Loss of dlx5a/dlx6a Locus Alters Non-Canonical Wnt Signaling and Meckel’s Cartilage Morphology
Abstract
1. Introduction
2. Materials and Methods
2.1. gRNA Construction and Germline Transformation
2.2. Animal Care and Husbandry
2.3. Histology Staining
2.4. Whole Mount In Situ Hybridization
2.5. BrdU Treatment
2.6. Acridine Orange Staining
2.7. MTOC Sample Preparation and Immunohistochemistry
2.8. RNA Extraction, cDNA Synthesis and RT-qPCR
2.9. Imaging
2.10. Cell Counting and Statistical Analysis
3. Results
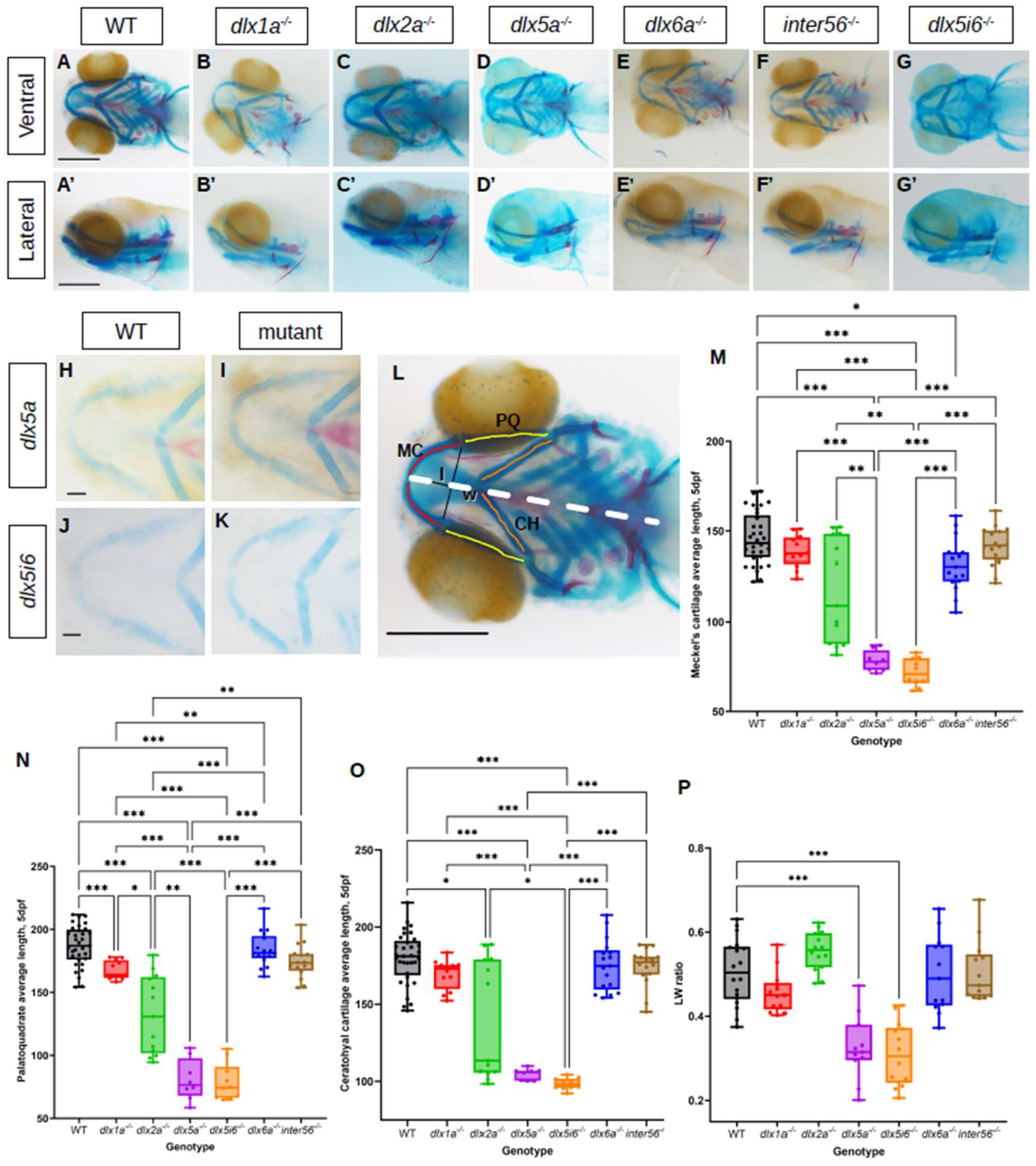
3.1. Loss of Function Mutants Are Viable and Do Not Exhibit Severe Morphological Defects
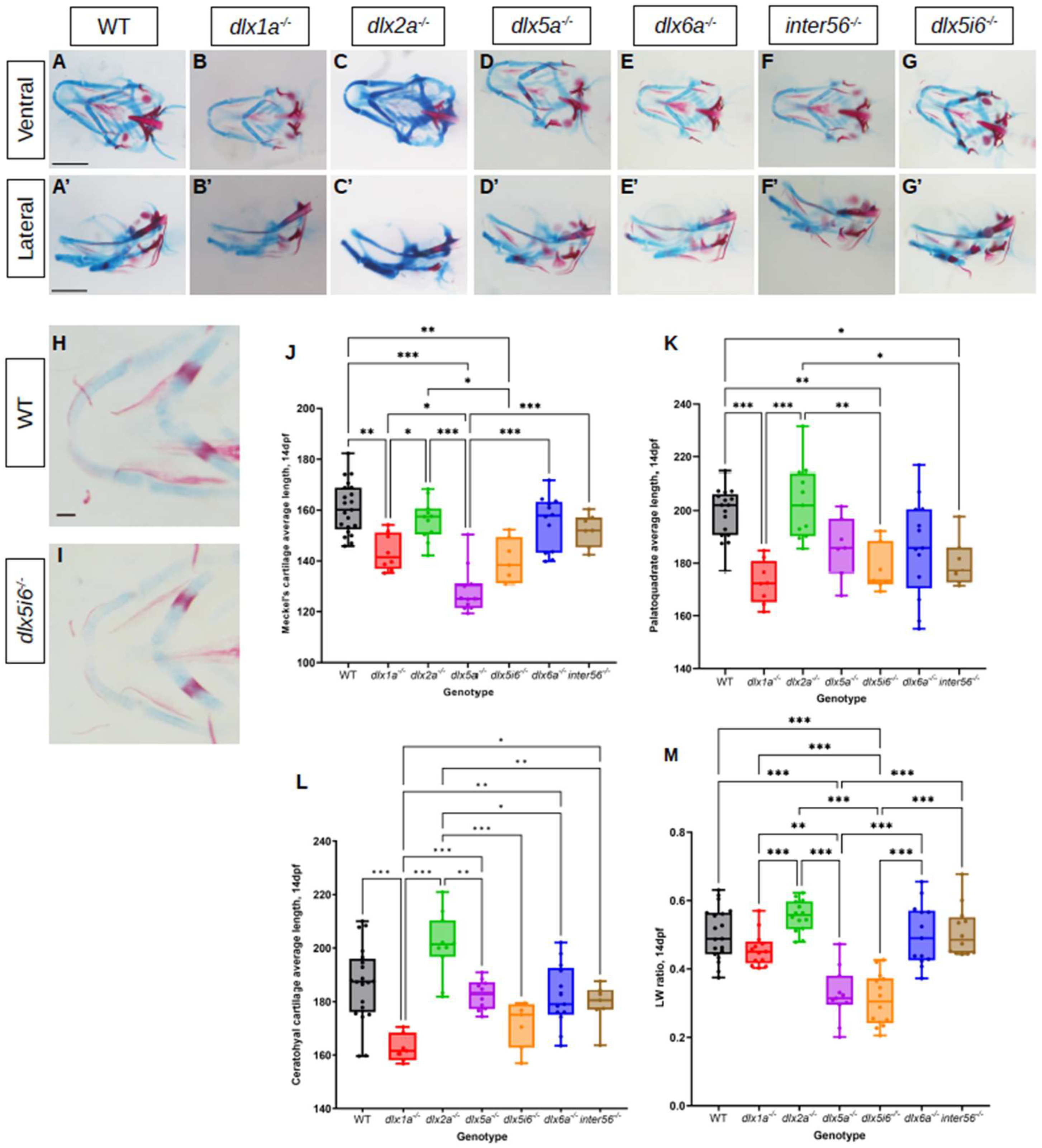
3.2. Morphology of Jaw Structures Are Altered in dlx Mutants at 5 dpf and 14 dpf
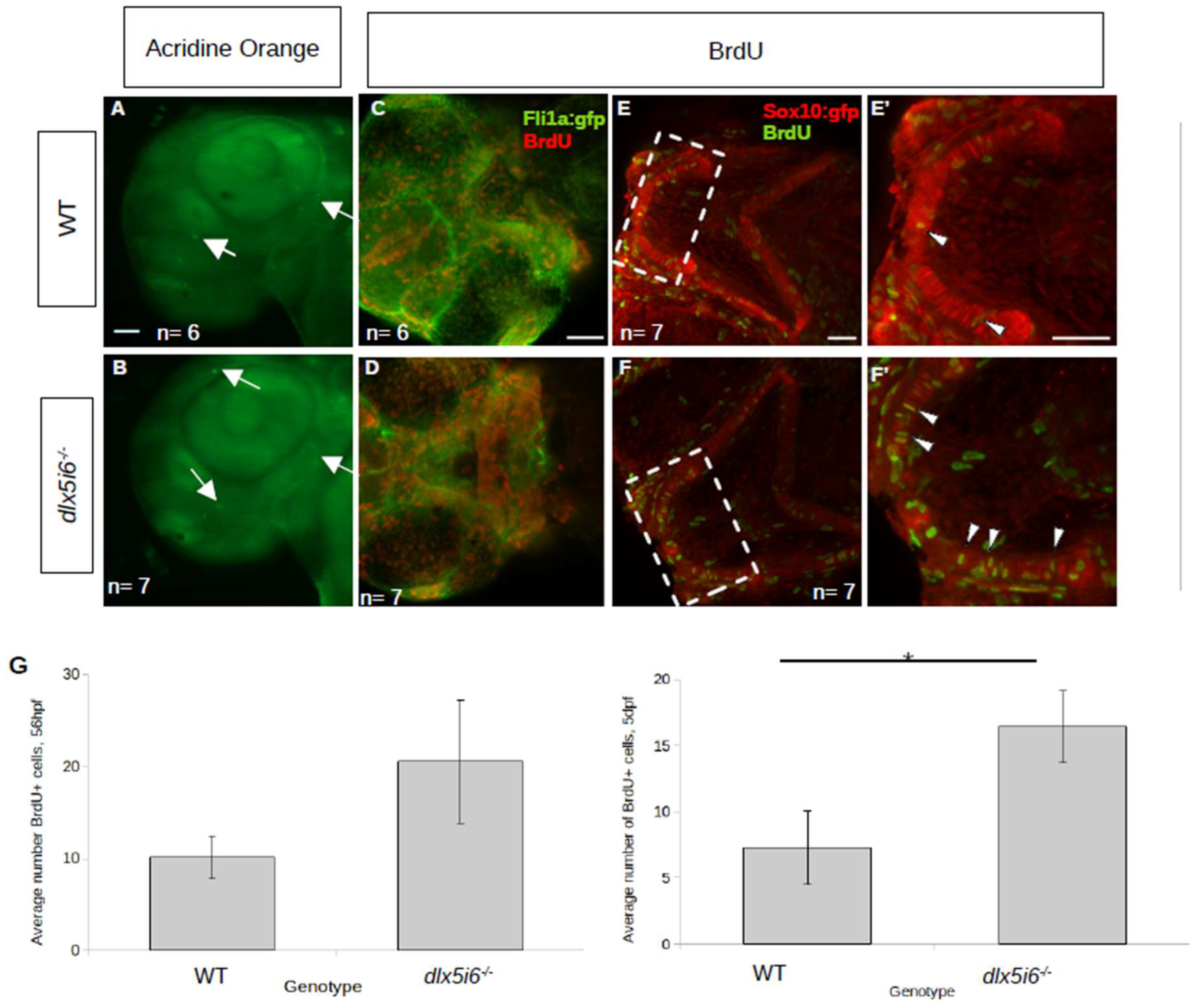
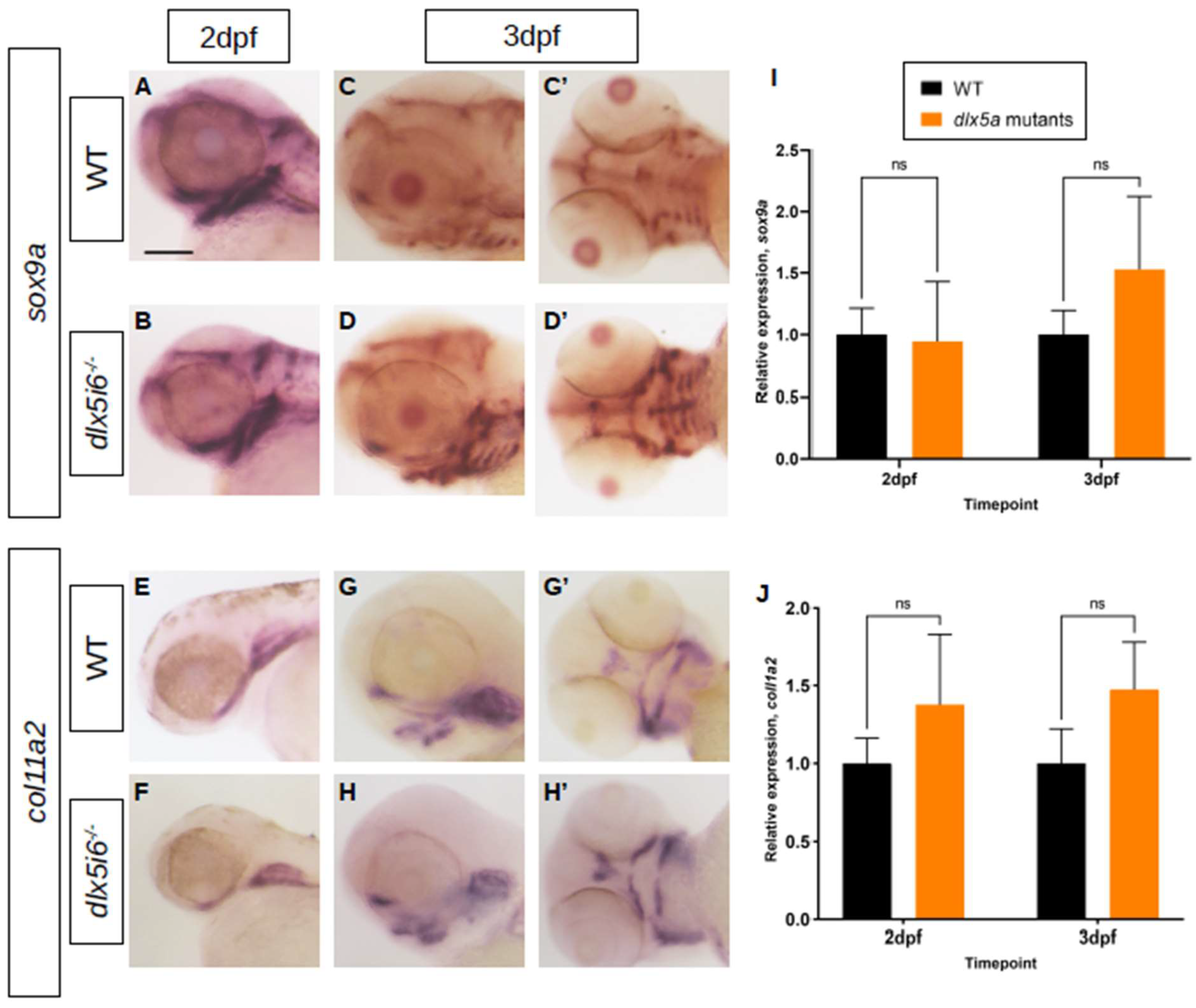
3.3. Altered Expression of Chondrocyte Markers and Increased Proliferation Observed in dlx5i6 Mutants
3.4. Non-Canonical Wnt Signaling in MC Is Abnormal in dlx5i6−/−
4. Discussion
4.1. Reduced Length of Meckel’s, Palatoquadrate and Ceratohyal Cartilages in dlx Mutants
4.2. Increased Proliferation Observed in dlx5i6−/− during Early Pharyngeal Skeletal Morphogenesis
4.3. Novel Interaction between dlx5i6 Locus and Non-Canonical Wnt Signaling during MC Morphogenesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lumsden, A.; Krumlauf, R. Patterning the Vertebrate Neuraxis. Science 1996, 274, 1109–1115. [Google Scholar] [CrossRef]
- Kulesa, P.; Ellies, D.L.; Trainor, P.A. Comparative Analysis of Neural Crest Cell Death, Migration, and Function during Vertebrate Embryogenesis. Dev. Dyn. 2004, 229, 14–29. [Google Scholar] [CrossRef]
- Piotrowski, T.; Schilling, T.F.; Brand, M.; Jiang, Y.J.; Heisenberg, C.P.; Beuchle, D.; Grandel, H.; Van Eeden, F.J.M.; Furutani-Seiki, M.; Granato, M.; et al. Jaw and Branchial Arch Mutants in Zebrafish II: Anterior Arches and Cartilage Differentiation. Development 1996, 123, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.F.; Kimmel, C.B. Segment and Cell Type Lineage Restrictions during Pharyngeal Arch Development in the Zebrafish Embryo. Development 1994, 120, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.F.; Le Pabic, P. Neural Crest Cells in Craniofacial Skeletal Development. In Neural Crest Cells: Evolution, Development and Disease; Trainor, P.A., Ed.; Elsevier: Oxford, UK, 2014; pp. 127–151. [Google Scholar] [CrossRef]
- Amores, A.; Force, A.; Yan, Y.L.; Joly, L.; Amemiya, C.; Fritz, A.; Ho, R.K.; Langeland, J.; Prince, V.; Wang, Y.L.; et al. Zebrafish Hox Clusters and Vertebrate Genome Evolution. Science 1998, 282, 1711–1714. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.G.; Swartz, M.E.; Eberhart, J.K.; Kimmel, C.B. Moz-Dependent Hox Expression Controls Segment-Specific Fate Maps of Skeletal Precursors in the Face. Development 2006, 133, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Depew, M.J.; Lufkin, T.; Rubenstein, J.L.R. Specification of Jaw Subdivisions by Dlx Genes. Science 2002, 298, 381–385. [Google Scholar] [CrossRef]
- Miller, C.T.; Maves, L.; Kimmel, C.B. Moz Regulates Hox Expression and Pharyngeal Segmental Identity in Zebrafish. Development 2004, 131, 2443–2461. [Google Scholar] [CrossRef]
- Baggiolini, A.; Varum, S.; Mateos, J.M.; Bettosini, D.; John, N.; Bonalli, M.; Ziegler, U.; Dimou, L.; Clevers, H.; Furrer, R.; et al. Premigratory and Migratory Neural Crest Cells Are Multipotent in Vivo. Cell Stem Cell 2015, 16, 314–322. [Google Scholar] [CrossRef]
- Ling, I.T.; Rochard, L.; Liao, E.C. Distinct Requirements of Wls, Wnt9a, Wnt5b and Gpc4 in Regulating Chondrocyte Maturation and Timing of Endochondral Ossification. Dev. Biol. 2017, 421, 219–232. [Google Scholar] [CrossRef]
- Jussila, M.; Ciruna, B. Zebrafish Models of Non-Canonical Wnt/Planar Cell Polarity Signalling: Fishing for Valuable Insight into Vertebrate Polarized Cell Behavior. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e267. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Tan, X.; Zhang, H.; Liu, C.; Zhao, B.; Li, Y.; Lu, L.; Liu, Y.; Zhou, J. Ror2 Receptor Mediates Wnt11 Ligand Signaling and Affects Convergence and Extension Movements in Zebrafish. J. Biol. Chem. 2014, 289, 20664–20676. [Google Scholar] [CrossRef]
- Curtin, E.; Hickey, G.; Kamel, G.; Davidson, A.J.; Liao, E.C. Zebrafish Wnt9a Is Expressed in Pharyngeal Ectoderm and Is Required for Palate and Lower Jaw Development. Mech. Dev. 2011, 128, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Dranow, D.B.; Le Pabic, P.; Schilling, T.F. The Non-Canonical Wnt Receptor Ror2 Is Required for Cartilage Cell Polarity and Morphogenesis of the Craniofacial Skeleton in Zebrafish. Development 2023, 150, dev201273. [Google Scholar] [CrossRef] [PubMed]
- Sisson, B.E.; Dale, R.M.; Mui, S.R.; Topczewska, J.M.; Topczewski, J. A Role of Glypican4 and Wnt5b in Chondrocyte Stacking Underlying Craniofacial Cartilage Morphogenesis. Mech. Dev. 2015, 138, 279–290. [Google Scholar] [CrossRef]
- Wu, B.T.; Wen, S.H.; Hwang, S.P.L.; Huang, C.J.; Kuan, Y.S. Control of Wnt5b Secretion by Wntless Modulates Chondrogenic Cell Proliferation through Fine-Tuning Fgf3 Expression. J. Cell Sci. 2015, 128, 2328–2339. [Google Scholar] [CrossRef]
- Zerucha, T.; Ekker, M. Distal-Less-Related Homeobox Genes of Vertebrates: Evolution, Function, and Regulation. Biochem. Cell Biol. 2000, 78, 593–601. [Google Scholar] [CrossRef]
- Anderson, S.A.; Qiu, M.; Bulfone, A.; Eisenstat, D.D.; Meneses, J.; Pedersen, R.; Rubenstein, J.L.R. Mutations of the Homeobox Genes Dlx-1 and Dlx-2 Disrupt the Striatal Subventricular Zone and Differentiation of Late Born Striatal Neurons. Neuron 1997, 19, 27–37. [Google Scholar] [CrossRef]
- Beverdam, A.; Merlo, G.R.; Paleari, L.; Mantero, S.; Genova, F.; Barbieri, O.; Janvier, P.; Levi, G. Jaw Transformation with Gain of Symmetry after Dlx5/Dlx6 Inactivation: Mirror of the Past? Genesis 2002, 34, 221–227. [Google Scholar] [CrossRef]
- Depew, M.J.; Simpson, C.A.; Morasso, M.; Rubenstein, J.L.R. Reassessing the Dlx Code: The Genetic Regulation of Branchial Arch Skeletal Pattern and Development. J. Anat. 2005, 207, 501–561. [Google Scholar] [CrossRef]
- Talbot, J.; Johnson, S.L.; Kimmel, C.B. Hand2 and Dlx Genes Specify Dorsal, Intermediate and Ventral Domains within Zebrafish Pharyngeal Arches. Development 2010, 137, 2507–2517. [Google Scholar] [CrossRef] [PubMed]
- Sperber, S.M.; Saxena, V.; Hatch, G.; Ekker, M. Zebrafish Dlx2a Contributes to Hindbrain Neural Crest Survival, Is Necessary for Differentiation of Sensory Ganglia and Functions with Dlx1a in Maturation of the Arch Cartilage Elements. Dev. Biol. 2008, 314, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.O.; Shin, M.; Ni, C.-W.; Gupta, A.; Grosse, A.S.; Van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Article Reverse Genetic Screening Reveals Poor Correlation between Morpholino-Induced and Mutant Phenotypes in Zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef]
- Yu, E.P.Y.; Perin, S.; Saxena, V.; Ekker, M. Novel Cross-Regulation Interactions between Dlx Genes in Larval Zebrafish. Gene 2021, 801, 145848. [Google Scholar] [CrossRef]
- Varshney, G.K.; Pei, W.; Lafave, M.C.; Idol, J.; Xu, L.; Gallardo, V.; Carrington, B.; Bishop, K.; Jones, M.; Li, M.; et al. High-Throughput Gene Targeting and Phenotyping in Zebrafish Using CRISPR/Cas9. Genome Res. 2015, 25, 1030–1042. [Google Scholar] [CrossRef]
- Walker, M.B.; Kimmel, C.B. A Two-Color Acid-Free Cartilage and Bone Stain for Zebrafish Larvae. Biotech. Histochem. 2009, 82, 23–28. [Google Scholar] [CrossRef]
- Chiang, E.F.L.; Pai, C.I.; Wyatt, M.; Yan, Y.L.; Postlethwait, J.; Chung, B. chu Two Sox9 Genes on Duplicated Zebrafish Chromosomes: Expression of Similar Transcription Activators in Distinct Sites. Dev. Biol. 2001, 231, 149–163. [Google Scholar] [CrossRef]
- Thisse, C.; Thisse, B. High-Resolution in Situ Hybridization to Whole-Mount Zebrafish Embryos. Nat. Protoc. 2008, 3, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Nikaido, M.; Horiuchi, N.; Kawakami, K.; Hatta, K. The Enteric Nervous System in Zebrafish Larvae Can Regenerate via Migration into the Ablated Area and Proliferation of Neural Crest-Derived Cells. Development 2021, 148, dev195339. [Google Scholar] [CrossRef]
- Kalyn, M.; Ekker, M. Cerebroventricular Microinjections of MPTP on Adult Zebrafish Induces Dopaminergic Neuronal Death, Mitochondrial Fragmentation, and Sensorimotor Impairments. Front. Neurosci. 2021, 15, 718244. [Google Scholar] [CrossRef]
- McCurley, A.T.; Callard, G.V. Characterization of Housekeeping Genes in Zebrafish: Male-Female Differences and Effects of Tissue Type, Developmental Stage and Chemical Treatment. BMC Mol. Biol. 2008, 9, 102. [Google Scholar] [CrossRef]
- Mukherjee, D.; Wagh, G.; Mokalled, M.H.; Kontarakis, Z.; Dickson, A.L.; Rayrikar, A.; Günther, S.; Poss, K.D.; Stainier, D.Y.R.; Patra, C. Ccn2a Is an Injury-Induced Matricellular Factor That Promotes Cardiac Regeneration in Zebrafish. Development 2021, 148, dev193219. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Poobalan, Y.; Tan, E.K.; Tao, S.; Ong, S.; Wehner, P.; Schwenty-Lara, J.; Lim, C.Y.; Sadasivam, A.; Lovatt, M.; et al. The PDZ Domain Protein Mcc Is a Novel Effector of Non-Canonical Wnt Signaling during Convergence and Extension in Zebrafish. Development 2014, 141, 3505–3516. [Google Scholar] [CrossRef] [PubMed]
- Linkert, M.; Rueden, C.T.; Allan, C.; Burel, J.M.; Moore, W.; Patterson, A.; Loranger, B.; Moore, J.; Neves, C.; MacDonald, D.; et al. Metadata Matters: Access to Image Data in the Real World. J. Cell Biol. 2010, 189, 777. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bell, D.M.; Leung, K.K.H.; Wheatley, S.C.; Ng, L.J.; Zhou, S.; Ling, K.W.; Sham, M.H.; Wing, K.; Har, M.; Koopman, P.; et al. SOX9 Directly Regulates the Type-II Collagen Gene. Nat. Genet. 1997, 16, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Mori-Akiyama, Y.; Akiyama, H.; Rowitch, D.H.; De Crombrugghe, B. Sox9 Is Required for Determination of the Chondrogenic Cell Lineage in the Cranial Neural Crest. Proc. Natl. Acad. Sci. USA 2003, 100, 9360–9365. [Google Scholar] [CrossRef]
- Yan, Y.L.; Miller, C.T.; Nissen, R.; Singer, A.; Liu, D.; Kirn, A.; Draper, B.; Willoughby, J.; Morcos, P.A.; Amsterdam, A.; et al. A Zebrafish Sox9 Gene Required for Cartilage Morphogenesis. Development 2002, 129, 5065–5079. [Google Scholar] [CrossRef]
- Fang, M.; Adams, J.S.; McMahan, B.L.; Brown, R.J.; Oxford, J.T. The Expression Patterns of Minor Fibrillar Collagens during Development in Zebrafish. Gene Expr. Patterns 2010, 10, 315–322. [Google Scholar] [CrossRef]
- Kamel, G.; Hoyos, T.; Rochard, L.; Dougherty, M.; Kong, Y.; Tse, W.; Shubinets, V.; Grimaldi, M.; Liao, E.C. Requirement for Frzb and Fzd7a in Cranial Neural Crest Convergence and Extension Mechanisms during Zebrafish Palate and Jaw Morphogenesis. Dev. Biol. 2013, 381, 423–433. [Google Scholar] [CrossRef]
- Randall, R.M.; Shao, Y.Y.; Wang, L.; Ballock, R.T. Activation of Wnt Planar Cell Polarity (PCP) Signaling Promotes Growth Plate Column Formation in Vitro. J. Orthop. Res. 2012, 30, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Baye, L.M.; Westfall, T.A.; Slusarski, D.C. Wnt5b-Ryk Pathway Provides Directional Signals to Regulate Gastrulation Movement. J. Cell Biol. 2010, 190, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Borovina, A.; Superina, S.; Voskas, D.; Ciruna, B. Vangl2 Directs the Posterior Tilting and Asymmetric Localization of Motile Primary Cilia. Nat. Cell Biol. 2010, 12, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Kilian, B.; Mansukoski, H.; Barbosa, F.C.; Ulrich, F.; Tada, M.; Heisenberg, C.P. The Role of Ppt/Wnt5 in Regulating Cell Shape and Movement during Zebrafish Gastrulation. Mech. Dev. 2003, 120, 467–476. [Google Scholar] [CrossRef]
- Robledo, R.F.; Rajan, L.; Li, X.; Lufkin, T. The Dlx5 and Dlx6 Homeobox Genes Are Essential for Craniofacial, Axial, and Appendicular Skeletal Development. Genes Dev. 2002, 16, 1089–1101. [Google Scholar] [CrossRef]
- Shimizu, M.; Narboux-Nême, N.; Gitton, Y.; de Lombares, C.; Fontaine, A.; Alfama, G.; Kitazawa, T.; Kawamura, Y.; Heude, E.; Marshall, L.; et al. Probing the Origin of Matching Functional Jaws: Roles of Dlx5/6 in Cranial Neural Crest Cells. Sci. Rep. 2018, 8, 14975. [Google Scholar] [CrossRef]
- Eames, B.F.; Delaurier, A.; Ullmann, B.; Huycke, T.R.; Nichols, J.T.; Dowd, J.; McFadden, M.; Sasaki, M.M.; Kimmel, C.B. FishFace: Interactive Atlas of Zebrafish Craniofacial Development at Cellular Resolution. BMC Dev. Biol. 2013, 13, 23. [Google Scholar] [CrossRef]
- Kague, E.; Gallagher, M.; Burke, S.; Parsons, M.; Franz-Odendaal, T. Skeletogenic Fate of Zebrafish Cranial and Trunk Neural Crest. PLoS ONE 2012, 7, 47394. [Google Scholar] [CrossRef]
- MacKenzie, R.K.; Sankar, P.R.; Bendall, A.J. Dlx5 and Dlx6 Can Antagonize Cell Division at the G1/S Checkpoint. BMC Mol. Cell Biol. 2019, 20, 8. [Google Scholar] [CrossRef]
- Ridenour, D.A.; Mclennan, R.; Teddy, J.M.; Semerad, C.L.; Haug, J.S.; Kulesa, P.M. The Neural Crest Cell Cycle Is Related to Phases of Migration in the Head. Development 2014, 141, 1095–1103. [Google Scholar] [CrossRef][Green Version]
- Perkins, R.S.; Suthon, S.; Miranda-Carboni, G.A.; Krum, S.A. WNT5B in cellular signaling pathways. Semin. Cell Dev. Biol. 2022, 125, 11–16. [Google Scholar] [CrossRef]



| Target | gRNA 1 (5′-3′) | gRNA 2 (5′-3′) |
|---|---|---|
| dlx1a | GGACCAATCAGAGAGCACCT | GGTCCCCTGAACTGGGCCAT |
| dlx2a | GGCAATGATCAACGTGGCAT | GGAAACGCTTTCGGCCCCTA |
| dlx5a | GGCTATTACAGCCCTGCCGG | GGCTCTCGATATAATGGCAT |
| dlx6a | GGGAGCCGGAATGGAGACAG and GGCAGGTACGGACTGTGGTG | GGCGTGTCAATGGTCGACAA |
| inter56 | GGCGTGTCAATGGTCGACAA | GGCTCTCGATATAATGGCAT |
| dlx5i6 | GGGAGCCGGAATGGAGACAG | GGCTATTACAGCCCTGCCGG |
| Target | Forward Sequence (5′-3′) | Reverse Sequence | Product Size |
|---|---|---|---|
| dlx1a WT | CCTATGCGTCTCGGTCCATT | CGTAGTGCGTCGACAAAAGC | 740 bp |
| dlx1a mutant | GTGTTTCTTCTCCGGTGCGA | 320–350 bp | |
| dlx2a WT | GCATGAAACACATGGAAACCGA | AGTCTCCACCTCACCCCTCA | 629 bp |
| dlx2a mutant | TTGCTGACGCGACATTGC | CAAGTCCCAGGAATCGCCG | 130–160 bp |
| dlx5a WT | ATCAAGAACCAGGCGCATCT | GAGCCCACACAGAGATAGCA | 498 bp |
| dlx5a mutant | CCCTTTTCAGCTCTCCACGAT | 290–310 bp | |
| dlx6a WT | GGAGGCTCAAGACTCGTCAA | TTGCTGACATACGACGGGG | 429 bp |
| dlx6a mutant | AAAACGATTCGCTTCCTGTC | 200–250 bp | |
| inter56 WT | AGCTACATGCCCGGCTATTC | GGACAAGATGCGGCTCTATTC | 796 bp |
| inter56 mutant | ATAGCCCCCAACCTACAAGC | 350–400 bp | |
| dlx5i6 WT | GGAGGCTCAAGACTCGTCAA | TTGCTGACATACGACGGGG | 429 bp |
| dlx5i6 mutant | CCCTTTTCAGCTCTCCACGAT | 200–230 bp |
| Target | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| foxd3 | AATTAACCCTCACTAAAGGCTCAGTGGA | TAATACGACTCACTATAGCCATTTCGAT |
| ATCTGCGAGT | ACCGCTGCTG | |
| col11a2 | AATTAACCCTCACTAAAGAGACCTCGTTT | TAATACGACTCACTATAGACGGGCTCC |
| GTGCTCCTC | AAAAACAGTGA | |
| wls | AATTAACCCTCACTAAAGCGGATGGAGC | TAATACGACTCACTATAGCATTGCGCA |
| TTCGATCACC | GGCAGTAATCC | |
| wnt5b | AATTAACCCTCACTAAAGGTGACCCTCAT | TAATACGACTCACTATAGCATTGCGCA |
| CGTCTGCAA | GGCAGTAATCC |
| Target | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Amplicon Size |
|---|---|---|---|
| sox9a | GCGTCCAGCATGGGAGAAGT | GCCTCTCGTTTCAGATCCGC | 125 bp |
| col11a1 [33] | GATATTCGGAAGAAGCGGAGG | CGCAAAACATCTACTGGATCTG | 101 bp |
| wnt5b | TATTGGAGAGGGGGCGAAGA | ATATGCATGACACGGCCGAA | 114 bp |
| ror2 [34] | GAGGATTACAACTGGAGCTCAT | AGCCTCAATCTCTTCAGACTCG | 106 bp |
| frzd7a | GACAAATTCAGCGACGACGG | CCCGCTGAGAGAAACCATGT | 149 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, E.P.Y.; Saxena, V.; Perin, S.; Ekker, M. Loss of dlx5a/dlx6a Locus Alters Non-Canonical Wnt Signaling and Meckel’s Cartilage Morphology. Biomolecules 2023, 13, 1347. https://doi.org/10.3390/biom13091347
Yu EPY, Saxena V, Perin S, Ekker M. Loss of dlx5a/dlx6a Locus Alters Non-Canonical Wnt Signaling and Meckel’s Cartilage Morphology. Biomolecules. 2023; 13(9):1347. https://doi.org/10.3390/biom13091347
Chicago/Turabian StyleYu, Emily P. Y., Vishal Saxena, Sofia Perin, and Marc Ekker. 2023. "Loss of dlx5a/dlx6a Locus Alters Non-Canonical Wnt Signaling and Meckel’s Cartilage Morphology" Biomolecules 13, no. 9: 1347. https://doi.org/10.3390/biom13091347
APA StyleYu, E. P. Y., Saxena, V., Perin, S., & Ekker, M. (2023). Loss of dlx5a/dlx6a Locus Alters Non-Canonical Wnt Signaling and Meckel’s Cartilage Morphology. Biomolecules, 13(9), 1347. https://doi.org/10.3390/biom13091347






