Abstract
Zebrafish are increasingly becoming an important model organism for studying the pathophysiological mechanisms of human diseases and investigating how these mechanisms can be effectively targeted using compounds that may open avenues to novel treatments for patients. The zebrafish skeleton has been particularly instrumental in modeling bone diseases as—contrary to other model organisms—the lower load on the skeleton of an aquatic animal enables mutants to survive to early adulthood. In this respect, the axial skeletons of zebrafish have been a good read-out for congenital spinal deformities such as scoliosis and degenerative disorders such as osteoporosis and osteoarthritis, in which aberrant mineralization in humans is reflected in the respective zebrafish models. Interestingly, there have been several reports of hereditary multisystemic diseases that do not affect the vertebral column in human patients, while the corresponding zebrafish models systematically show anomalies in mineralization and morphology of the spine as their leading or, in some cases, only phenotype. In this review, we describe such examples, highlighting the underlying mechanisms, the already-used or potential power of these models to help us understand and amend the mineralization process, and the outstanding questions on how and why this specific axial type of aberrant mineralization occurs in these disease models.
1. Introduction
Over the last 20 years, zebrafish have become an important model organism for studying the development and diseases of the skeleton in basic and preclinical research [1,2]. In general, the popularity of zebrafish as a model system is associated with their rapid external development, short generation time, large offspring number (which increases statistical power due to higher sample numbers), and imaging possibilities that are facilitated by the transparency of zebrafish embryos and larvae [1,2,3,4,5]. Genetically, there is 71% homology between the human and zebrafish genome, with 82% of the human-disease-causing genes having a zebrafish orthologue [3,6]. This is also true for key bone development regulators, which are highly conserved and have similar expression patterns in zebrafish and humans [2,7]. An additional advantage compared to mouse models, wherein many mutants with bone defects are embryonic lethal or die soon after birth, is that zebrafish mutants usually survive to early adulthood, possibly because of the reduced load on the skeleton in aquatic animals [2,8].
The axial skeleton of the zebrafish has been put forward as a model for understanding common spinal deformities, such as (idiopathic) scoliosis, and degenerative diseases like osteoarthritis and osteoporosis [9]. To interpret the effects of gene mutations and expression patterns and establish correlations between genes and morphology, a comprehensive understanding of the development of the axial skeleton is necessary. Methods such as systematic genome-wide mutagenesis screens can be used to understand the early patterning of the embryonic tissue required to build and pattern the embryonic spine [10]. Forward screens using N-ethyl-N-nitrosourea (ENU) have increased our knowledge on the postembryonic maturation and homeostasis of the spine, but the involved mechanisms remain poorly understood [10,11,12,13,14,15,16].
For many multisystem disorders, that is, diseases that affect multiple body systems, zebrafish have been instrumental in understanding the pathophysiological mechanisms of disease and the preclinical evaluation of the effects of drugs on one or more affected organ systems. Further, the partitioning of the expression patterns and functions of the duplicated zebrafish genome can be particularly helpful in untangling the different (patho)physiological roles of a gene [17,18,19]. Interestingly, several zebrafish models for hereditary multisystem disorders that do not affect the vertebral column in humans are characterized by abnormalities in the mineralization and morphology of the axial skeleton. In this review, we provide an overview of such zebrafish models, including the involved genes and signaling pathways. We illustrate how the axial skeleton can be a valuable read-out for the modeling of and therapeutic intervention in complex multisystemic disorders and how these disease models can be useful for providing insights into the biology of normal and pathological (skeletal) development in zebrafish.
2. Materials and Methods
A literature search was conducted via the Pubmed, ScienceDirect, Embase, and Google Scholar databases, limited to papers published in English and using the following key words: ‘zebrafish’, ‘disease model’, ‘axial skeleton’, ‘vertebral column’, ‘vertebra’, ‘mineralization’, and ‘calcification’. Exclusion criteria included papers on a bone disease or on a vertebral deformation disease (e.g., scoliosis). The remaining papers were further reviewed to ascertain whether the modeled disease was a multisystemic disorder. For each of the disorders that were retained, an additional search was conducted to find additional papers on zebrafish models, using the names of the diseases and ‘zebrafish’ as keywords.
3. A Brief Overview of the Normal Development of the Axial Skeleton in Zebrafish
The development of the teleost skeletal system is a complex spatiotemporally regulated process that—although very similar to that for amniotes—has several distinct characteristics [9,20,21,22,23,24,25,26,27,28,29]. These include the facts that osteocytes are not present in all bones, that certain structures such as the skull show greater complexity compared to mammals, and that many different bone and cartilage types with different cellularity and matrix compositions exist in physiological conditions while often only occurring in diseases in vertebrates [30,31]. For a detailed review of the bone development in zebrafish, we would like to refer the reader to several excellent reviews [4,5,9,20,27,31,32,33,34,35,36]. Here, we will focus on the development of the axial skeleton of teleosts.
As part of the endoskeleton of zebrafish, the axial skeleton consists of the vertebral column, ribs, intermuscular bones, and unpaired fins (Figure 1) [4,20]. The vertebral column contains 31 vertebrae in total: 4 vertebrae that form the Weberian apparatus, 10 abdominal vertebrae, 14 caudal vertebrae, and 3 caudal fin vertebrae [20]. The vertebrae—each containing a centrum, hemal and neural arches, and a spine—are connected by a ring-shaped ligament built up from intervertebral soft tissue [3,20]. The developmental timing and origin of the skeletal elements in the axial skeleton are complex; we would like to refer the reader to the work of Bird et al. [4] for a detailed overview, but we touch on a few essential principles below.
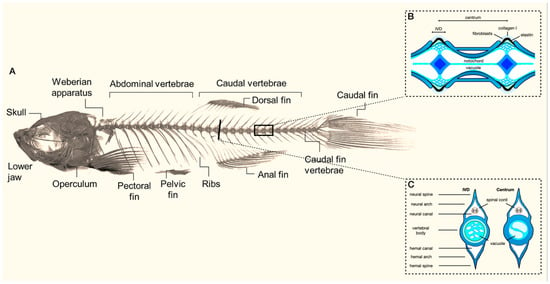
Figure 1.
(A) Overview of the composition of the axial skeleton of adult zebrafish (modified from Dietrich et al. (2021)) [20]. Inserts illustrate a sagittal (B) and transversal (C) view of the morphology of the vertebral body and the intervertebral disc (IVD) (adapted from Cotti et al. (2022)) [21].
Physiological ossification starts around 3 days post fertilization (dpf), beginning with the cranial cartilage. At the same time, the cleithrum, fifth branchial arch, and opercle form through dermal ossification [2,37]. Next, the centra of the axial skeleton are formed, starting with the third and fourth centrums at around 6 dpf [2,4,6,37]. Additional centra are then added bidirectionally [4]. Contrary to the other centra and unlike other fish, the Weberian centra remain unfused throughout development in zebrafish [4].
In contrast to humans, initial vertebral column development in zebrafish is not derived from the sclerotome but takes place through the direct mineralization of the notochord [23,38,39,40,41]. The notochord is composed of chordocytes, which provide a hydrostatic core, and an outer epithelial layer of chordoblasts. The chordoblasts secrete a collagenous matrix during early development that builds the notochord sheath and surrounds the notochord. The segmental mineralization of the notochord sheath induced by BMP and retinoid acid signals leads to the construction of the chordacentrum of the vertebral body [41,42]. Subsequently, sclerotome-derived cells form bone around the chordacentrum, creating the autocentrum.
Though originating from different cell types, it is noteworthy that this process in the vertebral column of teleosts is very similar to what is seen in typical intramembranous bone formation, in which mesenchymal cells condense and differentiate into osteoblasts. This process is initiated by the gene expression of RUNX2, which is involved in osteoblast differentiation; its orthologues in zebrafish, runx2a and runx2b, show 86% amino acid sequence conservation with mammals [37,43]. RUNX2 interacts with bone morphogenetic proteins (BMPs), which initiates osteoblast differentiation and activates genes such as BGLAP and SPP1, both involved in the deposition of the bone extracellular matrix [37]. The osteoblasts will produce an osteoid, uncalcified bone matrix consisting of collagen type I and osteocalcin, which subsequently mineralize via the precipitation of hydroxyapatite (HA) upon the removal of the mineralization inhibitor inorganic pyrophosphate (PPi) via the enzyme alkaline phosphatase, secreted by the osteoblasts [3,44,45,46].
4. Pseudoxanthoma Elasticum
Pseudoxanthoma elasticum (PXE; OMIM# 264800) is an autosomal recessive metabolic disease caused by loss-of-function pathogenic variants in the ABCC6 (ATP-binding cassette, subfamily C, member 6) gene and—to a much lesser extent—the ENPP1 (ectonucleotide pyrophosphatase/phosphodiesterase 1) gene [47,48]. In humans, ABCC6 encodes an ATP-dependent ABC transporter, ABCC6, which is mainly present in the liver and kidneys [49]. Though its substrates remain elusive, it was shown to be indirectly involved in ATP homeostasis, as ABCC6 deficiency was associated with decreased ATP efflux [50]. Circulatory ATP is then metabolized into AMP and PPi by the ENPP1 enzyme [51]. Due to ABCC6 or ENPP1 deficiency, progressive mineralization and fragmentation of the elastic fibers can occur, which results in skin, ophthalmological, and cardiovascular symptoms in PXE patients [52].
Two orthologs of ABCC6 have been described in zebrafish, abcc6a and abcc6b [50]. There are several reports that describe the effects of abcc6a deficiency caused by morpholino knock-down or a CRISPR/Cas9-mediated knockout of abcc6a. In 2010, Li et al. reported that abcc6a was required for normal zebrafish development [53]. The morpholino-mediated knock-down of the abcc6a gene revealed a shortening of the body, delayed development of the head, decreased tail length, and curving of the caudal part of the morphants at 1 dpf (Figure 2). At 3 dpf, cardiac edema was noted, which eventually led to demise at 8 dpf. It must be noted that many of these changes are rather unspecific and frequently encountered in morpholino-edited fish in a dose-dependent manner [53,54,55,56]. Also, the authors did not provide information on aberrant mineralization in their model [53]. In the same study, the knockdown of abcc6b did not lead to an aberrant phenotype. Via in situ hybridization, the authors reported that abcc6a was expressed in forerunner cells upon gastrulation and then in the Kupffer’s vesicle (which derives from forerunner cells) as well as in the tail bud, while abcc6b was mainly expressed in the anterior part of the embryonic kidney proximal straight tubule [53].
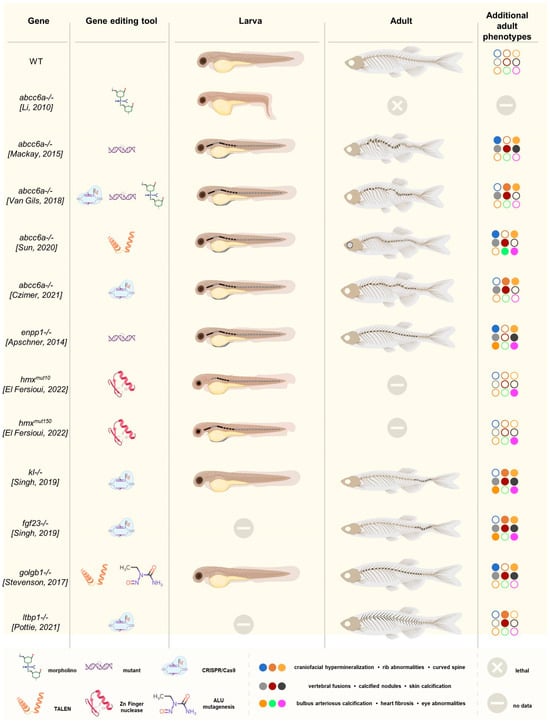
Figure 2.
Graphical overview of the discussed zebrafish models, including the targeted gene, the genome-editing tool(s) used, and a schematic representation of the larval and adult ectopic mineralization phenotype (indicated in black). All PXE/GACI models showed advanced vertebral mineralization in the larvae [55,57,58,59,60], except for the model developed by Li et al [53]. In the adult models, excessive (inter)vertebral calcification was observed. Similarly, excessive vertebral mineralization was seen in the hmx1−/− larvae [37]. This recurring phenotype was not present in the klotho−/− or golgb1−/− larvae, but the adult fish showed extensive vertebral mineralization [61,62]. This was also observed in the adult ltbp1-knockout fish, including calcification of the ribs [63]. Finally, the color scheme indicates the similarities and differences between the different adult models in terms of more detailed phenotypic characteristics such as craniofacial hypermineralization, rib abnormalities, the presence of a curved spine, vertebral fusions and calcified nodules on the vertebrae and/or ribs, skin and bulbus arteriosus calcification, heart fibrosis, and eye abnormalities. Filled circles indicate that the corresponding phenotypic feature is present.
Mackay et al. described a gräte mutant abcc6a zebrafish harboring a missense substitution p.(L1429R) in the second nucleotide-binding domain (NBD2) [57]. At 8 dpf, hypermineralization along the vertebral column was noted, which lead to a curved spine and reduced length (Figure 2). They also noted enhanced mineralization of craniofacial elements and skin mineralization, though the latter was rare. Mineralization of the vertebrae proceeded faster than in the wild type, leading to vertebral fusions. At 6 weeks post fertilization (wpf), mineralized nodules were observed on the margins of the intervertebral space along the thickened, curved spine. In situ hybridization at 5 dpf showed abcc6a expression in regions of developing bone but not in the liver or kidney. Using reporter constructs, the authors observed the co-expression of osterix and, to some extent, osteocalcin with abcc6a starting at 4 dpf in the operculum and cleithrum. At 20 dpf, abcc6a was mainly expressed in the intervertebral disc regions. Based on this, Mackay et al. believed abcc6a to be expressed in a population of mature osteoblasts, which is not the case for human ABCC6. Interestingly, abcc6b expression was confined to the operculum, parasphenoid, and ear cartilage [57].
In 2018, the first CRISPR/Cas9-edited abcc6a zebrafish model was described, the editing of which entailed the introduction of a four-base-pair deletion in exon 2, c.180delTCGG [55]. At 10 dpf, advanced mineralization of the vertebrae was seen (Figure 2). At 4.5 and 7.5 months post fertilization (mpf), the fish were shorter, had undergone hypermineralization as well as fusion of vertebral bodies, and developed calcified nodular lesions in the intervertebral spaces and broader and bifid ribs. A similar phenotype was also found in an abcc6a splice-site mutant (c.2250+1G>A) and—in the larval stage—in a morphant model. In contrast, no calcification of the eyes or heart was seen at 13.5 mpf. Van Gils et al. did not investigate the abcc6a expression pattern [55].
A fourth model, where abcc6a knockout was achieved by introducing 8 bp and 17 bp deletions leading to a truncated abcc6a protein via TALEN, was reported by Sun et al. [58]. The mutants were indistinguishable from the WT at 4 dpf, but at young-adult stages, they were shorter and more curved, with fusions of the vertebral bodies and intervertebral spaces (Figure 2). Also, calcified nodular lesions in the vertebrae were seen; quantification analysis of bone volume and BMD showed increased mineralization in the vertebrae and suborbital and supraorbital bones. Contrary to the previous models, the authors also found fibrosis in the heart and sclera, ocular calcifications, and electron-dense material in the thickened mutant Bruch’s membrane of the eye. In early embryonic stages, abcc6a was expressed ubiquitously; at 48 h post fertilization (hpf) and 4 dpf, expression was noted in the heart, operculum, and cleithrum as well as in the ear and notochord [58].
Finally, Czimer et al. used CRISPR/Cas9 editing to create frameshift alleles, p.Arg60Serfs*183 and p.Cys205Leufs*4, in the abcc6a and abcc6b genes, respectively, both leading to a premature termination codon [59]. Abcc6a−/− larvae showed advanced vertebral calcification in both larvae and adult fish, contrary to what was observed for abcc6b mutants, and these findings were similar to those reported by MacKay et al. and Van Gils et al. (Figure 2) [55,57]. No gene expression analyses were performed [59].
5. Generalized Arterial Calcification of Infancy
Mutations in the ENPP1 gene were first described in generalized arterial calcification of infancy (GACI, OMIM #208000, #614473), an autosomal recessive disorder characterized by extensive vascular calcification, resulting in cardiovascular complications such as heart failure, respiratory distress, edema, cyanosis, hypertension, and cardiomegaly [64]. Other possible manifestations in the skin and eyes are similar to PXE, and periarticular calcifications, hearing loss, and the development of rickets after infancy have also been reported [64].
More recently, Nitschke et al. discovered that some GACI patients harbor ABCC6 pathogenic variants, further emphasizing that PXE and GACI are part of one spectrum [47]. Interestingly, an identical ABCC6 genotype can lead to PXE in one patient and GACI in another, suggesting that other (epi)genetic factors must be at play in determining the definitive phenotype [47].
ENPP1 has but one orthologue in zebrafish and the enpp1 mutant, coined dragonfish (dgf), which recapitulates features of both GACI and PXE, as they undergo ectopic calcification in soft tissues such as the skin, cartilage, heart, and intracranial space as well as perichondral ossification as early as 4 dpf in some (Figure 2) [60]. Further, hypermineralization of the vertebral column with fusion of vertebral centra occurred in juvenile and young adult fish, together with the calcification of the heart and its outflow (bulbus arteriosus). Of interest and contrary to what is observed in some abcc6a zebrafish, these calcifications occur independently of the expression of typical osteoblast or cartilage markers such as osterix and have been suggested to result from passive calcium deposition [60].
The ubiquitous expression of enpp1 was noted, with some of the highest expression levels observed in bone elements, such as the operculum and cleithrum, similar to the expression pattern in mice. Besides the local function of the phosphodiesterase, it was shown that enpp1 also acts in areas that are remote from its expression site, probably reflecting the anti-mineralizing effect of PPi [60].
6. Schorderet–Munier–Franceschetti Syndrome
Also known as oculoauricular syndrome (OAS; OMIM#612109), Schorderet–Munier–Franceschetti syndrome is a rare autosomal recessive disease that has been described in two consanguineous families [65,66]. It is characterized by complex ophthalmological and external ear anomalies as well as aberrant orofacial development with underdeveloped and asymmetric jaws [37]. OAS is caused by homozygous pathogenic variants in HMX1 (H6 Family Homeobox 1), encoding a homeobox transcription factor with high affinity toward a consensus HMX-binding site harboring a 5′-CAAGTG-3′ element required for sensory organ development [65,66,67,68]. HMX1 is highly conserved across orthologs and contains three essential domains: a homeobox domain (HD) and conserved domains SD1 and SD2 [37,65,66]. HD and SD1 are involved in the dimerization of HMX1, while the function of SD2 remains unknown.
Two zebrafish mutants have been generated to ascertain the OAS phenotype using zinc-finger nucleases that target two different conserved regions of the orthologue hmx1 gene [37]. Hmx1mut10 zebrafish harbor a frameshift variant resulting in a premature termination codon in the SD1 domain, while hmx1mut150 zebrafish have an indel in the HD domain that obliterates this domain’s function. Both mutants prevent the dimerization of SD1 and HD—an essential prerequisite for proper hmx1 function—leading to a loss-of-function effect [37]. Hmx1 is mainly expressed during the development of the eyes and ears, starting at the 10-somite stage, which correlates with 14 hpf [33,69]. The mutant zebrafish developed smaller eyes and presented with delayed neurogenesis paired with increased apoptosis in the retina and brain [68,70]. Interestingly, hmx1 knockdown resulted in defects in regions where hmx1 is not expressed, such as the vertebral column [70]. Both mutants presented normal cartilage and bone development until 5 dpf, but premature mineralization was observed from day 7 onward (Figure 2) [37]. After this point, 70% of the wild-type zebrafish at 8 dpf showed one mineralized vertebra, while 80% of the hmx1mut10 and hmx1mut150 zebrafish presented with five mineralized vertebrae [37].
To explain the advanced mineralization phenotype, a dysregulation of the BMP gradient that regulates dorsal/ventral patterning in zebrafish was put forward [71]. Physiologically, the concentration of BMP is highest ventrally and is inhibited dorsally by antagonists such as chordin, noggin, and follistatin, which play essential roles in the formation of the axial skeleton [37,71,72,73,74]. In hmx1 mutants, reduced RNA levels of chordin and noggin1 result in a pro-osteogenic environment with increased expression levels of bmp2b and bmp4b and the subsequent upregulation of osteogenic markers runx2b and spp1 [37]. Runx2b initiates endochondral and intramembranous ossification, and its complex with BMP initiates osteoblast differentiation. The treatment of the mutants with the selective BMP inhibitor DMH1 (dorsomorphin homolog 1) restricted vertebral mineralization, indicating that this mineralization is indeed BMP-driven, possibly resulting from increased bmp2b and bmp4b activity [37]. While it is unlikely that hmx1 directly regulates bmp2b and bmp4b since they do not have the appropriate CAAGTG binding element, it is possible that hmx1 is involved in the regulation of noggin1 and chordin expression, as the promotor regions of these BMP inhibitors contain hmx1 binding sites, but there is currently no experimental evidence to confirm this hypothesis [37]. Another known hmx1 target with a regulatory function is uhrf1 (Ubiquitin-Like With PHD and Ring Finger Domains 1), a multi-domain key epigenetic regulator protein involved in chromatin modifications and cellular proliferation. Hmx1 was shown to inhibit uhrf1 in mutant zebrafish, though this effect was mainly limited to the cranial region and therefore seems less likely to be involved [37,68].
7. Hyperphosphatemic Familial Tumoral Calcinosis
Hyperphosphatemic familial tumoral calcinosis (HFTC, OMIM# 211900, 617993, 617994) is an autosomal recessive metabolic disorder characterized by the progressive deposition of calcium phosphate crystals in periarticular soft tissues, particularly after repetitive trauma or pressure, and painful swellings [75]. Dental anomalies and—less frequently—vascular, testicular, and retinal calcifications can also occur. The biochemical hallmark of tumoral calcinosis, hyperphosphatemia, is caused by increased renal absorption of phosphate due to pathogenic variants in the FGF23, KLOTHO (KL), or GALNT3 genes. Under physiological conditions, serum calcium and phosphate concentrations are strictly regulated through hormonal mechanisms via the coordinated action of parathyroid hormone (PTH), vitamin D, and fibroblast growth factor-23 (FGF23) [76,77]. FGF23 is a hormone that downregulates renal phosphate reabsorption and promotes urinary phosphate excretion [45,78]. αKlotho is a single-pass transmembrane protein encoded by the KL gene, which is predominantly expressed in the distal convoluted tubules of the kidney where it binds to FGF receptors and functions as an FGF23 co-receptor [78,79]. The GALNT3 gene encodes for the enzyme N-acetylgalactosaminyltransferase 3, responsible for the O-glycosylation of FGF23 [80,81,82,83]. The absence of this post-translational modification leads to poor FGF23 secretion. In humans, FGF23 is secreted by osteocytes in bone, but in zebrafish, it originates from teleost-specific kidney-associated glands, namely, the corpuscles of Stannius, and from the gills [61,78]. Even though the origins of this hormone are different, the zebrafish fgf23 gene is evolutionary conserved, and its function in maintaining mineral homeostasis has been preserved [61,84]. This is also true for the kl gene, which is expressed in the brain, pancreas, liver, and kidneys in embryonic and larval zebrafish but only in the liver and kidney in adults [61,84].
αKlotho and fgf23 CRISPR/Cas9-mediated knockout zebrafish were reported to have an indistinguishable phenotype from each other, with adult-onset morbidity and mortality, starting at 4 to 5 months of age, with loss of fin integrity, eye overgrowth, widespread ectopic calcification (especially in the outflow tract of the heart but also in the vasculature of the dermis and muscles), and spinal deformities (Figure 2) [61,78]. The last features were most prominent in the caudal region, with multifocal areas of hyperostosis and chondrodysplasia. These regions of bone overgrowth were accompanied by areas of dystrophic calcification, connective tissue proliferation, and immune cell infiltration [61,78].
There have been no reports on galnt3 mutant zebrafish to date, and little is known about its expression sites. However, a near-complete loss of function of galnt3 was observed in the bone of zebrafish knockout models of giantin, a golgin protein (encoded by the golgb1 gene) important for Golgi organization and vesicular transport [62]. Two golgb1 mutants with a premature stop codon mutation due to ENU mutagenesis and TALEN site-directed mutagenesis, respectively, did not display any gross developmental defects and had normal lifespans but presented ectopic mineralization of soft tissues in adulthood. Specifically, in the axial skeleton, hyperostosis and ectopic mineralization of the intervertebral discs were seen, leading to a reduction in vertebral spacing and vertebral fusion as well as ectopic calcified deposits in multiple vertebrae [62].
Mechanistically, the greatest amount of attention has been paid to vascular calcification in the bulbus arteriosus of klotho and fgf23 mutants, where an osteogenic gene expression profile was observed in the heart and vascular smooth muscle cells, with increased expression of mmp9, mmp13, spp1, and, more modestly, runx2b [61,85]. Besides pathways involved in bone formation and remodeling, inflammatory pathways were also upregulated in the kl−/− mutants. More recently, it was proposed that cellular senescence contributes to the ectopic calcification and premature death of these mutants, opening the possibility of using these mutants to model (premature) aging [86,87,88].
8. LTBP1-Related Cutis Laxa Syndrome
Cutis laxa (CL) comprises a large group of inherited and acquired diseases characterized by reduced elastic recoil leading to a loose, saggy, and aged appearance [89]. The inherited forms of CL (OMIM# 150390) can be autosomal dominant or recessive and are induced by pathogenic variants in a variety of genes, including ALDH18A1, ATP6V0A2, ATP6V1E1, ATP7A, EFEMP2, ELN, FBLN5, GORAB, LTBP1, MAP kinases, PYCR1, and RIN2. Among the CL zebrafish models that were generated, only ltbp mutants displayed an axial skeletal phenotype [63].
LTBPs (Latent transforming growth factor β (TGF-β)-binding proteins) are microfibril-associated extracellular matrix (ECM) proteins that anchor TGF-β onto the ECM and regulate its storage, release, and activation [63,90]. They are also responsible for the correct assembly of ECM proteins and are considered structural components of connective tissue [63,90]. In patients, bi-allelic LTBP1 pathogenic variants cause an autosomal recessive CL syndrome featuring cutis laxa, facial dysmorphism, cardiac defects, and skeletal abnormalities such as short stature and craniosynostosis [63].
Human LTBP1 has two isoforms: the long isoform (LTBP1L) is essential for TGF-β signaling and cardiovascular development, while the short isoform (LTBP1S) might play a role in craniofacial development [63]. This protein is both localized in the ECM, where it interacts with fibrillin-1 and fibronectin via its C- and N-terminal regions, respectively, [63], and in newly forming bone (osteoid), illustrating that LTBP1 might play a role in bone and connective tissue [90].
In contrast to humans, zebrafish only have one highly conserved long isoform of ltbp1, which is expressed in the bulbus arteriosus, pancreas, brain, liver, and cranial bones [63,90,91]. Several models have been reported, among which number ltbp1−/− Δ29 (c.3526del) and ltbp1−/− Δ35(c.4294_4303del), which were generated using CRISPR/Cas9 and harbor a premature stop codon [63]. Both mutants had normal viability but showed a significant increase in ectopic bone formation of intramembranous origin on the neural and hemal arches of the vertebrae at 4 mpf, especially at the base of the arches (Figure 2). This was accompanied by decreased tissue mineral density in both vertebrae as well as vertebral and hemal arches in the ltbp1−/− Δ29 mutant but not in the ltbp1−/− Δ35 mutant. Additionally, bone volume and thickness were increased in the ltbp1−/− Δ29 mutant only, possibly because the C-terminal TGF-β-binding domains are only affected in this mutant [63].
For the two other ltbp1 zebrafish models, a morphant knock-down and a TALEN-induced knockout, respectively, skeletal mineralization was not investigated, and the models were only evaluated as larvae [90,91]. Xiong et al. described the morphants as being shorter, so an axial phenotype might have remained undetected [90]. It is hypothesized that the collagen maturation defect that was seen in ltbp1 mutants contributes to ectopic bone formation, but no experimental evidence is available to date to confirm or refute this hypothesis [63].
9. Discussion
The zebrafish has been an outstanding research organism for studying molecular mechanisms, cellular signaling, and the treatment possibilities for a wide variety of diseases [92]. Among these, the axial skeleton has proven to be very useful for modeling diseases that primarily affect the vertebral column in humans, such as scoliosis and osteoporosis [23,93,94]. Further, zebrafish models for various bone disorders, such as osteogenesis imperfecta and fibrodysplasia ossificans progressive (FOP), feature abnormalities of the axial skeleton, and their respective causal genes usually have a well-documented role in bone development and repair (Table 1) [8,95,96,97,98,99,100,101,102,103,104,105]. Remarkably, the zebrafish models that are discussed in this review feature a striking axial skeletal phenotype, while the human disease they model does not show vertebral column abnormalities, even though the human phenotype affects multiple organ systems.
For some of these disorders, such as PXE or GACI, the vertebral phenotype is the main—if not the only—feature of the zebrafish mutants and knockouts [53,55,57,58,59,60]. PXE is considered a hallmark disease with respect to studying ectopic soft tissue mineralization, c.q., hydroxyapatite precipitation, occurring in several genetic and acquired diseases as well as during aging [50,60,106]. As a multisystemic disorder, PXE affects several organs, but the vertebrae remain unaltered. Indeed, bone development and morphology have been studied in PXE patients and were found to be normal, except for a potentially higher propensity for osteoarthritis in the knees and acromioclavicular joints [107,108]. A variety of zebrafish models have been created for PXE, and though differences exist—regardless of whether they related to the underlying genetic defect or genome-editing technology—the vertebral phenotype is rather consistent and can therefore be considered a true consequence of abcc6a deficiency [53,55,57,58,59,60]. Several groups have demonstrated that this phenotype is readily amendable to experimental perturbation via drugs using vitamin K as an example, enabling its use as a screening read-out for compounds such as bisphosphonates, sodium thiosulphate, magnesium citrate, and the PARP inhibitor minocycline [57,58,109,110]. Except for vitamin K, the effects of the evaluated drugs were similar to what was observed in PXE mouse models or patients, proving that zebrafish are a good preclinical intermediate model for anti-mineralizing drug screening [111]. Zebrafish models can be used after in vitro experiments to screen and select compounds that may target (ectopic) calcification before evaluating a drug’s effect in other vertebrates such as rodents [57,58,109,110]. The results obtained with vitamin K, which could not be replicated in mice, however, indicate that screening results for teleosts are not always applicable to vertebrates and highlight why rodent experiments—though they can be reduced in number—remain necessary [112,113]. Similarly, drug interventions were also conducted in the enpp1 and hmx1 models using axial skeleton mineralization as a read-out, although more modestly than in abcc6a mutants [37,60]. Altogether, these first pharmacological experiments confirm the great potential of zebrafish in drug discovery and evaluation for the treatment of multisystemic diseases. The relatively easy and robust evaluation and quantification of a vertebral phenotype, both in larvae and adult fish, add to the great potential of these animal models. In addition, it is possible to study drug activity at single-cell resolution within the complexity of an entire animal, across tissues and over an extended timescale, when combined with cell-specific or tissue-specific reporters and gene-editing technologies [114].
A challenge for most of the models addressed in this review was attaining a comprehensive understanding of how the axial skeleton phenotype occurs. When looking at the sites of expression of the different genes and proteins involved, most are not expressed at the sites of hypermineralization. Abcc6a may be an exception, as it was suggested to be expressed in osteoblasts in zebrafish, though discrepancies exist between different studies [53,57,58]. This proposed expression pattern completely differs from that of human and murine ABCC6, which is mainly expressed in the liver and kidneys, though species-, population-, and context-specific expression is not unusual [49,115]. Interestingly, in the Abcc6−/− mouse model, Boneski et al. and Kauffenstein et al. independently revealed the presence of progressive vertebral osteopenia but not intervertebral disc or other axial ectopic calcifications. This murine phenotype is essentially characterized by trabecular bone loss and mainly occurs in older knockout mice. The authors suggested it to be closely linked to increased osteoclastic activity, but, at the same time, these observations pose the question of the importance of PPi/Pi balance in bone homeostasis (Figure 3) [111,116]. Moreover, the ABCC6 transporter facilitates ATP efflux, and PXE patients have lowered plasma levels of PPi compared to healthy controls and heterozygous carriers [49,117,118,119,120]. The existence of a subset of adult GACI patients with pathogenic variants in ABCC6 or ENPP1 that have a complete lack of PPi in their plasma and suffer from hypophosphatemic rickets strengthens the belief that PPi/Pi balance may have an influence on bone, though there is currently little additional experimental evidence for this hypothesis [121]. The abcc6a and enpp1 zebrafish provide excellent opportunities for studying the roles of ABCC6 and ENPP1, their downstream signal transduction proteins, and PPi in bone since they are required for the generation of PPi [53]. As the effects on bone may be the most impactful over time, further study of the vertebral phenotype in these models can expand our knowledge about (bone) aging and how it can be linked to cellular senescence. Senescence plays a role in childhood bone-growth-associated bone mass acquisition and contributes to the development of osteoarthritis and osteoporosis, but a detailed characterization taking into account the heterogeneity of senescence-related signaling and differences depending on cell type and senescence-inducing stimuli remains elusive [122,123]. Abcc6, but also fgf23 and kl, have been previously associated with downstream senescence signaling pathways, and their respective models may therefore hold answers to some of these outstanding questions [124,125,126,127].
As for the other genes discussed in this paper, their expression profiles resemble those of their human counterparts, and their downstream signaling mediators—TGF-β and BMP-RUNX2—have established roles in bone formation and homeostasis (Figure 3) [128]. In physiological circumstances, TGF-β and BMP signal through a canonical SMAD pathway and a noncanonical SMAD-independent pathway [129,130,131,132,133]. Both pathways lead to the expression of osteogenic transcription factors such as RUNX2 and regulate mesenchymal stem cell differentiation, bone formation, and bone homeostasis [128,129,134]. Continuous activation of the BMP pathway leads to ectopic bone formation, as illustrated by FOP, caused by gain-of-function pathogenic variants in the type 1 BMP receptor (encoded by the ACVR1 gene) [95,135,136,137]. Furthermore, inhibitors of BMP2 signaling, such as matrix gla protein (MGP) and fetuin-A, also play a role in maintaining the balance between a pro- and anti-osteogenic environment. By binding together in calciprotein particles (CPP), they capture hydroxyapatite in body fluids [138,139,140,141,142]. These so-called primary CPPs are initially spherical, amorphous, and soft but can spontaneously transform into secondary CPPs that are larger, crystalline, and less soluble [143,144]. The intricate relations between these pro- and anti-calcification mediators have been well-documented in, e.g., murine and human PXE, with low serum levels of MGP and Fetuin-A, contributing to the upregulation of RUNX2 through the BMP2-SMAD-RUNX2 and TGFB2-SMAD2/3 pathways [128,138,145,146,147,148] but also affecting the calcification propensity T50 test, which reflects the time required to convert 50% of primary CPPs in a serum sample into secondary CPPs [149,150,151]. The latter was significantly prolonged and associated with disease severity in PXE [146]. In the two hmx1 mutants for Schorderet–Munier–Franceschetti syndrome, bmp also plays a crucial role, as loss of inhibition via chordin and noggin1 creates a pro-osteogenic environment with increased expression of bmp2b and bmp4b and upregulation of runx2b, initiating mineralization through endochondral and intramembranous ossification and osteoblast differentiation [37,152,153,154,155]. Conversely, TGF-β plays a central role in the pathophysiology of both hyperphosphatemic tumoral calcinosis and the LTBP1-related CL syndrome. In the former, the complex interplay between TGF-β and FGF23, influencing each other directly and indirectly via the co-receptor Klotho and the active vitamin D metabolite 1,25(OH)2D3 in a regulatory feedback mechanism, results in osteoblast proliferation [156,157,158,159,160,161,162,163,164,165]. Additionally, mouse studies have revealed that Klotho indirectly inhibits Egr-1 expression, a transcription factor involved in several signaling pathways such as apoptosis and angiogenesis, by inhibiting the TGF-β pathway [166,167]. This transcription factor stimulates osteogenic differentiation and upregulates osteogenic markers such as Runx2 and Sox9 [168]. Klotho-deficient mice present an upregulation of the TGF-β pathway, followed by upregulated Egr-1, Runx2, and Sox9 as a result. The LTBP1-related CL syndrome reduces the anchorage of TGF-β in the ECM, leading to the continuous activation of the TGF-β signaling pathway and, again, Runx2 expression [63,169,170].
This documented involvement of ubiquitously active key mineralization mediators brings us to a final intriguing, outstanding question: why is only the vertebral skeleton affected in these models? Such compartmentalized effects suggest the existence of tissue-specific regulatory mechanisms of mineralization. While it is a well-documented concept that the mineralization process is unique to mineralized tissues, e.g., bone versus dentine or enamel, the mechanisms underlying the diversity of mineralization within a specific tissue are less well described. In bone, the unravelment of the complex mixture of heterogeneous cell types has only just begun; many discrete populations, e.g., mesenchymal cells, seem to exist, the dynamics of which are influenced by external stimuli and impact bone formation [114]. Such compartmentalization is also seen in pathological mineralization in hereditary or acquired diseases and aging, e.g., in the selective elastic fiber mineralization in PXE, but the underlying mechanisms remain largely unknown. Again, this makes the zebrafish strains discussed in this paper outstanding models with which to gain insights into the tissue- and cell-specific regulatory mechanisms of mineralization via, e.g., the use of single-cell transcriptomic profiling.
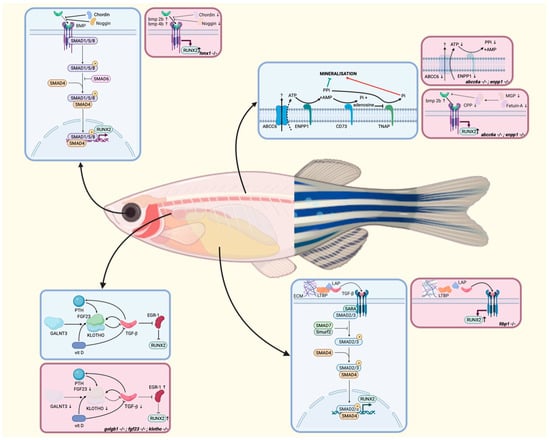
Figure 3.
Signaling pathways involved in ectopic mineralization in zebrafish in physiological circumstances (blue) and disease (pink) for each of the discussed models, depicted at the site of highest expression for each gene. In the pathophysiological illustrations, arrows indicate increased or decreased expression of the involved mediators. AMP: adenosine monophosphate; ATP: adenosine triphosphate; BMP: bone morphogenetic protein; CPP: calciprotein particle; ECM: extracellular matrix; LAP: latency-associated peptide; LTBP: latent transforming growth factor β-binding proteins; PTH: parathyroid hormone; P: phosphorylated; Pi: inorganic phosphate; PPi: inorganic pyrophosphate; SARA: smad anchor for receptor activation; vit D: vitamin D. Adapted from [37,90,128,129,138,152,153,155].

Table 1.
Zebrafish models for bone disorders with a vertebral phenotype.
Table 1.
Zebrafish models for bone disorders with a vertebral phenotype.
| Disease | Human Gene | Zebrafish Orthologue | Knockout Vertebral Phenotype | References |
|---|---|---|---|---|
| Bruck Syndrome type II | PLOD2 | plod2 | Shortened body axis, kyphoscoliosis, compressed vertebrae, excess bone at vertebral end plates that results in loss of hourglass shape of vertebra, increased TMD and thickness | [8] |
| FOP | ACVR1 | acvr1l | Dorsalization of the embryonic axis | [95,96,97] |
| Craniosynostosis | CYP26B1 | cyp26b1 | Outgrowth of cartilaginous endochondral disc in pectoral fins, coronal craniosynostosis | [171,172] |
| Osteoporosis | ATP6V1H | atp6v1h | Premature death, reduction in/absence of bone cells, almost complete absence of mineralized bone | [94] |
| Osteogenesis imperfecta | COL1A1 COL1A2 BMP1 SP7 | col1a1a, col1a1b col1a2 bmp1a, bmp1b sp7 | Variable phenotype including callus formation, bending of ribs, short stature, craniofacial abnormalities, malformation of vertebral column | [8,99,100,101,102,103,104,105] |
| Ehlers-Danlos syndrome | B4GALT7 | b4galt7 | Scoliosis; small, bent pectoral fins; reduced or absent mineralized bone | [10,173] |
| Spinal curvature disorders | COL8A1 KIF6 PTK7 TBX6 | col8a1a, col8a1b kif6 ptk7a tbx6 | Extensive scoliosis in thoracic and caudal parts of the spine, deformed and fused vertebrae, fused neural and hemal arches | [10,174,175,176,177] |
| Gaucher disease | GBA1 | gba1 | Reduced osteoblast differentiation and bone mineralization, slight curvature of the trunk | [178] |
10. Conclusions
The axial skeletal phenotype of zebrafish models offers a good read-out for compound detection and preclinical evaluation in hereditary multisystemic diseases. This clear and great potential of zebrafish should lead to increased efforts to identify and screen drugs for these complex disorders that are currently often intractable. Furthermore, they can be a valuable aid in untangling the mechanisms—in general but also in tissue- and cell-specific cases—that contribute to physiological and pathological mineralization. Aside from the many advantages of using zebrafish as a model system, there are limitations to their use. Besides the fact that not all human genes have a zebrafish orthologue and that parts of their physiology and anatomy are not exact replicas of those in humans, a major drawback is the difficulty of blood drawing, particularly in the field of ectopic mineralization, as circulatory mineralization regulators cannot be reliably evaluated. Further, skeletal development in teleosts is very prone to environmental factors, contrary to the case for mammals. Though zebrafish cannot, therefore, completely replace murine models in modeling and studying human mineralization, they have become an indispensable intermediate model for performing mechanistic studies and targeted and high-throughput drug-screening and pharmaceutical pilot studies, thereby contributing to reducing the use of mammals in biomedical research.
Author Contributions
J.V.W. writing—original draft preparation, O.M.V. writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Figures were created using BioRender.com, accessed on 21 September 2023. J.V.W. and O.M.V. are members of the International Network on Ectopic Calcification (INTEC www.itnintec.com). O.M.V. is a Senior Clinical Investigator of the Fund for Scientific Research Flanders.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tonelli, F.; Bek, J.W.; Besio, R.; De Clercq, A.; Leoni, L.; Salmon, P.; Coucke, P.J.; Willaert, A.; Forlino, A. Zebrafish: A Resourceful Vertebrate Model to Investigate Skeletal Disorders. Front. Endocrinol. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Spoorendonk, K.M.; Hammond, C.L.; Huitema, L.F.A.; Vanoevelen, J.; Schulte-Merker, S. Zebrafish as a unique model system in bone research: The power of genetics and in vivo imaging. J. Appl. Ichthyol. 2010, 26, 219–224. [Google Scholar] [CrossRef]
- Kwon, R.Y.; Watson, C.J.; Karasik, D. Using zebrafish to study skeletal genomics. Bone 2019, 126, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Bird, N.C.; Mabee, P.M. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev. Dyn. 2003, 228, 337–357. [Google Scholar] [CrossRef]
- Kimmel, C.B. Genetics and early development of zebrafish. Trends Genet. 1989, 5, 283–288. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Du, S.J.; Frenkel, V.; Kindschi, G.; Zohar, Y. Visualizing normal and defective bone development in zebrafish embryos using the fluorescent chromophore calcein. Dev. Biol. 2001, 238, 239–246. [Google Scholar] [CrossRef]
- Gistelinck, C.; Witten, P.E.; Huysseune, A.; Symoens, S.; Malfait, F.; Larionova, D.; Simoens, P.; Dierick, M.; Van Hoorebeke, L.; De Paepe, A.; et al. Loss of Type I Collagen Telopeptide Lysyl Hydroxylation Causes Musculoskeletal Abnormalities in a Zebrafish Model of Bruck Syndrome. J. Bone Miner. Res. 2016, 31, 1930–1942. [Google Scholar] [CrossRef]
- Carnovali, M.; Banfi, G.; Mariotti, M. Zebrafish Models of Human Skeletal Disorders: Embryo and Adult Swimming Together. BioMed Res. Int. 2019, 2019, 1253710. [Google Scholar] [CrossRef]
- Gray, R.S.; Gonzalez, R.; Ackerman, S.D.; Minowa, R.; Griest, J.F.; Bayrak, M.N.; Troutwine, B.; Canter, S.; Monk, K.R.; Sepich, D.S.; et al. Postembryonic screen for mutations affecting spine development in zebrafish. Dev. Biol. 2021, 471, 18–33. [Google Scholar] [CrossRef]
- Driever, W.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.; Malicki, J.; Stemple, D.L.; Stainier, D.Y.; Zwartkruis, F.; Abdelilah, S.; Rangini, Z.; et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 1996, 123, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Solnica-Krezel, L.; Stemple, D.L.; Mountcastle-Shah, E.; Rangini, Z.; Neuhauss, S.C.; Malicki, J.; Schier, A.F.; Stainier, D.Y.; Zwartkruis, F.; Abdelilah, S.; et al. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development 1996, 123, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, A.; Burgess, S.; Golling, G.; Chen, W.; Sun, Z.; Townsend, K.; Farrington, S.; Haldi, M.; Hopkins, N. A large-scale insertional mutagenesis screen in zebrafish. Genes. Dev. 1999, 13, 2713–2724. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.C.; Hammerschmidt, M.; Haffter, P.; Nüsslein-Volhard, C. Large-scale mutagenesis in the zebrafish: In search of genes controlling development in a vertebrate. Curr. Biol. 1994, 4, 189–202. [Google Scholar] [CrossRef]
- Henke, K.; Farmer, D.T.; Niu, X.; Kraus, J.M.; Galloway, J.L.; Youngstrom, D.W. Genetically engineered zebrafish as models of skeletal development and regeneration. Bone 2023, 167, 116611. [Google Scholar] [CrossRef] [PubMed]
- Kettleborough, R.N.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; de Bruijn, E.; van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef]
- Pasquier, J.; Braasch, I.; Batzel, P.; Cabau, C.; Montfort, J.; Nguyen, T.; Jouanno, E.; Berthelot, C.; Klopp, C.; Journot, L.; et al. Evolution of gene expression after whole-genome duplication: New insights from the spotted gar genome. J. Exp. Zool. B Mol. Dev. Evol. 2017, 328, 709–721. [Google Scholar] [CrossRef]
- Ogawa, Y.; Corbo, J.C. Partitioning of gene expression among zebrafish photoreceptor subtypes. Sci. Rep. 2021, 11, 17340. [Google Scholar] [CrossRef]
- Yan, Y.L.; Titus, T.; Desvignes, T.; BreMiller, R.; Batzel, P.; Sydes, J.; Farnsworth, D.; Dillon, D.; Wegner, J.; Phillips, J.B.; et al. A fish with no sex: Gonadal and adrenal functions partition between zebrafish NR5A1 co-orthologs. Genetics 2021, 217, iyaa030. [Google Scholar] [CrossRef]
- Dietrich, K.; Fiedler, I.A.; Kurzyukova, A.; López-Delgado, A.C.; McGowan, L.M.; Geurtzen, K.; Hammond, C.L.; Busse, B.; Knopf, F. Skeletal Biology and Disease Modeling in Zebrafish. J. Bone Miner. Res. 2021, 36, 436–458. [Google Scholar] [CrossRef]
- Cotti, S.; Huysseune, A.; Larionova, D.; Koppe, W.; Forlino, A.; Witten, P.E. Compression Fractures and Partial Phenotype Rescue with a Low Phosphorus Diet in the Chihuahua Zebrafish Osteogenesis Imperfecta Model. Front. Endocrinol. 2022, 13, 851879. [Google Scholar] [CrossRef] [PubMed]
- Prince, V.E.; Joly, L.; Ekker, M.; Ho, R.K. Zebrafish hox genes: Genomic organization and modified colinear expression patterns in the trunk. Development 1998, 125, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Lleras Forero, L.; Narayanan, R.; Huitema, L.F.; VanBergen, M.; Apschner, A.; Peterson-Maduro, J.; Logister, I.; Valentin, G.; Morelli, L.G.; Oates, A.C.; et al. Segmentation of the zebrafish axial skeleton relies on notochord sheath cells and not on the segmentation clock. eLife 2018, 7, e33843. [Google Scholar] [CrossRef]
- Sheth, R.; Bastida, M.F.; Kmita, M.; Ros, M. “Self-regulation,” a new facet of Hox genes’ function. Dev. Dyn. 2014, 243, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Sordino, P.; van der Hoeven, F.; Duboule, D. Hox gene expression in teleost fins and the origin of vertebrate digits. Nature 1995, 375, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.; Ho, R.K. Tri-phasic expression of posterior Hox genes during development of pectoral fins in zebrafish: Implications for the evolution of vertebrate paired appendages. Dev. Biol. 2008, 322, 220–233. [Google Scholar] [CrossRef]
- Mork, L.; Crump, G. Zebrafish Craniofacial Development: A Window into Early Patterning. Curr. Top. Dev. Biol. 2015, 115, 235–269. [Google Scholar] [CrossRef]
- Le Pabic, P.; Dranow, D.B.; Hoyle, D.J.; Schilling, T.F. Zebrafish endochondral growth zones as they relate to human bone size, shape and disease. Front. Endocrinol. 2022, 13, 1060187. [Google Scholar] [CrossRef]
- Witten, P.E.; Huysseune, A. A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol. Rev. Camb. Philos. Soc. 2009, 84, 315–346. [Google Scholar] [CrossRef]
- Weigele, J.; Franz-Odendaal, T.A. Functional bone histology of zebrafish reveals two types of endochondral ossification, different types of osteoblast clusters and a new bone type. J. Anat. 2016, 229, 92–103. [Google Scholar] [CrossRef]
- Witten, P.E.; Harris, M.P.; Huysseune, A.; Winkler, C. Small teleost fish provide new insights into human skeletal diseases. Methods Cell Biol. 2017, 138, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Cubbage, C.C.; Mabee, P.M. Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, Cyprinidae). J. Morphol. 1996, 229, 121–160. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; DeLaurier, A.; Ullmann, B.; Dowd, J.; McFadden, M. Modes of developmental outgrowth and shaping of a craniofacial bone in zebrafish. PLoS ONE 2010, 5, e9475. [Google Scholar] [CrossRef]
- Nguyen, S.V.; Lanni, D.; Xu, Y.; Michaelson, J.S.; McMenamin, S.K. Dynamics of the Zebrafish Skeleton in Three Dimensions During Juvenile and Adult Development. Front. Physiol. 2022, 13, 875866. [Google Scholar] [CrossRef] [PubMed]
- Giffin, J.L.; Gaitor, D.; Franz-Odendaal, T.A. The Forgotten Skeletogenic Condensations: A Comparison of Early Skeletal Development Amongst Vertebrates. J. Dev. Biol. 2019, 7, 4. [Google Scholar] [CrossRef]
- El Fersioui, Y.; Pinton, G.; Allaman-Pillet, N.; Schorderet, D.F. Premature Vertebral Mineralization in hmx1-Mutant Zebrafish. Cells 2022, 11, 1088. [Google Scholar] [CrossRef]
- Witten, P.; Hall, B. The Ancient Segmented Active and Permanent Notochord. In Ancient Fishes and their Living Relatives: A Tribute to John G. Maisey; Verlag Dr. Friedrich Pfeil: Munich, Germany, 2021. [Google Scholar]
- Pogoda, H.M.; Riedl-Quinkertz, I.; Löhr, H.; Waxman, J.S.; Dale, R.M.; Topczewski, J.; Schulte-Merker, S.; Hammerschmidt, M. Direct activation of chordoblasts by retinoic acid is required for segmented centra mineralization during zebrafish spine development. Development 2018, 145, dev159418. [Google Scholar] [CrossRef]
- Wopat, S.; Bagwell, J.; Sumigray, K.D.; Dickson, A.L.; Huitema, L.F.A.; Poss, K.D.; Schulte-Merker, S.; Bagnat, M. Spine Patterning Is Guided by Segmentation of the Notochord Sheath. Cell Rep. 2018, 22, 2026–2038. [Google Scholar] [CrossRef]
- Pogoda, H.M.; Riedl-Quinkertz, I.; Hammerschmidt, M. Direct BMP signaling to chordoblasts is required for the initiation of segmented notochord sheath mineralization in zebrafish vertebral column development. Front. Endocrinol. 2023, 14, 1107339. [Google Scholar] [CrossRef]
- Huxley, F.R.S. On the Structure and Development of the Vertebrate Skeleton. Chic. Med. J. 1863, 20, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.V.; Tsang, V.W.; Hu, W.; Kalev-Zylinska, M.; Postlethwait, J.; Crosier, P.; Crosier, K.; Fisher, S. Duplicate zebrafish runx2 orthologues are expressed in developing skeletal elements. Gene Expr. Patterns 2004, 4, 573–581. [Google Scholar] [CrossRef]
- Sims, N.; Baron, R. Bone Cells and Their Function; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000; pp. 1–16. [Google Scholar]
- Cotti, S.; Huysseune, A.; Koppe, W.; Rücklin, M.; Marone, F.; Wölfel, E.M.; Fiedler, I.A.K.; Busse, B.; Forlino, A.; Witten, P.E. More Bone with Less Minerals? The Effects of Dietary Phosphorus on the Post-Cranial Skeleton in Zebrafish. Int. J. Mol. Sci. 2020, 21, 5429. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.P. Calcium and phosphate: A duet of ions playing for bone health. J. Am. Coll. Nutr. 2011, 30, 438s–448s. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, Y.; Baujat, G.; Botschen, U.; Wittkampf, T.; du Moulin, M.; Stella, J.; Le Merrer, M.; Guest, G.; Lambot, K.; Tazarourte-Pinturier, M.F.; et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am. J. Hum. Genet. 2012, 90, 25–39. [Google Scholar] [CrossRef]
- Ralph, D.; Levine, M.A.; Richard, G.; Morrow, M.M.; Flynn, E.K.; Uitto, J.; Li, Q. Mutation update: Variants of the ENPP1 gene in pathologic calcification, hypophosphatemic rickets, and cutaneous hypopigmentation with punctate keratoderma. Hum. Mutat. 2022, 43, 1183–1200. [Google Scholar] [CrossRef]
- Bergen, A.A.; Plomp, A.S.; Hu, X.; de Jong, P.T.; Gorgels, T.G. ABCC6 and pseudoxanthoma elasticum. Pflugers Arch. 2007, 453, 685–691. [Google Scholar] [CrossRef]
- Shimada, B.K.; Pomozi, V.; Zoll, J.; Kuo, S.; Martin, L.; Le Saux, O. ABCC6, Pyrophosphate and Ectopic Calcification: Therapeutic Solutions. Int. J. Mol. Sci. 2021, 22, 4555. [Google Scholar] [CrossRef]
- Li, Q.; van de Wetering, K.; Uitto, J. Pseudoxanthoma Elasticum as a Paradigm of Heritable Ectopic Mineralization Disorders: Pathomechanisms and Treatment Development. Am. J. Pathol. 2019, 189, 216–225. [Google Scholar] [CrossRef]
- Terry, S.F.; Uitto, J. Pseudoxanthoma Elasticum. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Li, Q.; Sadowski, S.; Frank, M.; Chai, C.; Váradi, A.; Ho, S.Y.; Lou, H.; Dean, M.; Thisse, C.; Thisse, B.; et al. The abcc6a gene expression is required for normal zebrafish development. J. Investig. Dermatol. 2010, 130, 2561–2568. [Google Scholar] [CrossRef]
- Van Gils, M.; Vanakker, O.M. Morpholino-Mediated Gene Knockdown in Zebrafish: It Is All About Dosage and Validation. J. Investig. Dermatol. 2019, 139, 1599–1600. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, M.; Willaert, A.; De Vilder, E.Y.G.; Coucke, P.J.; Vanakker, O.M. Generation and Validation of a Complete Knockout Model of abcc6a in Zebrafish. J. Investig. Dermatol. 2018, 138, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.O.; Shin, M.; Ni, C.W.; Gupta, A.; Grosse, A.S.; van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Mackay, E.W.; Apschner, A.; Schulte-Merker, S. Vitamin K reduces hypermineralisation in zebrafish models of PXE and GACI. Development 2015, 142, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; She, P.; Liu, X.; Gao, B.; Jin, D.; Zhong, T.P. Disruption of Abcc6 Transporter in Zebrafish Causes Ocular Calcification and Cardiac Fibrosis. Int. J. Mol. Sci. 2020, 22, 278. [Google Scholar] [CrossRef] [PubMed]
- Czimer, D.; Porok, K.; Csete, D.; Gyüre, Z.; Lavró, V.; Fülöp, K.; Chen, Z.; Gyergyák, H.; Tusnády, G.E.; Burgess, S.M.; et al. A New Zebrafish Model for Pseudoxanthoma Elasticum. Front. Cell Dev. Biol. 2021, 9, 628699. [Google Scholar] [CrossRef]
- Apschner, A.; Huitema, L.F.; Ponsioen, B.; Peterson-Maduro, J.; Schulte-Merker, S. Zebrafish enpp1 mutants exhibit pathological mineralization, mimicking features of generalized arterial calcification of infancy (GACI) and pseudoxanthoma elasticum (PXE). Dis. Models Mech. 2014, 7, 811–822. [Google Scholar] [CrossRef]
- Singh, A.P.; Sosa, M.X.; Fang, J.; Shanmukhappa, S.K.; Hubaud, A.; Fawcett, C.H.; Molind, G.J.; Tsai, T.; Capodieci, P.; Wetzel, K.; et al. αKlotho Regulates Age-Associated Vascular Calcification and Lifespan in Zebrafish. Cell Rep. 2019, 28, 2767–2776.e2765. [Google Scholar] [CrossRef]
- Stevenson, N.L.; Bergen, D.J.M.; Skinner, R.E.H.; Kague, E.; Martin-Silverstone, E.; Robson Brown, K.A.; Hammond, C.L.; Stephens, D.J. Giantin-knockout models reveal a feedback loop between Golgi function and glycosyltransferase expression. J. Cell Sci. 2017, 130, 4132–4143. [Google Scholar] [CrossRef]
- Pottie, L.; Adamo, C.S.; Beyens, A.; Lütke, S.; Tapaneeyaphan, P.; De Clercq, A.; Salmon, P.L.; De Rycke, R.; Gezdirici, A.; Gulec, E.Y.; et al. Bi-allelic premature truncating variants in LTBP1 cause cutis laxa syndrome. Am. J. Hum. Genet. 2021, 108, 2386–2388. [Google Scholar] [CrossRef]
- Ziegler, S.G.; Gahl, W.A.; Ferreira, C.R. Generalized Arterial Calcification of Infancy. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Schorderet, D.F.; Nichini, O.; Boisset, G.; Polok, B.; Tiab, L.; Mayeur, H.; Raji, B.; de la Houssaye, G.; Abitbol, M.M.; Munier, F.L. Mutation in the human homeobox gene NKX5-3 causes an oculo-auricular syndrome. Am. J. Hum. Genet. 2008, 82, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, R.L.; Urquhart, J.; Lovell, S.C.; Biswas, S.; Parry, N.R.; Schorderet, D.F.; Lloyd, I.C.; Clayton-Smith, J.; Black, G.C. Abrogation of HMX1 function causes rare oculoauricular syndrome associated with congenital cataract, anterior segment dysgenesis, and retinal dystrophy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Amendt, B.A.; Sutherland, L.B.; Russo, A.F. Transcriptional antagonism between Hmx1 and Nkx2.5 for a shared DNA-binding site. J. Biol. Chem. 1999, 274, 11635–11642. [Google Scholar] [CrossRef] [PubMed]
- El Fersioui, Y.; Pinton, G.; Allaman-Pillet, N.; Schorderet, D.F. Hmx1 regulates urfh1 expression in the craniofacial region in zebrafish. PLoS ONE 2021, 16, e0245239. [Google Scholar] [CrossRef]
- Marcelli, F.; Boisset, G.; Schorderet, D.F. A dimerized HMX1 inhibits EPHA6/epha4b in mouse and zebrafish retinas. PLoS ONE 2014, 9, e100096. [Google Scholar] [CrossRef][Green Version]
- Boisset, G.; Schorderet, D.F. Zebrafish hmx1 promotes retinogenesis. Exp. Eye Res. 2012, 105, 34–42. [Google Scholar] [CrossRef]
- Pomreinke, A.P.; Soh, G.H.; Rogers, K.W.; Bergmann, J.K.; Bläßle, A.J.; Müller, P. Dynamics of BMP signaling and distribution during zebrafish dorsal-ventral patterning. eLife 2017, 6, e25861. [Google Scholar] [CrossRef]
- Fisher, S.; Halpern, M.E. Patterning the zebrafish axial skeleton requires early chordin function. Nat. Genet. 1999, 23, 442–446. [Google Scholar] [CrossRef]
- Stafford, D.A.; Monica, S.D.; Harland, R.M. Follistatin interacts with Noggin in the development of the axial skeleton. Mech. Dev. 2014, 131, 78–85. [Google Scholar] [CrossRef]
- Wijgerde, M.; Karp, S.; McMahon, J.; McMahon, A.P. Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev. Biol. 2005, 286, 149–157. [Google Scholar] [CrossRef]
- Chefetz, I.; Heller, R.; Galli-Tsinopoulou, A.; Richard, G.; Wollnik, B.; Indelman, M.; Koerber, F.; Topaz, O.; Bergman, R.; Sprecher, E.; et al. A novel homozygous missense mutation in FGF23 causes Familial Tumoral Calcinosis associated with disseminated visceral calcification. Hum. Genet. 2005, 118, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kurpas, A.; Supeł, K.; Idzikowska, K.; Zielińska, M. FGF23: A Review of Its Role in Mineral Metabolism and Renal and Cardiovascular Disease. Dis. Markers 2021, 2021, 8821292. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Kakitani, M.; Yamazaki, Y.; Hasegawa, H.; Takeuchi, Y.; Fujita, T.; Fukumoto, S.; Tomizuka, K.; Yamashita, T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J. Clin. Investig. 2004, 113, 561–568. [Google Scholar] [CrossRef]
- Ogura, Y.; Kaneko, R.; Ujibe, K.; Wakamatsu, Y.; Hirata, H. Loss of αklotho causes reduced motor ability and short lifespan in zebrafish. Sci. Rep. 2021, 11, 15090. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.C.; Moe, O.W.; et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef]
- Ten Hagen, K.G.; Fritz, T.A.; Tabak, L.A. All in the family: The UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferases. Glycobiology 2003, 13, 1R–16R. [Google Scholar] [CrossRef]
- Tiwari, V.; Zahra, F. Hyperphosphatemic Tumoral Calcinosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kato, K.; Jeanneau, C.; Tarp, M.A.; Benet-Pagès, A.; Lorenz-Depiereux, B.; Bennett, E.P.; Mandel, U.; Strom, T.M.; Clausen, H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 2006, 281, 18370–18377. [Google Scholar] [CrossRef]
- Gok, F.; Chefetz, I.; Indelman, M.; Kocaoglu, M.; Sprecher, E. Newly discovered mutations in the GALNT3 gene causing autosomal recessive hyperostosis-hyperphosphatemia syndrome. Acta Orthop. 2009, 80, 131–134. [Google Scholar] [CrossRef]
- Mangos, S.; Amaral, A.P.; Faul, C.; Jüppner, H.; Reiser, J.; Wolf, M. Expression of fgf23 and αklotho in developing embryonic tissues and adult kidney of the zebrafish, Danio rerio. Nephrol. Dial. Transplant. 2012, 27, 4314–4322. [Google Scholar] [CrossRef]
- Kuro-o, M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat. Rev. Nephrol. 2013, 9, 650–660. [Google Scholar] [CrossRef]
- Nollet, L.L.; Vanakker, O.M. Mitochondrial Dysfunction and Oxidative Stress in Hereditary Ectopic Calcification Diseases. Int. J. Mol. Sci. 2022, 23, 15288. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.G.; Krizhanovsky, V. Physiological and pathological consequences of cellular senescence. Cell. Mol. Life Sci. 2014, 71, 4373–4386. [Google Scholar] [CrossRef] [PubMed]
- Eren, M.; Boe, A.E.; Murphy, S.B.; Place, A.T.; Nagpal, V.; Morales-Nebreda, L.; Urich, D.; Quaggin, S.E.; Budinger, G.R.; Mutlu, G.M.; et al. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc. Natl. Acad. Sci. USA 2014, 111, 7090–7095. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Voet, M.; Gardeitchik, T.; Morava, E. Cutis Laxa. Adv. Exp. Med. Biol. 2014, 802, 161–184. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, R.; Li, J.; Wu, Y.; Zhang, J. Latent TGF-beta binding protein-1 plays an important role in craniofacial development. J. Appl. Oral. Sci. 2020, 28, e20200262. [Google Scholar] [CrossRef]
- Abrial, M.; Basu, S.; Huang, M.; Butty, V.; Schwertner, A.; Jeffrey, S.; Jordan, D.; Burns, C.E.; Burns, C.G. Latent TGFβ-binding proteins 1 and 3 protect the larval zebrafish outflow tract from aneurysmal dilatation. Dis. Models Mech. 2022, 15, dmm046979. [Google Scholar] [CrossRef]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Ban, H.; Yokota, D.; Otosaka, S.; Kikuchi, M.; Kinoshita, H.; Fujino, Y.; Yabe, T.; Ovara, H.; Izuka, A.; Akama, K.; et al. Transcriptional autoregulation of zebrafish tbx6 is required for somite segmentation. Development 2019, 146, dev177063. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Zhao, G.; Yokoyama, T.; Vega, H.; Huang, Y.; Sood, R.; Bishop, K.; Maduro, V.; Accardi, J.; et al. ATP6V1H Deficiency Impairs Bone Development through Activation of MMP9 and MMP13. PLoS Genet. 2017, 13, e1006481. [Google Scholar] [CrossRef]
- Shen, Q.; Little, S.C.; Xu, M.; Haupt, J.; Ast, C.; Katagiri, T.; Mundlos, S.; Seemann, P.; Kaplan, F.S.; Mullins, M.C.; et al. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J. Clin. Investig. 2009, 119, 3462–3472. [Google Scholar] [CrossRef]
- Mintzer, K.A.; Lee, M.A.; Runke, G.; Trout, J.; Whitman, M.; Mullins, M.C. Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development 2001, 128, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.; Lele, Z.; Rauch, G.J.; Geisler, R.; Hammerschmidt, M. The type I serine/threonine kinase receptor Alk8/Lost-a-fin is required for Bmp2b/7 signal transduction during dorsoventral patterning of the zebrafish embryo. Development 2001, 128, 849–858. [Google Scholar] [CrossRef] [PubMed]
- LaBonty, M.; Pray, N.; Yelick, P.C. A Zebrafish Model of Human Fibrodysplasia Ossificans Progressiva. Zebrafish 2017, 14, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Henke, K.; Daane, J.M.; Hawkins, M.B.; Dooley, C.M.; Busch-Nentwich, E.M.; Stemple, D.L.; Harris, M.P. Genetic Screen for Postembryonic Development in the Zebrafish (Danio rerio): Dominant Mutations Affecting Adult Form. Genetics 2017, 207, 609–623. [Google Scholar] [CrossRef]
- Gistelinck, C.; Kwon, R.Y.; Malfait, F.; Symoens, S.; Harris, M.P.; Henke, K.; Hawkins, M.B.; Fisher, S.; Sips, P.; Guillemyn, B.; et al. Zebrafish type I collagen mutants faithfully recapitulate human type I collagenopathies. Proc. Natl. Acad. Sci. USA 2018, 115, E8037–E8046. [Google Scholar] [CrossRef]
- Enderli, T.A.; Burtch, S.R.; Templet, J.N.; Carriero, A. Animal models of osteogenesis imperfecta: Applications in clinical research. Orthop. Res. Rev. 2016, 8, 41–55. [Google Scholar] [CrossRef]
- Fisher, S.; Jagadeeswaran, P.; Halpern, M.E. Radiographic analysis of zebrafish skeletal defects. Dev. Biol. 2003, 264, 64–76. [Google Scholar] [CrossRef]
- Fiedler, I.A.K.; Schmidt, F.N.; Wölfel, E.M.; Plumeyer, C.; Milovanovic, P.; Gioia, R.; Tonelli, F.; Bale, H.A.; Jähn, K.; Besio, R.; et al. Severely Impaired Bone Material Quality in Chihuahua Zebrafish Resembles Classical Dominant Human Osteogenesis Imperfecta. J. Bone Miner. Res. 2018, 33, 1489–1499. [Google Scholar] [CrossRef]
- Asharani, P.V.; Keupp, K.; Semler, O.; Wang, W.; Li, Y.; Thiele, H.; Yigit, G.; Pohl, E.; Becker, J.; Frommolt, P.; et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am. J. Hum. Genet. 2012, 90, 661–674. [Google Scholar] [CrossRef]
- Kague, E.; Witten, P.E.; Soenens, M.; Campos, C.L.; Lubiana, T.; Fisher, S.; Hammond, C.; Brown, K.R.; Passos-Bueno, M.R.; Huysseune, A. Zebrafish sp7 mutants show tooth cycling independent of attachment, eruption and poor differentiation of teeth. Dev. Biol. 2018, 435, 176–184. [Google Scholar] [CrossRef]
- Giachelli, C.M. Ectopic calcification: Gathering hard facts about soft tissue mineralization. Am. J. Pathol. 1999, 154, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Hoppé, E.; Kauffenstein, G.; Omarjee, L.; Navasiolava, N.; Henni, S.; Willoteaux, S.; Leftheriotis, G. Early arterial calcification does not correlate with bone loss in pseudoxanthoma elasticum. Bone 2017, 103, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Gielis, W.P.; de Jong, P.A.; Bartstra, J.W.; Foppen, W.; Spiering, W.; den Harder, A.M. Osteoarthritis in Pseudoxanthoma Elasticum Patients: An Explorative Imaging Study. J. Clin. Med. 2020, 9, 3898. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, M.; Willaert, A.; Coucke, P.J.; Vanakker, O.M. The Abcc6a Knockout Zebrafish Model as a Novel Tool for Drug Screening for Pseudoxanthoma Elasticum. Front. Pharmacol. 2022, 13, 822143. [Google Scholar] [CrossRef]
- Nollet, L.; Van Gils, M.; Willaert, A.; Coucke, P.J.; Vanakker, O.M. Minocycline Attenuates Excessive DNA Damage Response and Reduces Ectopic Calcification in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2022, 142, 1629–1638.e6. [Google Scholar] [CrossRef]
- Kauffenstein, G.; Chappard, D.; Leftheriotis, G.; Martin, L. ABCC6 deficiency and bone loss: A double benefit of etidronate for patient presenting with pseudoxanthoma elasticum? Exp. Dermatol. 2022, 31, 1635–1637. [Google Scholar] [CrossRef]
- Brampton, C.; Yamaguchi, Y.; Vanakker, O.; Van Laer, L.; Chen, L.H.; Thakore, M.; De Paepe, A.; Pomozi, V.; Szabó, P.T.; Martin, L.; et al. Vitamin K does not prevent soft tissue mineralization in a mouse model of pseudoxanthoma elasticum. Cell Cycle 2011, 10, 1810–1820. [Google Scholar] [CrossRef]
- Gorgels, T.G.; Waarsing, J.H.; Herfs, M.; Versteeg, D.; Schoensiegel, F.; Sato, T.; Schlingemann, R.O.; Ivandic, B.; Vermeer, C.; Schurgers, L.J.; et al. Vitamin K supplementation increases vitamin K tissue levels but fails to counteract ectopic calcification in a mouse model for pseudoxanthoma elasticum. J. Mol. Med. 2011, 89, 1125–1135. [Google Scholar] [CrossRef]
- Greenblatt, M.B.; Ono, N.; Ayturk, U.M.; Debnath, S.; Lalani, S. The Unmixing Problem: A Guide to Applying Single-Cell RNA Sequencing to Bone. J. Bone Miner. Res. 2019, 34, 1207–1219. [Google Scholar] [CrossRef]
- Alvarez, M.; Schrey, A.W.; Richards, C.L. Ten years of transcriptomics in wild populations: What have we learned about their ecology and evolution? Mol. Ecol. 2015, 24, 710–725. [Google Scholar] [CrossRef]
- Boneski, P.K.; Madhu, V.; Tomlinson, R.E.; Shapiro, I.M.; van de Wetering, K.; Risbud, M.V. Abcc6 Null Mice-a Model for Mineralization Disorder PXE Shows Vertebral Osteopenia without Enhanced Intervertebral Disc Calcification with Aging. Front. Cell Dev. Biol. 2022, 10, 823249. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, M.; Depauw, J.; Coucke, P.J.; Aerts, S.; Verschuere, S.; Nollet, L.; Vanakker, O.M. Inorganic Pyrophosphate Plasma Levels Are Decreased in Pseudoxanthoma Elasticum Patients and Heterozygous Carriers but Do Not Correlate with the Genotype or Phenotype. J. Clin. Med. 2023, 12, 1893. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.S.; Duijst, S.; Mahakena, S.; Sommer, D.; Szeri, F.; Váradi, A.; Plomp, A.; Bergen, A.A.; Oude Elferink, R.P.; Borst, P.; et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1985–1989. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tévar, A.M.; García-Fernández, M.; Murcia-Casas, B.; Rioja-Villodres, J.; Carrillo, J.L.; Camacho, M.; Van Gils, M.; Sánchez-Chaparro, M.A.; Vanakker, O.; Valdivielso, P. Plasma inorganic pyrophosphate and alkaline phosphatase in patients with pseudoxanthoma elasticum. Ann. Transl. Med. 2019, 7, 798. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Navasiolava, N.; Clotaire, L.; Duranton, C.; Le Saux, O.; Bendahhou, S.; Laurain, A.; Rubera, I.; Martin, L. Relationships between Plasma Pyrophosphate, Vascular Calcification and Clinical Severity in Patients Affected by Pseudoxanthoma Elasticum. J. Clin. Med. 2022, 11, 2588. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Kintzinger, K.; Hackbarth, M.E.; Botschen, U.; Nitschke, Y.; Mughal, M.Z.; Baujat, G.; Schnabel, D.; Yuen, E.; Gahl, W.A.; et al. Ectopic Calcification and Hypophosphatemic Rickets: Natural History of ENPP1 and ABCC6 Deficiencies. J. Bone Miner. Res. 2021, 36, 2193–2202. [Google Scholar] [CrossRef]
- Li, C.; Chai, Y.; Wang, L.; Gao, B.; Chen, H.; Gao, P.; Zhou, F.Q.; Luo, X.; Crane, J.L.; Yu, B.; et al. Programmed cell senescence in skeleton during late puberty. Nat. Commun. 2017, 8, 1312. [Google Scholar] [CrossRef]
- Wan, M.; Gray-Gaillard, E.F.; Elisseeff, J.H. Cellular senescence in musculoskeletal homeostasis, diseases, and regeneration. Bone Res. 2021, 9, 41. [Google Scholar] [CrossRef]
- Miglionico, R.; Ostuni, A.; Armentano, M.F.; Milella, L.; Crescenzi, E.; Carmosino, M.; Bisaccia, F. ABCC6 knockdown in HepG2 cells induces a senescent-like cell phenotype. Cell. Mol. Biol. Lett. 2017, 22, 7. [Google Scholar] [CrossRef]
- Tiemann, J.; Wagner, T.; Lindenkamp, C.; Plümers, R.; Faust, I.; Knabbe, C.; Hendig, D. Linking ABCC6 Deficiency in Primary Human Dermal Fibroblasts of PXE Patients to p21-Mediated Premature Cellular Senescence and the Development of a Proinflammatory Secretory Phenotype. Int. J. Mol. Sci. 2020, 21, 9665. [Google Scholar] [CrossRef]
- Sato, C.; Iso, Y.; Mizukami, T.; Otabe, K.; Sasai, M.; Kurata, M.; Sanbe, T.; Sekiya, I.; Miyazaki, A.; Suzuki, H. Fibroblast growth factor-23 induces cellular senescence in human mesenchymal stem cells from skeletal muscle. Biochem. Biophys. Res. Commun. 2016, 470, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Cheikhi, A.; Barchowsky, A.; Sahu, A.; Shinde, S.N.; Pius, A.; Clemens, Z.J.; Li, H.; Kennedy, C.A.; Hoeck, J.D.; Franti, M.; et al. Klotho: An Elephant in Aging Research. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Hosen, M.J.; Coucke, P.J.; Le Saux, O.; De Paepe, A.; Vanakker, O.M. Perturbation of specific pro-mineralizing signalling pathways in human and murine pseudoxanthoma elasticum. Orphanet J. Rare Dis. 2014, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.F. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef]
- Feng, X.H.; Derynck, R. Specificity and versatility in tgf-beta signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005, 21, 659–693. [Google Scholar] [CrossRef]
- Bernabeu, C.; Lopez-Novoa, J.M.; Quintanilla, M. The emerging role of TGF-beta superfamily coreceptors in cancer. Biochim. Biophys. Acta 2009, 1792, 954–973. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Koba, T.; Watanabe, K.; Goda, S.; Kitagawa, M.; Mutoh, N.; Hamada, N.; Tani-Ishii, N. The Effect of Transforming Growth Factor Beta 1 on the Mineralization of Human Cementoblasts. J. Endod. 2021, 47, 606–611. [Google Scholar] [CrossRef]
- Payne, T.L.; Postlethwait, J.H.; Yelick, P.C. Functional characterization and genetic mapping of alk8. Mech. Dev. 2001, 100, 275–289. [Google Scholar] [CrossRef]
- Tylzanowski, P.; Verschueren, K.; Huylebroeck, D.; Luyten, F.P. Smad-interacting protein 1 is a repressor of liver/bone/kidney alkaline phosphatase transcription in bone morphogenetic protein-induced osteogenic differentiation of C2C12 cells. J. Biol. Chem. 2001, 276, 40001–40007. [Google Scholar] [CrossRef]
- Zhang, D.; Schwarz, E.M.; Rosier, R.N.; Zuscik, M.J.; Puzas, J.E.; O’Keefe, R.J. ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development. J. Bone Miner. Res. 2003, 18, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Hendig, D.; Zarbock, R.; Szliska, C.; Kleesiek, K.; Götting, C. The local calcification inhibitor matrix Gla protein in pseudoxanthoma elasticum. Clin. Biochem. 2008, 41, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Nguyen, T.M.; Williamson, M.K. Biochemical characterization of the serum fetuin-mineral complex. J. Biol. Chem. 2003, 278, 22153–22160. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Iwazu, Y.; Shiizaki, K.; Akimoto, T.; Kotani, K.; Kurabayashi, M.; Kurosu, H.; Kuro, O.M. Identification and quantification of plasma calciprotein particles with distinct physical properties in patients with chronic kidney disease. Sci. Rep. 2018, 8, 1256. [Google Scholar] [CrossRef]
- Gelli, R.; Pucci, V.; Ridi, F.; Baglioni, P. A study on biorelevant calciprotein particles: Effect of stabilizing agents on the formation and crystallization mechanisms. J. Colloid Interface Sci. 2022, 620, 431–441. [Google Scholar] [CrossRef]
- Smith, E.R.; Hewitson, T.D.; Jahnen-Dechent, W. Calciprotein particles: Mineral behaving badly? Curr. Opin. Nephrol. Hypertens. 2020, 29, 378–386. [Google Scholar] [CrossRef]
- Kutikhin, A.G.; Feenstra, L.; Kostyunin, A.E.; Yuzhalin, A.E.; Hillebrands, J.L.; Krenning, G. Calciprotein Particles: Balancing Mineral Homeostasis and Vascular Pathology. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1607–1624. [Google Scholar] [CrossRef]
- Köppert, S.; Büscher, A.; Babler, A.; Ghallab, A.; Buhl, E.M.; Latz, E.; Hengstler, J.G.; Smith, E.R.; Jahnen-Dechent, W. Cellular Clearance and Biological Activity of Calciprotein Particles Depend on Their Maturation State and Crystallinity. Front. Immunol. 2018, 9, 1991. [Google Scholar] [CrossRef]
- Hendig, D.; Schulz, V.; Arndt, M.; Szliska, C.; Kleesiek, K.; Götting, C. Role of serum fetuin-A, a major inhibitor of systemic calcification, in pseudoxanthoma elasticum. Clin. Chem. 2006, 52, 227–234. [Google Scholar] [CrossRef]
- Nollet, L.; Van Gils, M.; Fischer, S.; Campens, L.; Karthik, S.; Pasch, A.; De Zaeytijd, J.; Leroy, B.P.; Devos, D.; De Backer, T.; et al. Serum Calcification Propensity T50 Associates with Disease Severity in Patients with Pseudoxanthoma Elasticum. J. Clin. Med. 2022, 11, 3727. [Google Scholar] [CrossRef] [PubMed]
- Gheduzzi, D.; Boraldi, F.; Annovi, G.; DeVincenzi, C.P.; Schurgers, L.J.; Vermeer, C.; Quaglino, D.; Ronchetti, I.P. Matrix Gla protein is involved in elastic fiber calcification in the dermis of pseudoxanthoma elasticum patients. Lab. Investig. 2007, 87, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Blazquez-Medela, A.M.; Guihard, P.J.; Yao, J.; Jumabay, M.; Lusis, A.J.; Boström, K.I.; Yao, Y. ABCC6 deficiency is associated with activation of BMP signaling in liver and kidney. FEBS Open Bio 2015, 5, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Pasch, A. Novel assessments of systemic calcification propensity. Curr. Opin. Nephrol. Hypertens. 2016, 25, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Pasch, A.; Farese, S.; Gräber, S.; Wald, J.; Richtering, W.; Floege, J.; Jahnen-Dechent, W. Nanoparticle-based test measures overall propensity for calcification in serum. J. Am. Soc. Nephrol. 2012, 23, 1744–1752. [Google Scholar] [CrossRef]
- Pasch, A.; Jahnen-Dechent, W.; Smith, E.R. Phosphate, Calcification in Blood, and Mineral Stress: The Physiologic Blood Mineral Buffering System and Its Association with Cardiovascular Risk. Int. J. Nephrol. 2018, 2018, 9182078. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, H.J.; Li, Q.L.; Chi, X.Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.G.; Choi, J.Y.; Ryoo, H.M.; et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 2000, 20, 8783–8792. [Google Scholar] [CrossRef]
- van der Meulen, T.; Kranenbarg, S.; Schipper, H.; Samallo, J.; van Leeuwen, J.L.; Franssen, H. Identification and characterisation of two runx2 homologues in zebrafish with different expression patterns. Biochim. Biophys. Acta 2005, 1729, 105–117. [Google Scholar] [CrossRef]
- Liu, D.D.; Zhang, C.Y.; Liu, Y.; Li, J.; Wang, Y.X.; Zheng, S.G. RUNX2 Regulates Osteoblast Differentiation via the BMP4 Signaling Pathway. J. Dent. Res. 2022, 101, 1227–1237. [Google Scholar] [CrossRef]
- Feger, M.; Hase, P.; Zhang, B.; Hirche, F.; Glosse, P.; Lang, F.; Föller, M. The production of fibroblast growth factor 23 is controlled by TGF-β2. Sci. Rep. 2017, 7, 4982. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Ito, Y.; Bringas, P., Jr.; Chou, S.; Urata, M.M.; Slavkin, H.; Chai, Y. TGFbeta-mediated FGF signaling is crucial for regulating cranial neural crest cell proliferation during frontal bone development. Development 2006, 133, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. Renal Physiol. 2010, 299, F882–F889. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Wu, X.; Zhang, F.; Clemens, T.L.; Wan, M.; Cao, X. TGF-beta type II receptor phosphorylates PTH receptor to integrate bone remodelling signalling. Nat. Cell Biol. 2010, 12, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Zerr, P.; Vollath, S.; Palumbo-Zerr, K.; Tomcik, M.; Huang, J.; Distler, A.; Beyer, C.; Dees, C.; Gela, K.; Distler, O.; et al. Vitamin D receptor regulates TGF-β signalling in systemic sclerosis. Ann. Rheum. Dis. 2015, 74, e20. [Google Scholar] [CrossRef]
- Irani, M.; Seifer, D.B.; Grazi, R.V.; Julka, N.; Bhatt, D.; Kalgi, B.; Irani, S.; Tal, O.; Lambert-Messerlian, G.; Tal, R. Vitamin D Supplementation Decreases TGF-β1 Bioavailability in PCOS: A Randomized Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2015, 100, 4307–4314. [Google Scholar] [CrossRef]
- Subramaniam, N.; Leong, G.M.; Cock, T.A.; Flanagan, J.L.; Fong, C.; Eisman, J.A.; Kouzmenko, A.P. Cross-talk between 1,25-dihydroxyvitamin D3 and transforming growth factor-beta signaling requires binding of VDR and Smad3 proteins to their cognate DNA recognition elements. J. Biol. Chem. 2001, 276, 15741–15746. [Google Scholar] [CrossRef]
- Doi, S.; Zou, Y.; Togao, O.; Pastor, J.V.; John, G.B.; Wang, L.; Shiizaki, K.; Gotschall, R.; Schiavi, S.; Yorioka, N.; et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 2011, 286, 8655–8665. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef]
- de Las Rivas, M.; Paul Daniel, E.J.; Narimatsu, Y.; Compañón, I.; Kato, K.; Hermosilla, P.; Thureau, A.; Ceballos-Laita, L.; Coelho, H.; Bernadó, P.; et al. Molecular basis for fibroblast growth factor 23 O-glycosylation by GalNAc-T3. Nat. Chem. Biol. 2020, 16, 351–360. [Google Scholar] [CrossRef]
- Li, Y.; Hu, F.; Xue, M.; Jia, Y.J.; Zheng, Z.J.; Wang, L.; Guan, M.P.; Xue, Y.M. Klotho down-regulates Egr-1 by inhibiting TGF-β1/Smad3 signaling in high glucose treated human mesangial cells. Biochem. Biophys. Res. Commun. 2017, 487, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xue, M.; Hu, F.; Jia, Y.; Zheng, Z.; Yang, Y.; Liu, X.; Yang, Y.; Wang, Y. Klotho prevents epithelial-mesenchymal transition through Egr-1 downregulation in diabetic kidney disease. BMJ Open Diabetes Res. Care 2021, 9, e002038. [Google Scholar] [CrossRef] [PubMed]
- Toan, N.K.; Tai, N.C.; Kim, S.A.; Ahn, S.G. Soluble Klotho regulates bone differentiation by upregulating expression of the transcription factor EGR-1. FEBS Lett. 2020, 594, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; McCune, B.K.; Sporn, M.B. TGF-beta: Regulation of extracellular matrix. Kidney Int. 1992, 41, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.B.; Rifkin, D.B. Regulation of the Bioavailability of TGF-β and TGF-β-Related Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021907. [Google Scholar] [CrossRef]
- Laue, K.; Pogoda, H.M.; Daniel, P.B.; van Haeringen, A.; Alanay, Y.; von Ameln, S.; Rachwalski, M.; Morgan, T.; Gray, M.J.; Breuning, M.H.; et al. Craniosynostosis and multiple skeletal anomalies in humans and zebrafish result from a defect in the localized degradation of retinoic acid. Am. J. Hum. Genet. 2011, 89, 595–606. [Google Scholar] [CrossRef]
- Spoorendonk, K.M.; Peterson-Maduro, J.; Renn, J.; Trowe, T.; Kranenbarg, S.; Winkler, C.; Schulte-Merker, S. Retinoic acid and Cyp26b1 are critical regulators of osteogenesis in the axial skeleton. Development 2008, 135, 3765–3774. [Google Scholar] [CrossRef]
- Delbaere, S.; Van Damme, T.; Syx, D.; Symoens, S.; Coucke, P.; Willaert, A.; Malfait, F. Hypomorphic zebrafish models mimic the musculoskeletal phenotype of β4GalT7-deficient Ehlers-Danlos syndrome. Matrix Biol. 2020, 89, 59–75. [Google Scholar] [CrossRef]
- Buchan, J.G.; Gray, R.S.; Gansner, J.M.; Alvarado, D.M.; Burgert, L.; Gitlin, J.D.; Gurnett, C.A.; Goldsmith, M.I. Kinesin family member 6 (kif6) is necessary for spine development in zebrafish. Dev. Dyn. 2014, 243, 1646–1657. [Google Scholar] [CrossRef]
- Grimes, D.T.; Boswell, C.W.; Morante, N.F.; Henkelman, R.M.; Burdine, R.D.; Ciruna, B. Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science 2016, 352, 1341–1344. [Google Scholar] [CrossRef]
- Gray, R.S.; Wilm, T.P.; Smith, J.; Bagnat, M.; Dale, R.M.; Topczewski, J.; Johnson, S.L.; Solnica-Krezel, L. Loss of col8a1a function during zebrafish embryogenesis results in congenital vertebral malformations. Dev. Biol. 2014, 386, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Van Gennip, J.L.M.; Boswell, C.W.; Ciruna, B. Neuroinflammatory signals drive spinal curve formation in zebrafish models of idiopathic scoliosis. Sci. Adv. 2018, 4, eaav1781. [Google Scholar] [CrossRef] [PubMed]
- Zancan, I.; Bellesso, S.; Costa, R.; Salvalaio, M.; Stroppiano, M.; Hammond, C.; Argenton, F.; Filocamo, M.; Moro, E. Glucocerebrosidase deficiency in zebrafish affects primary bone ossification through increased oxidative stress and reduced Wnt/β-catenin signaling. Hum. Mol. Genet. 2015, 24, 1280–1294. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).