Chemical Composition, In Vitro Antioxidant Activities, and Inhibitory Effects of the Acetylcholinesterase of Liparis nervosa (Thunb.) Lindl. Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. EO Isolation
2.3. GC-MS and GC-FID Analysis
2.4. Antioxidant Activities Determination
2.4.1. DPPH Method
2.4.2. ABTS Method
2.4.3. Ferric-Reducing Antioxidant Power (FRAP) Method
2.5. Anti-Acetylcholinesterase Activity Test
3. Results and Discussion
3.1. EO Yield and Component Analysis
3.2. Antioxidant Activities Evaluation
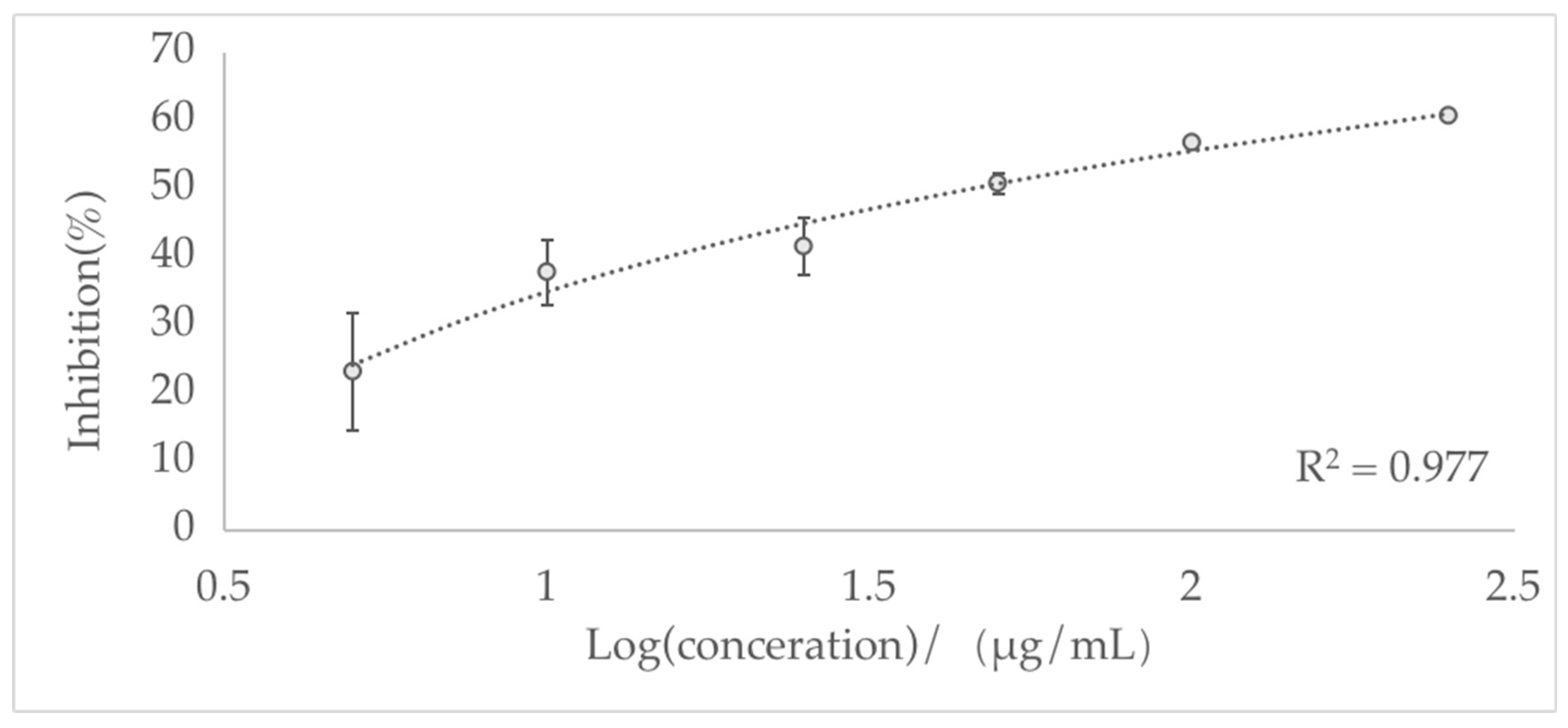
3.3. Acetylcholinesterase Inhibitory Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.; Chung, S.W.; Li, B.; Zeng, S.J.; Yan, H.F.; Li, S.J. New insight into the molecular phylogeny of the genus Liparis s.l. (Orchidaceae: Malaxideae) with a new generic segregate: Blepharoglossum. Plant Syst. Evol. 2020, 306, 1–10. [Google Scholar] [CrossRef]
- Liang, W.; Guo, X.; Nagle, D.G.; Zhang, W.D.; Tian, X.H. Genus Liparis: A review of its traditional uses in China, phytochemistry and pharmacology. J. Ethnopharmacol. 2019, 234, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Zhong, Y.; Zheng, Q.; Hu, P.Y.; Huang, X.Y.; Yang, M.; Ren, G.L.; Du, Q.; Luo, J.; Zhang, K.N. Sedative and hypnotic effects of compound Anshen essential oil inhalation for insomnia. BMC Complement. Altern. Med. 2019, 19, 306. [Google Scholar] [CrossRef] [PubMed]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Nematollahi, N.; Weinberg, J.L.; Flattery, J.; Goodman, N.; Kolev, S.D.; Steinemann, A. Volatile chemical emissions from essential oils with therapeutic claims. Air Qual. Atmos. Health 2021, 14, 365–369. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Greeough, M.A.; Camakaris, J.; Bush, A.I. Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem. Int. 2012, 62, 540–555. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, E. Antioxidants: An approach for restricting oxidative stress induced neurodegeneration in Alzheimer’s disease. Inflammopharmacology 2023, 31, 717–730. [Google Scholar] [CrossRef]
- Reddy, P.H.; Tripathi, R.; Troung, Q.; Tirumala, K.; Reddy, T.P.; Anekonda, V.; Shirendeb, U.P.; Calkins, M.J.; Reddy, A.P.; Mao, P.; et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: Implications to mitochondria-targeted antioxidant therapeutics. Biochim. Biophys. Acta 2012, 1822, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Sgarbossa, A.; Giacomazza, D.; Di Carlo, M. Ferulic acid: A hope for Alzheimer’s disease therapy from plants. Nutrients 2015, 7, 5764–5782. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Uriarte-Pueyo, I.; Calvo, M. Flavonoids as acetylcholinesterase inhibitors. Curr. Med. Chem. 2011, 18, 5289–5302. [Google Scholar] [CrossRef] [PubMed]
- Benny, A.; Thomas, J. Essential Oils as Treatment Strategy for Alzheimer’s Disease: Current and Future Perspectives. Planta Med. 2019, 85, 239–248. [Google Scholar] [PubMed]
- Jiang, N.; Wang, Y.; Zhou, J.; Zheng, R.; Yuan, X.; Wu, M.; Bao, J.; Wu, C. A novel mannose-binding lectin from Liparis nervosa with anti-fungal and anti-tumor activities. Acta Biochim. Biophys. Sin. 2020, 52, 1081–1092. [Google Scholar] [CrossRef]
- Huang, S.; Pan, M.F.; Zhou, X.L.; Zhou, Z.L.; Wang, C.J.; Shan, L.H.; Weng, J. Five new nervogenic acid derivatives from Liparis nervosa. Chin. Chem. Lett. 2013, 24, 734–736. [Google Scholar] [CrossRef]
- Liu, L.; Zou, M.; Yin, Q.; Zhang, Z.; Zhang, X. Phenylpropanoids from Liparis nervosa and their in vitro antioxidant andα-glucosidase inhibitory activities. Med. Chem. Res. 2021, 30, 1005–1010. [Google Scholar] [CrossRef]
- Huang, S.; Zhong, D.; Shan, L.; Zheng, Y.; Zhang, Z.; Bu, Y.; Ma, H.; Zhou, X. Three new pyrrolizidine alkaloids derivatives from Liparis nervosa. Chin. Chem. Lett. 2016, 27, 757–760. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Huang, S.; Zhou, X.L. Two new pairs of epimeric pyrrolizidine alkaloids from Liparis nervosa. Chem. Nat. Compd. 2019, 55, 305–308. [Google Scholar] [CrossRef]
- Sarhadi, E.; Ebrahimi, S.N.; Hadjiakhoondi, A.; Manayi, A. Chemical Composition and Antioxidant Activity of Root Essential Oil of Different Salvia leriifolia Populations. J. Essent. Oil-Bear. Plants 2021, 24, 209–217. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, P.; Liu, D.; Song, W.; Zhu, L.; Liu, X. Chemical Composition and In Vitro Antioxidant Activity of Sida rhombifolia L. Volatile Organic Compounds. Molecules 2022, 27, 7067. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, A.; Fakhfakh, J.; Brieudes, V.; Halabalaki, M.; Fontanay, S.; Duval, R.E.; Mliki, K.; Sayadi, S.; Allouche, N. Chemical composition, antibacterial activity using micro-broth dilution method and antioxidant activity of essential oil and water extract from aerial part of Tunisian Thymus algeriensis Boiss & Reut. J. Essent. Oil-Bear. Plants 2022, 24, 1349–1364. [Google Scholar]
- Andrade, M.A.; Das Graças Cardoso, M.; De Andrade, J.; Silva, L.F.; Teixeira, M.L.; Valério Resende, J.M.; Da Silva Figueiredo, A.C.; Barroso, J.G. Chemical Composition and Antioxidant Activity of Essential Oils from Cinnamodendron dinisii Schwacke and Siparuna guianensis Aublet. Antioxidants 2013, 2, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Sripahco, T.; Khruengsai, S.; Charoensup, R.; Tovaranonte, J.; Pripdeevech, P. Chemical composition, antioxidant, and antimicrobial activity of Elsholtzia beddomei C. B. Clarke ex Hook. f. essential oil. Sci. Rep. 2022, 12, 2225. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, P.; Ren, X.; Liu, X. Thermal Treatment (Hydrodistillation) on The Biomass of Ficus hispida L. f.: Volatile Organic Compounds Yield, Phytochemical Composition, and Antioxidant Activity Evaluation. Energies 2022, 15, 8092. [Google Scholar] [CrossRef]
- Szafranska, K.; Szewczyk, R.; Janas, K.M. Involvement of melatonin applied to Vigna radiata L. seeds in plant response to chilling stress. Cent. Eur. J. Biol. 2014, 9, 1117–1126. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Ingkaninan, K.; Temkitthawon, P.; Chuenchom, K.; Yuyaem, T.; Thongnoi, W. Screening for acetylcholinesterase inhibitory activity in plants used in Thai traditional rejuvenating and neurotonic remedies. J. Ethnopharmacol. 2003, 89, 261–264. [Google Scholar] [CrossRef]
- Aodah, A.H.; Balaha, M.F.; Jawaid, T.; Khan, M.M.; Ansari, M.J.; Alam, A. Aegle marvels (L.) Correa Leaf Essential Oil and Its Phytoconstituents as an Anticancer and Anti-Streptococcus mutans Agent. Antibiotics 2023, 12, 835. [Google Scholar] [CrossRef]
- Robustelli della Cuna, F.S.; Calevo, J.; Bari, E.; Giovannini, A.; Boselli, C.; Tava, A. Characterization and Antioxidant Activity of Essential Oil of Four Sympatric Orchid Species. Molecules 2019, 24, 3878. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, G.F.; Wang, Z.H. Chemical Var-iation in Essential Oil of Cymbidium sinense Flowers from Six Cultivars. J. Essent. Oil Bear. Plants 2017, 20, 385–394. [Google Scholar] [CrossRef]
- Robustelli della Cuna, F.S.; Cortis, P.; Esposito, F.; De Agostini, A.; Sottani, C.; Sanna, C. Chemical Composition of Essential Oil from Four Sympatric Orchids in NW-Italy. Plants 2022, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Hadjiakhoondi, A.; Vatandoost, H.; Khanavi, M.; Sadeghipour-Roodsari, H.R.; Vosoughi, M.; Motahareh Kazemi, M.; Abaib, M.R. Fatty acid composition and toxicity of Melia azedarach L. fruits against malaria vector Anopheles stephensi. Iran. J. Pharmceutical Sci. 2006, 9, 97–102. [Google Scholar]
- Khaldi, R.; Rehimi, N.; Kharoubi, R.; Soltani, N. Phytochemical composition of almond oil from Melia azedarach L. and its larvicidal, ovicidal, repellent and enzyme activities in Culex pipiens L. Trop. Biomed. 2022, 39, 531–538. [Google Scholar]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer. Res. 2002, 22, 2587–2590. [Google Scholar]
- Perumalsamy, H.; Jang, M.J.; Kim, J.-R.; Kadarkarai, M.; Ahn, Y.-J. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasites Vectors 2015, 8, 237. [Google Scholar] [CrossRef]
- Muema, J.M.; Bargul, J.L.; Mutunga, J.M.; Obonyo, M.A.; Asudi, G.O.; Njeru, S.N. Neurotoxic Zanthoxylum chalybeum root constituents invoke mosquito larval growth retardation through ecdysteroidogenic CYP450s transcriptional perturbations. Pestic. Biochem. Physiol. 2021, 178, 104912. [Google Scholar] [CrossRef]
- Ruan, J.H.; Wan, X.C.; Quan, P.; Liu, C.; Fang, L. Investigation of Effect of Isopropyl Palmitate on Drug Release from Transdermal Patch and Molecular Dynamics Study. AAPS PharmSciTech 2019, 20, 174. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Hashemi, M.; Ghahfarokhi, F.T. Essential Oil and Chemical Compositions of Wild and Cultivated Thymus Daenensis Celak and Thymus vulgaris L. Ind. Crops Prod. 2013, 48, 43–48. [Google Scholar] [CrossRef]
- Périno-Issartier, S.; Ginies, C.; Cravotto, G.; Chemat, F. A comparison of essential oils obtained from lavandin via different extraction processes: Ultrasound, microwave, turbohydrodistillation, steam and hydrodistillation. J. Chromatogr. A 2013, 1305, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Comp. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2, 2-diphenyl-1-picrylhydrazyl method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef] [PubMed]
- Jianu, C.; Rusu, L.-C.; Muntean, I.; Cocan, I.; Lukinich-Gruia, A.T.; Goleț, I.; Horhat, D.; Mioc, M.; Mioc, A.; Șoica, C.; et al. In Vitro and In Silico Evaluation of the Antimicrobial and Antioxidant Potential of Thymus pulegioides Essential Oil. Antioxidants 2022, 11, 2472. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.G.A.; dos Reis, R.B.; Souza, A.S.D.; Canuto, K.M.; Castro, K.N.D.; Pereira, A.M.L.; Diniz, F.M. Essential oil composition, antioxidant and antibacterial activity against Vibrio parahaemolyticus from five Lamiaceae species. J. Essent. Oil Res. 2022, 34, 313–321. [Google Scholar] [CrossRef]
- Rashid, S.; Ahmad, M.; Amin, W.; Ahmad, B. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.A.B.d.; Costa, K.S.d.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J.G.d. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Houghton, P.J.; Ren, Y.; Howes, M.-J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z. Für Nat. C 2016, 71, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Khadri, A.; Serralheiro, M.; Nogueira, J.; Neffati, M.; Smiti, S.; Araújo, M. Antioxidant and antiacetylcholinesterase activities of essential oils from Cymbopogon schoenanthus L. Spreng. Determination of chemical composition by GC–mass spectrometry and 13C NMR. Food Chem. 2008, 109, 630–637. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Razzaghirad, Y.; Ostad, S.N.; Hadjiakhoondi, A.; Shams Ardekani, M.R.; Hajimehdipoor, H.; Attar, H.; Samadi, M.; Jovel, E.; Khanavi, M. Cytotoxic, acetylcholinesterase inhibitor and antioxidant activity of Nepeta menthoides Boiss & Buhse essential oil. J. Essent. Oil-Bear. Plants 2014, 17, 544–552. [Google Scholar]
- Falé, P.L.; Borges, C.; Madeira, P.J.A.; Ascensão, L.; Araújo, M.E.M.; Florêncio, M.H.; Serralheiro, M.L.M. Rosmarinic acid, scutellarein 4′-methyl ether 7-O-glucuronide and (16S)-coleon E are the main compounds responsible for the antiacetylcholinesterase and antioxidant activity in herbal tea of Plectranthus barbatus (“falso boldo”). Food Chem. 2009, 114, 798–805. [Google Scholar] [CrossRef]
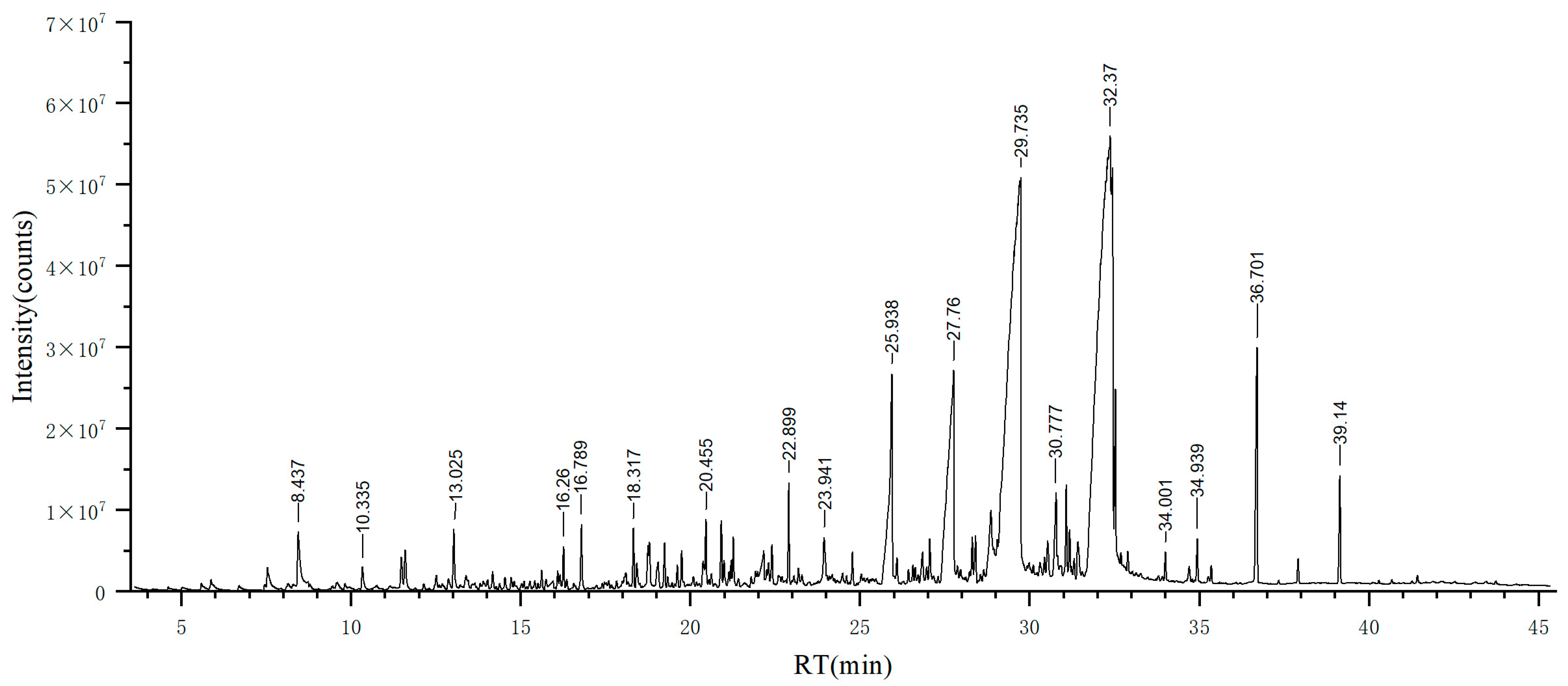

| No. | Retention Time, tR (min) | Compound | RI a | RI b | Area (%) | Identification Method | CAS ID |
|---|---|---|---|---|---|---|---|
| 1 | 5.867 | Heptanal | 907 | 901 | 0.22% | RRI, MS | 111-71-7 |
| 2 | 7.531 | Benzaldehyde | 968 | 962 | 0.43% | RRI, MS | 100-52-7 |
| 3 | 8.437 | 2-Pentylfuran | 995 | 993 | 1.08% | RRI, MS | 3777-69-3 |
| 4 | 9.583 | 2-Octyn-1-ol | 1038 | - | 0.14% | MS | 20739-58-6 |
| 5 | 10.335 | (E)-2-Octenal | 1065 | 1060 | 0.25% | RRI, MS | 2548-87-0 |
| 6 | 11.481 | Linalool | 1103 | 1099 | 0.29% | RRI, MS | 78-70-6 |
| 7 | 11.59 | Nonanal | 1108 | 1104 | 0.39% | RRI, MS | 124-19-6 |
| 8 | 12.507 | 3-Nonen-2-one | 1127 | 1142 | 0.17% | RRI, MS | 14309-57-0 |
| 9 | 12.872 | Cucumber aldehyde | 1159 | 1155 | 0.16% | RRI, MS | 557-48-2 |
| 10 | 13.025 | (E)-2-Nonenal | 1165 | 1162 | 0.56% | RRI, MS | 18829-56-6 |
| 11 | 13.39 | 4-Ethylbenzaldehyde | 1178 | 1180 | 0.18% | RRI, MS | 4748-78-1 |
| 12 | 14.171 | Decanal | 1209 | 1206 | 0.17% | RRI, MS | 112-31-2 |
| 13 | 15.949 | Nonanoic acid | 1283 | 1273 | 0.17% | RRI, MS | 112-05-0 |
| 14 | 16.091 | Anethole | 1290 | 1286 | 0.14% | RRI, MS | 104-46-1 |
| 15 | 16.26 | 2-Undecanone | 1296 | 1294 | 0.31% | RRI, MS | 112-12-9 |
| 16 | 16.789 | (E,E)-2,4-Decadienal | 1320 | 1317 | 0.48% | RRI, MS | 25152-84-5 |
| 17 | 18.093 | n-Decanoic acid | 1379 | 1373 | 0.19% | RRI, MS | 334-48-5 |
| 18 | 18.317 | β-Damascenone | 1388 | 1386 | 0.39% | RRI, MS | 23726-93-4 |
| 19 | 18.419 | (+)-Sativen | 1393 | 1396 | 0.23% | RRI, MS | 3650-28-0 |
| 20 | 18.748 | β-Longipinene | 1408 | 1403 | 0.33% | RRI, MS | 41432-70-6 |
| 21 | 18.791 | Longifolene | 1411 | 1405 | 0.34% | RRI, MS | 475-20-7 |
| 22 | 19.048 | Dihydrodehydro-β- ionone | 1424 | 1424 | 0.32% | RRI, MS | 20483-36-7 |
| 23 | 19.239 | α-Ionone | 1433 | 1426 | 0.31% | RRI, MS | 127-41-3 |
| 24 | 19.615 | Acenaphthylene | 1451 | 1454 | 0.15% | RRI, MS | 208-96-8 |
| 25 | 19.74 | Dihydropseudoionone | 1457 | 1456 | 0.25% | RRI, MS | 689-67-8 |
| 26 | 20.373 | Curcumene | 1487 | 1483 | 0.27% | RRI, MS | 644-30-4 |
| 27 | 20.455 | β-Ionone | 1491 | 1491 | 0.45% | RRI, MS | 14901-07-6 |
| 28 | 20.908 | Tridecanal | 1513 | 1512 | 0.47% | RRI, MS | 10486-19-8 |
| 29 | 20.990 | Dibenzofuran | 1518 | 1514 | 0.15% | RRI, MS | 132-64-9 |
| 30 | 21.197 | δ-Cadinene | 1528 | 1524 | 0.13% | RRI, MS | 483-76-1 |
| 31 | 22.163 | Dodecanoic acid | 1577 | 1568 | 0.55% | RRI, MS | 143-07-7 |
| 32 | 22.304 | Fluorene | 1584 | 1583 | 0.15% | RRI, MS | 86-73-7 |
| 33 | 22.403 | (Z)-α-Bisabolene epoxide | 1589 | 1586 | 0.30% | RRI, MS | 111536-37-9 |
| 34 | 22.899 | Tetradecanal | 1615 | 1613 | 0.66% | RRI, MS | 124-25-4 |
| 35 | 23.183 | Oxacyclotetradeca-4,11-diyne | 1630 | 1639 | 0.14% | RRI, MS | 6568-32-7 |
| 36 | 23.941 | Tridecanoic acid | 1671 | 1666 | 0.63% | RRI, MS | 638-53-9 |
| 37 | 24.776 | Pentadecanal | 1717 | 1715 | 0.23% | RRI, MS | 2765-11-9 |
| 38 | 25.938 | Tetradecanoic acid | 1782 | 1768 | 4.01% | RRI, MS | 544-63-8 |
| 39 | 26.085 | Phenanthrene | 1790 | 1775 | 0.22% | RRI, MS | 85-01-8 |
| 40 | 26.56 | Hexadecanal | 1818 | 1817 | 0.13% | RRI, MS | 629-80-1 |
| 41 | 26.843 | Isopropyl myristate | 1835 | 1827 | 0.32% | MS | 117-27-0 |
| 42 | 27.051 | Hexahydrofarnesyl acetone | 1847 | 1844 | 0.35% | RRI, MS | 502-69-2 |
| 43 | 27.76 | Propyl tetradecanoate | 1889 | 1896 | 7.20% | RRI, MS | 14303-70-9 |
| 44 | 28.311 | Farnesyl acetone | 1923 | 1919 | 0.33% | RRI, MS | 1117-52-8 |
| 45 | 28.404 | Methyl palmitate | 1929 | 1926 | 0.34% | RRI, MS | 112-39-0 |
| 46 | 28.856 | 11-Hexadecenoic acid | 1957 | 1953 | 1.66% | RRI, MS | 2416-20-8 |
| 47 | 29.042 | Dodecenyl succinic anhydride | 1969 | 1968 | 0.36% | RRI, MS | 19780-11-1 |
| 48 | 29.538 | n-Hexadecanoic acid | - | 1970 | 15.08% | MS | 57-10-3 |
| 49 | 29.735 | Isopropyl palmitate | 2012 | 2023 | 12.44% | RRI, MS | 142-91-6 |
| 50 | 30.531 | Fluoranthene | 2063 | 2054 | 0.37% | RRI, MS | 206-44-0 |
| 51 | 30.777 | Heptadecanoic acid | 2079 | 2071 | 0.87% | RRI, MS | 506-12-7 |
| 52 | 31.077 | Methyl linoleate | 2099 | 2092 | 0.67% | RRI, MS | 112-63-0 |
| 53 | 31.175 | Methyl linolenate | 2105 | 2098 | 0.33% | RRI, MS | 301-00-8 |
| 54 | 31.427 | Phytol | 2112 | 2114 | 0.39% | RRI, MS | 150-86-7 |
| 55 | 32.370 | methyl (9E,11E)-octadeca-9,11-dienoate | 2186 | 2187 | 31.69% | RRI, MS | 13038-47-6 |
| 56 | 32.441 | 17-Octadecynoic acid | 2190 | 2199 | 3.71% | RRI, MS | 34450-18-5 |
| 57 | 32.528 | 2-Methyl-Z,Z-3,13- octadecadienol | 2196 | - | 1.62% | MS | 519002-96-1 |
| 58 | 32.686 | Isopropyl linolenate | 2207 | 2200 | 0.20% | RRI, MS | 83918-59-6 |
| 59 | 32.752 | 10-trans,12-cis-Linoleic acid | 2213 | 2222 | 0.17% | RRI, MS | 2420-56-6 |
| 60 | 32.893 | 2,4,5,7-Tetramethylphenanthrene | 2223 | - | 0.21% | MS | 7396-38-5 |
| 61 | 34.001 | Tricosane | 2300 | 2300 | 0.18% | RRI, MS | 638-67-5 |
| 62 | 34.699 | Diroleuton | 2352 | 2346 | 0.19% | RRI, MS | 1783-84-2 |
| 63 | 34.939 | Octadecanamide | 2370 | 2374 | 0.34% | RRI, MS | 124-26-5 |
| 64 | 35.348 | Tetracosane | 2399 | 2400 | 0.13% | RRI, MS | 646-31-1 |
| 65 | 36.701 | Pentacosane | 2503 | 2500 | 2.24% | RRI, MS | 629-99-2 |
| 66 | 37.907 | Hexacosane | 2599 | 2600 | 0.18% | RRI, MS | 630-01-3 |
| 67 | 39.140 | Heptacosane | 2699 | 2700 | 0.81% | RRI, MS | 593-49-7 |
| Tested Samples | DPPH 50% Effective Concentration (μg/mL) | ABTS 50% Effective Concentration (μg/mL) | FRAP Antioxidant Capacity (μM/g) |
|---|---|---|---|
| L. nervosa EOs | >10,000 | 748.3 | 39.64 ± 3.38 |
| BHT | 37.02 ± 2.21 | 14.69 ± 1.32 | - |
| Trolox | 18.23 ± 1.12 | 9.47 ± 1.21 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Xu, Z.; Gao, P.; Liu, X. Chemical Composition, In Vitro Antioxidant Activities, and Inhibitory Effects of the Acetylcholinesterase of Liparis nervosa (Thunb.) Lindl. Essential Oil. Biomolecules 2023, 13, 1089. https://doi.org/10.3390/biom13071089
Zhao J, Xu Z, Gao P, Liu X. Chemical Composition, In Vitro Antioxidant Activities, and Inhibitory Effects of the Acetylcholinesterase of Liparis nervosa (Thunb.) Lindl. Essential Oil. Biomolecules. 2023; 13(7):1089. https://doi.org/10.3390/biom13071089
Chicago/Turabian StyleZhao, Jiayi, Ziyue Xu, Peizhong Gao, and Xu Liu. 2023. "Chemical Composition, In Vitro Antioxidant Activities, and Inhibitory Effects of the Acetylcholinesterase of Liparis nervosa (Thunb.) Lindl. Essential Oil" Biomolecules 13, no. 7: 1089. https://doi.org/10.3390/biom13071089
APA StyleZhao, J., Xu, Z., Gao, P., & Liu, X. (2023). Chemical Composition, In Vitro Antioxidant Activities, and Inhibitory Effects of the Acetylcholinesterase of Liparis nervosa (Thunb.) Lindl. Essential Oil. Biomolecules, 13(7), 1089. https://doi.org/10.3390/biom13071089






