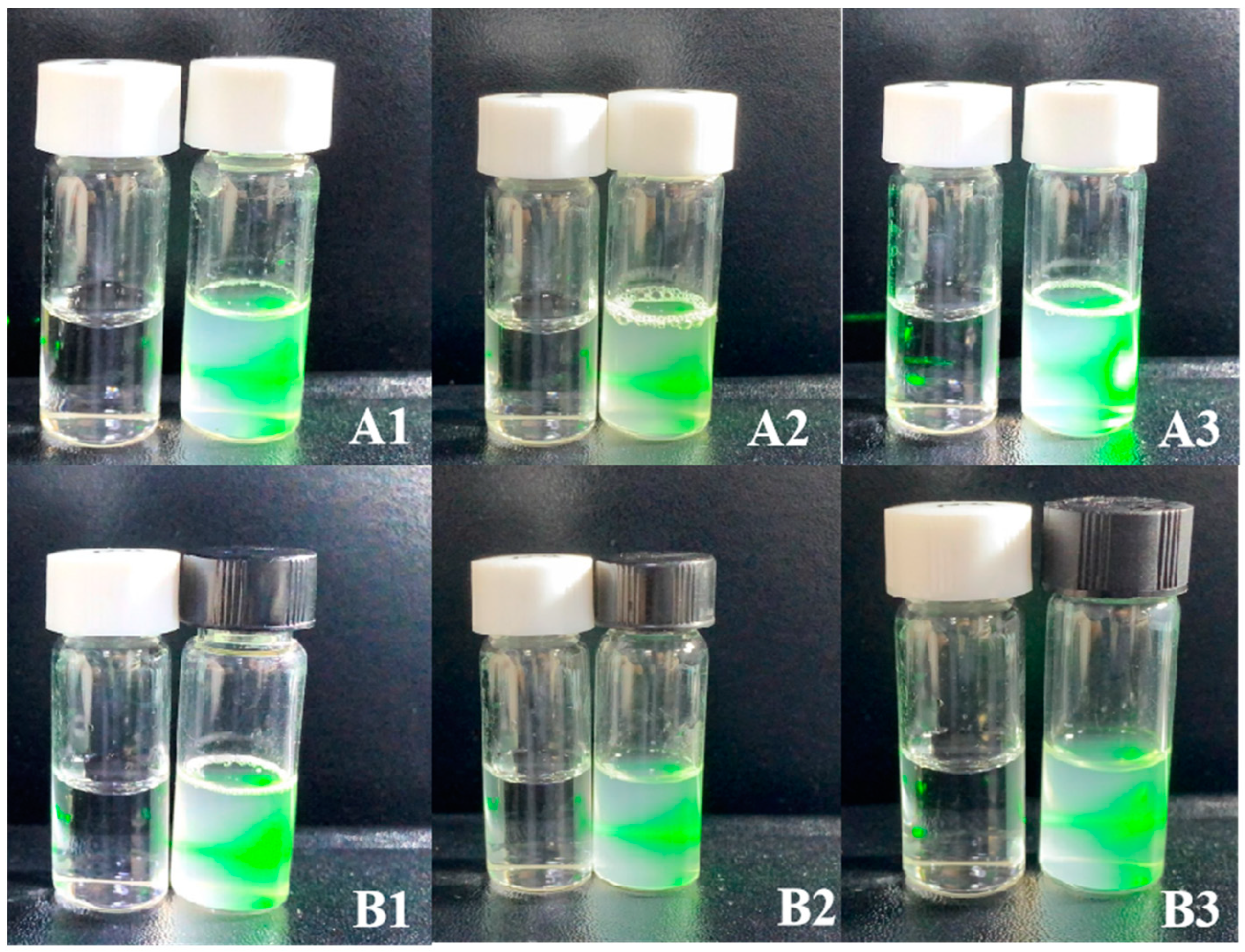


| Method | Formulation | Entrapment Efficiency (%) | Loading Capacity (%) |
|---|---|---|---|
| Co-solvency | A1 (1:1) | 56.89 ± 3.09 | 51.71 ± 3.29 |
| A2 (1:2) | 71.19 ± 4.88 | 62.91 ± 3.11 | |
| A3 (1:3) | 65.57 ± 3.49 | 61.85 ± 0.21 | |
| Thin-layer film | B1 (1:1) | 43.14 ± 2.86 | 36.50 ± 3.64 |
| B2 (1:2) | 79.98 ± 1.45 | 69.17 ± 0.14 | |
| B3 (1:3) | 57.90 ± 1.87 | 54.98 ± 4.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Xu, S.; Yang, L.; Qu, A.; Li, D.; Yu, M.; Wu, Y.; Zheng, S.; Ruan, X.; Wang, Q. Preparing a Phytosome for Promoting Delivery Efficiency and Biological Activities of Methyl Jasmonate-Treated Dendropanax morbifera Adventitious Root Extract (DMARE). Biomolecules 2024, 14, 1273. https://doi.org/10.3390/biom14101273
Xu F, Xu S, Yang L, Qu A, Li D, Yu M, Wu Y, Zheng S, Ruan X, Wang Q. Preparing a Phytosome for Promoting Delivery Efficiency and Biological Activities of Methyl Jasmonate-Treated Dendropanax morbifera Adventitious Root Extract (DMARE). Biomolecules. 2024; 14(10):1273. https://doi.org/10.3390/biom14101273
Chicago/Turabian StyleXu, Fengjiao, Shican Xu, Li Yang, Aili Qu, Dongbin Li, Minfen Yu, Yongping Wu, Shaojian Zheng, Xiao Ruan, and Qiang Wang. 2024. "Preparing a Phytosome for Promoting Delivery Efficiency and Biological Activities of Methyl Jasmonate-Treated Dendropanax morbifera Adventitious Root Extract (DMARE)" Biomolecules 14, no. 10: 1273. https://doi.org/10.3390/biom14101273
APA StyleXu, F., Xu, S., Yang, L., Qu, A., Li, D., Yu, M., Wu, Y., Zheng, S., Ruan, X., & Wang, Q. (2024). Preparing a Phytosome for Promoting Delivery Efficiency and Biological Activities of Methyl Jasmonate-Treated Dendropanax morbifera Adventitious Root Extract (DMARE). Biomolecules, 14(10), 1273. https://doi.org/10.3390/biom14101273







