Abstract
Numerous chemical probes have been used to measure or image oxidative, nitrosative and related stress induced by free radicals in biology and biochemistry. In many instances, the chemical pathways involved are reasonably well understood. However, the rate constants for key reactions involved are often not yet characterized, and thus it is difficult to ensure the measurements reflect the flux of oxidant/radical species and are not influenced by competing factors. Key questions frequently unanswered are whether the reagents are used under ‘saturating’ conditions, how specific probes are for particular radicals or oxidants and the extent of the involvement of competing reactions (e.g., with thiols, ascorbate and other antioxidants). The commonest-used probe for ‘reactive oxygen species’ in biology actually generates superoxide radicals in producing the measured product in aerobic systems. This review emphasizes the need to understand reaction pathways and in particular to quantify the kinetic parameters of key reactions, as well as measure the intracellular levels and localization of probes, if such reagents are to be used with confidence.
1. Introduction
While most papers in this Special Issue focus on modified endogenous biological molecules as markers of oxidative, nitrosative or other changes initiated by free radicals, such damage has also been routinely assessed using exogenous, xenobiotic molecular probes. Thus, as described below, a reduced fluorescein dye has been widely used to detect the production of (usually unspecified) ‘reactive oxygen species’ in biological material or biomolecular models—astonishingly, in over 6000 studies to date—despite the obvious shortcomings discussed below. Hence, it is important not to take a too narrow or literal viewpoint in considering ‘biomarkers’ of oxidative stress and radical damage.
There will often be competing pathways in the production of endogenous biomarkers of oxidative or other radical damage, with the yield of biomarker reflecting not only the production of radical reactants but also the concentrations and availability of potentially protective substances such as thiols, ascorbate or phenolic antioxidants (for example). Similarly, the ‘signal’ reflecting chemical change in exogenous markers or probes, whether measured concentrations or image intensity (e.g., in fluorescence readers or microscopy), may reflect not only the extent of the production of radicals or oxidants but also the involvement of competing reactions. The outcome of such competing processes will be defined by the Law of Mass Action, and so the important characteristics will be the product of concentration and rate constant for all the competing reactions. Hence, whether damage is assessed by endogenous biomarkers or by added chemical probes, chemical kinetics is central to an informed assessment of the damage pathways, and the availability of rate constants for specific reactions involving free radicals (in particular) and potential biomolecular targets (or reasonable models for them) is central to an informed discussion about competing pathways in free-radical biology. Of course, the intracellular concentrations of reactants or probes are equally important, and cellular heterogeneity is a daunting challenge to the accurate chemical modelling of biological pathways, as discussed elsewhere in the context of radiation damage [1]. As an example, ‘molecular crowding’ can modulate reaction rates (or effective rate constants), as illustrated recently [2]. As a first step, though, analyzing potential competing reactions by simple homogenous competition kinetics is far better than nothing, even if, ultimately, more sophisticated approaches are desirable, such as applying Monte Carlo numerical simulation methods to a heterogenous model of cellular compartments.
This review aims to illustrate the importance of kinetic factors in using chemical probes for radical-initiated damage, although the concepts apply equally to discussing the production of endogenous biomarkers. It will also help readers to access sources of pertinent kinetic information in both contexts. Kinetic information (rate constants) can be estimated by measuring the final product yields analyzed by competition kinetics in model systems where a reference reaction has been characterized separately, but the direct observation of reactive intermediates—radical or product—is usually the most reliable. This involves the production of sufficient radicals in a time significantly shorter than the reaction timescale to follow the reactions and enable detection, e.g., by kinetic spectrophotometry. The technique of flash photolysis, introduced around 1950, made this possible for photochemically initiated reactions. The analogous technique of pulse radiolysis, introduced a decade later, is much more relevant for free-radical reactions, since ionizing radiation generates free radical pathways which, in the case of water as a solvent, can be easily manipulated to monitor reactions of specific radicals of biological interest. Hence, radiation chemistry has provided, as a ‘spinoff’, much kinetic information concerning reactions involving free radicals and biomolecules. It is not necessary to understand the complexity of radiation damage to appreciate the application of the specialized techniques to general redox chemistry [3].
It is not intended here to give an extensive overview of the chemistry and/or methodology of markers and/or probes for ‘reactive oxygen species’ or oxidative damage, which has recently been reviewed with specific recommendations [4]. The present author reviewed the chemistry of fluorescent and luminescent probes for oxidative and nitrosative stress in some detail [5], and numerous more recent reviews in this area updated and expanded the earlier survey [6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Rather, the intention here is to illustrate the importance of understanding the reaction pathways involved in the use of these probes and, especially, the importance of kinetics in controlling these pathways.
We first have to consider the terms used in labelling these probes; following this, a discussion of the chemistry of some of the most commonly used probes serves as a ‘template’ or ‘worked example’ against which other probes can be assessed. Finally, the questions which all users of chemical probes should ask themselves before commencing on a study are briefly summarized.
2. ‘Oxidative Stress’ and ‘Reactive Oxygen Species’ (‘ROS’): Their Definitions (or Lack of)
If we are to discuss sensibly probes for chemical species, then we need to be clear about the molecules involved. While the term ‘oxidative stress’ is reasonably well understood and quite well defined [50,51,52,53], the related but not synonymous term ‘reactive oxygen species’ and its acronym ‘ROS’ are mere ‘catch-all’ phrases lacking usefulness because of their vague, all-encompassing nature. Indeed, experts in free-radical biology are increasingly warning about the indiscriminate use of ‘ROS’. Thus, it was recommended: ‘The use of ROS or RNS should be … only when it is clearly stated that the species is unknown or one of several implicated molecules without certainty’ [54]; it was commented: ‘The ubiquitous use of these terms seems to provide a screen to hide the detailed chemistry of these species’ [55]. Another group of experts noted: ‘Reactive oxygen species’ (ROS) is a generic term that defines a wide variety of oxidant molecules with vastly different properties and biological functions … The generic term ROS should not be used to describe specific molecular agents’ [43]. The first recommendation of an expert review group [4] was ‘… wherever possible, the actual chemical species involved in a biological process should be stated, and consideration given to whether the observed effect is compatible with its reactivity, lifespan, products generated and fate in vivo’.
The difficulty in beginning to assess the reactions of vague ‘ROS’ in kinetic terms is apparent if we compare the rate constants for reactions of the common biological antioxidant, the thiol, glutathione (GSH), with specific radical or molecular oxidants, all containing oxygen, at physiological pH. Thus, the rate constants (units of M−1 s−1) for reactions of GSH with different ‘ROS’ span over ten orders of magnitude, with the rate constants for •OH (~1 × 1010 [56]), HOCl (~1 × 108 [57]), NO2• (~2 × 107 [58]) and CO3•− (~5 × 106 [59]) being several orders of magnitude higher than those for reactions of O2•− (~2 × 102 [60]) and H2O2 (~9 × 10−1 [61]).
In the context of chemical probes for oxidizing radicals derived from biomolecules, it should also be noted that radicals centered on sulfur (thiyl radicals, RS•, and not normally viewed as ‘reactive oxygen species’) can oxidize targets directly: the mid-point reduction potential of the couple GS•,H+/GSH is ~0.90 V at pH ~7 [62], only ~0.04 V lower than that of the tyrosine phenoxyl radical, TyrO•,H+/TyrOH [63]. Thiols are often viewed as protective antioxidants, but they do have the potential to elevate ‘ROS’/oxidative stress following the chemical ‘repair’ of diverse radical sites via the sequence of reactions [64,65]:
radical damage + RSH → ‘repaired’ (or modified) damage + RS•
RS• + RS− ⇌ (RSSR)•−
(RSSR)•− + O2 → RSSR + O2•−.
However, ascorbate (AscH−) can disrupt this pathway by intercepting thiyl radicals [66]:
but it is important to note that many in vitro cell culture models lack ascorbate and so are poor models for tissues. The potential reaction pathways of thiyl radicals in cells are multiple and complex [67,68,69] and include the catalysis of the cis/trans isomerization of unsaturated lipids [70,71,72,73]. Since thiyl radicals can form reactive thiylperoxyl (RSOO•) and sulphonyl radicals in the presence of oxygen [74,75], thiol radical chemistry should be included in any discussion of ‘ROS’.
RS• + AscH− → RSH + Asc•−,
It has also long been recognized that ‘ROS’ should not be viewed in isolation, in particular, reactive nitrogen-based oxidants include peroxynitrous acid/peroxynitrite (ONOOH/ONOO−, from the reaction between •NO and O2•−), which in turn can source •OH and •NO2 as well as carbonate radicals (CO3•−) [76,77]. CO3•− and NO2• are fairly powerful oxidants: the reduction potentials of the couples CO3•−/CO32− and •NO2/NO2− are ~1.57 and 1.04 V, respectively [78], higher than the midpoint potentials at pH 7 of the radical/reductant couples of tyrosine, glutathione or ascorbate [3]. Hence, the spectrum of radical oxidants potentially reactive towards chemical probes is quite wide—and of course the non-radical oxidants H2O2 and HOCl cannot be ignored, particularly since H2O2 can form oxidizing intermediates in peroxidase chemistry or upon reaction with cytochrome c, as noted below. Overall, then, a whole battery of oxidants are potentially reactive towards some chemical probes for ‘ROS’ or oxidative stress, presenting a major challenge to the informed use of probes.
3. Dichlorodihydrofluorescein (DCFH2): By Far, the Most Widely Used—And Certainly the Most Abused—Probe for ‘ROS’ or Cellular Oxidative Stress
It is sensible—and instructive—to illustrate the importance of mapping reaction pathways of molecular probes and to characterize them kinetically by discussing the chemistry of the most widely used probe for oxidative events associated with free radicals in biology, not least because the reactivity of this probe has been kinetically characterized quite well. However, as discussed below, there remain many gaps in our knowledge, and the results of any study using this or similar probes must continue to be viewed with skepticism.
Assays based on the fluorescence of a probe or its product are both sensitive and facilitate imaging. Fluorescein is perhaps the archetypical fluorophore, and its reduced form (dihydrofluorescein) is a non-fluorescent or ‘leuco’ dye. The variant 2′,7′-dichlorodihydrofluorescein (DCFH2, Figure 1, 1) is the most commonly used probe for vague ‘ROS’ or general oxidative damage in biology associated with free radicals, with the fluorescent product dichlorofluorescein (DCF) measured; an appropriate PubMed search revealed almost 7000 ‘hits’ in May 2023 for papers referencing these dyes. Even accounting for the papers which simply mention or review the use of DCFH2 or related probes, it seems likely that well over 6000 studies have utilized such probes. Despite well-known shortcomings, which have been repeatedly stressed by numerous authors, e.g., [5,6,8,9,13,19,23,24,27,30,54,55,79,80,81,82,83,84,85,86,87,88,89], these probes continue to be widely recommended and used in many investigations, e.g., [90,91,92,93,94,95], including a recent study that attracted quite widespread press coverage [96].
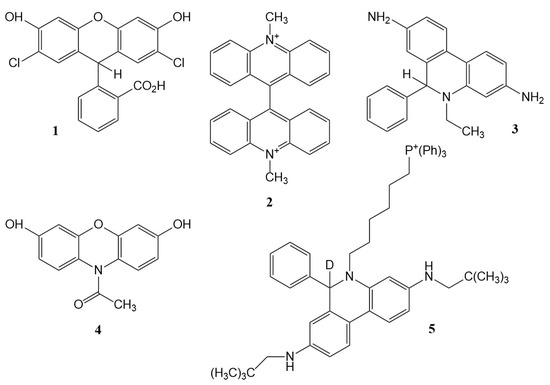
Figure 1.
Structures of the probes discussed: 1, dichlorodihydrofluorescein (DCFH2); 2, lucigenin; 3, hydroethidine (HE); 4, Amplex Red; 5, ‘MitoNeoD’.
There are three basic problems with this and similar ‘leuco’ dyes such as the chloromethyl analogue and other variants promoted by manufacturers; while DCFH2 is the best characterized, it seems likely that all exhibit many of the problems discussed below. First, oxidation may reflect the level of the catalyst rather than that of the oxidant; second, the rate of oxidation by radicals varies widely, and radicals other than ‘ROS’ react rapidly with the probe; and third, the radical intermediate produced during oxidation—either directly by radicals or by a catalyst activated by H2O2—reacts rapidly with oxygen to generate superoxide radicals.
Consider first oxidation by non-radical species, of which H2O2 is the most studied. For a start, the direct reaction between H2O2 and DCFH2 is very slow, and the reaction is most unlikely to compete with the destruction of H2O2 by cellular peroxidases, peroxiredoxins, etc.: oxidation by H2O2 requires a catalyst, as shown in early studies with DCFH2 or analogues where horseradish peroxidase (HRP) or hematin was used [97], and the signal measured, therefore, may reflect the level of catalyst rather than (or as well as) the level of H2O2 generation. Fluorescent probes for catalytic iron(II) were developed [34]. Another potent catalyst is cytochrome c [98], very important because it is released from the mitochondria during apoptosis. Further, diverse hydroperoxides can substitute for H2O2 in the catalyzed oxidation of DCFH2 [79]. The peroxidase- or cytochrome c-catalyzed oxidation is also subject to interference by antioxidants, including uric acid, ascorbate and α-tocopherol [79], as well as NADH and thiols [99]. Overall, then, associating fluorescence with changes in H2O2 production rather than with the levels of catalyst or antioxidants is difficult, and confusion with both apoptotic pathways and hydroperoxide formation rather than H2O2 is quite possible.
Other non-radical oxidants have been investigated in this context: hypochlorite reacts inefficiently with DCFH2 [100]; peroxynitrite (ONOO−) is much more efficient, but kinetic studies [101] showed this probably reflects radical oxidation involving NO2•, •OH and/or CO3•− radicals, decomposition products of peroxynitrite under physiological conditions [76,77].
The rates of oxidation of DCFH2 by radical oxidants vary widely: superoxide has very low reactivity, while •OH radicals react at the diffusion-controlled limit (rate constant k ~1.3 × 1010 M−1 s−1; intermediate reactivity is seen with NO2• (k ~1.3 × 107 M−1 s−1) and CO3•− (k ~2.6 × 108 M−1 s−1, pH 8.2)) [102]. (Unless otherwise indicated, the rate constants here refer to physiological pH; the pH value can be important, since DCFH2 dissociates with pKa values estimated as ~7.9 and 9.2 [102], and oxidants may show pH-dependent reactivity. NO2•, for example, may oxidize a phenolate species considerably faster than the corresponding phenol [103,104].) Sulfur-centered radicals (RS•, thiyl) oxidize DCFH2 with rate constants of ~3.7 × 107 (GSH) or 1.7 × 107 (cysteine) M−1 s−1 [85]; since such radicals are commonly produced in diverse radical ‘repair’ reactions (e.g., the donation of H to carbon-centered radicals, as noted above), the lack of specificity of DCFH2 towards oxygen-based radicals is obvious. Note, however, that the involvement of thiols in probe chemistry is more complex than the direct oxidation of DCFH2 by thiyl radicals: the oxidized probe DCF has a phenolic moiety and is a substrate for peroxidases, the resulting phenoxyl radical DCF(–O•) oxidizing GSH (as well as NADH and ascorbate), generating superoxide radicals via further reactions [81]:
DCF + oxidant → DCF(–O•)
DCF(–O•) + GSH (or NADH) → DCF + GS• (or NAD•)
GS• + GSH ⇌ (GSSG)•−
(GSSG)•− + O2 → GSSG + O2•−
NAD• + O2 → NAD+ + O2•−.
The oxidized probe also reacts rapidly with some radicals, e.g., the rate constant for the reaction of CO3•− radicals with DCF is almost identical to that for the reaction of DCFH2; however, DCF is much less reactive towards NO2• than is DCFH2 [105].
By the rule of spin conservation, the oxidation of DCFH2 to the fluorescent product DCF by radicals must produce a radical intermediate, DCFH•. Further, the catalyzed oxidation of DCFH2 also proceeds via this radical intermediate. In landmark studies [80,81,83], it was shown that this radical also reacts with oxygen to produce superoxide:
leading the authors to describe such probes for oxidative stress as a ‘self-fulfilling prophesy’ [83]. A direct observation of the reaction of DCFH• with O2 showed k ~5.3 × 108 M−1 s−1 [102]. The rate of the possible competing reaction:
is pH-dependent around physiological pH because of prototropic equilibria, but the effective rate constant is 2k ~2.8 × 108 M−1 s−1 at pH 7.4. Since the two rate constants are of the same order, and it is easily calculated that in most biological systems, the concentration of O2 far exceeds that of DCFH• at a steady state, superoxide formation seems an inevitable consequence of DCFH2 oxidation, whether by (catalyzed) peroxides or directly by radicals [102].
DCFH• + O2 → DCF + O2•− (+ H+)
2 DCFH• → DCFH2 + DCF
The extent of DCFH2 oxidation by both H2O2 (or hydroperoxides) and radicals will reflect competition kinetics (the Law of Mass Action), since there are obviously competing pathways for the species to react, but this presents an immediate difficulty: the reaction pathways reflect the products of rate constant and concentration, but hardly any users of these probes have measured the intracellular concentration of DCFH2. Usually, the diacetate derivative is used, relying on cellular esterases to cleave the diacetate and relying on the resulting negative charge on the dissociated carboxylic acid to trap the probe intracellularly. In the author’s Laboratory, L K Folkes measured ~300 µM DCFH2 as the average intracellular concentration after incubating hamster fibroblasts in cell suspension (5 × 106/mL) at pH 7.4 with DCFH2 diacetate (10 µM) for 15 min at 37 °C [102].
There will be intracellular concentration gradients typical of a weak acid, as well as lipid/water partitioning of both probe and product [106]; this study reported DCFH2 was ‘totally partitioned into the octanol’ using octanol/water 1:1 at pH 6, while DCF had a partition coefficient of 2:1 (the octanol:/water partition coefficient of DCFH2 at an unspecified pH was quoted elsewhere as 2.62 [107], but it will be pH-dependent because of prototropic equilibria [102]). There may also be leakage of product into the extracellular medium [108]. Further, the nature of the culture medium must be considered, including the presence of catalytic metals, antioxidants, pH indicators, etc. [109,110].
Considering, as an example, competing reactions between CO3•− and either DCFH2 or GSH present together, the rate constant for the reaction of CO3•− and GSH is ~5.3 × 106 M−1 s−1 at pH ~7 [59], i.e., ~50–fold lower than that for the reaction between CO3•− and DCFH2, but the intracellular concentration of GSH is typically around 10-fold higher than that of DCFH2 if the concentration of the latter is similar to that in the measurements with hamster cells described above. (Ascorbate reacts with CO3•− ~260 × faster than does GSH [111].) Nominally, just considering the one competing reaction with GSH, the probe will ‘capture’ a good fraction—but not all—CO3•− radicals, but since other free thiols are present along with other targets for CO3•− radicals (protein thiols, tyrosine residues, etc., and ascorbate in tissues), it seems likely that the probe is often not being used in ‘saturating’ conditions, making quantitation difficult. This is more clear-cut if we consider competing reactions of NO2• radicals. The rate constants for the oxidation of GSH or cysteine at pH 7.4 by NO2• are ~2 or 5 × 107 M−1 s−1, respectively [58], i.e., both higher than that for the reaction of NO2• with DCFH2 (~1.3 × 107 M−1 s−1). Hence, ~0.3 mM DCFH2 will ‘pick up’ only a very small fraction of any NO2• generated. The total ‘scavenging capacity (Σk[scavenger]) for •OH radicals in mammalian cells has been estimated as ~8 × 108 s−1, compared with the corresponding value for •OH reacting with 0.3 mM DCFH2, i.e., ~4 × 106 s−1; so, clearly it is most unlikely that DCFH2 (and indeed most probes at realistic intracellular concentrations) can intercept a significant fraction of •OH radicals.
Kinetic competition may also be exhibited between the activated peroxidase intermediate (such as HRP Compound I) reacting with either the probe or endogenous antioxidants; so, for a full understanding of probe chemistry, quite extensive studies must be undertaken. These are likely to involve several sophisticated techniques; in this case, electron paramagnetic resonance spectroscopy to identify reaction intermediates, stopped-flow rapid mixing to measure the rates of non-radical reactions, flash photolysis to monitor reactions of excited states (see below), high-performance liquid chromatography to measure intracellular uptake, preferably in specific organelles, and (especially) pulse radiolysis to generate specific ‘ROS’ in known amounts and monitor their reactions in real time.
Even leaving aside the obvious contraindication of the generation of one specific ‘ROS’ (superoxide radical) via the obligate involvement of an oxygen-reactive radical in the formation of the fluorescent product, without measurements of both the rate constants of the reactions of putative reactants with a probe and the estimates of the intracellular (or better, intra-organelle or localized) concentrations of the probe, it is obvious that users are working in the dark in seeking to quantify reactive oxidants using such probes. Actually, users had best work in the dark, because a further complication is that visible light can generate an excited state of DCF, quenched by cellular reductants such as GSH or NADH (and doubtless other reactants such as ascorbate) to generate DCFH• and hence O2•− [112]. Photooxidation of Amplex® Red has also been reported, once again involving the production of superoxide radicals [113].
4. Some Kinetic Studies of Other Chemical Probes for Oxidants and Biological Radicals
DCFH2 is not the only ‘ROS’ probe to be a ‘self-fulfilling prophesy’. As discussed in more detail previously [5], the bis-N-methyl acridinium salt, lucigenin (LC2+, Figure 1, 2), is reduced to a radical LC•+, probably by flavoprotein reductases, and exhibits chemiluminescence on reaction of this radical intermediate with superoxide radicals via an unstable intermediate producing an excited state of N-methylacridone [114]. However, the obligate radical intermediate LC•+ reacts with oxygen to generate superoxide [115,116] with a rate constant of ~3 × 106 M−1 s−1:
and since the reduction potential of the LC2+/LC•+ couple is –0.28 V vs. NHE, the equilibrium constant of the reaction is ~50; the reverse reaction producing LC•+ from superoxide must have a rate constant of ~6 × 104 M−1 s−1 and is unlikely to compete with the reaction of O2•− with superoxide dismutase in experiments involving loading LC2+ into mammalian cells [117]. Again, despite such an obvious contraindication, the probe is still being used: thus, in a recent study ‘… superoxide production in the brain, heart tissue, and aorta was measured using lucigenin-enhanced chemiluminescence’ [118].
LC•+ + O2 ⇌ LC2+ + O2•−
Even if repeated warnings about the shortcomings of commonly used probes continue to be ignored, some groups have exhibited a clear understanding of the problems; the collaboration between the Institute of Applied Radiation Chemistry at Łodz University of Technology and the Medical College of Wisconsin has been particularly fruitful (e.g., [9,27,30,39]), and considerable progress is being made in the kinetic characterization of some probes. Some examples are outlined very briefly below. Nonetheless, it is not at all encouraging that, while a widely consulted Handbook of Fluorescent Probes (see: https://www.thermofisher.com/uk/en/home/global/forms/mp-handbook-download-request-form-2014.html, accessed on 27 May 2023) has a 27-page chapter devoted to ‘Probes for Reactive Oxygen Species, Including Nitric Oxide’, the terms ‘rate’ or ‘rate constant’ are both conspicuously absent.
Progress is also illustrated by a discussion of the extent of intercepting oxidants using the more recently introduced boronate probes for nucleophilic oxidants, which has been the subject of intense activity [36], and the reactivity of probes for hypochlorous acid [44]. Earlier, a much clearer understanding of the reactions involved in the use of hydroethidine as a probe for superoxide radicals emerged after a series of careful studies [119,120], and the insightful review by Zielonka and Kalyanaraman [30] is an essential reading for users of luminescent probes for cellular oxidizing and nitrating species.
In particular, the generation of specific radicals by pulse radiolysis was used to estimate key rate constants involved in the detection of superoxide radicals by dihydroethidium (hydroethidine, HE, Figure 1, 3), which superficially is similar to that of lucigenin in that the oxidized probe reacts with superoxide to form a detectable product. The oxidation of hydroethidine by O2•− has a rate constant around 2 × 106 M−1 s−1; the rate constant for the reaction of (HE)•+ with O2•− to form the measured product, 2-hydroxyethidium, is orders of magnitude higher; so, the first reaction may be rate-limiting, depending on the concentrations of HE and (HE)•+—at least in systems where only O2•− is the oxidant: obviously, other oxidants can achieve the first step, as directly observed using radiolytically produced radicals [120].
‘Amplex® Red’, a dihydroxyphenoxazine (Figure 1, 4), has been quite widely used to assay hydrogen peroxide, peroxidase-catalyzed oxidation yielding the fluorescent dye resorufin. However, other oxidants, including biological oxidants, are also reactive: stopped-flow kinetic spectrophotometry was used to demonstrate that peroxynitrite-derived oxidants, but not peroxynitrite, also oxidized the probe, and pulse radiolysis quantified the reactivity towards carbonate radicals, although at pH 10.3 (where the reactivity might be higher than at pH 7.4 because of prototropic properties that the authors demonstrated [121]). Intermediates formed during the conversion of Amplex® Red into resorufin were characterized. An earlier study [10] discussed possible chain reactions.
The reactivity towards superoxide of a new mitochondria-targeted probe, the phenanthridine derivative ‘MitoNeoD’ (Figure 1, 5), was also characterized using pulse radiolysis, with a rate constant for the reaction of the probe with O2•− of ~1.4 × 107 M−1 s−1 measured [122]. The details of this study are outside the scope of the present paper but are well worth studying as they demonstrate the depth of understanding now being applied in the development of new probes.
5. Some Compilations of Rate Constants for Reactions of Free Radicals Relevant to Biology
While there are very few rate constants measured for reactions of radicals with the specific chemical probes of the general type discussed here, there are useful, if dated, compilations of expertly evaluated rate constants for reactions of •OH and H• [123,124], HO2•/O2•− [125], peroxyl radicals [126], inorganic radicals including CO3•−, •NO2 and ClO2• [103], phenoxyl radicals [127] and aliphatic carbon-centered radicals [128]. It may be possible to estimate the likely rate constants in a few instances by comparison with the simpler chemical models that have been studied, and these compilations are invaluable in considering the chemical kinetics of possible competing reactions with endogenous reactants. However, the publication dates should be noted; further, while the former database of the University of Notre Dame Radiation Chemistry Data Center (which hosted the production of these compilations) has been transferred to the website of the National Institute of Standards and Technology (see: https://kinetics.nist.gov/solution/, accessed on 23 May 2023), it too suffers from not being updated and has strict syntax. It should be noted that PubMed will often miss relevant studies of a purely chemical nature. The majority of the rate constants involving reactions of interest involving free radicals have been obtained using radiation–chemical methods, and the inclusion of the term ‘pulse radiolysis’ as a search term may help filter literature searches for relevant kinetic data in both PubMed and Chemical Abstracts (SciFinder®). However, in recent years several pulse radiolysis facilities in Europe have closed down, and this is a barrier to the future application of the technique in this area.
6. Key Points to Consider When Using Chemical Probes in Free-Radical Biology
Biochemists familiar with enzyme-catalyzed reactions will take for granted that an assay for an enzyme will involve conditions such that the catalyst is rate-limiting, rather than the concentration of the substrate(s) (or vice versa if a substrate is to be assayed using an enzyme-catalyzed assay): saturating conditions characterized by Michaelis–Menten kinetics. Yet, in attempting to assay ‘ROS’ and other biological oxidants, the necessary parameters to evaluate whether a probe is used under saturating conditions or to estimate the fraction of the oxidant that is being detected—the products of rate constants and concentrations for reactions of both probe and competing reactants—have often been ignored or are simply not available.
The applications of chemical probes vary widely, and the criteria for using them in cell-free systems may be very different from those for cellular models and especially tissues. However, it may be useful to reiterate briefly the main questions that researchers should ask before using a probe, based on earlier discussion [5,13,30]:
- Where is the probe located, and what is its concentration? Consider, if appropriate, whether there are likely to be extracellular–intracellular concentration gradients or inter-organelle or other local variations in the concentration of the probe (driven by, e.g., lipid/water partitioning, trans-membrane pH differentials or binding to macromolecules). Measure, or at least estimate, the probe concentration(s) in the region(s) of interest.
- What are the species likely to react with the probe? Consider all the putative species being generated and their reactivities towards the probe, as estimated by the product of rate constant and concentration, preferably under relevant conditions (pH; solvent polarity; ideally, also temperature, although competing reactions may exhibit broadly similar temperature effects).
- What are the reaction pathways involved in the generation of the final product being measured? Identify reaction intermediates if possible, noting that spin conservation is likely to dictate the obligate formation of intermediate free radicals from radical oxidants.
- Is a catalyst involved in the reaction(s)? Consider whether the concentration of the catalyst may be rate-limiting and whether the presence of the catalyst results from the treatment being assessed, e.g., the release of cytochrome c from mitochondria as a result of apoptosis initiated by the oxidative challenge.
- What are possible competing reactions, firstly involving kinetic competition between the probe and cellular antioxidants for the species of interest (e.g., oxidizing radicals or molecules)? The Law of Mass Action will dictate the extent of the competition involving reactions of the species being assessed with endogenous reactants, especially with antioxidants: thiols, ascorbate, urate, NADH, α-tocopherol, etc., and other redox-active reagents (including oxygen, which can also modulate thiol radical chemistry).
- Do the intermediates in probe chemistry react with endogenous molecules in competition with pathways leading to the final, measured product? Again, the Law of Mass Action is central to an analysis, and quantifying the redox properties of reactive intermediates will help suggest possible reactants. The archetypical example is the reaction with oxygen of the obligate intermediate radical obtained on oxidation of reduced fluorescein dyes—oxidation either by radical species or by intermediates formed in the catalyzed reaction with hydrogen peroxide—to generate superoxide radicals.
- Are there any potential effects of the cell culture medium (if used)? Consider comparing the results in full medium (proteins, amino acids, redox- or light-sensitive pH indicator, metals, ascorbate, etc.) with those from cells suspended/plated in as pure phosphate-buffered saline as can be obtained.
- Could there be artefacts arising from the photochemical properties of the probes and products? Visible light (including ambient room light or instrumental light sources) may initiate photochemical reactions; photochemically induced excited states may be much more reactive towards cellular reductants than the probes themselves. Consider whether the product build-up is sufficient to initiate inner-filter effects by absorbing a significant fraction of the incident light, e.g., in fluorescence plate readers.
- How far is the measured product likely to diffuse from the site of interest or ‘leak’ from cells? This will be time-dependent and, within a specific organelle, can be estimated from the likely diffusion coefficient and the Einstein–Smoluchowski equation, but trans-membrane transport should also be considered.
- Does the probe itself affect cellular function? Probe and/or product may bind to macromolecules or change the mitochondrial membrane potential or may initiate apoptosis and lead to potential catalysts released during the experiment (e.g., cytochrome c in apoptosis).
7. Conclusions
To design, synthesize and use with confidence a chemical probe for reactants of interest in free-radical biology is a daunting prospect, requiring skills spanning disciplines, and techniques and instrumentation unlikely to be found in a single laboratory. The chemical obstacles to the use of the commonest probe for ‘ROS’ have been discussed previously (and repeatedly) but were summarized here with emphasis on kinetic factors, as a pointer to some of the properties which must be considered in the rational use of probes.
Some readers may find the discussion above of the language of free-radical biology and the advice to avoid the term ‘ROS’ wherever possible to be unrealistic, pedantic and repetitive. Yet, in the view of the present author, the language is a major part of the problem. The term ‘reactive oxygen species’ may have arisen in the early days of free-radical biology, when the emphasis was on Fenton chemistry and the term simply avoided distinguishing between free hydroxyl radicals as a product of iron/hydrogen peroxide chemistry, or whether an iron-oxo species was the reactant. The index to the second (1989) edition of a standard text discussing free radicals in biology and medicine does not include ‘reactive oxygen’ or ‘ROS’ [129]. Now, though, the term and its acronym retrieved over 327,000 ‘hits’ in PubMed at the time of writing, and it would seem impossible to avoid this vague term. One is reminded of the old computer adage, ‘GIGO’: ‘garbage in, garbage out’; in the context of chemical probes for ‘ROS’, the vagueness of the input term almost guarantees a vague output in the use of the probes. As a group of experts recently noted [4], ‘Multiple roles of reactive oxygen species (ROS) … have led researchers unfamiliar with the complexities of ROS and their reactions to employ commercial kits and probes to measure ROS and oxidative damage inappropriately, treating ROS (a generic abbreviation) as if it were a discrete molecular entity’.
In the present article, the author has again stressed the pitfalls in the use of the commonest probe for ‘ROS’ to illustrate what can go wrong if a careful study of the literature is outweighed by the convenience of purchasing and using a commercial reagent where the manufacturer has glossed over the problems. This was followed by a brief mention of some recent studies which show that leading workers in the field do have a clear grasp of the problems to be addressed. Kinetic measurements in which specific chemical entities are generated and their reactions followed directly to quantify reactivity are central to the successful application of chemical probes for oxidants and radicals in biology, but the rate constants are only part of the story; the local concentrations of the probe and possible endogenous reactants at specific sites in the cell are equally important. It is hoped the list above of questions that users should first consider in the use of chemical probes may, at the very least, help scientists to be aware of the uncertainties which arise if these factors are ignored.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Wardman, P. Approaches to modeling chemical reaction pathways in radiobiology. Int. J. Radiat. Biol. 2022, 98, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lemus, E.; Reyes, J.S.; Gamon, L.F.; López-Alarcón, C.; Davies, M.J. Effect of macromolecular crowding on protein oxidation: Consequences on the rate, extent and oxidation pathways. Redox Biol. 2021, 48, 102202. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Initiating redox reactions by ionizing radiation: A versatile, selective and quantitative tool. Redox Biochem. Chem. 2023, 5-6, 100004. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic. Biol. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Srikun, D.; Chang, C.J. Mitochondrial-targeted fluorescent probes for reactive oxygen species. Curr. Opin. Chem. Biol. 2010, 14, 50–56. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Oxidative chemistry of fluorescent dyes: Implications in the detection of reactive oxygen and nitrogen species. Biochem. Soc. Trans. 2011, 39, 1221–1225. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Darley-Usmar, V.; Davies, K.J.; Dennery, P.A.; Forman, H.J.; Grisham, M.B.; Mann, G.E.; Moore, K.; Roberts, L.J., 2nd; Ischiropoulos, H. Measuring reactive oxygen and nitrogen species with fluorescent probes: Challenges and limitations. Free Radic. Biol. Med. 2012, 52, 1–6. [Google Scholar] [CrossRef]
- Liochev, S.I. Free radicals: How do we stand them? Anaerobic and aerobic free radical (chain) reactions involved in the use of fluorogenic probes and in biological systems. Med. Princ. Pract. 2013, 23, 195–203. [Google Scholar] [CrossRef]
- Woolley, J.F.; Stanicka, J.; Cotter, T.G. Recent advances in reactive oxygen species measurement in biological systems. Trends Biochem. Sci. 2013, 38, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Joseph, J.; Sikora, A.; Kalyanaraman, B. Real-time monitoring of reactive oxygen and nitrogen species in a multiwell plate using the diagnostic marker products of specific probes. Methods Enzymol. 2013, 526, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim. Biophys. Acta 2014, 1840, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.I.; Harrison, D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 2014, 20, 372–382. [Google Scholar] [CrossRef]
- Nauseef, W.M. Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases. Biochim. Biophys. Acta 2014, 1840, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Barzegar Amiri Olia, M.; Schiesser, C.H.; Taylor, M.K. New reagents for detecting free radicals and oxidative stress. Org. Biomol. Chem. 2014, 12, 6757–6766. [Google Scholar] [CrossRef]
- Adegoke, O.; Forbes, P.B. Challenges and advances in quantum dot fluorescent probes to detect reactive oxygen and nitrogen species: A review. Anal. Chim. Acta 2015, 862, 1–13. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, F. Imaging mitochondrial reactive oxygen species with fluorescent probes: Current applications and challenges. Free Radic. Res. 2015, 49, 374–382. [Google Scholar] [CrossRef]
- Debowska, K.; Debski, D.; Hardy, M.; Jakubowska, M.; Kalyanaraman, B.; Marcinek, A.; Michalski, R.; Michalowski, B.; Ouari, O.; Sikora, A.; et al. Toward selective detection of reactive oxygen and nitrogen species with the use of fluorogenic probes—Limitations, progress, and perspectives. Pharmacol. Rep. 2015, 67, 756–764. [Google Scholar] [CrossRef]
- Kolanowski, J.L.; Kaur, A.; New, E.J. Selective and reversible approaches toward imaging redox signaling using small-molecule probes. Antioxid. Redox Signal. 2016, 24, 713–730. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Hyun, J.Y.; Wei, T.; Qiang, J.; Ren, X.; Shin, I.; Yoon, J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2016, 45, 2976–3016. [Google Scholar] [CrossRef]
- Ribou, A.C. Synthetic sensors for reactive oxygen species detection and quantification: A critical review of current methods. Antioxid. Redox Signal. 2016, 25, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Żamojć, K.; Zdrowowicz, M.; Jacewicz, D.; Wyrzykowski, D.; Chmurzyński, L. Fluorescent probes used for detection of hydrogen peroxide under biological conditions. Crit. Rev. Anal. Chem. 2016, 46, 171–200. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Hardy, M.; Podsiadly, R.; Cheng, G.; Zielonka, J. Recent developments in detection of superoxide radical anion and hydrogen peroxide: Opportunities, challenges, and implications in redox signaling. Arch. Biochem. Biophys. 2017, 617, 38–47. [Google Scholar] [CrossRef]
- Andina, D.; Leroux, J.C.; Luciani, P. Ratiometric fluorescent probes for the detection of reactive oxygen species. Chemistry 2017, 23, 13549–13573. [Google Scholar] [CrossRef]
- Lü, R. Reaction-based small-molecule fluorescent probes for dynamic detection of ROS and transient redox changes in living cells and small animals. J. Mol. Cell. Cardiol. 2017, 110, 96–108. [Google Scholar] [CrossRef]
- Hardy, M.; Zielonka, J.; Karoui, H.; Sikora, A.; Michalski, R.; Podsiadły, R.; Lopez, M.; Vasquez-Vivar, J.; Kalyanaraman, B.; Ouari, O. Detection and characterization of reactive oxygen and nitrogen species in biological systems by monitoring species-specific products. Antioxid. Redox Signal. 2018, 28, 1416–1432. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Y.; Niu, J.; Xie, X.; Wang, X.; Tang, B. Small-molecule fluorescent probes for imaging and detection of reactive oxygen, nitrogen, and sulfur species in biological systems. Anal. Chem. 2018, 90, 533–555. [Google Scholar] [CrossRef]
- Erard, M.; Dupré-Crochet, S.; Nüße, O. Biosensors for spatiotemporal detection of reactive oxygen species in cells and tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R667–r683. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Kalyanaraman, B. Small-molecule luminescent probes for the detection of cellular oxidizing and nitrating species. Free Radic. Biol. Med. 2018, 128, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Pan, W.; Li, N.; Tang, B. Fluorescent probes for organelle-targeted bioactive species imaging. Chem. Sci. 2019, 10, 6035–6071. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ng, K.K.; Hu, J.J.; Ye, S.; Yang, D. Small-molecule-based fluorescent sensors for selective detection of reactive oxygen species in biological systems. Annu. Rev. Biochem. 2019, 88, 605–633. [Google Scholar] [CrossRef]
- Wu, L.; Sedgwick, A.C.; Sun, X.; Bull, S.D.; He, X.P.; James, T.D. Reaction-based fluorescent probes for the detection and imaging of reactive oxygen, nitrogen, and sulfur species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T. Fluorescent probes for the detection of catalytic Fe(II) ion. Free Radic. Biol. Med. 2019, 133, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, W.; Li, P.; Zhang, W.; Wang, X.; Tang, B. Versatile fluorescent probes for imaging the superoxide anion in living cells and in vivo. Angew. Chem. Int. Ed. Engl. 2020, 59, 4216–4230. [Google Scholar] [CrossRef]
- Sikora, A.; Zielonka, J.; Dębowska, K.; Michalski, R.; Smulik-Izydorczyk, R.; Pięta, J.; Podsiadły, R.; Artelska, A.; Pierzchała, K.; Kalyanaraman, B. Boronate-based probes for biological oxidants: A novel class of molecular tools for redox biology. Front. Chem. 2020, 8, 580899. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Pitfalls of reactive oxygen species (ROS) measurements by fluorescent probes and mitochondrial superoxide determination using MitoSox. In Measuring Oxidants and Oxidative Stress in Biological Systems; Berliner, L.J., Parinandi, N.L., Eds.; Springer: Cham, Switzerland, 2020; pp. 7–9. [Google Scholar]
- Gardiner, B.; Dougherty, J.A.; Ponnalagu, D.; Singh, H.; Angelos, M.; Chen, C.A.; Khan, M. Measurement of oxidative stress markers in vitro using commercially available kits. In Measuring Oxidants and Oxidative Stress in Biological Systems; Berliner, L.J., Parinandi, N.L., Eds.; Springer: Cham, Switzerland, 2020; pp. 39–60. [Google Scholar]
- Michalski, R.; Thiebaut, D.; Michałowski, B.; Ayhan, M.M.; Hardy, M.; Ouari, O.; Rostkowski, M.; Smulik-Izydorczyk, R.; Artelska, A.; Marcinek, A.; et al. Oxidation of ethidium-based probes by biological radicals: Mechanism, kinetics and implications for the detection of superoxide. Sci. Rep. 2020, 10, 18626. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Das, A. Fluorescent probes for imaging bioactive species in subcellular organelles. Chem. Commun. 2021, 57, 12058–12073. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, W.; Zhang, B. ROS-responsive probes for low-background optical imaging: A review. Biomed. Mater. 2021, 16, 022002. [Google Scholar] [CrossRef]
- Li, J.; LoBue, A.; Heuser, S.K.; Leo, F.; Cortese-Krott, M.M. Using diaminofluoresceins (DAFs) in nitric oxide research. Nitric Oxide 2021, 115, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Pierzchała, K.; Pięta, M.; Rola, M.; Świerczyńska, M.; Artelska, A.; Dębowska, K.; Podsiadły, R.; Pięta, J.; Zielonka, J.; Sikora, A.; et al. Fluorescent probes for monitoring myeloperoxidase-derived hypochlorous acid: A comparative study. Sci. Rep. 2022, 12, 9314. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Yue, Y.; Yin, C.; Huo, F. Design of dual-responsive ROS/RSS fluorescent probes and their application in bioimaging. Chem. Asian J. 2022, 17, e202200907. [Google Scholar] [CrossRef]
- Niu, P.; Zhu, J.; Wei, L.; Liu, X. Application of fluorescent probes in reactive oxygen species disease model. Crit. Rev. Anal. Chem. 2022, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cao, X.; Lu, W.; Wei, Y.; Kong, L.; Chen, W.; Shao, X.; Wang, Y. Recent advances in fluorescent probes of peroxynitrite: Structural, strategies and biological applications. Theranostics 2023, 13, 1716–1744. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Z.; Zhou, J.; Zhu, M.; Liu, J.; James, T.D. Recent progress in the development of fluorescent probes for imaging pathological oxidative stress. Chem. Soc. Rev. 2023, 52, 3873–3926. [Google Scholar] [CrossRef]
- Yin, J.; Zhan, J.; Hu, Q.; Huang, S.; Lin, W. Fluorescent probes for ferroptosis bioimaging: Advances, challenges, and prospects. Chem. Soc. Rev. 2023, 52, 2011–2030. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Halliwell, B. Mini-review: Oxidative stress, redox stress or redox success? Biochem. Biophys. Res. Commun. 2018, 502, 183–186. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Radi, R.; Roberts, L.J.; Vina, J.; et al. Even free radicals should follow some rules: A guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef]
- Buettner, G.R. Moving free radical and redox biology ahead in the next decade(s). Free Radic. Biol. Med. 2015, 78, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Quintiliani, M.; Badiello, R.; Tamba, M.; Esfandi, A.; Gorin, G. Radiolysis of glutathione in oxygen-containing solutions of pH 7. Int. J. Radiat. Biol. 1977, 32, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Storkey, C.; Davies, M.J.; Pattison, D.I. Reevaluation of the rate constants for the reaction of hypochlorous acid (HOCl) with cysteine, methionine, and peptide derivatives using a new competition kinetic approach. Free Radic. Biol. Med. 2014, 73, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.; Hughes, M.N.; Wardman, P. Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH. Free Radic. Biol. Med. 2002, 32, 1314–1323. [Google Scholar] [CrossRef]
- Chen, S.N.; Hoffman, M.Z. Rate constants for the reaction of the carbonate radical with compounds of biochemical interest in neutral aqueous solution. Radiat. Res. 1973, 56, 40–47. [Google Scholar] [CrossRef]
- Jones, C.M.; Lawrence, A.; Wardman, P.; Burkitt, M.J. Electron paramagnetic resonance spin-trapping investigation into the kinetics of glutathione oxidation by the superoxide radical: Re-evaluation of the rate constant. Free Radic. Biol. Med. 2002, 32, 982–990. [Google Scholar] [CrossRef]
- Abedinzadeh, Z.; Gardes-Albert, M.; Ferradini, C. Kinetic study of the oxidation mechanism of glutathione by hydrogen peroxide in neutral aqueous medium. Can. J. Chem. 1989, 67, 1247–1255. [Google Scholar] [CrossRef]
- Madej, E.; Wardman, P. The oxidizing power of the glutathione thiyl radical as measured by its electrode potential at physiological pH. Arch. Biochem. Biophys. 2007, 462, 94–102. [Google Scholar] [CrossRef]
- DeFelippis, M.R.; Murthy, C.P.; Faraggi, M.; Klapper, M.H. Pulse radiolytic measurement of redox potentials: The tyrosine and tryptophan radicals. Biochemistry 1989, 28, 4847–4853. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.E.; McNaughton, G.S.; Michael, B.D. Pulse radiolysis of sulphur compounds. Part 2.—Free radical “repair” by hydrogen transfer from sulphydryl compounds. Trans. Faraday Soc. 1968, 64, 902–910. [Google Scholar] [CrossRef]
- Willson, R.L. Pulse radiolysis studies of electron transfer in aqueous disulphide solutions. Chem. Commun. 1970, 1425–1426. [Google Scholar] [CrossRef]
- Forni, L.G.; Mönig, J.; Mora-Arellano, V.O.; Willson, R.L. Thiyl free radicals: Direct observations of electron transfer reactions with phenothiazines and ascorbate. J. Chem. Soc. Perkin Trans. 1983, 2, 961–965. [Google Scholar] [CrossRef]
- Wardman, P.; von Sonntag, C. Kinetic factors that control the fate of thiyl radicals in cells. Methods Enzymol. 1995, 251, 31–45. [Google Scholar] [CrossRef]
- Schöneich, C. Thiyl radicals, perthiyl radicals, and oxidative reactions. In Biothiols in Health and Disease; Packer, L., Cadenas, E., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 21–47. [Google Scholar]
- Schöneich, C. Thiyl radicals: Formation, properties, and detection. In Redox Chemistry and Biology of Thiols; Alvarez, B., Comini, M.A., Salinas, G., Trujillo, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 115–132. [Google Scholar]
- Chatgilialoglu, C.; Ferreri, C.; Ballestri, M.; Mulazzani, Q.G.; Landi, L. Cis-trans isomerization of monounsaturated fatty acid residues in phospholipids by thiyl radicals. J. Am. Chem. Soc. 2000, 122, 4593–4601. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C. Trans lipids: The free radical path. Acc. Chem. Res. 2005, 38, 441–448. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Lykakis, I.N.; Wardman, P. Trans-fatty acids and radical stress: What are the real culprits? Bioorg. Med. Chem. 2006, 14, 6144–6148. [Google Scholar] [CrossRef]
- Mihaljević, B.; Tartaro, I.; Ferreri, C.; Chatgilialoglu, C. Linoleic acid peroxidation vs. isomerization: A biomimetic model of free radical reactivity in the presence of thiols. Org. Biomol. Chem. 2011, 9, 3541–3548. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.D.; Becker, D.; Yan, M. The formation and structure of the sulfoxyl radicals RSO•, RSOO•, RSO2•, and RSO2OO• from the reaction of cysteine, glutathione and penicillamine thiyl radicals with molecular oxygen. Int. J. Radiat. Biol. 1990, 57, 65–81. [Google Scholar] [CrossRef]
- Schöneich, C.; Dillinger, U.; Bruchhausen, V.; Asmus, K.-D. Oxidation of polyunsaturated fatty acids and lipids through thiyl and sulfonyl radicals: Reaction kinetics, and influence of oxygen and structure of thiyl radicals. Arch. Biochem. Biophys. 1992, 292, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Augusto, O.; Goldstein, S.; Hurst, J.K.; Lind, J.; Lymar, S.V.; Merenyi, G.; Radi, R. Carbon dioxide-catalyzed peroxynitrite reactivity—The resilience of the radical mechanism after two decades of research. Free Radic. Biol. Med. 2019, 135, 210–215. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merényi, G.; Neta, P.; Ruscica, B.; Stanburyb, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals (IUPAC technical report). Pure App. Chem. 2015, 87, 1139–1150. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2¢,7¢-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.; Chignell, C.F.; Mason, R.P. Evidence for free radical formation during the oxidation of 2¢-7¢-dichlorofluorescin to the fluorescent dye 2¢-7¢-dichlorofluorescein by horseradish peroxidase: Possible implications for oxidative stress measurements. Free Rad. Biol. Med. 1999, 27, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Rota, C.; Fann, Y.C.; Mason, R.P. Phenoxyl free radical formation during the oxidation of the fluorescent dye 2¢,7¢-dichlorofluorescein by horseradish peroxidase. J. Biol. Chem. 1999, 274, 28161–28168. [Google Scholar] [CrossRef]
- O’Malley, Y.Q.; Reszka, K.J.; Britigan, B.E. Direct oxidation of 2′,7′-dichlorodihydrofluorescein by pyocyanin and other redox-active compounds independent of reactive oxygen species production. Free Radic. Biol. Med. 2004, 36, 90–100. [Google Scholar] [CrossRef]
- Bonini, M.G.; Rota, C.; Tomasi, A.; Mason, R.P. The oxidation of 2′,7′-dichlorofluorescin to reactive oxygen species: A self-fulfilling prophesy? Free Radic. Biol. Med. 2006, 40, 968–975. [Google Scholar] [CrossRef]
- Wardman, P. Use of the dichlorofluorescein assay to measure “reactive oxygen species”. Radiat. Res. 2008, 170, 406–408. [Google Scholar] [CrossRef]
- Wrona, M.; Patel, K.B.; Wardman, P. The roles of thiol-derived radicals in the use of 2¢,7¢-dichlorodihydrofluorescein as a probe for oxidative stress. Free Radic. Biol. Med. 2008, 44, 56–62, Erratum in Free Radic. Biol. Med. 2008, 45, 547. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Holmgren, A.; Larsson, N.G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M. Concerns in the application of fluorescent probes DCDHF-DA, DHR 123 and DHE to measure reactive oxygen species in vitro. Toxicol. In Vitro 2015, 30, 578–582. [Google Scholar] [CrossRef] [PubMed]
- De Haan, L.R.; Reiniers, M.J.; Reeskamp, L.F.; Belkouz, A.; Ao, L.; Cheng, S.; Ding, B.; van Golen, R.F.; Heger, M. Experimental conditions that influence the utility of 2¢,7¢-dichlorodihydrofluorescein diacetate (DCFH2-DA) as a fluorogenic biosensor for mitochondrial redox status. Antioxidants 2022, 11, 1424. [Google Scholar] [CrossRef]
- Kalyanaraman, B. NAC, NAC, Knockin’ on heaven’s door: Interpreting the mechanism of action of N-acetylcysteine in tumor and immune cells. Redox Biol. 2022, 57, 102497. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. In Advanced Protocols in Oxidative Stress II; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 57–72. [Google Scholar]
- Kim, H.; Xue, X. Detection of total reactive oxygen species in adherent cells by 2¢,7¢-dichlorodihydrofluorescein diacetate staining. J. Vis. Exp. 2020, e60682. [Google Scholar] [CrossRef]
- Hans, C.; Saini, R.; Sachdeva, M.U.S.; Sharma, P. 2¢,7¢-dichlorofluorescein (DCF) or 2¢,7¢- dichlorodihydrofluorescein diacetate (DCFH2DA) to measure reactive oxygen species in erythrocytes. Biomed. Pharm. 2021, 138, 111512. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, X.; Zeng, W.; Qin, X.; Miao, B.; Gao, S.; Liu, J.; Li, Z. Berberine inhibits the progression of renal cell carcinoma cells by regulating reactive oxygen species generation and inducing DNA damage. Mol. Biol. Rep. 2023, 50, 5697–5707. [Google Scholar] [CrossRef]
- Binjawhar, D.N.; Alhazmi, A.T.; Bin Jawhar, W.N.; MohammedSaeed, W.; Safi, S.Z. Hyperglycemia-induced oxidative stress and epigenetic regulation of ET-1 gene in endothelial cells. Front. Genet. 2023, 14, 1167773. [Google Scholar] [CrossRef]
- Ezequiel, J.; Nitschke, M.R.; Laviale, M.; Serôdio, J.; Frommlet, J.C. Concurrent bioimaging of microalgal photophysiology and oxidative stress. Photosynth. Res. 2023, 155, 177–190. [Google Scholar] [CrossRef]
- Huang, J.; Yu, P.; Liao, M.; Dong, X.; Xu, J.; Ming, J.; Bin, D.; Wang, Y.; Zhang, F.; Xia, Y. A self-charging salt water battery for antitumor therapy. Sci. Adv. 2023, 9, eadf3992. [Google Scholar] [CrossRef]
- Cathcart, R.; Schwiers, E.; Ames, B.N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal. Biochem. 1983, 134, 111–116. [Google Scholar] [CrossRef]
- Burkitt, M.J.; Wardman, P. Cytochrome c is a potent catalyst of dichlorofluorescin oxidation: Implications for the role of reactive oxygen species in apoptosis. Biochem. Biophys. Res. Commun. 2001, 282, 329–333. [Google Scholar] [CrossRef]
- Lawrence, A.; Jones, C.M.; Wardman, P.; Burkitt, M.J. Evidence for the role of a peroxidase compound-I type intermediate in the oxidation of glutathione, NADH, ascorbate, and dichlorofluorescin by cytochrome c/H2O2. Implications for oxidative stress during apoptosis. J. Biol. Chem. 2003, 278, 29410–29419. [Google Scholar] [CrossRef]
- Crow, J.P. Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: Implications for intracellular measurements of reactive nitrogen and oxygen species. Nitric Oxide 1997, 1, 145–157. [Google Scholar] [CrossRef]
- Glebska, J.; Koppenol, W.H. Peroxynitrite-mediated oxidation of dichlorodihydrofluorescein and dihydrorhodamine. Free Radic. Biol. Med. 2003, 35, 676–682. [Google Scholar] [CrossRef]
- Wrona, M.; Wardman, P. Properties of the radical intermediate obtained on oxidation of 2¢,7¢-dichlorodihydrofluorescein, a probe for oxidative stress. Free Radic.Biol. Med. 2006, 41, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Wardman, P. Nitrogen dioxide in biology: Correlating chemical kinetics with biological effects. In The Chemistry of N-Centered Radicals; Alfassi, Z.B., Ed.; Wiley: New York, NY, USA, 1998; pp. 155–179. [Google Scholar]
- Wrona, M.; Patel, K.B.; Wardman, P. Reactivity of 2¢,7¢-dichlorodihydrofluorescein and dihydrorhodamine 123 and their oxidized forms towards carbonate, nitrogen dioxide, and hydroxyl radicals. Free Radic. Biol. Med. 2005, 38, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Afri, M.; Frimer, A.A.; Cohen, Y. Active oxygen chemistry within the liposomal bilayer. Part IV: Locating 2′,7′-dichlorofluorescein (DCF), 2′,7′-dichlorodihydrofluorescein (DCFH) and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) in the lipid bilayer. Chem. Phys. Lipids 2004, 131, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J.L. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods. 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Royall, J.A.; Ischiropoulos, H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 1993, 302, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Zielonka, M.; Sikora, A.; Adamus, J.; Joseph, J.; Hardy, M.; Ouari, O.; Dranka, B.P.; Kalyanaraman, B. Global profiling of reactive oxygen and nitrogen species in biological systems: High-throughput real-time analyses. J. Biol. Chem. 2012, 287, 2984–2995. [Google Scholar] [CrossRef] [PubMed]
- Huie, R.E.; Shoute, L.C.T.; Neta, P. Temperature dependence of the rate constants for reactions of the carbonate radical with organic and inorganic reductants. Int. J. Chem. Kinet. 1991, 23, 541–552. [Google Scholar] [CrossRef]
- Marchesi, E.; Rota, C.; Fann, Y.C.; Chignell, C.F.; Mason, R.P. Photoreduction of the fluorescent dye 2¢-7¢-dichlorofluorescein: A spin trapping and direct electron spin resonance study with implications for oxidative stress measurements. Free Radic. Biol. Med. 1999, 26, 148–161. [Google Scholar] [CrossRef]
- Summers, F.A.; Zhao, B.; Ganini, D.; Mason, R.P. Photooxidation of Amplex Red to resorufin: Implications of exposing the Amplex Red assay to light. Methods Enzymol. 2013, 526, 1–17. [Google Scholar] [CrossRef]
- Faulkner, K.; Fridovich, I. Luminol and lucigenin as detectors for O2•−. Free Radic. Biol. Med. 1993, 15, 447–451. [Google Scholar] [CrossRef]
- Liochev, S.I.; Fridovich, I. Lucigenin (bis-N-methylacridinium) as a mediator of superoxide anion production. Archi.Biochem. Biophys. 1996, 337, 115–120. [Google Scholar] [CrossRef]
- Vásquez-Vivar, J.; Hogg, N.; Pritchard, K.A., Jr.; Martasek, P.; Kalyanaraman, B. Superoxide anion formation from lucigenin: An electron spin resonance spin-trapping study. FEBS Lett. 1997, 403, 127–130. [Google Scholar] [CrossRef]
- Wardman, P.; Burkitt, M.J.; Patel, K.B.; Lawrence, A.; Jones, C.M.; Everett, S.A.; Vojnovic, B. Pitfalls in the use of common luminescent probes for oxidative and nitrosative stress. J. Fluoresc. 2002, 12, 65–68. [Google Scholar] [CrossRef]
- Wu, Q.; Gurpinar, A.; Roberts, M.; Camelliti, P.; Ruggieri, M.R., Sr.; Wu, C. Identification of the NADPH oxidase (Nox) subtype and the source of superoxide production in the micturition centre. Biology 2022, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Zhao, H.; Xu, Y.; Kalyanaraman, B. Mechanistic similarities between oxidation of hydroethidine by Fremy’s salt and superoxide: Stopped-flow optical and EPR studies. Free Radic. Biol. Med. 2005, 39, 853–863. [Google Scholar] [CrossRef]
- Zielonka, J.; Sarna, T.; Roberts, J.E.; Wishart, J.F.; Kalyanaraman, B. Pulse radiolysis and steady-state analyses of the reaction between hydroethidine and superoxide and other oxidants. Arch. Biochem. Biophys. 2006, 456, 39–47. [Google Scholar] [CrossRef]
- Dębski, D.; Smulik, R.; Zielonka, J.; Michałowski, B.; Jakubowska, M.; Dębowska, K.; Adamus, J.; Marcinek, A.; Kalyanaraman, B.; Sikora, A. Mechanism of oxidative conversion of Amplex® Red to resorufin: Pulse radiolysis and enzymatic studies. Free Radic. Biol. Med. 2016, 95, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Shchepinova, M.M.; Cairns, A.G.; Prime, T.A.; Logan, A.; James, A.M.; Hall, A.R.; Vidoni, S.; Arndt, S.; Caldwell, S.T.; Prag, H.A.; et al. MitoNeoD: A mitochondria-targeted superoxide probe. Cell Chem. Biol. 2017, 24, 1285–1298.e1212. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Madden, K.P.; Mezyk, S.P. Critical review of aqueous solution reaction rate constants for hydrogen atoms. J. Phys. Chem. Ref. Data 2011, 40, 023103. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L. Reactivity of HO2•/O2•− radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of peroxyl radicals in fluid solutions. J. Phys. Chem. Ref. Data 1990, 19, 413–513. [Google Scholar] [CrossRef]
- Neta, P.; Grodkowski, J. Rate constants for reactions of phenoxyl radicals in solution. J. Phys. Chem. Ref. Data 2005, 34, 109–199. [Google Scholar] [CrossRef]
- Neta, P.; Grodkowski, J.; Ross, A.B. Rate constants for reactions of aliphatic carbon-centered radicals in aqueous solution. J. Phys. Chem. Ref. Data 1996, 25, 709–1050. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 2nd ed.; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).