Abstract
Stroke is a major cause of mortality and disability globally, with ischemic stroke (IS) accounting for over 80% of all stroke cases. The pathological process of IS involves numerous signal molecules, among which are the highly conserved nicotinamide adenine dinucleotide (NAD+)-dependent enzymes known as sirtuins (SIRTs). SIRTs modulate various biological processes, including cell differentiation, energy metabolism, DNA repair, inflammation, and oxidative stress. Importantly, several studies have reported a correlation between SIRTs and IS. This review introduces the general aspects of SIRTs, including their distribution, subcellular location, enzyme activity, and substrate. We also discuss their regulatory roles and potential mechanisms in IS. Finally, we describe the current therapeutic methods based on SIRTs, such as pharmacotherapy, non-pharmacological therapeutic/rehabilitative interventions, epigenetic regulators, potential molecules, and stem cell-derived exosome therapy. The data collected in this study will potentially contribute to both clinical and fundamental research on SIRTs, geared towards developing effective therapeutic candidates for future treatment of IS.
1. Introduction
Stroke is a major cause of global mortality and morbidity, with two-thirds of stroke survivors developing disabilities, which pose a heavy burden on families and society [1]. Ischemic stroke (IS), which accounts for more than 80% of all strokes, is the most common type of stroke [2]. The sudden cessation of cerebral blood flow results in a complex cascade of events that lead to neuronal cell death and brain damage. The current and the only approved medical therapy for ischemic stroke is the recombinant tissue plasminogen activator (rt-PA); however, it should be administered within 4.5 h of stroke onset [1,3]. Mechanical thrombectomy, an alternative or additional method to rt-PA, has expanded the treatment time window producing therapeutic effects [4,5]. Nonetheless, ischemia-reperfusion injury after these clot-targeting therapies may lead to serious neurological deficits and prognosis. Therefore, it is crucial to elucidate the underlying mechanisms of brain damage associated with ischemic stroke and develop novel therapeutic targets.
Recent evidence suggests that histone modifications regulate the pathological mechanisms that cause ischemic stroke [6,7]. Histone acetylation, dynamically regulated by histone acetyltransferase (HATs) and histone deacetylase (HDACs), is important for global changes in gene transcription and protein expression [8,9]. Sirtuins (SIRTs) are a class of nicotinamide adenine dinucleotide (NAD+) dependent HDACs with a highly conserved central catalytic core. Increasing NAD+ levels can improve the activity of SIRTs. The sirtuin family has seven members, i.e., SIRT1-SIRT7. They have highly conserved NAD+ binding and catalytic domains [10]. The various subcellular localizations, binding sites, and functions of SIRTs are partially explained by differences in their sequence and length in their N and C-terminal domains [11]. SIRTs modulate cell differentiation, energy metabolism, DNA repair, inflammation, and oxidative stress by catalyzing NAD+-dependent deacetylation, desuccinylation, and ADP glycosylation reactions [10,12,13]. SIRTs have been associated with neurodegenerative diseases, cardiovascular diseases, diabetic kidney disease, and other aging-related diseases [14,15,16]. Importantly, recent preclinical and clinical studies have reported a correlation between SIRTs and IS, suggesting that SIRTs may be a promising target for IS treatment [17,18,19].
In this review, we present a systematic overview of recent advances in SIRTs research in ischemic stroke and discuss their therapeutic potential. We hope to generate novel ideas for the development of clinical therapy and medications for ischemic stroke treatment.
2. Sirtuins in Ischemic Stroke
2.1. General Aspects Regarding Sirtuins
2.1.1. SIRT1
Among the sirtuin family members, SIRT1 has been extensively studied and is expressed in a wide range of human tissues, including the brain, liver, muscle, endothelium, pancreas, and adipose tissue [20,21,22]. In the central nervous system, SIRT1 is widely expressed in neurons of the cortex, hippocampus, cerebellum, and thalamus [23,24]. SIRT1 expression at the subcellular level, i.e., in mitochondria, centrosomes, Golgi bodies, and nuclei, varies depending on the tissue and cell type, and occurs through nucleocytoplasmic shuttling, along with growth, development, and stress responses [25]. SIRT1 is expressed in the nucleus and cytoplasm of cultured brain vascular endothelial cells, mainly in the nucleus [26]. The biological effects of SIRT1 are largely attributed to its capacity to deacetylate target proteins. SIRT1 has been shown to deacetylate histone H4 lysine 16 (H4K16) and H3K9, leading to the subsequent suppression of transcription [27]. Moreover, SIRT1 interacts with and deacetylates H1K26, H3K14, H3K18, and H4K6 [27,28]. Other than the histone deacetylation, SIRT1 can drive the deacetylation of various non-histone proteins, including p53, forkhead-box transcription factor (FOXO), proliferator-activated receptor γ coactivator 1α (PGC-1α), nuclear factor kappa B (NF-κB), hypoxia-inducible factor-1a (HIF-1α), nuclear factor E2-related factor 2 (Nrf2), and AMP-activated protein kinase (AMPK) [29,30]. The broad range of targets influenced by SIRT1 endows it with a critical role in regulating various biological processes, including cellular differentiation, inflammation, oxidative stress, cell apoptosis, mitochondrial function, and autophagy [31,32,33].
SIRT1 exerts a neuroprotective effect in IS. In an experimental study, neurons in the ipsilesional cortex of a mouse brain expressed more SIRT1 upon exposure to middle cerebral artery occlusion (MCAO). Furthermore, treatment with a SIRT1 activator could further reduce the infarct volume [34]. Similarly, clinical studies have shown that serum SIRT1 concentrations in patients with acute ischemic stroke (AIS) are significantly higher than normal and SIRT1 may act as a possible diagnostic marker for AIS patients [35]. The increased expression of SIRT1 after cerebral ischemia may represent an endogenous defense mechanism as a stress response. Additionally, evidence suggests that mice overexpressing SIRT1 show minimal hippocampal damage and preserved cerebral blood flow after bilateral common carotid artery stenosis (BCAO) [36]. However, SIRT1 knockout (KO) mice showed larger infarct volumes after ischemia [34].
2.1.2. SIRT2
SIRT2 is the first sirtuin to be identified; it is extensively expressed in multiple tissues, especially in metabolically active ones, like the brain, liver, heart, and adipose tissue [37,38]. Although SIRT2 mainly localizes in the cytoplasm, it can shuttle to the nucleus under certain conditions to participate in a series of biological processes [38,39]. Existing findings have shown that SIRT2 is specifically enriched in brain oligodendroglia [40]. In MCAO mice, SIRT2 mRNA induction was discovered in the entire hemisphere following the stroke and highly expressed in myelin formed by oligodendrocytes [41]. Similar to this, upregulated SIRT2 expression and nuclear translocation in cerebral stroke patients’ ischemic penumbra were identified [42].
Unlike SIRT1, SIRT2 appears to have a negative impact on IS. SIRT2 inhibition increases neural tolerance to oxidative stress by enhancing the activation of cytoprotective genes [43]. SIRT2 was upregulated during neuronal ischemia in the oxygen-glucose deprivation (OGD)-induced cell model and the MCAO-induced mouse model; notably, downregulating SIRT2 could significantly protect neurons against cerebral ischemia [42]. According to Lea’s study, SIRT2-deficient mice exhibited significantly reduced neurological impairments upon MCAO compared to wild-type animals [41]. Moreover, the level of serum SIRT2 significantly increased in AIS patients compared to controls and negatively correlated with patient prognosis [44].
2.1.3. SIRT3
SIRT3 is predominantly found in the mitochondrial matrix and is widely expressed in highly metabolic tissues such as the brain, heart, liver, and brown adipose tissue [45,46]. Studies indicate that SIRT3 is also present in the nucleus and cytoplasm [47,48,49]. Mitochondria, i.e., the energy center of cells, manufacture adenosine triphosphate (ATP) to fuel cells and are also essential for regulating the production of reactive oxygen species (ROS), maintaining Ca2+ homeostasis, controlling osmotic pressure, and transmitting cell signals. SIRT3 is essential for mitochondrial energy metabolism, substrate oxidation, and mitophagy through its deacetylation activity. By deacetylating ATP synthase proteins via SIRT3, acetylome signaling helps maintain energy balance in the mitochondria [50]. Moreover, SIRT3 increases the activity of the antioxidant enzyme superoxide dismutase 2 (SOD2) by deacetylating FOXO3a, resulting in ROS reduction and protecting cells from oxidative stress [51]. However, SIRT3 deficiency can lead to disruption of mitochondrial homeostasis and inhibit mitochondrial autophagy [52].
SIRT3 plays an important role in the regulation of ischemic stroke processes. Previous studies have shown that upregulated SIRT3 expression reduces the infarct volume and improves neurological function, via the SIRT3-FOXO3a-SOD2 pathway in MCAO mice [17]. FOXO3a, which inhibits astrocyte proliferation and cytokine-mediated astrocyte proliferation, is also a crucial mediator of astrogliosis [53]. Astrocyte activation can form a scar after a stroke. Reports indicate that SIRT3 deficiency attenuates the inhibitory effect of adjudin’s (a SIRT3 activator) in the formation of the glial scar, whereas SIRT3 overexpression could further reduce the glial scar by inhibiting astrogliosis through the SIRT3- FOXO3a pathway activation [54].
2.1.4. SIRT4
SIRT4 exists in the mitochondrial matrix of several organs, including the brain, heart, kidney, liver, and muscle [55,56,57]. Although SIRT4 has little deacetylation activity, it functions primarily as an ADP ribosyltransferase [58]. SIRT4 transfers an ADP-ribosyl group from NAD+ to the glutamate dehydrogenase enzyme (GDH), which in turn represses GDH activity [55]. Another significant role of SIRT4 is its regulation of glutamine metabolism and promotion of the damage response of deoxyribonucleic acid (DNA) [59]. Furthermore, SIRT4 can regulate the expression of genes involved in fatty acid oxidation in muscle mitochondria and hepatocytes [60].
Although information regarding the role of SIRT4 in ischemic stroke is limited, its function can still provide valuable insights. Excessive glutamate accumulation in neurons causes excitotoxicity, which is the underlying cause of brain injury following IS. Studies have shown that SIRT4 deficiency causes a more severe response to the potent excitotoxin kainic acid, whereas SIRT4 overexpression can increase the protein levels of glutamate transporter 1 (GLT-1) and inhibits the level of glutamine synthetase, thereby preventing excitotoxicity [61,62]. Furthermore, SIRT4 was downregulated in healthy human umbilical vein endothelial cells (HUVECs) during acute hypoxia, resulting in increased oxidative stress and inflammation [63]. These results imply that SIRT4 may be a potential new target for the therapy of acute vascular events including IS.
2.1.5. SIRT5
SIRT5 is expressed widely in various organs, including the brain, heart, liver, kidney, muscle, and testicles, primarily located in mitochondria, but also present in the cytoplasm and nucleus [64,65,66]. In the human brain, SIRT5 transcripts are expressed in all cortical layers, with layer II exhibiting the highest levels [67]. Unlike other sirtuins, SIRT5 has weak deacetylase activity but possesses strong lysine desuccinylation, desmalonylation, and desglutarylase activity due to the larger lysine acyl binding pocket in its structure [68,69]. SIRT5 can reduce excessive ROS levels by binding and succinate SOD, thereby enhancing cells’ ability to resist oxidative stress [70].
The role of SIRT5 in ischemic stroke remains controversial. Studies have shown that SIRT5 maintained mitochondrial respiration and protected against metabolic stressors and cell death after cerebral ischemia [71]. However, some researchers reported that compared to wild-type mice, infarct volume decreased and neurological impairments improved 48 h after 45 min of MCAO in SIRT5 KO mice [72]. Furthermore, upregulated levels of SIRT5 gene expression were observed in peripheral blood monocytes (PBMCs) of AIS patients 6 h after the initial stroke compared to healthy controls [72]. Additional research is necessary to elucidate the precise role of SIRT5 in the brain after ischemia.
2.1.6. SIRT6
SIRT6 is primarily located in the nucleus of the liver, brain, thymus, muscle, and heart and deacetylates histone H3 at lysine sites 9, 18, and 56 sites in a site-specific manner [73,74]. However, the first enzymatic activity discovered in SIRT6 is its NAD+-dependent mono-ADP-ribosyltransferase activity [75]. Additionally, SIRT6 can deacetylate various non-histone substrates, such as FOXO1, histone acetyltransferase 5 (GCN5), and C-terminal binding protein interacting protein (CTIP) [76,77,78]. By regulating the activity of key proteins, SIRT6 plays an important role in maintaining genome stability and telomere integrity, promoting DNA repair, preventing aging, and maintaining glucose homeostasis [79].
SIRT6 is highly expressed in the cortex and hippocampus of the brain [80,81]. It protects the brain from DNA damage, neurological impairment, and neurodegeneration [82]. SIRT6 KO brains showed accumulated DNA damage and increased apoptosis [82]. Luca and colleagues constructed the MCAO injury model in vascular endothelial cell (VEC)-specific SIRT6 knockout mice and discovered that these mice exhibited increased cerebral infarction volume, increased neuronal mortality, and aggravated neurological function damage [18]. Conversely, post-ischemic SIRT6 overexpression mitigated neurological deficiency and infarct size [18]. Several studies suggest that SIRT6 is a protective molecule in IS; however, other studies maintain opposing opinions. For example, Shao et al. reported that suppressing SIRT6 reduced oxidative stress-induced neuronal damage [83].
2.1.7. SIRT7
SIRT7 is primarily located in the nucleolus, although evidence suggests that it also exists in the cytoplasm of primary fibroblasts [84]. All mouse tissues investigated expressed SIRT7 mRNA, except for skeletal muscle. In non-proliferating tissues, such as muscle, heart, and brain, SIRT7 levels were very low, but they were high in metabolically active tissues such as the liver, spleen, and testicles [85]. SIRT7 is involved in various cellular processes, including mitochondrial homeostasis, genomic stability, chromatin regulation, and ribosome biogenesis [86].
Studies on the function of SIRT7 in ischemic stroke are scarce. Using high-throughput databases and a stroke systems biological model, Wong et al. identified SIRT7 as a potentially effective target for first aid and emergency treatment within 24 h post-stroke [87]. Lv et al. reported that after OGD reoxygenation treatment, SIRT7 expression in neurons significantly increased, whereas SIRT7 knockout aggravated the OGD-induced injury [88] (Figure 1).
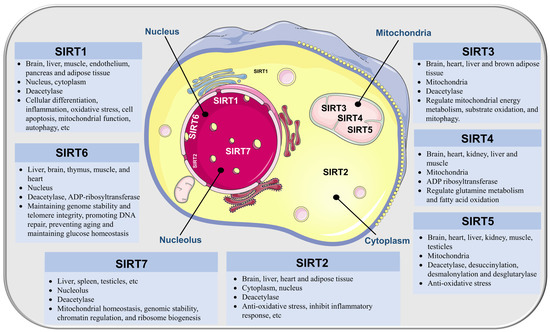
Figure 1.
Distribution, subcellular location, enzyme activity, and functions of SIRTs.
2.2. Regulation of Sirtuins in Ischemic Stroke
2.2.1. Energy Metabolism
The brain primarily relies on glucose to produce energy [89], and impaired energy metabolism is a key pathological sign of IS [90,91]. An abrupt reduction in cerebral blood flow causes decreased glucose and oxygen delivery, insufficient NAD+ synthesis, and a drop in the NAD+/NADH ratio, all of which pose substantial risks to the survival and function of neurons. Mitochondria are the major sites for ATP production, and the integrity of their structure and function is the basis for smooth energy metabolism [92].
ATP synthesis in the electron transport chain is mildly uncoupled by uncoupling protein 2 (UCP2), and high levels of UCP2 expression may prevent cells from producing ATP [93]. Preconditioning with a SIRT1 agonist can significantly reduce the area of cerebral infarction in mice with focal cerebral ischemia, and its mechanism is related to the downregulation of mitochondrial UCP2 [94]. Kevin and colleagues discovered that in neurons of SIRT1 knockout mice, the rate of glycolysis decreased and ATP production was impaired [95]. AMPK is an essential factor that regulates mitochondrial biogenesis and plays a crucial role in regulating energy metabolism and mitochondrial function via proliferator-activated receptor gamma coactivator-1α (PGC-1α) signaling [96,97]. AMPK and SIRT1 can activate each other and have a synergistic relationship in response to ischemic injury [98]. Wan and colleagues reported that AMPK activator pretreatment reduces ATP energy consumption during cerebral ischemia by activating SIRT1, thus showing beneficial effects in protecting neurons [99]. Other studies have shown that SIRT3 overexpression can promote mitochondrial biogenesis and induce autophagy by activating the AMPK pathway, thereby alleviating OGD-induced neuronal damage [100].
Protein kinase C epsilon (PKCε) is an important signaling molecule that protects mitochondrial function and nerve cells and plays a vital role in reducing ischemic injury [101,102]. PKCε enhances the expression of nicotinamide phosphoribosyl transferase (Nampt), an enzyme primarily expressed in neurons that catalyzes the rate-limiting step of NAD+. The lack of Nampt after cerebral ischemia can exacerbate the aging of nerve cells [103]. Morris-Blanco et al. discovered that Nampt was integral to PKCε-mediated maintenance of mitochondrial respiration. The activation of PKCε can enhance the desuccinylation of SIRT5. After the deletion of SIRT5, the oxygen consumption in mitochondria significantly reduced, and PKCε could not prevent cortical degeneration after cerebral ischemia injury, strongly suggesting that SIRT5 plays an important role in the regulation of mitochondrial energy generation and the neuroprotective effect after cerebral ischemia mediated by PKCε [71]. Collectively, these studies indicate that SIRTs play an important role in maintaining energy metabolism.
2.2.2. Oxidative Stress
The brain tissue contains high levels of lipids, including unsaturated fatty acids, and possesses weak antioxidant defenses, rendering it susceptible to oxidative stress caused by oxygen free radicals [104]. Neurons experience oxygen and energy depletion after ischemic stroke. When free radical generation, including ROS and reactive nitrogen species (RNS), exceeds the endogenous capacity to counteract them, redox equilibrium is disrupted, causing oxidative stress, which causes extensive damage to neurons [105]. Additional effects of oxidative stress include neuroinflammation, apoptosis, disruption of the blood–brain barrier (BBB), and exacerbation of neurological disability [106].
Previous research has demonstrated that SIRT3 overexpression can decrease the infarct volume and improve the neurologic function of MCAO mice. This mechanism could be associated with the deacetylating of FOXO3a, increasing the activity of the antioxidant enzyme SOD2 and reducing ROS production [17]. Similarly, recent evidence indicates that SIRT3 overexpression effectively attenuates ROS overproduction induced by oxygen–glucose deprivation/reperfusion (OGD/R) in HUVECs [107].
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a redox-sensitive transcription factor that is broadly expressed and regulates the expression of a range of antioxidant genes by interacting with antioxidant response elements (AREs) in the nucleus [108,109,110]. The Nrf2 signaling system is an important antioxidant defense system that removes overproduced free radicals. Increasing evidence suggests that Nrf2 expression upregulates after an ischemic stroke to protect against damage caused by excessive ROS generation [111,112]. However, the absence of Nrf2 exacerbates neurological impairments and cerebral infarction [113,114]. Nrf2 protein and its downstream antioxidant proteins, including HO-1 and NQO1, were upregulated in both the OGD/R and MCAO models [115]. However, SIRT1 silencing blocked these effects and exacerbated the oxidative stress that follows an ischemic stroke. SIRT1 may exert beneficial effects after cerebral I/R by upregulating the Nrf2 protein [115]. SIRT6 was also found to have an antioxidant effect by promoting Nrf2 expression. SIRT6 expression in the cerebral cortex decreased after cerebral I/R, whereas SIRT6 overexpression using in vivo gene transfer improved Nrf2 signaling, decreased oxidative stress, and alleviated the neurological impairments and brain tissue damage caused by cerebral I/R [116]. These data suggest that suppressing oxidative stress via SIRTs is a potentially valuable therapeutic strategy for IS in the future.
2.2.3. Autophagy
Autophagy is a vital process by which cells remove damaged proteins and organelles via lysosomes to maintain normal physiological function and homeostasis. The sudden interruption of cerebral blood flow results in energy deprivation, followed by the activation of autophagy with a biphasic effect on ischemic stroke. As a key process of self-repair, moderate activation of autophagy can protect nerve cells and reduce brain damage. However, excessive or insufficient autophagy can cause autophagic cell death and aggravate brain damage [2]. Various autophagy-related (ATG) regulate different stages of autophagy formation, including isolation membrane formation, autophagy maturation, autophagy-lysosome fusion, and autophagy degradation [117,118].
AMPK is a key cellular energy sensor, and its activation can induce autophagy [119,120]. Conversely, the mammalian target of rapamycin (mTOR) is a strong inhibitor of autophagy, and AKT is an upstream signaling molecule that activates mTOR [121]. Evidence suggests that AMPK inhibits mTOR, thereby causing autophagy [122]. SIRT3 protects against neuronal ischemia in OGD-induced cortical neurons by increasing the number of LC3-positive puncta and autophagosomes by interacting with the AMPK-mTOR pathway [100]. Conversely, SIRT6 can activate autophagy by upregulating the number of LC3 and ATG5 by weakening the AKT signal; however, this aggravates neuronal damage induced by oxidative stress [83]. Moreover, SIRT1 deacetylates FOXO1 to inhibit the expression of autophagy-associated factors, improving cerebral injury in MCAO rats [123]. Taken together, the role of autophagy in acute cerebral ischemia remains controversial. Autophagy activation during cerebral ischemia may either have a neuroprotective impact or promote brain damage [119,124]. Additional studies are necessary to elucidate the crosstalk between SIRTs and autophagy.
2.2.4. Neuroinflammation
An inflammatory reaction is a key pathological factor that contributes to neurological impairment in ischemic stroke. The release of a large number of necrotic substances after an ischemic stroke causes peripheral white blood cells to infiltrate into the brain parenchyma. The activation of microglial cells and astrocytes in the brain parenchyma further triggers a series of strong inflammatory cascade reactions, which destroy the BBB, leading to brain edema and neuronal death [125].
Nuclear factor κB (NF-κB), a transcription factor, is crucial in triggering the inflammatory response. TNF-α, IL-1β, and IL-6 are among the inflammatory cytokines and chemokines that are released when NF-κB is activated [126,127]. SIRT1 can inhibit the transcription activity of NF-κB by deacetylation, resulting in a reduction in vascular inflammation [128]. TRAF6 is an essential adaptor protein that connects upstream receptors to downstream effector molecules in cells, allowing for the transmission of multiple receptor signaling pathways on cell membranes and, consequently, exerting biological effects [129]. Following an ischemic stroke, the expression of TRAF6 in brain homogenates increases in a time-dependent manner [130]. The activation of TRAF6 leads to the activation of the NF-κb signaling pathway, which then triggers the inflammatory response. In a mouse model of MCAO-induced stroke, a decrease in the level of SIRT1 protein increased ROS expression, as mentioned earlier [131]. Interestingly, ischemia-induced TRAF6 accumulation and injury were reduced when the ROS levels dropped, whereas a burst of ROS was accompanied by an increase in TRAF6 protein [131]. In short, according to Yan’s report, the decrease in SIRT1 protein levels leads to an increase in ROS production, and the burst of ROS further leads to an increase in TRAF6 protein levels. The disruption of this mechanism may be related to oxidative stress, inflammation, and injury repair processes in MCAO-induced cerebral ischemic injury in mice [131]. The SIRT1-ROS-TRAF6 pathway is a potential therapeutic target against IS.
High-mobility group box 1 (HMGB1) is a DNA-binding protein that exists widely in the nucleus and cytoplasm of eukaryotes, and it can act as an inflammatory trigger when passively released from necrotic cells [132]. As a key histone deacetylase, SIRT6 decreased significantly during cerebral ischemia, promoting the release of HMGB1 [133]. This was also confirmed in SH-SY5Y neuronal cells induced by OGD [133].
Microglia play a dual role in cerebral ischemia. On the one hand, they produce inflammatory mediators that promote the inflammatory response; on the other hand, they protect neurons from excitotoxicity [134]. Following cerebral ischemia, microglia expressing the fractalkine receptor (CX3CR1) are activated and drawn to the ischemic area. By upregulating CX3CR1, Cao et al. discovered that SIRT3 encouraged microglia to migrate to the ischemic brain [135]. Future research should focus on deeply investigating the complex relationship between SIRT3 and microglia.
Additionally, regulatory T cells (Tregs), a component of the adaptive immune system, serve as an inbuilt defense mechanism to reduce inflammation in the brain following an ischemic stroke. Tregs can prevent the recruitment of peripheral inflammatory cells and the infiltration of microglia in the ischemic penumbra [136]. SIRT2 was significantly upregulated in infiltrating Tregs at day 3 post-MCAO, but this change weakened the anti-inflammatory effect of Tregs [137]. In summary, SIRTs play an important role in regulating neuroinflammation to ameliorate ischemic injury.
2.2.5. Apoptosis
Ischemic neuronal apoptosis is primarily a mitochondrial-centered process that involves genes from the B-cell lymphoma (Bcl-2) family and the cysteine protease family. Anti-apoptotic factor Bcl-2 and pro-apoptotic factor Bax are two crucial regulatory proteins of the apoptotic pathway. During cerebra ischemia, glucose/energy metabolism disorder leads to a decrease in Na+/K+-ATPase activity, which results in an ion homeostasis imbalance, cell membrane depolarization, and a large influx of Ca2+. The high concentration of calcium ions cleaves the Bcl-2 interaction domain (Bid) to produce truncated Bit (tBid), which migrates to the mitochondria and interacts with pro-apoptotic proteins such as Bax, inducing the opening of mitochondrial transition pores (MTP) and the subsequent release of cytochrome c (Cytc) [138]. Cytc induces the transformation of Apaf1 (apoptotic protease activating factor 1), which then binds with procaspase-9 to form an apoptosome, that spontaneously activates the caspase-9 complex. The Cyt-c, Apaf1, and caspase-9 complex continue to activate downstream caspase-3 resulting in the formation of apoptotic bodies. The activated caspase3 hydrolyzes structural and functional proteins related to cell disintegration, leading to DNA fragmentation and apoptosis [139].
Reduction in caspase-3 and other apoptotic markers like Bim and Bad suggest that SIRT2 downregulation may prevent the apoptosis of OGD-induced neurons. The underlying mechanisms may be linked to the suppression of the AKT/FOXO3a and MAPK pathways caused by SIRT2 inhibition [140]. SIRT7 may protect neurons from OGD/R-induced damage by regulating the p53-mediated proapoptotic signal pathway, according to research by Lv et al. [88]. In experimentally generated cerebral ischemic rats, estrogen deficiency caused by ovariectomy exacerbates brain infarction. However, estrogen pretreatment suppresses apoptosis and reduces ischemia-induced cerebral damage. Importantly, the estrogen-induced neuroprotection effect was abolished in SIRT1 knockout mice and AMPK-inhibited rats. It is reasonable to speculate that estrogen protects against ischemic injury through the SIRT1/AMPK pathway, with SIRT1 being a key mediator in this process [141]. Additionally, Yan et al. found that SIRT1 deacetylates NF-κB to reduce the apoptosis rate and improve cerebral infarction conditions in IS rats [142]. It should be noted that, as mentioned earlier, SIRT1 is primarily localized in the nucleus, and its high expression in the nucleus is associated with anti-apoptotic effects, whereas overexpression in the cytoplasm does not possess this anti-apoptotic function [143]. In short, these findings suggest a possible link between apoptosis and SIRTs, involving neuroprotection. This opens up a new avenue for targeting SIRTs in the treatment of cerebral ischemia.
2.2.6. Blood–Brain Barrier Integrity and Angiogenesis
The BBB, an important physical and biochemical barrier between blood and brain tissue, majorly comprises brain microvascular endothelial cells (BMECs) and is wrapped by pericytes and astrocytes [144]. The BBB can protect brain tissue from toxic substances and control the stability of the central nervous system. The destruction of the BBB is one of the significant pathological features of ischemic stroke, playing an important role in promoting neurological deficits [145]. Therefore, it is urgent to develop new therapeutic strategies to preserve the integrity of the BBB after ischemic stroke.
The tight junctions (TJs) proteins expressed by BMECs can restrict cell bypass permeability and are crucial regulators of BBB integrity [146,147]. HIF-1α activation can disrupt TJs and increase BBB permeability [148]. SIRT3 helps in relieving brain edema and BBB damage after ischemic stroke, which might be achieved by increasing TJ proteins ZO-1 and Occludin by regulating the HIF-1α signaling pathway [149]. Diaz-Cañestro’s study revealed that SIRT5 is an important promoter of brain injury and neurological deficits. This effect is mediated by increasing BBB permeability and reducing the expression of TJ proteins through the PI3K/AKT pathway in MCAO mice models and primary human brain microvascular endothelial cells [72]. Additionally, SIRT6 has been found to attenuate BBB damage in an MCAO-induced mice model [18]. Using mice models lacking SIRT6, researchers found that mice lacking SIRT6 exhibited larger stroke size, more severe neurological deficits, and increased blood–brain barrier disruption [18].
There is evidence to suggest that increased microvessel density in the peri-infarct region is associated with longer survival in patients with ischemic stroke [150]. This indicates that enhancement of angiogenesis is one of the effective strategies for promoting functional recovery after ischemic stroke [151,152]. The vascular endothelial growth factor (VEGF) is one of the main targets of HIF-1α and is a potent growth factor that plays an important role in angiogenesis by activating extracellular regulated protein kinases (ERK) [153]. Indeed, the pro-migratory effect of vascular endothelial growth factor (VEGF) has been found to be mediated by the inhibition of thioredoxin-interacting protein (TXNIP) [154]. In the context of cerebral ischemia, energy restriction-induced SIRT6 has been found to inhibit TXNIP transcription and promote angiogenesis, which is critical for tissue repair and recovery after cerebral ischemia [155]. However, VEGF is also a vascular permeability factor that controls paracellular permeability and is responsible for disrupting BBB function [148,156]. Therefore, VEGF plays a dual role following an ischemic stroke [157]. During the acute phase of ischemic stroke, the levels of HIF-1α and VEGF proteins in SIRT3-deficient mice increased, resulting in more severe BBB damage [149]. Moreover, in the I/R model constructed using human BMECs, SIRT3 overexpression reduced the permeability of the BBB by regulating the expression of tight junctions [158]. However, during the recovery period of cerebral ischemia, SIRT3 KO mice showed a notable decrease in VEGF levels and ERK phosphorylation, and the infarct striatum exhibited lower vascular density [159]. Hence, it is important to note that the effectiveness of targeting SIRT3 to enhance angiogenesis as a therapeutic approach for IS recovery may vary depending on the intervention time. Further research is needed to optimize and validate this strategy and determine the long-term benefits and potential risks.
2.2.7. Neurogenesis
In recent decades, several neurorestorative therapies have been developed to activate brain plasticity and support functional recovery in both experimental and clinical investigations. Neurogenesis, which offers essential support for remodeling and restoring brain architecture and function, has attracted significant attention in the last decade [160]. Neurogenesis includes the proliferation, migration, and differentiation of neural stem cells (NSCs). Research has demonstrated that adult neural stem cells located in the subventricular zone and the subgranular zone of the hippocampal dentate gyrus can be activated and generate neuroblasts. These neuroblasts are recruited to the ischemic area and participate in the repair of ischemic brain tissue [161,162,163]. Zhao and colleagues found that SIRT1 and SIRT2 contribute to NSC proliferation, whereas SIRT1, SIRT2, and SIRT6 contribute to NSC differentiation [164]. Specifically, an increase in the proportion of young neurons and a decrease in the proportion of mature neurons, without impacting glial differentiation, have been discovered to be effects of SIRT6 on neuronal maturation and differentiation within the hippocampus [80]. SIRT6 is one of several key players that regulate the complexity of adult hippocampal neurogenesis.
In summary, we discussed the regulatory mechanisms of SIRTs in ischemic stroke, including energy metabolism, oxidative stress, autophagy, neuroinflammation, apoptosis, BBB disruption, angiogenesis, and neurogenesis. We can obtain that SIRTs play important roles in both the acute and long-term stages of post-ischemic stroke. SIRTs can exert a neuroprotective effect in the acute stage following a stroke. They help reduce inflammation, oxidative stress, and neuronal apoptosis, promoting cell survival and preserving the integrity of the BBB. SIRTs also regulate energy metabolism and enhance cellular resilience to ischemic injury. In the chronic stage after a stroke, SIRTs play a regulatory role in neurorepair and angiogenesis, contributing to the promotion of functional recovery. It should be noted that the beneficial effects of SIRTs are not solely attributed to their activation. The role of certain SIRTs, such as SIRT4, SIRT5, and SIRT7, in IS, is still unclear. Targeting SIRTs appropriately holds promise in the treatment of IS (Figure 2).
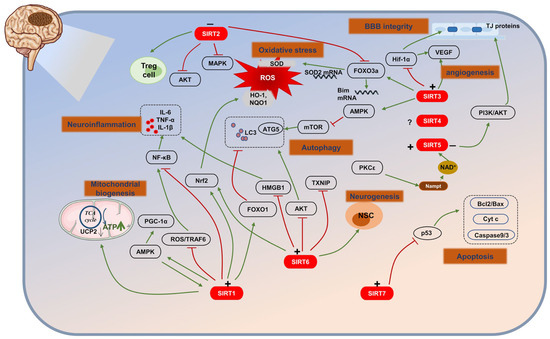
Figure 2.
The role of SIRTs in ischemic stroke (IS). (+) means activation; (−) means inhibition. SIRTs, by acting on multiple targets, regulate several pathological processes including energy metabolism, oxidative stress, autophagy, neuroinflammation, apoptosis, BBB disruption, angiogenesis, and neurogenesis.
3. Therapeutic Research
3.1. Pharmacotherapy
Drug therapy is an important component of modern medicine, which helps individuals combat diseases and improve their health. With the continuous progress of science and technology, researchers are constantly seeking new drugs and treatment methods to address the challenges posed by diseases. In the following section, we will review drug therapies targeting SIRT, including NAD+ enhancers, SIRTs activators, and SIRTs inhibitors (Table 1). This section aims to provide valuable insights for further development and innovation in the field of drug therapy.
3.1.1. Mitigating Mitochondrial Injury
Melatonin is a hormone secreted by the pineal gland and a promising neuroprotective agent. Yang and colleagues reported that melatonin treatment activates SIRT1 signaling and thus increases Bcl2 expression, decreases the level of Bax, and attenuates mitochondrial dysfunction in MCAO-induced mice [165]. In addition, melatonin retained the mitochondrial mass affected by OGD/R in mouse hippocampal HT22 cells, and restored the mitochondrial fusion/division kinetics. Interestingly, this phenomenon is accompanied by an increase in SIRT3 levels [166]. Promoting SIRTs activity by increasing the concentration of NAD+ is also a beneficial therapy. Research has shown that administering nicotinamide mononucleotide (NMN), a precursor to NAD+ synthesis, can significantly improve ischemic brain injury caused by global cerebral ischemia [167]. In a mouse model of cerebral ischemia induced by bilateral common carotid artery occlusion (CCAO), NMN treatment was found to enhance the activity of SIRT3, leading to the suppression of ischemia-induced mitochondrial fragmentation and the generation of ROS. This study has unraveled the importance of NAD+ and SIRT3 in maintaining mitochondrial health and functionality [168].
Resveratrol is the first natural compound to be discovered that could activate SIRT1, which plays an important role in protecting against neurological damage. Resveratrol could reduce ATP energy consumption during ischemia to exert a neuroprotection effect by activating the cAMP/AMPK/SIRT1 pathway [99]. Resveratrol’s translatability in the treatment of IS is encouraging in terms of clinical efficacy. A two-period, open-label, single-arm, within-subject control study showed that healthy participants tolerated trans-resveratrol 2000 mg twice daily with an acceptable exposure [169]. Notoginseng leaf triterpenes (PNGL) is a dammarane-type saponin purified from Panax notoginseng stem and leaf. In OGD/R-induced SH-SY5Y cells, PNGL remarkably reversed the decrease in intracellular NAD+ and NADH and the depletion of ATP production, reducing neuronal necrosis and improving neuronal survival under ischemia and hypoxia conditions. PNGL may exert neuroprotective effects by regulating the Nampt-NAD+- SIRT1 pathway [170].
3.1.2. Anti-Oxidative Stress Damage
Picetannol (Pic) is a natural compound with a strong antioxidant capacity found primarily in seeds, wines, and fruits [171]. Wang et al. reported that Pic treatment exerts strong antioxidant effects by upregulating the activities of antioxidant enzymes SOD, catalase (CAT), and glutathione peroxidase (GSH-Px) by activating the SIRT1/FOXO1 pathway [172]. Diosmetin, a bioflavonoid compound isolated from citrus fruits, displayed antioxidative stress effects on various organs. Administration of diosmetin before MCAO improved neurological outcomes and decreased the cerebral infarct volume and pathological lesions of rats by reducing the levels of lactate dehydrogenase (LDH) and ROS and inhibiting oxidative stress damage via SIRT1/Nrf2 signaling pathway activation [173]. Similarly, mangiferin inhibited LDH release and ROS generation to exert a neuroprotection effect by activating the SIRT1/PGC-1α pathway [174]. Alpha-lipoic acid (ALA) is a natural antioxidant isolated from plants and animals for use as a dietary supplement. ALA has been proven to increase the SOD level to protect the MCAO mice brain against ischemic damage by upregulating SIRT1-dependent PGC-1α [175]. In addition, Zhou and colleagues revealed that kaempferol (KFL), a natural flavonol, significantly upregulated SIRT1 protein and inhibited OGD-induced oxidative stress, resulting in a decrease in the levels of ROS and LDH and an increase in SOD activity and GSH content [176].
3.1.3. Regulating Autophagy
A few natural products exert neuroprotection effects by regulating autophagy. Betulinic acid (BA) is a biologically active pentacyclic triterpene compound extracted from Betula platyphylla. BA pretreatment was reported to notably reduce the ratio of autophagic cells, downregulate autophagy-associated factors Beclin 1 and LC3I/II, and upregulate p62 level in MCAO rats, as well as activate the SIRT1/ FOXO1 signal pathway [123]. In ischemic rats administrated with quaternary aporphine alkaloid magnoflorine, the fluorescence intensity of LC3 was remarkably downregulated, whereas p62, SIRT1, and AMPK were upregulated. Therefore, it is rational to conclude that magnoflorine relieved cerebral ischemia-induced neuronal damage by activating the SIRT1/AMPK pathway [177].
3.1.4. Inhibiting Neuroinflammation
MDL-811 is a superior SIRT6 activator with strong anti-inflammation in microglia/macrophages, which could attenuate brain injury and improve neurobehavioral deficits in MCAO mice by inhibiting the neuroinflammation via the SIRT6/EZH2/FOXC1 pathway [178]. Notably, MDL-811 treatment inhibits the expression of pro-inflammatory factors (TNF-α and IL-1β) in primary human monocytes of patients with ischemic stroke through activating SIRT6 [178]. Moreover, treatment with activator 3 (SIRT1 activator) can significantly reduce cerebral infarction volume in MCAO mice, possibly via inhibition of NF-κB induced inflammation and apoptosis pathways [34].
Research reports have shown that ginkgo biloba (GA) contains flavonoid-like compounds and terpenoids with potent anti-inflammatory, antioxidant, and free radical scavenging properties [179]. Xu et al. revealed that GA treatment ameliorates the neuroinflammation in the brain tissue of stroke rats by targeting the SIRT1/ NF-κB pathway [180]. Studies have shown that arctigenin (ARC), a phenylpropanoid dibenzylbutyrolactone lignan derived from Arctium lappa L, protects against cerebral ischemia injury in rats by inhibiting NLRP3 inflammasome activation and lowering the levels of inflammatory factors IL-1β and IL-18 by activating SIRT1 signaling in the brain [181]. Bergenin (BGN), a C-glycoside of 4-O-methylgallic acid, exerts anti-inflammatory, antioxidant, and tissue repair effects. BGN inhibited the activation of microglia, the phosphorylation of NF-κB, and the expression of inflammatory factors IL-1β, IL-6, and TNF-α. Moreover, BGN upregulates SIRT1 and FOXO3a, suggesting its potential in alleviating IS-mediated neuroinflammation by enhancing SIRT1/FOXO3a pathway [182]. Cycloastragenol can significantly upregulate the expression of SIRT1 in the ischemic brain and inhibit the mRNA expressions of pro-inflammatory factors TNF-α and IL-1β [183]. Besides these compounds, trilobatin (TLB), a small molecule monomer isolated from the Chinese herb Lithocarpus polystachyus Rehd, directly interacts with SIRT3 to increase the expression and activity of SIRT3, thereby inhibiting the toll-like receptor 4 (TLR4) signaling pathway and alleviating neuroinflammation after middle cerebral artery occlusion in rats [184]. Inflammatory cytokines and TLR-4 promote NF-κB activation, resulting in severe neurological damage [126].
3.1.5. Anti-Apoptosis
AK-7 is a SIRT2-specific inhibitor, which can remarkably decrease the infarction volume and promote the recovery of neurological function by activating p38 [185]. Additionally, SIRT2 inhibitors AK-1 and AGK2 have been proven to reduce apoptotic cell death by SIRT2 inhibition in OGD-induced cortical neurons [140].
Resuvastatin is a synthetic statin, which lowers lowering lipid levels, and exerts anti-inflammation as well as anti-oxidation. Resuvastatin may reduce cerebral infarction area and apoptosis rate in IS rats; the underlying mechanism may be related to SIRT1/NF-κB pathway regulation [142]. Melatonin has also been proven to increase SIRT3 expression and, via activating SIRT3, reduce neurological impairment and cell apoptosis after transient middle cerebral artery occlusion (tMCAO) in mice [186].
In MCAO rats, resveratrol pretreatment could enhance the SIRT1 activity, thus decreasing the levels of p53 and caspase3, and improving ischemic stroke by inhibiting cell apoptosis [187]. Calycosin-7-O-β-D-glucoside (CG), a representative isoflavone in Radix Astragali (RA), was found to upregulate Bcl-2 and downregulate Bax through activating the SIRT1/FOXO1/PGC-1α signaling pathway in OGD/R-induced hippocampal cells [188]. Similarly, Pic treatment significantly reduced the number of apoptotic hippocampal neurons in the cerebral ischemia/reperfusion injury (CIRI) mice model via activation of the Sirt1/FOXO1 pathway [172]. Kou et al. observed that magnolol could also increase the level of bcl-2 by activating SIRT1, thereby reducing brain edema and infarct volume and improving neurological scores [189]. According to Zhou et al.’s study, KFL reversed OGD-induced downregulation of SIRT1, concomitant with decreased caspase3, caspase9, and Bax, and enhanced Bcl-2 level [176]. Moreover, salvianolic acid B has also been proven to play a neuroprotective role by upregulating SIRT1 and Bcl-2 [190]. Stilbene glycoside is an ingredient extracted from Polygonum multiflorum. It upregulates SIRT3/AMPK expression, promotes mitochondrial autophagy, and inhibits cell apoptosis in ischemic neurons [191].
3.1.6. Protecting the Blood–Brain Barrier
Donepezil, an acetylcholinesterase inhibitor, has been approved for the treatment of Alzheimer’s disease. In recent years, the neuroprotective effects of donepezil have attracted significant attention. By activating SIRT1 and further deacetylating FOXO3a and NF-κB, donepezil increases the expressions of tight junction proteins, indicating that donepezil may act as a key therapeutic agent of IS [192].
After a stroke, the serine/threonine-specific protein kinase CaMKK, a key kinase caused by increased intracellular calcium, maintains the integrity of BBB [193,194]. CaMKK phosphorylates the well-known vascular defender SIRT1, making it more stable and active [195]. In MCAO mice models, CaMKKβ activity inhibition reduced the pSIRT1 levels, reducing brain endothelial cell damage and ischemia-induced BBB leakage [196].
TLB could upregulate VEGFA protein and its receptor VEGFR-2, thereby promoting the proliferation of cerebral microvascular endothelial cells and angiogenesis in mice with CIRI [197]. The underlying mechanism may be that TLB binds to SIRT7, thus activating the SIRT7/VEGFA signaling pathway. Zhu et al. discovered that the vascular length, vascular density, branching index, and the structure of brain microvascular were significantly improved after notoginsenoside R1 (R1) treatment in MCAO rats [198]. It is possible that R1 exerts its effect by regulating the Nampt-NAD+-SIRT1 cascade and Notch/VEGFR-2 signaling pathway. In OGD/R-induced HBMEC cells, R1 activated the Nampt-NAD+-SIRT1 cascade and upregulated VEGFR-2 [198]. The activation of Notch signaling and VEGFR-2 is necessary for angiogenesis [199].
3.1.7. Enhancing Neurogenesis
The Wnt signaling pathway is important for promoting neurogenesis and the development of neural tissues after cerebral ischemia [200]. Nuclear transfer and accumulation of β-catenin is an essential step in activating the Wnt/β-catenin signaling pathway [201]. Evidence suggests that SIRT1 promotes the deacetylation of β-catenin, resulting in β-catenin nuclear accumulation, which is essential for SIRT1 transcriptional activity [202,203]. Momordica charantia, an important multipurpose edible, medicinal plant, is the major source of bioactive compounds Momordica charantia polysaccharides (MCP). According to reports, MCP therapy promotes the differentiation of hippocampal SGZ neurons in MCAO rats, possibly by upregulating the SIRT1 level, thereby promoting the β-catenin deacetylation and nuclear accumulation [204,205]. This effect has also been confirmed through in vitro experiments.
In short, if SIRTs modulators with good pharmacokinetics and tolerance can be developed and converted into clinical applications, they can provide important treatment methods for IS. Therefore, research and development of effective drugs of SIRTs warrant urgent attention for IS treatment.
3.2. Non-Pharmacological Therapeutic/Rehabilitative Interventions
Acupuncture is an ancient traditional Chinese medicine technology, which is applied to treat various diseases, because of its advantages, including safety, potent therapeutic properties, low risks, and cost-effectiveness [206,207]. Electroacupuncture (EA) is an important form of acupuncture, in which it is easy to control and quantify the intensity and frequency of acupoint stimulation. It combines the advantages of acupuncture treatment and electrical stimulation and is widely used in clinical practice [208,209]. EA preconditioning significantly alleviates the neural damage caused by cerebral ischemia [210]. Ying’s study revealed that EA treatment at 24 h after ischemic stroke significantly inhibited the expression of autophagy-related proteins LC3II/I, Beclin1, and cell apoptosis, reducing brain injury in rats [211]. This neuroprotection may be achieved by promoting the expression of SIRT1, p-ERK, and p-JNK. However, autophagy is a double-edged sword in cerebral ischemia. The acetylation of H4K16 is associated with I/R-induced autophagic activation [212,213,214]. Another study showed that EA can trigger the molecular histone switch of SIRT1 and reduce the acetylation of H4K16, thus promoting the expression of LC3II/I and Beclin1 and reducing I/R damage [215].
In clinical trials, transcranial direct-current stimulation (tDCS), a non-invasive brain stimulation, is safe and with a neuroprotective benefit for stroke patients [216,217]. In the MCAO/R rat model, tDCs treatment has been found to upregulate SIRT6, thereby reducing DNA double strand-break and exerting neuroprotective effects [218].
The supplementary therapy known as hyperbaric oxygen therapy (HBO) is frequently used to treat various pathological disorders, mostly those caused by hypoxia and/or ischemic conditions. HBO can induce the deacetylation of HMGB1 by upregulating the level of SIRT1, thereby alleviating the neuroinflammatory reaction induced by OGD-R and improving ischemic brain injury [219]. In addition, HBO treatment also can increase the levels of ATP and NAD+ and consequently increase SIRT1 expression, leading to the attenuation of brain infarction volume, BBB integrity, and improvement of neurological functions in MCAO rats [220] (Table 1).

Table 1.
Pharmacotherapy and non-pharmacological therapeutic/rehabilitative interventions targeting SIRTs.
Table 1.
Pharmacotherapy and non-pharmacological therapeutic/rehabilitative interventions targeting SIRTs.
| Models/Subjects | Inducers | Therapeutics | Effects | Targets or Pathways | Reference |
|---|---|---|---|---|---|
| NAD+ enhancers | |||||
| Mice | CCAO | NMN | ↓Mitochondrial fragmentation; ↓ROS | ↑SIRT3 | [168] |
| SIRTs inhibitors | |||||
| Mice | MCAO/R | AK-7 | ↑Neurological function | ↓SIRT2; ↑p38 | [185] |
| Cortical neurons | OGD | AK-1; AGK2 | ↓Apoptosis | ↓SIRT2; ↓caspase3, Bim | [140] |
| SIRTs activators | |||||
| Mice | MCAO/R | Melatonin | ↓Mitochondrial dysfunction | ↑SIRT1 | [165] |
| Hippocampal HT22 cells | OGD/R | Melatonin | ↑Mitochondrial mass; ↑mitochondrial fusion/division kinetics | ↑SIRT3 | [166] |
| Rats | MCAO/R | Resveratrol | ↓ATP energy consumption | ↑cAMP/AMPK/SIRT1 pathway | [99] |
| SH-SY5Y cells | OGD/R | Notoginseng leaf triterpenes | ↓Mitochondria dysfunction | ↑Nampt-NAD+-SIRT1 pathway | [170] |
| Mice | MCAO/R | Picetannol | ↓Oxidative stress | ↑SIRT1/FOXO1; ↑SOD, CAT, GSH-Px | [172] |
| Rats | MCAO/R | Diosmetin | ↓Oxidative stress | ↑SIRT1/Nrf2; ↓LDH, ROS | [173] |
| Neuroblastoma cells | HR | Mangiferin | ↓Oxidative stress | ↑SIRT1/PGC-1α; ↓LDH, ROS | [174] |
| Mice | pMCAO | Alpha-lipoic acid | ↓Oxidative stress | ↑SIRT1/PGC-1α; ↑SOD | [175] |
| PC12 Cells | OGD/R | Kaempferol | ↓Oxidative stress | ↑SIRT1; ↓LDH, ROS; ↑SOD, GSH | [176] |
| Rats | MCAO/R | Betulinic acid | ↓Autophagy | ↑SIRT1/FOXO1; ↓Beclin 1, LC3I/II; ↑p62 | [123] |
| Rats | MCAO/R | Magnoflorine | ↓Autophagy | ↑SIRT1/AMPK; ↓LC3; ↑p62 | [177] |
| Mouse primary cortical microglia | OGD | MDL-811 | ↓Neuroinflammation | ↑SIRT6/EZH2/FOXC1 pathway | [178] |
| Mice | pMCAO | Activator 3 | ↓Neuroinflammation | ↑SIRT1; ↓NF-κb | [34] |
| Rats | MCAO/R | Ginkgo biloba | ↓Neuroinflammation | ↑SIRT1; ↓NF-κb | [180] |
| Rats | MCAO/R | Arctigenin | ↓Neuroinflammation | ↑SIRT1; ↓NLRP3, IL-1β, IL-18 | [181] |
| Mice | MCAO/R | Bergenin | ↓Neuroinflammation | ↑SIRT1/FOXO3a; ↓NF-κb, IL-1β, IL-6, TNF-α | [182] |
| Mice | MCAO/R | Cycloastragenol | ↓Neuroinflammation | ↑SIRT1; ↓TNF-α, IL-1β | [183] |
| Rats | MCAO/R | Trilobatin | ↓Neuroinflammation | ↑SIRT3; ↓TLR4, NF-κb | [184] |
| Rats | MCAO/R | Resuvastatin | ↓Apoptosis | ↑SIRT1; ↓NF-κb | [142] |
| Mice | MCAO/R | Melatonin | ↓Apoptosis | ↑SIRT3 | [186] |
| Rats | MCAO/R | Resveratrol | ↓Apoptosis | ↑SIRT1; ↓p53, caspase3 | [187] |
| Hippocampal cell | OGD/R | Calycosin-7-O-β-D-glucoside | ↓Apoptosis | ↑SIRT1/FOXO1/PGC-1α; ↑Bcl-2; ↓Bax | [188] |
| Mice | MCAO/R | Picetannol | ↓Apoptosis | ↑SIRT1/FOXO1; ↓apoptotic hippocampal neurons number | [172] |
| Rats | MCAO/R | Magnolol | ↓Apoptosis | ↑SIRT1; ↑Bcl-2 | [189] |
| PC12 Cells | OGD/R | Kaempferol | ↓Apoptosis | ↑SIRT1; ↑Bcl-2; ↓caspase3, caspase9, Bax | [176] |
| Rats | MCAO/R | Salvianolic acid B | ↓Apoptosis | ↑SIRT1; ↑Bcl-2 | [190] |
| Rat PC12 cells | OGD/R | Stilbene glycoside | ↓Apoptosis; ↑mitochondrial autophagy | ↑SIRT3/AMPK pathway | [191] |
| HBMECs | OGD/R | Donepezil | ↑BBB integrity | ↑SIRT1; ↓FOXO3a, NF-κb; ↑TJ proteins | [192] |
| Mice | MCAO/R | CaMKK | ↑BBB integrity | ↑SIRT1 | [196] |
| Mice | MCAO/R | Trilobatin | ↑Angiogenesis | ↑SIRT7/VEGFA pathway | [197] |
| Rats | MCAO/R | Notoginsenoside R1 | ↑Angiogenesis | ↑NAMPT-NAD+-SIRT1 pathway; ↑Notch/VEGFR-2 pathway | [198] |
| HBMECs | OGD/R | Notoginsenoside R1 | ↑Angiogenesis | ↑NAMPT-NAD+-SIRT1 pathway; ↑Notch/VEGFR-2 pathway | [198] |
| Rats | MCAO/R | Momordica charantia polysaccharides | ↑Neurogenesis | ↑SIRT1; ↑β-catenin nuclear accumulation | [204,205] |
| C17.2 cells | Glutamate | Momordica charantia polysaccharides | ↑Neurogenesis | ↑SIRT1; ↑β-catenin nuclear accumulation | [204] |
| C17.2 cells | OGD | Momordica charantia polysaccharides | ↑Neurogenesis | ↑SIRT1; ↑β-catenin nuclear accumulation | [205] |
| Non-pharmacological therapeutic/rehabilitative interventions | |||||
| Rats | MCAO/R | EA | ↓Autophagy, apoptosis | ↑SIRT1; p-ERK, p-JNK; ↓ Beclin 1, LC3I/II; ↑p62; ↓apoptotic cells | [211] |
| Rats | MCAO/R | EA | ↑Autophagy | ↑SIRT1; ↑Beclin 1, LC3I/II; | [215] |
| Rats | MCAO/R | tDCS | Neuroprotective effect | ↑SIRT6; ↓DNA double strand-break | [218] |
| N2a cells | OGD/R | HBO | ↓Neuroinflammation | ↑SIRT1; ↓HMGB1 | [219] |
| Rats | MCAO/R | HBO | ↑BBB integrity, neurological functions; ↓brain infarction volume | ↑NAD; ↑SIRT1; ↑ATP | [220] |
Abbreviations: middle cerebral artery occlusion (MCAO); middle cerebral artery occlusion/reperfusion (MCAO/R); common carotid artery occlusion (CCAO); oxygen–glucose deprivation/reperfusion (OGD/R); hypoxia 12 h/reoxygenation 12 h (HR); nicotinamide mononucleotide (NMN); permanent middle cerebral artery occlusion (pMCAO); rat pheochromocytoma (PC12); human neuroblastoma cells (SH-SY5Y cells); brain microvascular endothelial cells (HBMECs); electroacupuncture (EA); transcranial direct-current stimulation (tDCS); hyperbaric oxygen therapy (HBO); inhibition (↓); activation (↑).
3.3. Epigenetic Regulators
Epigenetic modification is a heritable change in gene expression and regulation that does not involve DNA sequence changes. These modifications can result in the loss of protein function, changes in body structure and function, and disease occurrence. The modification mechanism of epigenetics mainly includes DNA methylation, histone modification, and regulation of non-coding RNAs (ncRNAs). NcRNAs, which primarily comprise microRNAs (miRNA), long non-coding RNAs (lncRNA), and circular RNAs (circRNA), can influence gene expression at both the post-transcriptional and translational stages [221]. In this section, we mainly focus on the regulation of SIRTs by ncRNAs.
MiRNAs are endogenous non-coding RNAs about 20–25 nucleotides in length, which usually interact with their downstream target, a short sequence of mRNA’s 3′-UTR region, to either inhibit its translation or cause its degradation to regulate its expression [222]. According to Ruan et al.’s study, miR-370 was upregulated in brain tissues of MCAO rats and its knockdown decreased the volume of cerebral infarction, blocked cell apoptosis, and promoted the expression of Nrf2 and HO-1 in vivo [223]. Further investigation revealed that SIRT6 is miR-370’s direct target, and overexpressing SIRT6 partially counteracted the effect of miR-370 on OGD/R-induced SH-SY5Y cell death by activating the Nrf2 signaling pathway [223]. In addition, miR-19a/b-3p expression is significantly upregulated during cerebral ischemia/reperfusion injury [224]. Further experimental evidence demonstrated that the upregulated miR-19a/b-3p promotes the occurrence and development of inflammation during cerebral ischemia/reperfusion injury. MiR-19a/b-3p inhibits the expression of SIRT1, thereby activating FOXO3, which in turn activates the SPHK1 signaling pathway. Activation of this pathway subsequently increases the production of inflammatory factors and infiltration of inflammatory cells, exacerbating cerebral ischemia/reperfusion injury [224].
LncRNAs are non-coding RNAs with a length of more than 200 nucleotides but with no protein-coding function [225]. Experiments have shown that lncRNAs participate in the regulation of the posttranscriptional process through miRNA sponging [226]. LncRNAs are mainly involved in the pathogenesis of ischemic stroke through pathological processes, including oxidative stress, apoptosis, BBB permeability, and inflammatory response. LncRNA nuclear enriched abundant transcript 1 (NEAT1) upregulates SIRT3 by targeting the expression of mitofusin 2 (Mfn2), thereby exerting anti-oxidative stress and apoptosis effects caused by ischemia-reperfusion [227]. Small nucleolar RNA host gene 15 (Snhg15), a lncRNA, is a sponge of miR-141. Snhg15 was significantly upregulated in OGD/R-induced SH-SY5Y cells, and based on this finding, Kang discovered that Snhg15 may indirectly upregulate SIRT1 by inhibiting miR-141, resulting in a decrease of ROS, iNOS, IL-1β, and IL-6 [228]. In addition, the expression of Snhg7 was upregulated in the OGD/R-induced PC12 cells [229]. And Snhg7 overexpression could attenuate oxidative stress and cell damage brought on by OGD/R. The results of further research showed that Snhg7 may interact with miR-9 to restrict its expression while increasing the expression of SIRT1, which in turn reduced ROS generation, MDA level, and apoptotic rate [229]. Tian and colleagues revealed that Snhg8 could inhibit the inflammatory response of microglia and BBB injury by regulating the SIRT1-mediated NF-κB pathway by sponging miR-425-5p [230]. Additionally, Snhg8 inhibits microglia activation and BBB permeability by sponging miR-449c-5p to upregulate SIRT1 and FOXO1, with neuroprotective effects against ischemic brain injury [231]. Moreover, Xu et al. revealed that lncRNA H19 is markedly downregulated in MCAO mice and OGD-induced HT22 cells [232]. Knockdown of lncRNA H19 reversed the decline in OGD-induced miR-29b, SIRT1, and PGC-1α levels, causing a decrease in apoptotic cells and inflammatory cytokine concentration [232].
CircRNAs are a new class of ncRNAs characterized by a closed-loop structure. They help in the expression of functional genes by binding with corresponding microRNAs or directly interacting with proteins [233]. Chen et al. reported that circHIPK3 (circRNA homeodomain-interacting protein kinase 3) functions as an endogenous sponge of miR-148b-3p, leading to the downregulation of SIRT1. This study observed the overexpression of circHIPK3 in the brain tissue of MCAO mice and identified apoptosis and mitochondrial dysfunction in BMECs [234]. Their findings indicate that circHIPK3 may play a role in the pathogenesis of cerebral ischemia by modulating the miR-148b-3p/SIRT1 axis. Moreover, experiments by Yang et al. demonstrated that the expression of circ-Rps5 alleviated LPS-induced inflammation and apoptosis through the NF-κB signaling pathway by increasing the SIRT7 level. A further luciferase reporter revealed that the circ-Rps5 directly targeted the miR-124-3p. Therefore, it is reasonable to speculate that circ-Rps5 attenuated ischemic-stroke-induced brain injury via the miR-124-3p/SIRT7 signaling pathway [235] (Figure 3).
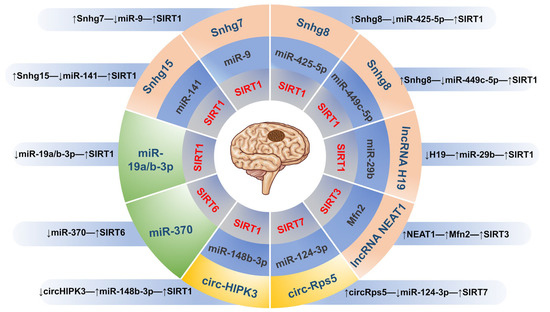
Figure 3.
The epigenetic regulatory mechanism of targeting SIRTs in IS. (↓) means inhibition; (↑) means activation.
3.4. Potential Molecules Targeting SIRTs
Besides the above treatment strategies, other molecules that can target SIRTs to confer neuroprotective effects in IS have been reported.
In OGD/R-induced hippocampal neuron cells, overexpression of C1q/tumor necrosis factor-related protein-3 (CTRP3) suppressed cell apoptosis and promoted mitochondrial biogenesis and physiological functions through a mechanism involving activation of the AMPK/SIRT1/PGC-1α pathway [236].
TGR5 is a plasma-membrane-bound G protein-coupled bile acid receptor [237]. Liang et al. reported that activation of TRG5 increased the tight junction (TJ) protein expression thereby improving the disrupted BBB in the ischemic brain via the BRCA1/Sirt1-related signaling pathway [238]. BRCA1 is a tumor suppressor gene expressed by endothelial cells and is a key regulator of SIRT1 [239,240,241].
Interferon (IFN) regulatory factors (IRFs) are transcription factors known to exert immunomodulatory roles by altering the expression of interferon genes. IRF9 was reported to be a negative transcriptional regulator of SIRT1. Chen and colleagues discovered that IRF9 is specifically activated in neurons of mice brains and this exacerbates brain injury upon I/R insult. The principal pathological effect of IRF9 is the downregulation of SIRT1 transcriptional expression, which leads to the activation of p53. This, in turn, results in an elevated number of TUNEL-positive cells and an increase in caspase3 levels [242].
The proto-oncogene c-myc encodes the transcription factor c-Myc, which is important for regulating cell growth and viability. A crucial regulator known as Myc coordinates various cell signals and mediates transcriptional processes. Upregulation of c-Myc can partially resolve the motor impairment induced by cerebral ischemic stroke [243]. Liu et al. discovered that c-Myc protects MCAO mice from neuronal damage by increasing miR-200b-5p-regulated SIRT1 expression [244].
Furthermore, overexpression of lanthionine synthetase C-like protein 1 (LanCL1) significantly reduced the release of LDH, increased the mitochondrial enzyme activity, and attenuated apoptosis in OGD-induced neuronal HT22 cells in a SIRT3-dependent manner [245]. This indicates that LanCL1 is a promising target for the therapy of IS.
3.5. Stem Cell-Derived Exosome Therapy
Research has found that the exosomes generated by bone marrow mesenchymal stem cells are rich in the transcription factor early growth response protein 2 (Egr2), which has the potential to treat IS [246]. Egr2 inhibits the activation of the Notch signaling pathway by upregulating the expression of SIRT6, thereby reducing MCAO/R-caused brain damage, and promoting angiogenesis in OGD/R-treated brain endothelial cells. This study provides a potential exosome-based therapy targeting SIRT6 for IS [246]. However, further research and clinical trials still need to verify the safety and effectiveness of this treatment strategy.
4. Concluding Remarks and Prospects
Limited therapeutic strategies are currently available to address ischemic stroke, hence it is a significant public health and socio-economic challenge. SIRTs are potential candidates for identifying novel molecular pathways and target molecules for drug development of ischemic stroke. Although David T. She reviewed the role and therapeutic significance of sirtuins in IS in 2017, this report updates research progress in recent years [247]. The broad introduction to SIRTs and IS in this review includes information on their expression, subcellular location, enzymatic action, and regulatory involvement in IS. The high expression of SIRTs in the brain and multiple targets changes multiple biological processes in response to ischemic stimuli, including regulating energy metabolism, inhibiting oxidative stress, modulating autophagy, mitigating inflammatory response, anti-apoptosis, protecting BBB integrity, promoting angiogenesis and enhancing neurogenesis. Further, we described the roles of SIRTs in the mechanisms of various treatment modalities including medical treatment, non-pharmacological therapeutic/rehabilitative interventions, epigenetic regulators, molecules regulation, and stem-cell-derived exosome therapy (Figure 4). Altogether, our results indicate that the sirtuin family holds great therapeutic potential in the treatment of IS.
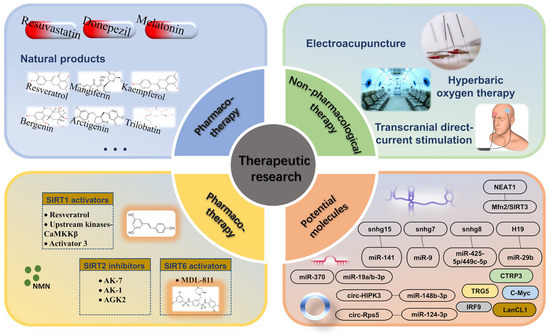
Figure 4.
Overview of IS treatment strategies targeting SIRTs.
In the development of strategies targeting sirtuins in ischemic stroke, it is important to consider the potential drawbacks. One major limitation is that the majority of SIRTs studies only focus on neurons or endothelial cells. However, the neurovascular unit (NVU) forms the fundamental building block of brain tissue, and the pathophysiology and neurovascular repair process of ischemic stroke are closely linked to the NVU’s steady-state [248]. Therefore, a treatment scheme that only targets neurons or endothelial cells alone and ignores the interaction of various nerve cells after cerebral ischemia injury may not be therapeutically effective. Secondly, SIRTs regulate several systemic diseases, and different SIRTs may exert different effects on various diseases, or even opposite outcomes [10]. Therefore, there is a need to investigate strategies to develop SIRT as a personalized therapeutic target for IS. In summary, although we have provided an overview of some pharmacological SIRT modulators, current research has not sufficiently addressed their pharmacokinetics, efficacy, and safety. Future research should fill these gaps and actively investigate the development of modulators that can cross the blood–brain barrier. To achieve this, a number of important questions need to be answered. For example, (1) SIRT1 has been widely studied, but the exact function and therapeutic effect of other SIRTs in IS are still unclear, and (2) is the role of SIRTs consistent between acute and chronic cerebral ischemia? In conclusion, despite the considerable amount of preclinical evidence presented on the role of sirtuins in ischemic stroke, the understanding of their clinical significance is limited. Therefore, additional clinical trials are necessary to more comprehensively investigate the potential of sirtuins as a viable treatment for ischemic stroke.
Author Contributions
Conceptualization, Y.L. and Y.Z.; writing—original draft preparation, Y.L.; writing—review and editing, L.W., G.Y. and X.C.; supervision, X.L. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine, grant number ZYYCXTD-C-202007, the China Academy of Chinese Medical Sciences Innovation Fund, grant numbers CI2021A01301 and CI2021A01311, the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences, grant number CI2021B006, and the Fundamental Research Funds for the Central public welfare research institutes, grant number 2020YJSZX-3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to express our appreciation to all authors of this review. The authors would like to thank all the reviewers who participated in the review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, S.R.; Phan, T.G.; Sobey, C.G. Targeting the Immune System for Ischemic Stroke. Trends Pharmacol. Sci. 2021, 42, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Wang, S.; Kroemer, G.; Penninger, J.M.; Uversky, V.N.; Pratico, D.; Henninger, N.; Reiter, R.J.; Bruno, A.; Joshipura, K.; et al. Targeting autophagy in ischemic stroke: From molecular mechanisms to clinical therapeutics. Pharmacol. Ther. 2021, 225, 107848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.G.; Chopp, M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol. Sci. 2012, 33, 415–422. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef]
- Kumar, A.; Misra, S.; Nair, P.; Algahtany, M. Epigenetics Mechanisms in Ischemic Stroke: A Promising Avenue? J. Stroke Cerebrovasc. Dis. 2021, 30, 105690. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Q.; Chen, J.; Ma, Y.; Liu, X. Updating a Strategy for Histone Deacetylases and Its Inhibitors in the Potential Treatment of Cerebral Ischemic Stroke. Dis. Markers 2020, 1, 8. [Google Scholar] [CrossRef]
- Fessler, E.B.; Chibane, F.L.; Wang, Z.; Chuang, D.M. Potential roles of HDAC inhibitors in mitigating ischemia-induced brain damage and facilitating endogenous regeneration and recovery. Curr. Pharm. Des. 2013, 19, 5105–5120. [Google Scholar] [CrossRef]
- Qiu, M.; Xu, E.; Zhan, L. Epigenetic Regulations of Microglia/Macrophage Polarization in Ischemic Stroke. Front. Mol. Neurosci. 2021, 14, 697416. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Carafa, V.; Rotili, D.; Forgione, M.; Cuomo, F.; Serretiello, E.; Hailu, G.S.; Jarho, E.; Lahtela-Kakkonen, M.; Mai, A.; Altucci, L. Sirtuin functions and modulation: From chemistry to the clinic. Clin. Epigenetics 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Van De Ven Ra, H.; Santos, D.; Haigis, M.C. Mitochondrial Sirtuins and Molecular Mechanisms of Aging. Trends Mol. Med. 2017, 23, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Sato, Y.; Sobuz, S.U.; Mizumoto, T.; Tsuyama, T.; Karim, M.F.; Miyata, K.; Tasaki, M.; Yamazaki, M.; Kariba, Y.; et al. SIRT7 suppresses energy expenditure and thermogenesis by regulating brown adipose tissue functions in mice. Nat. Commun. 2022, 13, 7439. [Google Scholar] [CrossRef] [PubMed]
- Donmez, G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol. Sci. 2012, 33, 494–501. [Google Scholar] [CrossRef]
- Winnik, S.; Auwerx, J.; Sinclair, D.A.; Matter, C.M. Protective effects of sirtuins in cardiovascular diseases: From bench to bedside. Eur. Heart J. 2015, 36, 3404–3412. [Google Scholar] [CrossRef]
- Bian, C.; Ren, H. Sirtuin Family and Diabetic Kidney Disease. Front. Endocrinol. 2022, 13, 901066. [Google Scholar] [CrossRef]
- Yin, J.; Han, P.; Tang, Z.; Liu, Q.; Shi, J. Sirtuin 3 mediates neuroprotection of ketones against ischemic stroke. J. Cereb. Blood Flow Metab. 2015, 35, 1783–1789. [Google Scholar] [CrossRef]
- Liberale, L.; Gaul, D.S.; Akhmedov, A.; Bonetti, N.R.; Nageswaran, V.; Costantino, S.; Pahla, J.; Weber, J.; Fehr, V.; Vdovenko, D.; et al. Endothelial SIRT6 blunts stroke size and neurological deficit by preserving blood-brain barrier integrity: A translational study. Eur. Heart J. 2020, 41, 1575–1587. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, S.; Liang, X.; Dong, M.; Xu, X.; Lu, C.; Wei, Y. Prognostic value of Sirtuin1 in acute ischemic stroke and its correlation with functional outcomes. Medicine 2018, 97, e12959. [Google Scholar] [CrossRef]
- Kadono, K.; Kageyama, S.; Nakamura, K.; Hirao, H.; Ito, T.; Kojima, H.; Dery, K.J.; Li, X.; Kupiec-Weglinski, J.W. Myeloid Ikaros-SIRT1 signaling axis regulates hepatic inflammation and pyroptosis in ischemia-stressed mouse and human liver. J. Hepatol. 2022, 76, 896–909. [Google Scholar] [CrossRef]
- Kou, G.; Li, P.; Shi, Y.; Traore, S.S.; Shi, X.; Amoah, A.N.; Cui, Z.; Lyu, Q. Sesamin Activates Skeletal Muscle FNDC5 Expression and Increases Irisin Secretion via the SIRT1 Signaling Pathway. J. Agric. Food Chem. 2022, 70, 7704–7715. [Google Scholar] [CrossRef]
- Carrizzo, A.; Iside, C.; Nebbioso, A.; Carafa, V.; Damato, A.; Sciarretta, S.; Frati, G.; Di Nonno, F.; Valenti, V.; Ciccarelli, M.; et al. SIRT1 pharmacological activation rescues vascular dysfunction and prevents thrombosis in MTHFR deficiency. Cell. Mol. Life Sci. 2022, 79, 410. [Google Scholar] [CrossRef]
- Ramadori, G.; Lee, C.E.; Bookout, A.L.; Lee, S.; Williams, K.W.; Anderson, J.; Elmquist, J.K.; Coppari, R. Brain SIRT1: Anatomical distribution and regulation by energy availability. J. Neurosci. 2008, 28, 9989–9996. [Google Scholar] [CrossRef] [PubMed]
- Zakhary, S.M.; Ayubcha, D.; Dileo, J.N.; Jose, R.; Leheste, J.R.; Horowitz, J.M.; Torres, G. Distribution analysis of deacetylase SIRT1 in rodent and human nervous systems. Anat. Rec. 2010, 293, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Tanno, M.; Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 2007, 282, 6823–6832. [Google Scholar] [CrossRef]
- Tajbakhsh, N.; Sokoya, E.M. Regulation of cerebral vascular function by sirtuin 1. Microcirculation 2012, 19, 336–342. [Google Scholar] [CrossRef]
- Vaquero, A.; Scher, M.; Lee, D.; Erdjument-Bromage, H.; Tempst, P.; Reinberg, D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol. Cell 2004, 16, 93–105. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Mostoslavsky, R.; Saito, S.; Manis, J.P.; Gu, Y.; Patel, P.; Bronson, R.; Appella, E.; Alt, F.W.; Chua, K.F. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. USA 2003, 100, 10794–10799. [Google Scholar] [CrossRef]
- Yu, Q.; Dong, L.; Li, Y.; Liu, G. SIRT1 and HIF1α signaling in metabolism and immune responses. Cancer Lett. 2018, 418, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef]
- Luo, J.; Nikolaev, A.Y.; Imai, S.; Chen, D.; Su, F.; Shiloh, A.; Guarente, L.; Gu, W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 2001, 107, 137–148. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K. SIRT1: Regulation of longevity via autophagy. Cell. Signal. 2009, 21, 1356–1360. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.; Hurtado, O.; Cuartero, M.I.; Ballesteros, I.; Moraga, A.; Pradillo, J.M.; McBurney, M.W.; Lizasoain, I.; Moro, M.A. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 2013, 44, 2333–2337. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Jia, S.; Xu, X.; Dong, M.; Wei, Y. SIRT1: The Value of Functional Outcome, Stroke-Related Dementia, Anxiety, and Depression in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 205–212. [Google Scholar] [CrossRef]
- Hattori, Y.; Okamoto, Y.; Nagatsuka, K.; Takahashi, R.; Kalaria, R.N.; Kinoshita, M.; Ihara, M. SIRT1 attenuates severe ischemic damage by preserving cerebral blood flow. Neuroreport 2015, 26, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Fleming Outeiro, T.; Cavadas, C. Emerging Role of Sirtuin 2 in the Regulation of Mammalian Metabolism. Trends Pharmacol. Sci. 2015, 36, 756–768. [Google Scholar] [CrossRef]
- North, B.J.; Marshall, B.L.; Borra, M.T.; Denu, J.M.; Verdin, E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 2003, 11, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, J.; Liao, M.; Hu, M.; Li, W.; Ouyang, H.; Wang, X.; Ye, T.; Zhang, Y.; Ouyang, L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2019, 161, 48–77. [Google Scholar] [CrossRef]
- Chamberlain, K.A.; Huang, N.; Xie, Y.; LiCausi, F.; Li, S.; Li, Y.; Sheng, Z.H. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron 2021, 109, 3456–3472.e8. [Google Scholar] [CrossRef] [PubMed]
- Krey, L.; Lühder, F.; Kusch, K.; Czech-Zechmeister, B.; Könnecke, B.; Fleming Outeiro, T.; Trendelenburg, G. Knockout of silent information regulator 2 (SIRT2) preserves neurological function after experimental stroke in mice. J. Cereb. Blood Flow Metab. 2015, 35, 2080–2088. [Google Scholar] [CrossRef]
- Xie, X.Q.; Zhang, P.; Tian, B.; Chen, X.Q. Downregulation of NAD-Dependent Deacetylase SIRT2 Protects Mouse Brain against Ischemic Stroke. Mol. Neurobiol. 2017, 54, 7251–7261. [Google Scholar] [CrossRef]
- Kaitsuka, T.; Matsushita, M.; Matsushita, N. SIRT2 inhibition activates hypoxia-inducible factor 1α signaling and mediates neuronal survival. Biochem. Biophys. Res. Commun. 2020, 529, 957–962. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Q.; Zhang, Y. Overexpression of sirtuin 2 and its association with prognosis in acute ischemic stroke patients. J. Clin. Lab. Anal. 2021, 35, e23707. [Google Scholar] [CrossRef] [PubMed]
- Schwer, B.; North, B.J.; Frye, R.A.; Ott, M.; Verdin, E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J. Cell Biol. 2002, 158, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Bao, J.; Lu, Z.; Joseph, J.J.; Carabenciov, D.; Dimond, C.C.; Pang, L.; Samsel, L.; McCoy, J.P., Jr.; Leclerc, J.; Nguyen, P.; et al. Characterization of the murine SIRT3 mitochondrial localization sequence and comparison of mitochondrial enrichment and deacetylase activity of long and short SIRT3 isoforms. J. Cell. Biochem. 2010, 110, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Scher, M.B.; Vaquero, A.; Reinberg, D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007, 21, 920–928. [Google Scholar] [CrossRef]
- Cooper, H.M.; Spelbrink, J.N. The human SIRT3 protein deacetylase is exclusively mitochondrial. Biochem. J. 2008, 411, 279–285. [Google Scholar] [CrossRef]
- Vassilopoulos, A.; Pennington, J.D.; Andresson, T.; Rees, D.M.; Bosley, A.D.; Fearnley, I.M.; Ham, A.; Flynn, C.R.; Hill, S.; Rose, K.L.; et al. SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress. Antioxid. Redox Signal. 2014, 21, 551–564. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Su, C.; Wu, F.; Sun, J.; Zhang, J.; Yang, X.; Zhang, C.; Zhou, Z.; Zhang, X.; et al. Inhibition of Mitochondrial Oxidative Damage Improves Reendothelialization Capacity of Endothelial Progenitor Cells via SIRT3 (Sirtuin 3)-Enhanced SOD2 (Superoxide Dismutase 2) Deacetylation in Hypertension. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1682–1698. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Song, L.; Yu, L.; Zhang, L.; Zhang, Y.; Xing, Y.; Yin, Y.; Ma, H. SIRT3 deficiency exacerbates p53/Parkin-mediated mitophagy inhibition and promotes mitochondrial dysfunction: Implication for aged hearts. Int. J. Mol. Med. 2018, 41, 3517–3526. [Google Scholar] [CrossRef]
- Cui, M.; Huang, Y.; Tian, C.; Zhao, Y.; Zheng, J. FOXO3a inhibits TNF-α- and IL-1β-induced astrocyte proliferation: Implication for reactive astrogliosis. Glia 2011, 59, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Geng, K.; Zhang, J.; Zhang, Y.; Shao, J.; Xia, W. Sirt3 Mediates the Inhibitory Effect of Adjudin on Astrocyte Activation and Glial Scar Formation following Ischemic Stroke. Front. Pharmacol. 2017, 8, 943. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Mostoslavsky, R.; Haigis, K.M.; Fahie, K.; Christodoulou, D.C.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Karow, M.; Blander, G.; et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell 2006, 126, 941–954. [Google Scholar] [CrossRef]
- Komlos, D.; Mann, K.D.; Zhuo, Y.; Ricupero, C.L.; Hart, R.P.; Liu, A.Y.; Firestein, B.L. Glutamate dehydrogenase 1 and SIRT4 regulate glial development. Glia 2013, 61, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.; Bentley, N.L.; Montgomery, M.K.; Turner, N. The role of mitochondrial sirtuins in health and disease. Free Radic. Biol. Med. 2016, 100, 164–174. [Google Scholar] [CrossRef]
- Rauh, D.; Fischer, F.; Gertz, M.; Lakshminarasimhan, M.; Bergbrede, T.; Aladini, F.; Kambach, C.; Becker, C.F.; Zerweck, J.; Schutkowski, M.; et al. An acetylome peptide microarray reveals specificities and deacetylation substrates for all human sirtuin isoforms. Nat. Commun. 2013, 4, 2327. [Google Scholar] [CrossRef]
- Jeong, S.M.; Xiao, C.; Finley, L.W.; Lahusen, T.; Souza, A.L.; Pierce, K.; Li, Y.H.; Wang, X.; Laurent, G.; German, N.J.; et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 2013, 23, 450–463. [Google Scholar] [CrossRef]
- Laurent, G.; German, N.J.; Saha, A.K.; de Boer, V.C.; Davies, M.; Koves, T.R.; Dephoure, N.; Fischer, F.; Boanca, G.; Vaitheesvaran, B.; et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol. Cell 2013, 50, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, G.D.; Colak, M. SIRT4 prevents excitotoxicity via modulating glutamate metabolism in glioma cells. Hum. Exp. Toxicol. 2020, 39, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.; Liu, L.; Mason, A.; Higashimori, H.; Donmez, G. Loss of SIRT4 decreases GLT-1-dependent glutamate uptake and increases sensitivity to kainic acid. J. Neurochem. 2014, 131, 573–581. [Google Scholar] [CrossRef]
- Pecher, S.J.; Potthast, A.B.; von Versen-Höynck, F.; Das, A.M. Impact of Short-Term Hypoxia on Sirtuins as Regulatory Elements in HUVECs. J. Clin. Med. 2020, 9, 2604. [Google Scholar] [CrossRef]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 2005, 16, 4623–4635. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Lomb, D.J.; Haigis, M.C.; Guarente, L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 2009, 137, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lombard, D.B. Functions of the sirtuin deacylase SIRT5 in normal physiology and pathobiology. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 311–334. [Google Scholar] [CrossRef]
- Glorioso, C.; Oh, S.; Douillard, G.G.; Sibille, E. Brain molecular aging, promotion of neurological disease and modulation by sirtuin 5 longevity gene polymorphism. Neurobiol. Dis. 2011, 41, 279–290. [Google Scholar] [CrossRef]
- Du, J.; Zhou, Y.; Su, X.; Yu, J.J.; Khan, S.; Jiang, H.; Kim, J.; Woo, J.; Kim, J.H.; Choi, B.H.; et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 2011, 334, 806–809. [Google Scholar] [CrossRef]
- Peng, C.; Lu, Z.; Xie, Z.; Cheng, Z.; Chen, Y.; Tan, M.; Luo, H.; Zhang, Y.; He, W.; Yang, K.; et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol. Cell Proteom. 2011, 10, M111.012658. [Google Scholar] [CrossRef]
- Lin, Z.F.; Xu, H.B.; Wang, J.Y.; Lin, Q.; Ruan, Z.; Liu, F.B.; Jin, W.; Huang, H.H.; Chen, X. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem. Biophys. Res. Commun. 2013, 441, 191–195. [Google Scholar] [CrossRef]
- Morris-Blanco, K.C.; Dave, K.R.; Saul, I.; Koronowski, K.B.; Stradecki, H.M.; Perez-Pinzon, M.A. Protein Kinase C Epsilon Promotes Cerebral Ischemic Tolerance Via Modulation of Mitochondrial Sirt5. Sci. Rep. 2016, 6, 29790. [Google Scholar] [CrossRef]
- Diaz-Cañestro, C.; Merlini, M.; Bonetti, N.R.; Liberale, L.; Wüst, P.; Briand-Schumacher, S.; Klohs, J.; Costantino, S.; Miranda, M.; Schoedon-Geiser, G.; et al. Sirtuin 5 as a novel target to blunt blood-brain barrier damage induced by cerebral ischemia/reperfusion injury. Int. J. Cardiol. 2018, 260, 148–155. [Google Scholar] [CrossRef]
- Chang, A.R.; Ferrer, C.M.; Mostoslavsky, R. SIRT6, a Mammalian Deacylase with Multitasking Abilities. Physiol. Rev. 2020, 100, 145–169. [Google Scholar] [CrossRef]
- Pereira, C.V.; Lebiedzinska, M.; Wieckowski, M.R.; Oliveira, P.J. Regulation and protection of mitochondrial physiology by sirtuins. Mitochondrion 2012, 12, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Liszt, G.; Ford, E.; Kurtev, M.; Guarente, L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J. Biol. Chem. 2005, 280, 21313–21320. [Google Scholar] [CrossRef]
- Dominy, J.E., Jr.; Lee, Y.; Jedrychowski, M.P.; Chim, H.; Jurczak, M.J.; Camporez, J.P.; Ruan, H.B.; Feldman, J.; Pierce, K.; Mostoslavsky, R.; et al. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol. Cell 2012, 48, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Zhang, R.; Wang, Y.; Li, J.; Song, Y.; Guo, D.; Gao, J.; Ren, H. Sirtuin 6 affects glucose reabsorption and gluconeogenesis in type 1 diabetes via FoxO1. Mol. Cell. Endocrinol. 2022, 547, 111597. [Google Scholar] [CrossRef]
- Ghosh, S.; Liu, B.; Wang, Y.; Hao, Q.; Zhou, Z. Lamin A Is an Endogenous SIRT6 Activator and Promotes SIRT6-Mediated DNA Repair. Cell Rep. 2015, 13, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, P.; Ge, J.; Li, H. SIRT6 in Aging, Metabolism, Inflammation and Cardiovascular Diseases. Aging Dis. 2022, 13, 1787–1822. [Google Scholar] [CrossRef]
- Okun, E.; Marton, D.; Cohen, D.; Griffioen, K.; Kanfi, Y.; Illouz, T.; Madar, R.; Cohen, H.Y. Sirt6 alters adult hippocampal neurogenesis. PLoS ONE 2017, 12, e0179681. [Google Scholar] [CrossRef]
- Hu, K.; Chen, H.; Gao, Y.; Hua, R.; Shi, X.; Li, L.; Yin, Y.; Zeng, C.; Liu, Q.; Qiu, Y.; et al. Astrocytic SIRT6 is a potential anti-depression and anti-anxiety target. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 123, 110702. [Google Scholar] [CrossRef] [PubMed]
- Kaluski, S.; Portillo, M.; Besnard, A.; Stein, D.; Einav, M.; Zhong, L.; Ueberham, U.; Arendt, T.; Mostoslavsky, R.; Sahay, A.; et al. Neuroprotective Functions for the Histone Deacetylase SIRT6. Cell Rep. 2017, 18, 3052–3062. [Google Scholar] [CrossRef]
- Shao, J.; Yang, X.; Liu, T.; Zhang, T.; Xie, Q.R.; Xia, W. Autophagy induction by SIRT6 is involved in oxidative stress-induced neuronal damage. Protein Cell 2016, 7, 281–290. [Google Scholar] [CrossRef]
- Kiran, S.; Chatterjee, N.; Singh, S.; Kaul, S.C.; Wadhwa, R.; Ramakrishna, G. Intracellular distribution of human SIRT7 and mapping of the nuclear/nucleolar localization signal. FEBS J. 2013, 280, 3451–3466. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.; Voit, R.; Liszt, G.; Magin, C.; Grummt, I.; Guarente, L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006, 20, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tang, H.; Tu, B.; Zhu, W.G. SIRT7: A sentinel of genome stability. Open Biol. 2021, 11, 210047. [Google Scholar] [CrossRef]
- Wong, Y.H.; Wu, C.C.; Lai, H.Y.; Jheng, B.R.; Weng, H.Y.; Chang, T.H.; Chen, B.S. Identification of network-based biomarkers of cardioembolic stroke using a systems biology approach with time series data. BMC Syst. Biol. 2015, 9 (Suppl. 6), S4. [Google Scholar] [CrossRef]
- Lv, J.; Tian, J.; Zheng, G.; Zhao, J. Sirtuin7 is involved in protecting neurons against oxygen-glucose deprivation and reoxygenation-induced injury through regulation of the p53 signaling pathway. J. Biochem. Mol. Toxicol. 2017, 31, e21955. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Rugarli, E.I.; Langer, T. Mitochondrial quality control: A matter of life and death for neurons. EMBO J. 2012, 31, 1336–1349. [Google Scholar] [CrossRef]
- Luo, J.Y.; Cheng, C.K.; He, L.; Pu, Y.; Zhang, Y.; Lin, X.; Xu, A.; Lau, C.W.; Tian, X.Y.; Ma, R.C.W.; et al. Endothelial UCP2 Is a Mechanosensitive Suppressor of Atherosclerosis. Circ. Res. 2022, 131, 424–441. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Dave, K.R.; Saul, I.; Camarena, V.; Thompson, J.W.; Neumann, J.T.; Young, J.I.; Perez-Pinzon, M.A. Resveratrol Preconditioning Induces a Novel Extended Window of Ischemic Tolerance in the Mouse Brain. Stroke 2015, 46, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Koronowski, K.B.; Khoury, N.; Saul, I.; Loris, Z.B.; Cohan, C.H.; Stradecki-Cohan, H.M.; Dave, K.R.; Young, J.I.; Perez-Pinzon, M.A. Neuronal SIRT1 (Silent Information Regulator 2 Homologue 1) Regulates Glycolysis and Mediates Resveratrol-Induced Ischemic Tolerance. Stroke 2017, 48, 3117–3125. [Google Scholar] [CrossRef]
- Choi, J.; Chandrasekaran, K.; Inoue, T.; Muragundla, A.; Russell, J.W. PGC-1α regulation of mitochondrial degeneration in experimental diabetic neuropathy. Neurobiol. Dis. 2014, 64, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Fernyhough, P. Mitochondrial dysfunction in diabetic neuropathy: A series of unfortunate metabolic events. Curr. Diabetes Rep. 2015, 15, 89. [Google Scholar] [CrossRef]
- Wang, L.; Quan, N.; Sun, W.; Chen, X.; Cates, C.; Rousselle, T.; Zhou, X.; Zhao, X.; Li, J. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc. Res. 2018, 114, 805–821. [Google Scholar] [CrossRef]
- Wan, D.; Zhou, Y.; Wang, K.; Hou, Y.; Hou, R.; Ye, X. Resveratrol provides neuroprotection by inhibiting phosphodiesterases and regulating the cAMP/AMPK/SIRT1 pathway after stroke in rats. Brain Res. Bull. 2016, 121, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.H.; Chen, T.; Li, X.; Yue, K.Y.; Luo, P.; Yang, L.K.; Zhu, J.; Wang, Y.H.; Fei, Z.; Jiang, X.F. Sirt3 confers protection against neuronal ischemia by inducing autophagy: Involvement of the AMPK-mTOR pathway. Free Radic. Biol. Med. 2017, 108, 345–353. [Google Scholar] [CrossRef]
- Della-Morte, D.; Raval, A.P.; Dave, K.R.; Lin, H.W.; Perez-Pinzon, M.A. Post-ischemic activation of protein kinase C ε protects the hippocampus from cerebral ischemic injury via alterations in cerebral blood flow. Neurosci. Lett. 2011, 487, 158–162. [Google Scholar] [CrossRef]
- Dave, K.R.; DeFazio, R.A.; Raval, A.P.; Torraco, A.; Saul, I.; Barrientos, A.; Perez-Pinzon, M.A. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J. Neurosci. 2008, 28, 4172–4182. [Google Scholar] [CrossRef]
- Wang, P.; Guan, Y.F.; Du, H.; Zhai, Q.W.; Su, D.F.; Miao, C.Y. Induction of autophagy contributes to the neuroprotection of nicotinamide phosphoribosyltransferase in cerebral ischemia. Autophagy 2012, 8, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Diaz de Barboza, G.; Guizzardi, S.; Moine, L.; Tolosa de Talamoni, N. Oxidative stress, antioxidants and intestinal calcium absorption. World J. Gastroenterol. 2017, 23, 2841–2853. [Google Scholar] [CrossRef]
- Orellana-Urzúa, S.; Rojas, I.; Líbano, L.; Rodrigo, R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr. Pharm. Des. 2020, 26, 4246–4260. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Zhang, Z.; Lu, J.; Pei, G.; Huang, S. Rescue of Mitochondrial SIRT3 Ameliorates Ischemia-like Injury in Human Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 9118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, M.; Wang, Y.; Xie, F.; Zhang, G.; Qin, X. Nrf2-a Promising Therapeutic Target for Defensing against Oxidative Stress in Stroke. Mol. Neurobiol. 2017, 54, 6006–6017. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef]
- Srivastava, S.; Alfieri, A.; Siow, R.C.; Mann, G.E.; Fraser, P.A. Temporal and spatial distribution of Nrf2 in rat brain following stroke: Quantification of nuclear to cytoplasmic Nrf2 content using a novel immunohistochemical technique. J. Physiol. 2013, 591, 3525–3538. [Google Scholar]
- Shah, Z.A.; Li, R.C.; Thimmulappa, R.K.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Doré, S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience 2007, 147, 53–59. [Google Scholar] [CrossRef]
- Shih, A.Y.; Li, P.; Murphy, T.H. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. 2005, 25, 10321–10335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, L.; Li, Y.; Zhao, Y. Silent information regulator 1 ameliorates oxidative stress injury via PGC-1α/PPARγ-Nrf2 pathway after ischemic stroke in rat. Brain Res. Bull. 2022, 178, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wei, R.; Zhang, L.; Tan, Y.; Qian, C. Sirtuin 6 protects the brain from cerebral ischemia/reperfusion injury through NRF2 activation. Neuroscience 2017, 366, 95–104. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Hansen, T.E.; Johansen, T. Following autophagy step by step. BMC Biol. 2011, 9, 39. [Google Scholar] [CrossRef]
- Sheng, R.; Zhang, L.S.; Han, R.; Liu, X.Q.; Gao, B.; Qin, Z.H. Autophagy activation is associated with neuroprotection in a rat model of focal cerebral ischemic preconditioning. Autophagy 2010, 6, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef]
- Arico, S.; Petiot, A.; Bauvy, C.; Dubbelhuis, P.F.; Meijer, A.J.; Codogno, P.; Ogier-Denis, E. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2001, 276, 35243–35246. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, X.; Wang, J.; Mang, J.; Xu, Z. Betulinic Acid Ameliorates Cerebral Injury in Middle Cerebral Artery Occlusion Rats through Regulating Autophagy. ACS Chem. Neurosci. 2021, 12, 2829–2837. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, Y.; Li, J.; Zhang, J.; Li, Y.; Dang, C.; Li, C.; Fan, Y.; Yu, J.; Pei, Z.; et al. Beclin 1 knockdown inhibits autophagic activation and prevents the secondary neurodegenerative damage in the ipsilateral thalamus following focal cerebral infarction. Autophagy 2012, 8, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 294. [Google Scholar]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway. J. Neuroinflamm. 2019, 16, 148. [Google Scholar] [CrossRef]
- Li, Y.; Chen, N.; Wu, C.; Lu, Y.; Gao, G.; Duan, C.; Yang, H.; Lu, L. Galectin-1 attenuates neurodegeneration in Parkinson’s disease model by modulating microglial MAPK/IκB/NFκB axis through its carbohydrate-recognition domain. Brain Behav. Immun. 2020, 83, 214–225. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Won, J.S.; Rahman, M.K.; Bae, E.J.; Cho, M.K. Modulation of Sirt1/NF-κB interaction of evogliptin is attributed to inhibition of vascular inflammatory response leading to attenuation of atherosclerotic plaque formation. Biochem. Pharmacol. 2019, 168, 452–464. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.C.; Cao, D.L.; Zhang, Z.J.; Zhang, X.; Ji, R.R.; Gao, Y.J. TRAF6 upregulation in spinal astrocytes maintains neuropathic pain by integrating TNF-α and IL-1β signaling. Pain 2014, 155, 2618–2629. [Google Scholar] [CrossRef]
- Li, T.; Qin, J.J.; Yang, X.; Ji, Y.X.; Guo, F.; Cheng, W.L.; Wu, X.; Gong, F.H.; Hong, Y.; Zhu, X.Y.; et al. The Ubiquitin E3 Ligase TRAF6 Exacerbates Ischemic Stroke by Ubiquitinating and Activating Rac1. J. Neurosci. 2017, 37, 12123–12140. [Google Scholar] [CrossRef]
- Yan, W.; Sun, W.; Fan, J.; Wang, H.; Han, S.; Li, J.; Yin, Y. Sirt1-ROS-TRAF6 Signaling-Induced Pyroptosis Contributes to Early Injury in Ischemic Mice. Neurosci. Bull. 2020, 36, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Scaffidi, P.; Misteli, T.; Bianchi, M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002, 418, 191–195. [Google Scholar] [CrossRef]
- Lee, O.H.; Kim, J.; Kim, J.M.; Lee, H.; Kim, E.H.; Bae, S.K.; Choi, Y.; Nam, H.S.; Heo, J.H. Decreased expression of sirtuin 6 is associated with release of high mobility group box-1 after cerebral ischemia. Biochem. Biophys. Res. Commun. 2013, 438, 388–394. [Google Scholar] [CrossRef]
- Szalay, G.; Martinecz, B.; Lénárt, N.; Környei, Z.; Orsolits, B.; Judák, L.; Császár, E.; Fekete, R.; West, B.L.; Katona, G.; et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 2016, 7, 11499. [Google Scholar] [CrossRef]
- Cao, R.; Li, S.; Yin, J.; Guo, L.; Shi, J. Sirtuin 3 promotes microglia migration by upregulating CX3CR1. Cell Adhes. Migr. 2019, 13, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Gan, Y.; Sun, B.L.; Zhang, F.; Lu, B.; Gao, Y.; Liang, W.; Thomson, A.W.; Chen, J.; Hu, X. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann. Neurol. 2013, 74, 458–471. [Google Scholar] [CrossRef]
- Shu, L.; Xu, C.Q.; Yan, Z.Y.; Yan, Y.; Jiang, S.Z.; Wang, Y.R. Post-Stroke Microglia Induce Sirtuin2 Expression to Suppress the Anti-inflammatory Function of Infiltrating Regulatory T Cells. Inflammation 2019, 42, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Culmsee, C.; Zhu, C.; Landshamer, S.; Becattini, B.; Wagner, E.; Pellecchia, M.; Blomgren, K.; Plesnila, N. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J. Neurosci. 2005, 25, 10262–10272. [Google Scholar] [CrossRef]
- Zou, H.; Li, Y.; Liu, X.; Wang, X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999, 274, 11549–11556. [Google Scholar] [CrossRef]
- She, D.T.; Wong, L.J.; Baik, S.H.; Arumugam, T.V. SIRT2 Inhibition Confers Neuroprotection by Downregulation of FOXO3a and MAPK Signaling Pathways in Ischemic Stroke. Mol. Neurobiol. 2018, 55, 9188–9203. [Google Scholar] [CrossRef]
- Guo, J.M.; Shu, H.; Wang, L.; Xu, J.J.; Niu, X.C.; Zhang, L. SIRT1-dependent AMPK pathway in the protection of estrogen against ischemic brain injury. CNS Neurosci. Ther. 2017, 23, 360–369. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, T. Effects of rosuvastatin on neuronal apoptosis in cerebral ischemic stroke rats via Sirt1/NF-kappa B signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5449–5455. [Google Scholar]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Bian, H.J.; Shu, S.; Xia, S.N.; Gu, Y.; Zhang, M.J.; Xu, Y.; Cao, X. AIM2 deletion enhances blood-brain barrier integrity in experimental ischemic stroke. CNS Neurosci. Ther. 2021, 27, 1224–1237. [Google Scholar] [CrossRef]
- Chen, J.T.; Lin, Y.L.; Chen, T.L.; Tai, Y.T.; Chen, C.Y.; Chen, R.M. Ketamine alleviates bradykinin-induced disruption of the mouse cerebrovascular endothelial cell-constructed tight junction barrier via a calcium-mediated redistribution of occludin polymerization. Toxicology 2016, 368–369, 142–151. [Google Scholar] [CrossRef]
- Maherally, Z.; Fillmore, H.L.; Tan, S.L.; Tan, S.F.; Jassam, S.A.; Quack, F.I.; Hatherell, K.E.; Pilkington, G.J. Real-time acquisition of transendothelial electrical resistance in an all-human, in vitro, 3-dimensional, blood-brain barrier model exemplifies tight-junction integrity. FASEB J. 2018, 32, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Al-Ahmad, A.J.; Gassmann, M.; Ogunshola, O.O. Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. J. Cell. Physiol. 2014, 229, 1096–1105. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Geng, K.; Yang, K.; Shao, J.; Xia, W. Sirt3 Protects Against Ischemic Stroke Injury by Regulating HIF-1α/VEGF Signaling and Blood-Brain Barrier Integrity. Cell Mol. Neurobiol. 2021, 41, 1203–1215. [Google Scholar] [CrossRef]
- Krupinski, J.; Kaluza, J.; Kumar, P.; Kumar, S.; Wang, J.M. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994, 25, 1794–1798. [Google Scholar] [CrossRef]
- Kanazawa, M.; Takahashi, T.; Ishikawa, M.; Onodera, O.; Shimohata, T.; Del Zoppo, G.J. Angiogenesis in the ischemic core: A potential treatment target? J. Cereb. Blood Flow Metab. 2019, 39, 753–769. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Ninomiya, I.; Kanazawa, M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen. Res. 2020, 15, 16–19. [Google Scholar]
- Miao, Z.; Dong, Y.; Fang, W.; Shang, D.; Liu, D.; Zhang, K.; Li, B.; Chen, Y.H. VEGF increases paracellular permeability in brain endothelial cells via upregulation of EphA2. Anat. Rec. 2014, 297, 964–972. [Google Scholar] [CrossRef]
- Ng, M.K.; Wu, J.; Chang, E.; Wang, B.Y.; Katzenberg-Clark, R.; Ishii-Watabe, A.; Cooke, J.P. A central role for nicotinic cholinergic regulation of growth factor-induced endothelial cell migration. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 106–112. [Google Scholar] [CrossRef]
- Song, M.Y.; Yi, F.; Xiao, H.; Yin, J.; Huang, Q.; Xia, J.; Yin, X.M.; Wen, Y.B.; Zhang, L.; Liu, Y.H.; et al. Energy restriction induced SIRT6 inhibits microglia activation and promotes angiogenesis in cerebral ischemia via transcriptional inhibition of TXNIP. Cell Death Dis. 2022, 13, 449. [Google Scholar] [CrossRef]
- Machein, M.R.; Kullmer, J.; Rönicke, V.; Machein, U.; Krieg, M.; Damert, A.; Breier, G.; Risau, W.; Plate, K.H. Differential downregulation of vascular endothelial growth factor by dexamethasone in normoxic and hypoxic rat glioma cells. Neuropathol. Appl. Neurobiol. 1999, 25, 104–112. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Jiang, Q.; Zhang, R.; Davies, K.; Powers, C.; Bruggen, N.; Chopp, M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J. Clin. Investig. 2000, 106, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, X.; Dai, Y.; Pan, K.; Deng, Y.; Meng, Y.; Xu, T. PPAR-γ promotes p38 MAP kinase-mediated endothelial cell permeability through activating Sirt3. BMC Neurol. 2019, 19, 289. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Geng, K.Y.; Zhang, Y.S.; Zhang, J.F.; Yang, K.; Shao, J.X.; Xia, W.L. Sirt3 deficiency impairs neurovascular recovery in ischemic stroke. CNS Neurosci. Ther. 2018, 24, 775–783. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Seki, T.; Imayoshi, I.; Tamamaki, N.; Hayashi, Y.; Tatebayashi, Y.; Hitoshi, S. Neural stem cells and neuro/gliogenesis in the central nervous system: Understanding the structural and functional plasticity of the developing, mature, and diseased brain. J. Physiol. Sci. 2016, 66, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Park, H.H. Neurogenesis in Stroke Recovery. Transl. Stroke Res. 2017, 8, 3–13. [Google Scholar] [CrossRef]
- Zhang, R.L.; Zhang, Z.G.; Zhang, L.; Chopp, M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 2001, 105, 33–41. [Google Scholar] [CrossRef]
- Jin, K.; Minami, M.; Lan, J.Q.; Mao, X.O.; Batteur, S.; Simon, R.P.; Greenberg, D.A. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. USA 2001, 98, 4710–4715. [Google Scholar] [CrossRef]
- Zhao, Y.; Guan, Y.F.; Zhou, X.M.; Li, G.Q.; Li, Z.Y.; Zhou, C.C.; Wang, P.; Miao, C.Y. Regenerative Neurogenesis After Ischemic Stroke Promoted by Nicotinamide Phosphoribosyltransferase-Nicotinamide Adenine Dinucleotide Cascade. Stroke 2015, 46, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015, 58, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Nasoni, M.G.; Carloni, S.; Canonico, B.; Burattini, S.; Cesarini, E.; Papa, S.; Pagliarini, M.; Ambrogini, P.; Balduini, W.; Luchetti, F. Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. J. Pineal Res. 2021, 71, e12747. [Google Scholar] [CrossRef]
- Park, J.H.; Long, A.; Owens, K.; Kristian, T. Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiol. Dis. 2016, 95, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Klimova, N.; Fearnow, A.; Long, A.; Kristian, T. NAD(+) precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms. Exp. Neurol. 2020, 325, 113144. [Google Scholar] [CrossRef] [PubMed]
- La Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef]
- Xie, W.; Zhu, T.; Zhou, P.; Xu, H.; Meng, X.; Ding, T.; Nan, F.; Sun, G.; Sun, X. Notoginseng Leaf Triterpenes Ameliorates OGD/R-Induced Neuronal Injury via SIRT1/2/3-Foxo3a-MnSOD/PGC-1α Signaling Pathways Mediated by the NAMPT-NAD Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 7308386. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, J.; Wang, Z.; Yu, L.L.; Wang, J. Piceatannol Protects Human Retinal Pigment Epithelial Cells against Hydrogen Peroxide Induced Oxidative Stress and Apoptosis through Modulating PI3K/Akt Signaling Pathway. Nutrients 2019, 11, 1515. [Google Scholar] [CrossRef]
- Wang, K.J.; Zhang, W.Q.; Liu, J.J.; Cui, Y.; Cui, J.Z. Piceatannol protects against cerebral ischemia/reperfusion-induced apoptosis and oxidative stress via the Sirt1/FoxO1 signaling pathway. Mol. Med. Rep. 2020, 22, 5399–5411. [Google Scholar] [CrossRef]
- Mei, Z.; Du, L.; Liu, X.; Chen, X.; Tian, H.; Deng, Y.; Zhang, W. Diosmetin alleviated cerebral ischemia/reperfusion injury in vivo and in vitro by inhibiting oxidative stress via the SIRT1/Nrf2 signaling pathway. Food Funct. 2022, 13, 198–212. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Zhou, W.; Lu, C.; Ji, T.; Yang, W.; Jin, Z.; Tian, Y.; Lei, W.; Wu, S.; et al. SIRT1/PGC-1α signaling activation by mangiferin attenuates cerebral hypoxia/reoxygenation injury in neuroblastoma cells. Eur. J. Pharmacol. 2021, 907, 174236. [Google Scholar] [CrossRef]
- Fu, B.; Zhang, J.; Zhang, X.; Zhang, C.; Li, Y.; Zhang, Y.; He, T.; Li, P.; Zhu, X.; Zhao, Y.; et al. Alpha-lipoic acid upregulates SIRT1-dependent PGC-1α expression and protects mouse brain against focal ischemia. Neuroscience 2014, 281, 251–257. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Li, G.C. Kaempferol Protects Cell Damage in In Vitro Ischemia Reperfusion Model in Rat Neuronal PC12 Cells. Biomed. Res. Int. 2020, 2020, 2461079. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Chang, X.; Xia, R.; Wu, W.; Guo, H.; Yang, M. Magnoflorine Attenuates Cerebral Ischemia-Induced Neuronal Injury via Autophagy/Sirt1/AMPK Signaling Pathway. Evid.-Based Complement. Altern. Med. 2022, 2022, 2131561. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Shang, J.; Gao, C.; Guan, X.; Chen, Y.; Zhu, L.; Zhang, L.; Zhang, C.; Zhang, J.; Pang, T. A novel SIRT6 activator ameliorates neuroinflammation and ischemic brain injury via EZH2/FOXC1 axis. Acta Pharm. Sin. B 2021, 11, 708–726. [Google Scholar] [CrossRef]
- Naik, S.R.; Pilgaonkar, V.W.; Panda, V.S. Evaluation of antioxidant activity of Ginkgo biloba phytosomes in rat brain. Phytother. Res. 2006, 20, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; He, X.; Sui, Y.; Wang, X.; Wang, X.; Ren, L.; Zhai, Y.X. Ginkgetin aglycone attenuates neuroinflammation and neuronal injury in the rats with ischemic stroke by modulating STAT3/JAK2/SIRT1. Folia Neuropathol. 2019, 57, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, L.; Che, F.; Lu, Y.; Xie, Z.; Wang, H. Arctigenin attenuates ischemic stroke via SIRT1-dependent inhibition of NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2017, 493, 821–826. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.; Yao, F.; Wang, P.; Xiong, Q.; Neng, P. Bergenin has neuroprotective effects in mice with ischemic stroke through antioxidative stress and anti-inflammation via regulating Sirt1/FOXO3a/NF-κB signaling. Neuroreport 2022, 33, 549–560. [Google Scholar] [CrossRef]
- Li, M.; Li, S.C.; Dou, B.K.; Zou, Y.X.; Han, H.Z.; Liu, D.X.; Ke, Z.J.; Wang, Z.F. Cycloastragenol upregulates SIRT1 expression, attenuates apoptosis and suppresses neuroinflammation after brain ischemia. Acta Pharmacol. Sin. 2020, 41, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, N.; Li, N.; Xu, F.; Wang, W.; Lei, Y.; Shi, J.; Gong, Q. Neuroprotective Effects of Trilobatin, a Novel Naturally Occurring Sirt3 Agonist from Lithocarpus polystachyus Rehd., Mitigate Cerebral Ischemia/Reperfusion Injury: Involvement of TLR4/NF-κB and Nrf2/Keap-1 Signaling. Antioxid. Redox Signal. 2020, 33, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lu, W.; Wei, Z.; Xu, M.; Liu, X. Neuroprotective Effect of Sirt2-specific Inhibitor AK-7 Against Acute Cerebral Ischemia is P38 Activation-dependent in Mice. Neuroscience 2018, 374, 61–69. [Google Scholar] [CrossRef]
- Liu, L.; Chen, H.; Jin, J.; Tang, Z.; Yin, P.; Zhong, D.; Li, G. Melatonin ameliorates cerebral ischemia/reperfusion injury through SIRT3 activation. Life Sci. 2019, 239, 117036. [Google Scholar] [CrossRef] [PubMed]
- Teertam, S.K.; Jha, S.; Prakash Babu, P. Up-regulation of Sirt1/miR-149-5p signaling may play a role in resveratrol induced protection against ischemia via p53 in rat brain. J. Clin. Neurosci. 2020, 72, 402–411. [Google Scholar] [CrossRef]
- Yan, X.; Yu, A.; Zheng, H.; Wang, S.; He, Y.; Wang, L. Calycosin-7-O-β-D-glucoside Attenuates OGD/R-Induced Damage by Preventing Oxidative Stress and Neuronal Apoptosis via the SIRT1/FOXO1/PGC-1α Pathway in HT22 Cells. Neural Plast. 2019, 2019, 8798069. [Google Scholar] [CrossRef]
- Kou, D.Q.; Jiang, Y.L.; Qin, J.H.; Huang, Y.H. Magnolol attenuates the inflammation and apoptosis through the activation of SIRT1 in experimental stroke rats. Pharmacol. Rep. 2017, 69, 642–647. [Google Scholar] [CrossRef]
- Lv, H.; Wang, L.; Shen, J.; Hao, S.; Ming, A.; Wang, X.; Su, F.; Zhang, Z. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res. Bull. 2015, 115, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, K.; Liang, M.; Yan, Q.; Huang, M.; Jin, L.; Chen, Y.; Yang, X.; Li, X. Stilbene glycoside upregulates SIRT3/AMPK to promotes neuronal mitochondrial autophagy and inhibit apoptosis in ischemic stroke. Adv. Clin. Exp. Med. 2021, 30, 139–146. [Google Scholar] [CrossRef]
- Sun, X.; Liu, B. Donepezil ameliorates oxygen-glucose deprivation/reoxygenation-induced brain microvascular endothelial cell dysfunction via the SIRT1/FOXO3a/NF-κB pathways. Bioengineered 2022, 13, 7760–7770. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yuan, H.; Denton, K.; Li, X.J.; McCullough, L.; Li, J. Calcium/calmodulin-dependent protein kinase kinase β is neuroprotective in stroke in aged mice. Eur. J. Neurosci. 2016, 44, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mccullough, L.; Li, J. Genetic deletion of calcium/calmodulin-dependent protein kinase kinase β (CaMKK β) or CaMK IV exacerbates stroke outcomes in ovariectomized (OVXed) female mice. BMC Neurosci. 2014, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Chen, Z.; Zhang, F.; Cui, X.; Sun, W.; Geary, G.G.; Wang, Y.; Johnson, D.A.; Zhu, Y.; Chien, S.; et al. Ca2+/calmodulin-dependent protein kinase kinase β phosphorylation of Sirtuin 1 in endothelium is atheroprotective. Proc. Natl. Acad. Sci. USA 2013, 110, E2420–E2427. [Google Scholar] [CrossRef]
- Sun, P.; Bu, F.; Min, J.W.; Munshi, Y.; Howe, M.D.; Liu, L.; Koellhoffer, E.C.; Qi, L.; McCullough, L.D.; Li, J. Inhibition of calcium/calmodulin-dependent protein kinase kinase (CaMKK) exacerbates impairment of endothelial cell and blood-brain barrier after stroke. Eur. J. Neurosci. 2019, 49, 27–39. [Google Scholar] [CrossRef]
- Huang, F.; Luo, L.; Wu, Y.; Xia, D.; Xu, F.; Gao, J.; Shi, J.; Gong, Q. Trilobatin promotes angiogenesis after cerebral ischemia-reperfusion injury via SIRT7/VEGFA signaling pathway in rats. Phytother. Res. 2022, 36, 2940–2951. [Google Scholar] [CrossRef]
- Zhu, T.; Xie, W.J.; Wang, L.; Jin, X.B.; Meng, X.B.; Sun, G.B.; Sun, X.B. Notoginsenoside R1 activates the NAMPT-NAD(+)-SIRT1 cascade to promote postischemic angiogenesis by modulating Notch signaling. Biomed. Pharmacother. 2021, 140, 111693. [Google Scholar] [CrossRef]
- Li, G.X.; Zhang, S.; Liu, R.; Singh, B.; Singh, S.; Quinn, D.I.; Crump, G.; Gill, P.S. Tetraspanin18 regulates angiogenesis through VEGFR2 and Notch pathways. Biol. Open 2021, 10, bio050096. [Google Scholar] [CrossRef]
- Xu, D.; Li, F.; Xue, G.; Hou, K.; Fang, W.; Li, Y. Effect of Wnt signaling pathway on neurogenesis after cerebral ischemia and its therapeutic potential. Brain Res. Bull. 2020, 164, 1–13. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.; Wang, X.; Wang, J.; Xi, G. lncRNA SNHG10 Promotes the Proliferation and Invasion of Osteosarcoma via Wnt/β-Catenin Signaling. Mol. Ther. Nucleic Acids 2020, 22, 957–970. [Google Scholar] [CrossRef]
- Chen, X.; Huan, H.; Liu, C.; Luo, Y.; Shen, J.; Zhuo, Y.; Zhang, Z.; Qian, C. Deacetylation of β-catenin by SIRT1 regulates self-renewal and oncogenesis of liver cancer stem cells. Cancer Lett. 2019, 463, 1–10. [Google Scholar] [CrossRef]
- Wang, S.H.; Li, N.; Wei, Y.; Li, Q.R.; Yu, Z.P. β-catenin deacetylation is essential for WNT-induced proliferation of breast cancer cells. Mol. Med. Rep. 2014, 9, 973–978. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, Z.; Li, F.; Zhou, X.; Zhang, F.; Huang, L.; Gu, B.; Shen, J.; Qi, S. Momordica charantia polysaccharides modulate the differentiation of neural stem cells via SIRT1/Β-catenin axis in cerebral ischemia/reperfusion. Stem Cell Res. Ther. 2020, 11, 485. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Fan, H.; Cai, H.; Hu, Z.; Zhou, X.; Li, F.; Chen, H.; Shen, J.; Qi, S. Promotion of Momordica Charantia polysaccharides on neural stem cell proliferation by increasing SIRT1 activity after cerebral ischemia/reperfusion in rats. Brain Res. Bull. 2021, 170, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.M.; Pach, D.; Brinkhaus, B.; Wruck, K.; Tag, B.; Mank, S.; Willich, S.N. Safety of acupuncture: Results of a prospective observational study with 229,230 patients and introduction of a medical information and consent form. Complement. Med. Res. 2009, 16, 91–97. [Google Scholar] [CrossRef]
- Mourad, F.; Yousif, M.S.; Maselli, F.; Pellicciari, L.; Meroni, R.; Dunning, J.; Puentedura, E.; Taylor, A.; Kerry, R.; Hutting, N.; et al. Knowledge, beliefs, and attitudes of spinal manipulation: A cross-sectional survey of Italian physiotherapists. Chiropr. Man. Ther. 2022, 30, 38. [Google Scholar] [CrossRef]
- Ulett, G.A.; Han, S.; Han, J.S. Electroacupuncture: Mechanisms and clinical application. Biol. Psychiatry 1998, 44, 129–138. [Google Scholar] [CrossRef]
- Tominaga, A.; Ishizaki, N.; Naruse, Y.; Kitakoji, H.; Yamamura, Y. Repeated application of low-frequency electroacupuncture improves high-fructose diet-induced insulin resistance in rats. Acupunct. Med. 2011, 29, 276–283. [Google Scholar] [CrossRef]
- Xiong, L.; Lu, Z.; Hou, L.; Zheng, H.; Zhu, Z.; Wang, Q.; Chen, S. Pretreatment with repeated electroacupuncture attenuates transient focal cerebral ischemic injury in rats. Chin. Med. J. 2003, 116, 108–111. [Google Scholar]
- Xing, Y.; Zhang, M.; Wang, M.M.; Feng, Y.S.; Dong, F.; Zhang, F. The Anti-apoptosis Effect of Single Electroacupuncture Treatment via Suppressing Neuronal Autophagy in the Acute Stage of Ischemic Stroke Without Infarct Alleviation. Front. Cell. Neurosci. 2021, 15, 633280. [Google Scholar] [CrossRef]
- Shogren-Knaak, M.; Ishii, H.; Sun, J.M.; Pazin, M.J.; Davie, J.R.; Peterson, C.L. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 2006, 311, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Oppikofer, M.; Kueng, S.; Martino, F.; Soeroes, S.; Hancock, S.M.; Chin, J.W.; Fischle, W.; Gasser, S.M. A dual role of H4K16 acetylation in the establishment of yeast silent chromatin. EMBO J. 2011, 30, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Füllgrabe, J.; Lynch-Day, M.A.; Heldring, N.; Li, W.; Struijk, R.B.; Ma, Q.; Hermanson, O.; Rosenfeld, M.G.; Klionsky, D.J.; Joseph, B. The histone H4 lysine 16 acetyltransferase hMOF regulates the outcome of autophagy. Nature 2013, 500, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Lv, H.Q.; Li, W.Q.; Hong, H.; Peng, Y.J.; Zhu, B.M. Electroacupuncture Alleviates Cerebral Ischemia/Reperfusion Injury in Rats by Histone H4 Lysine 16 Acetylation-Mediated Autophagy. Front. Psychiatry 2020, 11, 576539. [Google Scholar] [CrossRef]
- Hameed, M.Q.; Dhamne, S.C.; Gersner, R.; Kaye, H.L.; Oberman, L.M.; Pascual-Leone, A.; Rotenberg, A. Transcranial Magnetic and Direct Current Stimulation in Children. Curr. Neurol. Neurosci. Rep. 2017, 17, 11. [Google Scholar] [CrossRef]
- Li, L.; El-Hayek, Y.H.; Liu, B.; Chen, Y.; Gomez, E.; Wu, X.; Ning, K.; Li, L.; Chang, N.; Zhang, L.; et al. Direct-current electrical field guides neuronal stem/progenitor cell migration. Stem Cells 2008, 26, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Fan, Y.Q.; Jiang, H.X.; Chen, S.F.; Chen, J.; Liao, X.Y.; Zou, Y.Y.; Lan, H.Y.; Cui, Y.; Chen, Z.B.; et al. Transcranial direct-current stimulation protects against cerebral ischemia-reperfusion injury through regulating Cezanne-dependent signaling. Exp. Neurol. 2021, 345, 113818. [Google Scholar] [CrossRef]
- Zhao, P.C.; Xu, S.N.; Huang, Z.S.; Jiang, G.W.; Deng, P.C.; Zhang, Y.M. Hyperbaric oxygen via mediating SIRT1-induced deacetylation of HMGB1 improved cReperfusion inj/reperfusion injury. Eur. J. Neurosci. 2021, 54, 7318–7331. [Google Scholar] [CrossRef]
- Hu, Q.; Manaenko, A.; Bian, H.; Guo, Z.; Huang, J.L.; Guo, Z.N.; Yang, P.; Tang, J.; Zhang, J.H. Hyperbaric Oxygen Reduces Infarction Volume and Hemorrhagic Transformation Through ATP/NAD(+)/Sirt1 Pathway in Hyperglycemic Middle Cerebral Artery Occlusion Rats. Stroke 2017, 48, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Corey, D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017, 16, 167–179. [Google Scholar] [CrossRef]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.F.; Xie, M.; Gui, S.J.; Lan, F.; Wan, J.; Li, Y. MiR-370 accelerated cerebral ischemia reperfusion injury via targeting SIRT6 and regulating Nrf2/ARE signal pathway. Kaohsiung J. Med. Sci. 2020, 36, 741–749. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, Y.K.; Zhang, C.G.; Wu, B.Y. miR-19a/b-3p promotes inflammation during cerebral ischemia/reperfusion injury via SIRT1/FoxO3/SPHK1 pathway. J. Neuroinflamm. 2021, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Barangi, S.; Hayes, A.W.; Reiter, R.; Karimi, G. The therapeutic role of long non-coding RNAs in human diseases: A focus on the recent insights into autophagy. Pharmacol. Res. 2019, 142, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Liu, Z.; Shi, Z.S. Non-Coding RNA in Acute Ischemic Stroke: Mechanisms, Biomarkers and Therapeutic Targets. Cell Transpl. 2018, 27, 1763–1777. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Ren, X.; Zheng, L.J.; Li, A.P.; Zhou, W.S. LncRNA NEAT1 ameliorate ischemic stroke via promoting Mfn2 expression through binding to Nova and activates Sirt3. Metab. Brain Dis. 2022, 37, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ji, F.; Sun, X.; Liu, H.; Zhang, C. LncRNA SNHG15 Promotes Oxidative Stress Damage to Regulate the Occurrence and Development of Cerebral Ischemia/Reperfusion Injury by Targeting the miR-141/SIRT1 Axis. J. Healthc. Eng. 2021, 2021, 6577799. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, S.; Lu, K.; Yin, C. Long Non-Coding RNA SNHG7 Alleviates Oxygen and Glucose Deprivation/Reoxygenation-Induced Neuronal Injury by Modulating miR-9/SIRT1 Axis in PC12 Cells: Potential Role in Ischemic Stroke. Neuropsychiatr. Dis. Treat. 2020, 16, 2837–2848. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Y.; Wang, Z.; Zhang, S.; Yang, Y.; Zhu, Y.; Yang, C. LncRNA Snhg8 attenuates microglial inflammation response and blood-brain barrier damage in ischemic stroke through regulating miR-425-5p mediated SIRT1/NF-κB signaling. J. Biochem. Mol. Toxicol. 2021, 35, e22724. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, N.; Jiang, C.; Hao, M. LncRNA SNHG8 sponges miR-449c-5p and regulates the SIRT1/FoxO1 pathway to affect microglia activation and blood-brain barrier permeability in ischemic stroke. J. Leukoc. Biol. 2022, 111, 953–966. [Google Scholar] [CrossRef]
- Xu, J.; Wang, C.; Meng, F.; Xu, P. Long non-coding RNA H19 inhibition ameliorates oxygen-glucose deprivation-induced cell apoptosis and inflammatory cytokine expression by regulating the microRNA-29b/SIRT1/PGC-1α axis. Mol. Med. Rep. 2021, 23, 131. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Y.; Fan, Y.; Fang, N.; Wang, T.; Xu, T.; Shu, Y. CircRNAs in cancer metabolism: A review. J. Hematol. Oncol. 2019, 12, 90. [Google Scholar] [CrossRef]
- Chen, G.; Shan, X.; Li, L.; Dong, L.; Huang, G.; Tao, H. circHIPK3 regulates apoptosis and mitochondrial dysfunction induced by ischemic stroke in mice by sponging miR-148b-3p via CDK5R1/SIRT1. Exp. Neurol. 2022, 355, 114115. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tu, Z.; Yang, D.; Hu, M.; Zhou, L.; Li, Q.; Yu, B.; Hou, S. Exosomes from hypoxic pre-treated ADSCs attenuate acute ischemic stroke-induced brain injury via delivery of circ-Rps5 and promote M2 microglia/macrophage polarization. Neurosci. Lett. 2022, 769, 136389. [Google Scholar] [CrossRef]
- Gao, J.; Qian, T.; Wang, W. CTRP3 Activates the AMPK/SIRT1-PGC-1α Pathway to Protect Mitochondrial Biogenesis and Functions in Cerebral Ischemic Stroke. Neurochem. Res. 2020, 45, 3045–3058. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- Liang, H.; Matei, N.; McBride, D.W.; Xu, Y.; Tang, J.; Luo, B.; Zhang, J.H. Activation of TGR5 protects blood brain barrier via the BRCA1/Sirt1 pathway after middle cerebral artery occlusion in rats. J. Biomed. Sci. 2020, 27, 61. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Shukla, P.C.; Quan, A.; Al-Omran, M.; Lovren, F.; Pan, Y.; Brezden-Masley, C.; Ingram, A.J.; Stanford, W.L.; Teoh, H.; et al. BRCA1 is a novel target to improve endothelial dysfunction and retard atherosclerosis. J. Thorac. Cardiovasc. Surg. 2013, 146, 949–960.e944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.M.; Zhang, X.; Zhang, X.S.; Zhuang, Z.; Li, W.; Sun, Q.; Li, T.; Wang, C.X.; Zhu, L.; Shi, J.X.; et al. SIRT1 inhibition by sirtinol aggravates brain edema after experimental subarachnoid hemorrhage. J. Neurosci. Res. 2014, 92, 714–722. [Google Scholar] [CrossRef]
- Li, D.; Bi, F.F.; Chen, N.N.; Cao, J.M.; Sun, W.P.; Zhou, Y.M.; Li, C.Y.; Yang, Q. A novel crosstalk between BRCA1 and sirtuin 1 in ovarian cancer. Sci. Rep. 2014, 4, 6666. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Guo, S.; Li, Z.Z.; Lu, Y.; Jiang, D.S.; Zhang, R.; Lei, H.; Gao, L.; Zhang, X.; Zhang, Y.; et al. A critical role for interferon regulatory factor 9 in cerebral ischemic stroke. J. Neurosci. 2014, 34, 11897–11912. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yu, D.R.; Huang, J.; Liu, Q.; Wang, D.X.; Luo, N.; Jia, H.; Fan, H.F.; Liu, Q.B. Multimodal Rehabilitation Program Promotes Motor Function Recovery of Rats After Ischemic Stroke by Upregulating Expressions of GAP-43, SYN, HSP70, and C-MYC. J. Stroke Cerebrovasc. Dis. 2018, 27, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, H.; Wang, X.; Jiao, H.; Li, L.; Zheng, J. c-myc protects mice from ischemia stroke through elevating microRNA-200b-5p-regulated SIRT1 expression. Brain Res. Bull. 2021, 176, 76–84. [Google Scholar] [CrossRef]
- Xie, Z.; Cao, B.Q.; Wang, T.; Lei, Q.; Kang, T.; Ge, C.Y.; Gao, W.J.; Hui, H. LanCL1 attenuates ischemia-induced oxidative stress by Sirt3-mediated preservation of mitochondrial function. Brain Res. Bull. 2018, 142, 216–223. [Google Scholar] [CrossRef]
- Xiao, R.; Wang, Q.; Peng, J.; Yu, Z.; Zhang, J.; Xia, Y. BMSC-Derived Exosomal Egr2 Ameliorates Ischemic Stroke by Directly Upregulating SIRT6 to Suppress Notch Signaling. Mol. Neurobiol. 2023, 60, 1–17. [Google Scholar] [CrossRef]
- She, D.T.; Jo, D.G.; Arumugam, T.V. Emerging Roles of Sirtuins in Ischemic Stroke. Transl. Stroke Res. 2017, 8, 405–423. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, X.; Zhang, L.; Shen, J. Neurovascular Unit: A critical role in ischemic stroke. CNS Neurosci. Ther. 2021, 27, 7–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).