Temperature- and Diet-Induced Plasticity of Growth and Digestive Enzymes Activity in Spongy Moth Larvae
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Experimental Groups
2.3. Determination of Fitness-Related Traits
2.4. Enzyme Assays
2.5. Statistical Analyses
3. Results
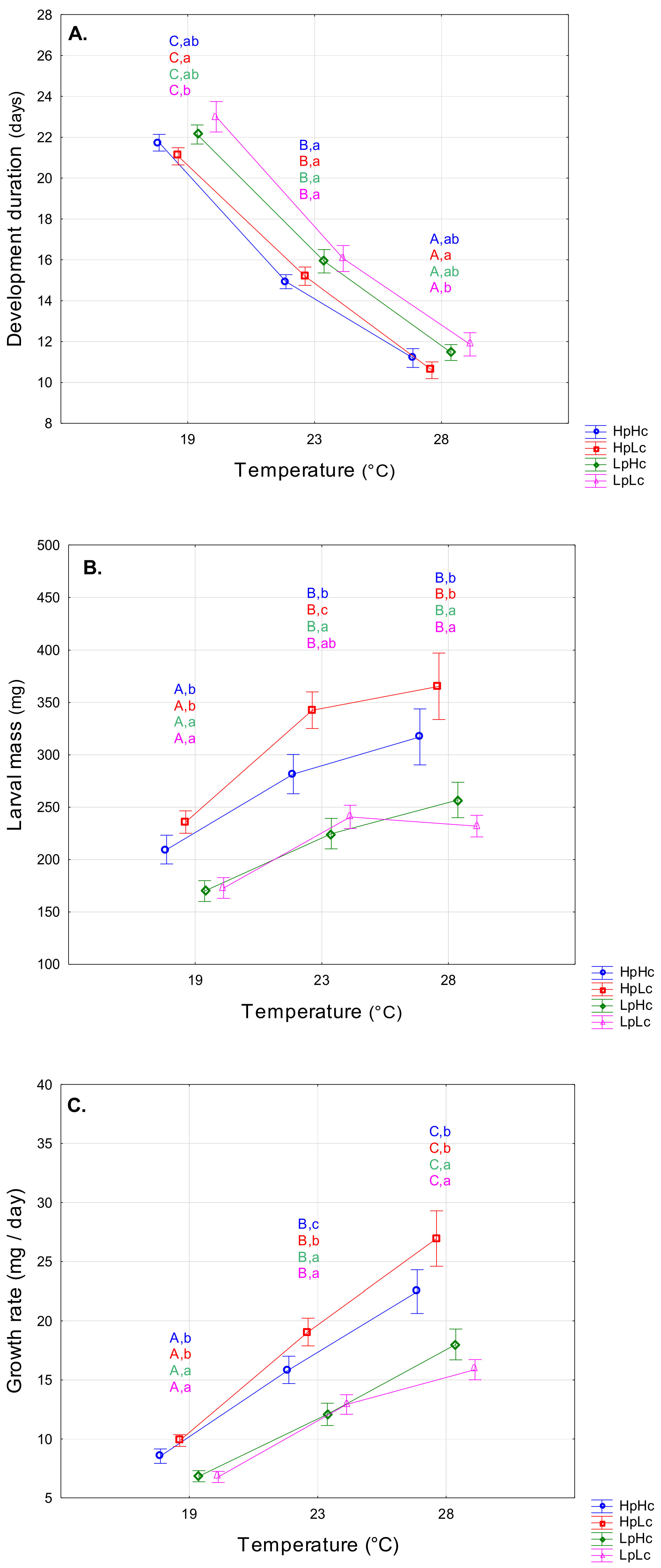
3.1. Influence of Rearing Temperature and Diet Quality on Larval Fitness-Related Traits
3.2. Influence of Rearing Temperature and Diet Quality on Activities of Digestive Enzymes
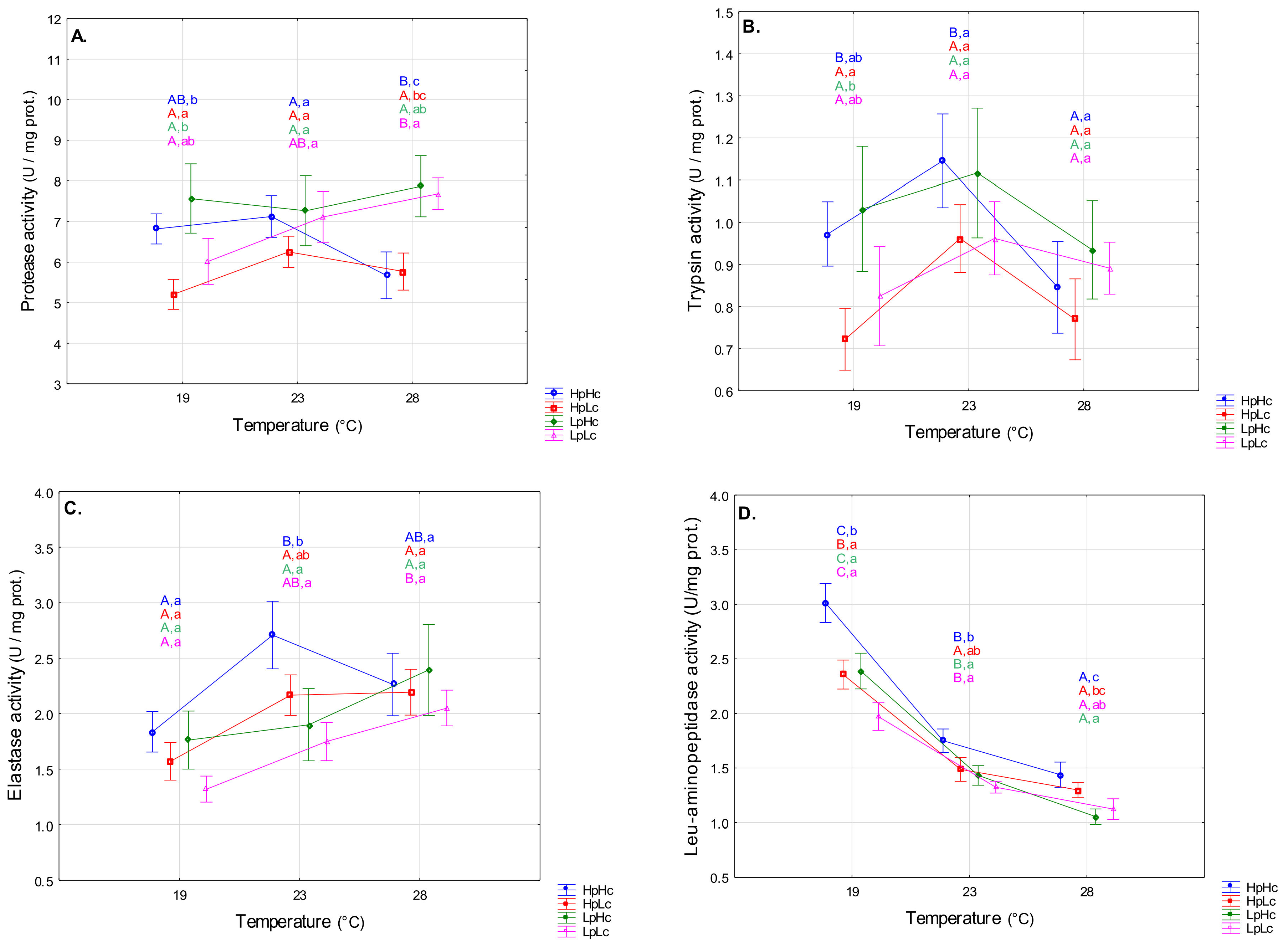
3.2.1. Proteolytic Enzymes
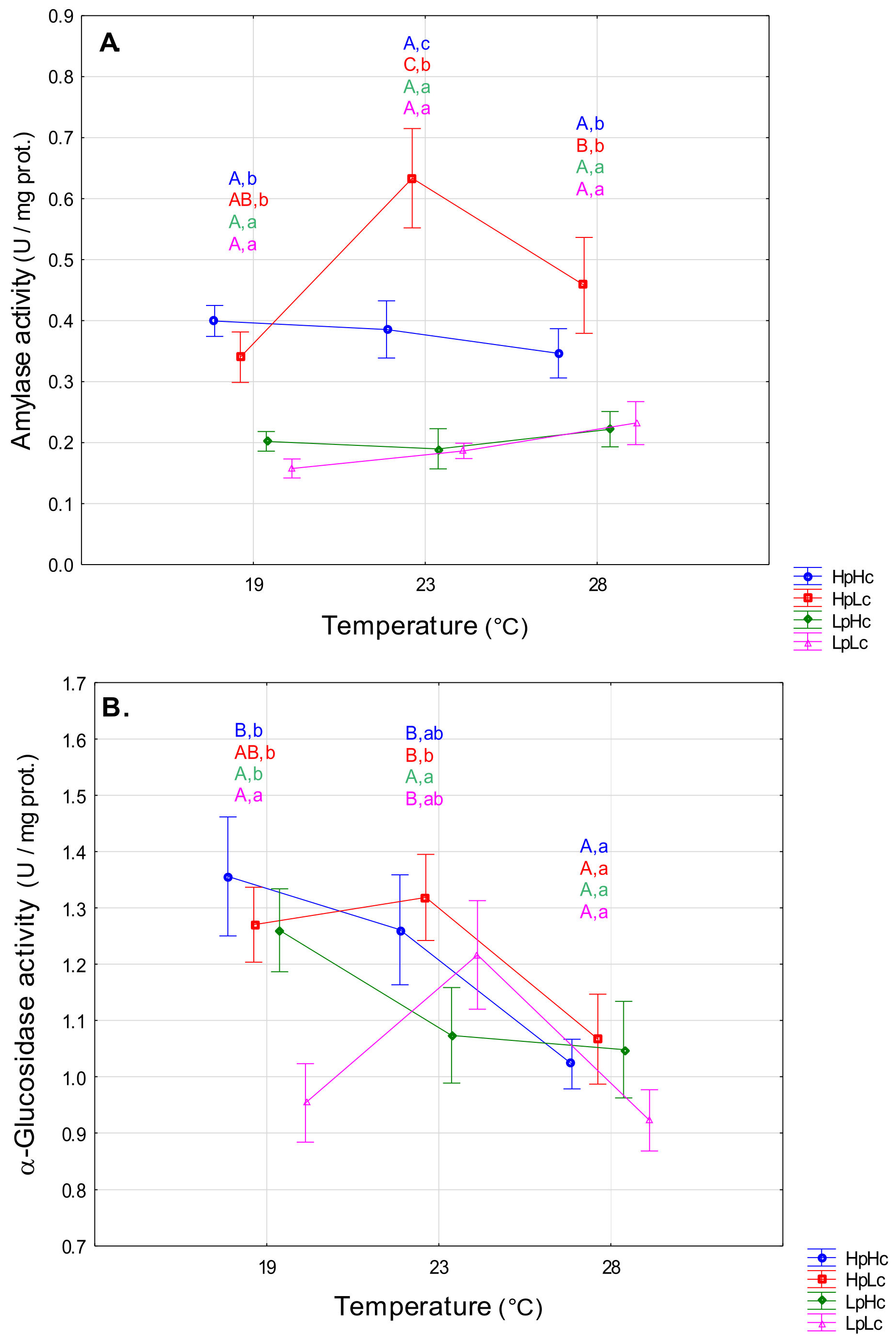
3.2.2. Carbohydrases
3.2.3. Lipase
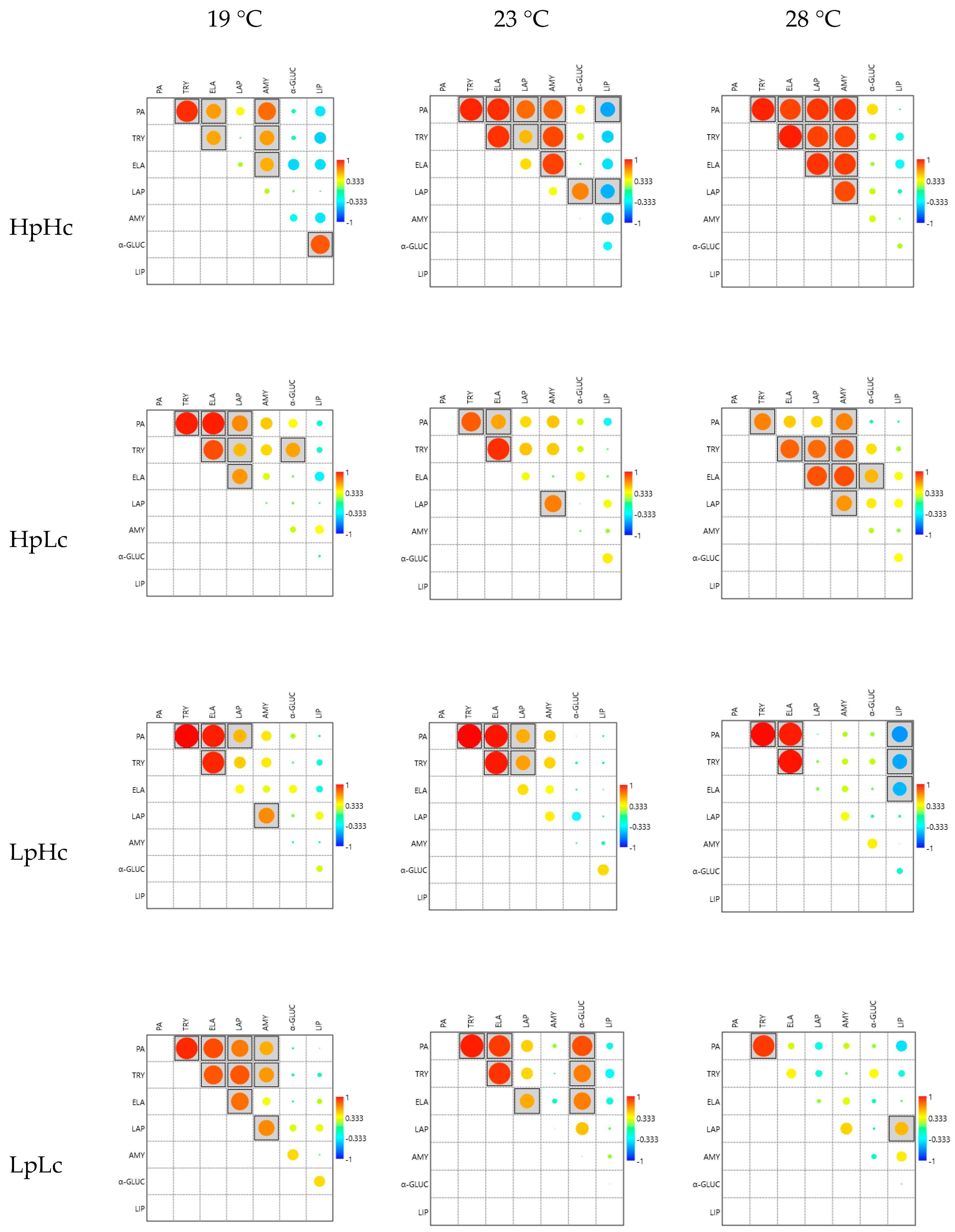
3.2.4. Correlations among Enzyme Activities
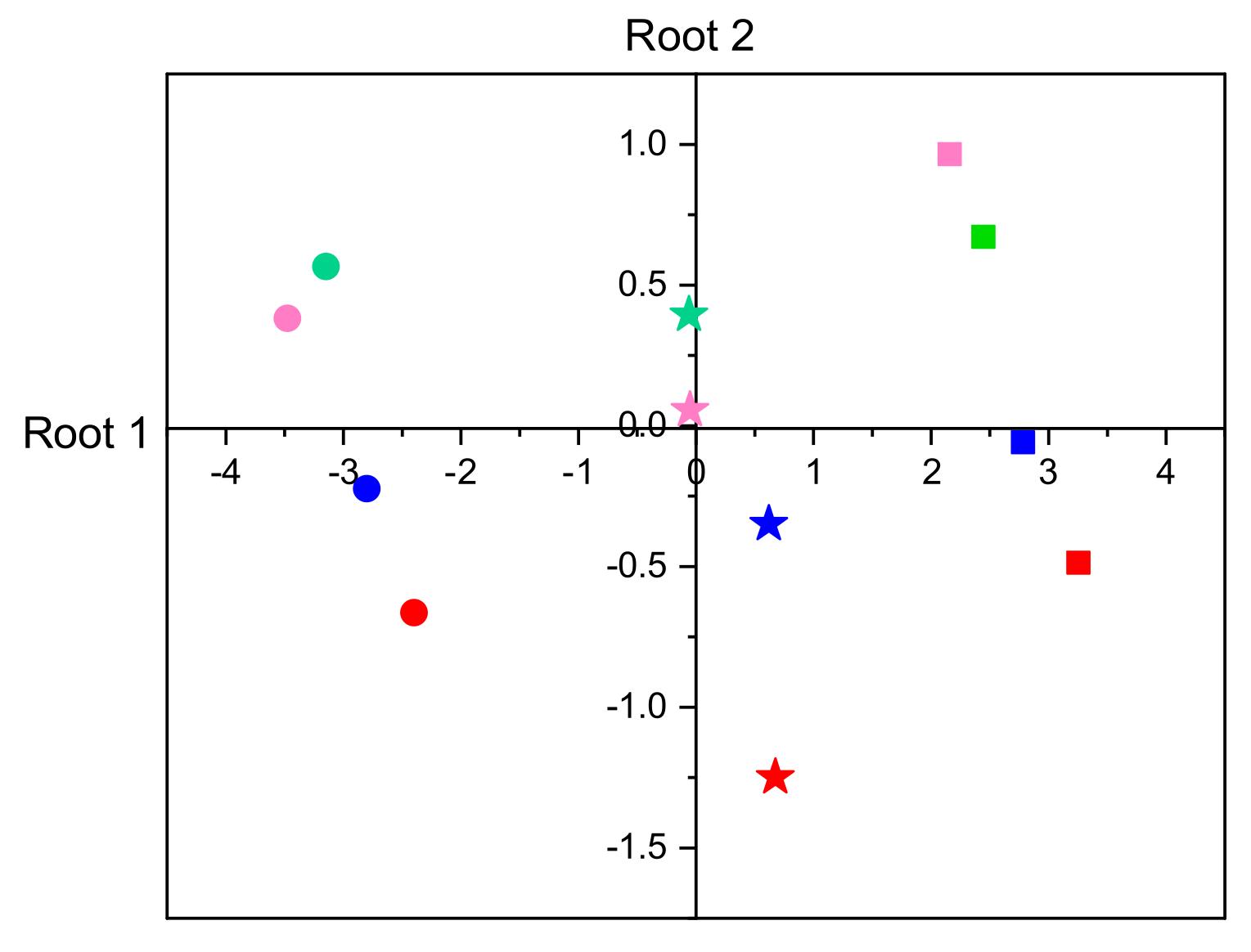
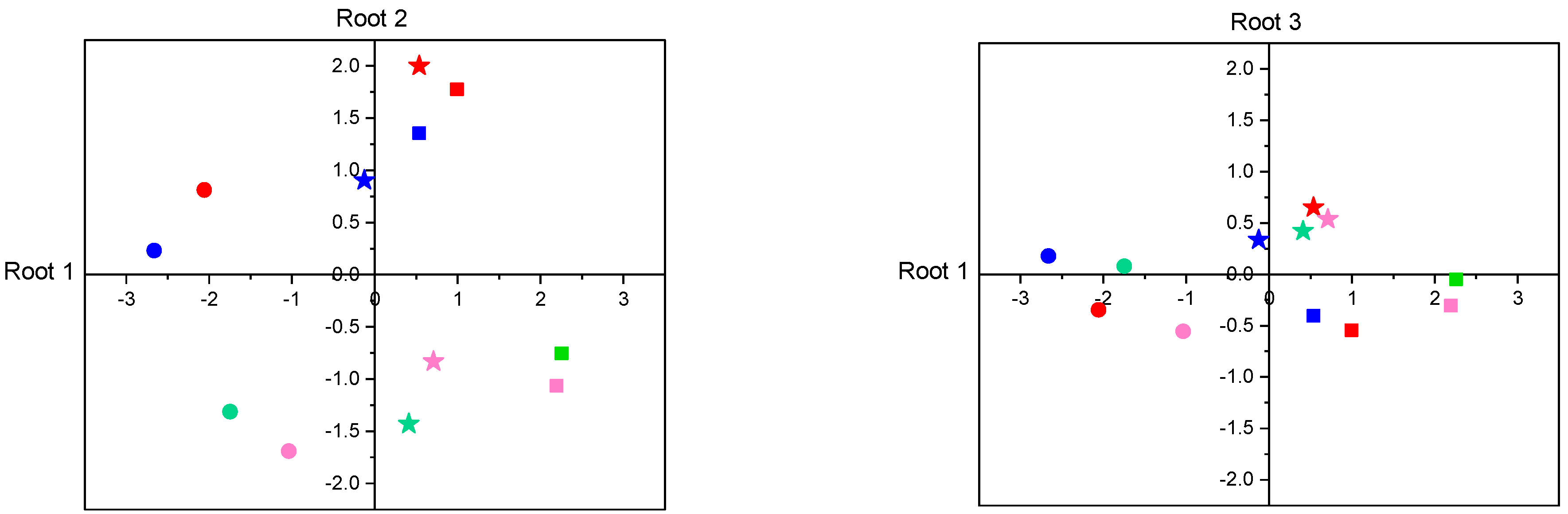
3.2.5. Discriminant Analysis of Enzyme Activities
3.3. Relationship between Fitness-Related Traits and Digestive Enzyme Activities
4. Discussion
4.1. Temperature- and Diet-Induced Plasticity of Fitness-Related Traits
4.2. Temperature- and Diet-Induced Plasticity of Digestive Enzyme Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clissold, F.J.; Simpson, S.J. Temperature, food quality and life history traits of herbivorous insects. Curr. Opin. Insect Sci. 2015, 11, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Abram, P.K.; Boivin, G.; Moiroux, J.; Brodeur, J. Behavioural effects of temperature on ectothermic animals: Unifying thermal physiology and behavioural plasticity. Biol. Rev. 2017, 92, 1859–1876. [Google Scholar] [CrossRef] [PubMed]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Jang, T.; Rho, M.S. Recent Trends in Integrative Insect Nutrition: A Nutritional Geometry Perspective. Korean J. Appl. Entomol. 2022, 61, 129–142. [Google Scholar]
- Chown, S.L.; Nicolson, S. Insect Physiological Ecology: Mechanisms and Patterns, online ed.; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Milanović, S.; Janković-Tomanić, M.; Kostić, I.; Kostić, M.; Morina, F.; Živanović, B.; Lazarević, J. Behavioural and physiological plasticity of gypsy moth larvae to host plant switching. Entomol. Exp. Appl. 2016, 158, 152–162. [Google Scholar] [CrossRef]
- Strilbytska, O.; Velianyk, V.; Burdyliuk, N.; Yurkevych, I.S.; Vaiserman, A.; Storey, K.B.; Pospisilik, A.; Lushchak, O. Parental dietary protein-to-carbohydrate ratio affects offspring lifespan and metabolism in drosophila. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 241, 110622. [Google Scholar] [CrossRef]
- Ilijin, L.; Grčić, A.; Mrdaković, M.; Vlahović, M.; Filipović, A.; Matić, D.; Mataruga, V.P. Tissue-specific responses of Lymantria dispar L. (Lepidoptera: Erebidae) larvae from unpolluted and polluted forests to thermal stress. J. Therm. Biol. 2021, 96, 102836. [Google Scholar] [CrossRef]
- Stockhoff, B.A. Ontogenetic change in dietary selection for protein and lipid by gypsy moth larvae. J. Insect Physiol. 1993, 39, 677–686. [Google Scholar] [CrossRef]
- Andersen, L.H.; Kristensen, T.N.; Loeschcke, V.; Toft, S.; Mayntz, D. Protein and carbohydrate composition of larval food affects tolerance to thermal stress and desiccation in adult Drosophila melanogaster. J. Insect Physiol. 2010, 56, 336–340. [Google Scholar] [CrossRef]
- Lee, K.P.; Kim, J.S.; Min, K.J. Sexual dimorphism in nutrient intake and life span is mediated by mating in Drosophila melanogaster. Anim. Behav. 2013, 86, 987–992. [Google Scholar] [CrossRef]
- Pascacio-Villafán, C.; Williams, T.; Birke, A.; Aluja, M. Nutritional and non-nutritional food components modulate phenotypic variation but not physiological trade-offs in an insect. Sci. Rep. 2016, 6, 29413. [Google Scholar] [CrossRef]
- Rendon, D.; Walton, V.; Tait, G.; Buser, J.; Lemos Souza, I.; Wallingford, A.; Loeb, G.; Lee, J. Interactions among morphotype, nutrition, and temperature impact fitness of an invasive fly. Ecol. Evol. 2019, 9, 2615–2628. [Google Scholar] [CrossRef]
- Little, C.M.; Chapman, T.W.; Hillier, N.K. Plasticity is key to success of Drosophila suzukii (Diptera: Drosophilidae) invasion. J. Insect Sci. 2020, 20, 5. [Google Scholar] [CrossRef]
- Strilbytska, O.; Strutynska, T.; Semaniuk, U.; Burdyliyk, N.; Bubalo, V.; Lushchak, O. Dietary sucrose determines stress resistance, oxidative damages, and antioxidant defense system in Drosophila. Scientifica 2022, 2022, 7262342. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. The geometric analysis of nutrient–allelochemical interactions: A case study using locusts. Ecology 2001, 82, 422–439. [Google Scholar]
- Saeed, S.; Sayyed, A.H.; Ahmad, I. Effect of host plants on life-history traits of Spodoptera exigua (Lepidoptera: Noctuidae). J. Pest. Sci. 2010, 83, 165–172. [Google Scholar] [CrossRef]
- Lee, K.P. Dietary protein: Carbohydrate balance is a critical modulator of lifespan and reproduction in Drosophila melanogaster: A test using a chemically defined diet. J. Insect Physiol. 2015, 75, 12–19. [Google Scholar] [CrossRef]
- Milanović, S.; Lazarević, J.; Popović, Z.; Miletić, Z.; Kostić, M.; Radulović, Z.; Karadžić, D.; Vuleta, A. Preference and performance of the larvae of Lymantria dispar (Lepidoptera: Lymantriidae) on three species of European oaks. Eur. J. Entomol. 2014, 111, 371–378. [Google Scholar] [CrossRef]
- Perkovich, C.; Ward, D. Protein: Carbohydrate ratios in the diet of gypsy moth Lymantria dispar affect its ability to tolerate tannins. J. Chem. Ecol. 2020, 46, 299–307. [Google Scholar] [CrossRef]
- Carey, M.R.; Archer, C.R.; Rapkin, J.; Castledine, M.; Jensen, K.; House, C.M.; Hosken, D.J.; Hunt, J. Mapping sex differences in the effects of protein and carbohydrates on lifespan and reproduction in Drosophila melanogaster: Is measuring nutrient intake essential? Biogerontology 2022, 23, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.; Uy, L.; Wong, A.C.N. Nutritional phenotype underlines the performance trade-offs of Drosophila suzukii on different fruit diets. Curr. Res. Insect Sci. 2022, 2, 100026. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Sibly, R.M.; Lee, K.P.; Behmer, S.T.; Raubenheimer, D. Optimal foraging when regulating intake of multiple nutrients. Anim. Behav. 2004, 68, 1299–1311. [Google Scholar] [CrossRef]
- Deans, C.A.; Sword, G.A.; Behmer, S.T. Revisiting macronutrient regulation in the polyphagous herbivore Helicoverpa zea (Lepidoptera: Noctuidae): New insights via nutritional geometry. J. Insect Physiol. 2015, 81, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jang, T.; Min, K.J.; Lee, K.P. Effects of dietary protein: Carbohydrate balance on life-history traits in six laboratory strains of Drosophila melanogaster. Entomol. Exp. Appl. 2020, 168, 482–491. [Google Scholar] [CrossRef]
- Rho, M.S.; Lee, K.P. Behavioural and physiological regulation of protein and carbohydrates in mealworm larvae: A geometric analysis. J. Insect Physiol. 2022, 136, 104329. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.L.; Joern, A. Dietary selection and nutritional regulation in a common mixed-feeding insect herbivore. Entomol. Exp. Appl. 2013, 148, 20–26. [Google Scholar] [CrossRef]
- Le Gall, M.; Behmer, S.T. Effects of protein and carbohydrate on an insect herbivore: The vista from a fitness landscape. Integr. Comp. Biol. 2014, 54, 942–954. [Google Scholar] [CrossRef]
- Strilbytska, O.; Semaniuk, U.; Bubalo, V.; Storey, K.B.; Lushchak, O. Dietary choice reshapes metabolism in Drosophila by affecting consumption of macronutrients. Biomolecules 2022, 12, 1201. [Google Scholar] [CrossRef]
- Keaton Wilson, J.; Ruiz, L.; Davidowitz, G. Dietary protein and carbohydrates affect immune function and performance in a specialist herbivore insect (Manduca sexta). Physiol. Biochem. Zool. 2019, 92, 58–70. [Google Scholar] [CrossRef]
- Deans, C.A.; Sword, G.A.; Vogel, H.; Behmer, S.T. Quantity versus quality: Effects of diet protein-carbohydrate ratios and amounts on insect herbivore gene expression. Insect Biochem. Mol. Biol. 2022, 145, 103773. [Google Scholar] [CrossRef]
- Sun, S.L.; Abudisilimu, N.; Yi, H.; Li, S.; Liu, T.X.; Jing, X. Understanding nutritive need in Harmonia axyridis larvae: Insights from nutritional geometry. Insect Sci. 2022, 29, 1433–1444. [Google Scholar] [CrossRef]
- Behmer, S.T. Insect herbivore nutrient regulation. Ann. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef]
- Waldbauer, G.P.; Friedman, S. Self-selection of optimal diets by insects. Ann. Rev. Entomol. 1991, 3, 43–63. [Google Scholar] [CrossRef]
- Hägele, B.F.; Rowell-Rahier, M. Dietary mixing in three generalist herbivores: Nutrient complementation or toxin dilution? Oecologia 1999, 119, 521–533. [Google Scholar] [CrossRef]
- Singer, M.S. Determinants of polyphagy by a woolly bear caterpillar: A test of the physiological efficiency hypothesis. Oikos 2001, 93, 194–204. [Google Scholar] [CrossRef]
- Karban, R.; Karban, C.; Huntzinger, M.; Pearse, I.A.N.; Crutsinger, G. Diet mixing enhances the performance of a generalist caterpillar, Platyprepia virginalis. Ecol. Entomol. 2010, 35, 92–99. [Google Scholar] [CrossRef]
- Bede, J.C.; McNeil, J.N.; Tobe, S.S. The role of neuropeptides in caterpillar nutritional ecology. Peptides 2007, 28, 185–196. [Google Scholar]
- Stockhoff, B.A. Diet-switching by gypsy moth: Effects of diet nitrogen history vs. switching on growth, consumption, and food utilization. Entomol. Exp. Appl. 1992, 64, 225–238. [Google Scholar]
- Thompson, S.N.; Borchardt, D.B.; Wang, L.W. Dietary nutrient levels regulate protein and carbohydrate intake, gluconeogenic/glycolytic flux and blood trehalose level in the insect Manduca sexta L. J. Comp. Physiol. B 2003, 173, 149–163. [Google Scholar] [CrossRef]
- Roeder, K.A.; Behmer, S.T. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 2014, 28, 1135–1143. [Google Scholar]
- Clissold, F.J.; Tedder, B.J.; Conigrave, A.D.; Simpson, S.J. The gastrointestinal tract as a nutrient-balancing organ. Proc. Royal Soc. B Biol. Sci. 2010, 277, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Van Broekhoven, S.; Oonincx, D.G.; Van Huis, A.; Van Loon, J.J. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Vassão, D.G.; Raguschke, B.; Furlong, M.J.; Zalucki, M.P. Balancing nutrients in a toxic environment: The challenge of eating. Insect Sci. 2022, 29, 289–303. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Woods, H.A. Thermal sensitivity of growth and feeding in Manduca sexta caterpillars. Physiol. Zool. 1997, 70, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Tomčala, A.; Sørensen, J.G.; Holmstrup, M.; Krogh, P.H.; Šimek, P.; Koštál, V. Effects of acclimation temperature on thermal tolerance and membrane phospholipid composition in the fruit fly Drosophila melanogaster. J. Insect Physiol. 2008, 54, 619–629. [Google Scholar] [CrossRef]
- Catalán, T.P.; Wozniak, A.; Niemeyer, H.M.; Kalergis, A.M.; Bozinovic, F. Interplay between thermal and immune ecology: Effect of environmental temperature on insect immune response and energetic costs after an immune challenge. J. Insect Physiol. 2012, 58, 310–317. [Google Scholar] [CrossRef]
- Wojda, I. Temperature stress and insect immunity. J. Therm. Biol. 2017, 68, 96–103. [Google Scholar] [CrossRef]
- Kobori, Y.; Hanboonsong, Y. Effect of temperature on the development and reproduction of the sugarcane white leaf insect vector, Matsumuratettix hiroglyphicus (Matsumura) (Hemiptera: Cicadellidae). J. Asia-Pac. Entomol. 2017, 20, 281–284. [Google Scholar] [CrossRef]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.H. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef]
- Du Plessis, H.; Schlemmer, M.L.; Van den Berg, J. The effect of temperature on the development of Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 228. [Google Scholar] [CrossRef]
- Eberle, S.; Schaden, L.M.; Tintner, J.; Stauffer, C.; Schebeck, M. Effect of temperature and photoperiod on development, survival, and growth rate of mealworms, Tenebrio molitor. Insects 2022, 13, 321. [Google Scholar] [CrossRef]
- Neven, L.G. Physiological responses of insects to heat. Postharvest Biol. Tec. 2000, 21, 103–111. [Google Scholar] [CrossRef]
- Zhao, L.; Jones, W.A. Expression of heat shock protein genes in insect stress responses. Invertebr. Surviv. J. 2012, 9, 93–101. [Google Scholar]
- Farahani, S.; Bandani, A.R.; Alizadeh, H.; Goldansaz, S.H.; Whyard, S. Differential expression of heat shock proteins and antioxidant enzymes in response to temperature, starvation, and parasitism in the Carob moth larvae, Ectomyelois ceratoniae (Lepidoptera: Pyralidae). PLoS ONE 2020, 15, e0228104. [Google Scholar] [CrossRef]
- Denlinger, D.L.; Yocum, G.D. Physiology of heat sensitivity. In Temperature Sensitivity in Insects and Application in Integrated Pest Management, 1st ed.; Hallman, G.J., Denlinger, D.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 7–53. [Google Scholar]
- Zhang, S.; Fu, W.; Li, N.; Zhang, F.; Liu, T.X. Antioxidant responses of Propylaea japonica (Coleoptera: Coccinellidae) exposed to high temperature stress. J. Insect Physiol. 2015, 73, 47–52. [Google Scholar] [CrossRef]
- Klepsatel, P.; Gáliková, M.; Xu, Y.; Kühnlein, R.P. Thermal stress depletes energy reserves in Drosophila. Sci. Rep. 2016, 6, 33667. [Google Scholar] [CrossRef]
- Stillwell, R.C.; Wallin, W.G.; Hitchcock, L.J.; Fox, C.W. Phenotypic plasticity in a complex world: Interactive effects of food and temperature on fitness components of a seed beetle. Oecologia 2007, 153, 309–321. [Google Scholar] [CrossRef]
- Cross, W.F.; Hood, J.M.; Benstead, J.P.; Huryn, A.D.; Nelson, D. Interactions between temperature and nutrients across levels of ecological organization. Glob. Chang. Biol. 2015, 21, 1025–1040. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Shlichta, J.G.; Ragland, G.J.; Massie, K.R. Thermal reaction norms for caterpillar growth depend on diet. Evol. Ecol. Res. 2006, 8, 703–715. [Google Scholar]
- Lee, K.P.; Roh, C. Temperature-by-nutrient interactions affecting growth rate in an insect ectotherm. Entomol. Exp. Appl. 2010, 136, 151–163. [Google Scholar] [CrossRef]
- Jang, T.; Rho, M.S.; Koh, S.H.; Lee, K.P. Host–plant quality alters herbivore responses to temperature: A case study using the generalist Hyphantria cunea. Entomol. Exp. Appl. 2015, 154, 120–130. [Google Scholar] [CrossRef]
- Lemoine, N.P.; Shantz, A.A. Increased temperature causes protein limitation by reducing the efficiency of nitrogen digestion in the ectothermic herbivore Spodoptera exigua. Physiol. Entomol. 2016, 41, 143–151. [Google Scholar] [CrossRef]
- Kutz, T.C.; Sgrò, C.M.; Mirth, C.K. Interacting with change: Diet mediates how larvae respond to their thermal environment. Funct. Ecol. 2019, 33, 1940–1951. [Google Scholar] [CrossRef]
- Singh, P.; van Bergen, E.; Brattström, O.; Osbaldeston, D.; Brakefield, P.M.; Oostra, V. Complex multi-trait responses to multivariate environmental cues in a seasonal butterfly. Evol. Ecol. 2020, 34, 713–734. [Google Scholar] [CrossRef]
- Quispe-Tarqui, R.; Yujra Pari, J.; Callizaya Condori, F.; Rebaudo, F. The effect of diet interacting with temperature on the development rate of a Noctuidae quinoa pest. Environ. Entomol. 2021, 50, 685–691. [Google Scholar] [CrossRef]
- Chakraborty, A.; Walter, G.M.; Monro, K.; Alves, A.N.; Mirth, C.K.; Sgrò, C.M. Within-population variation in body size plasticity in response to combined nutritional and thermal stress is partially independent from variation in development time. J. Evol. Biol. 2023, 36, 264–279. [Google Scholar] [CrossRef]
- Boukouvala, M.C.; Kavallieratos, N.G.; Skourti, A.; Pons, X.; Alonso, C.L.; Eizaguirre, M.; Fernandez, E.B.; Solera, E.D.; Fita, S.; Bohinc, T.; et al. Lymantria dispar (L.) (Lepidoptera: Erebidae): Current status of biology, ecology, and management in Europe with notes from North America. Insects 2022, 13, 854. [Google Scholar] [CrossRef]
- Lindroth, R.L.; Klein, K.A.; Hemming, J.D.; Feuker, A.M. Variation in temperature and dietary nitrogen affect performance of the gypsy moth (Lymantria dispar L.). Physiol. Entomol. 1997, 22, 55–64. [Google Scholar] [CrossRef]
- Janković-Tomanić, M.; Lazarević, J. Effects of temperature and dietary nitrogen on genetic variation and covariation in gypsy moth larval performance traits. Arch. Biol. Sci. 2012, 64, 1109–1116. [Google Scholar] [CrossRef]
- Sostak, B.E. Effects of Constant vs. Fluctuating Temperatures on Performance and Life History of the Herbivorous Pest Lymantria dispar (Lepidoptera: Eribidae). Graduate Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2015. [Google Scholar]
- O’Dell, T.M.; Butt, C.A.; Bridgeforth, A.W. Lymantria dispar. In Handbook of Insect Rearing; Singh, P., Moore, R.F., Eds.; Elsevier Science Publishing: New York, NY, USA, 1985; pp. 355–367. [Google Scholar]
- Stockhoff, B.A. Starvation resistance of gypsy moth, Lymantria dispar (L.) (Lepidoptera: Lymantriidae): Tradeoffs among growth, body size, and survival. Oecologia 1991, 88, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of sourdough fermentation on stabilisation, and chemical and nutritional characteristics of wheat germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- De Vasconcelos, M.C.B.M.; Bennett, R.; Castro, C.A.B.B.; Cardoso, P.; Saavedra, M.J.; Rosa, E.A. Study of composition, stabilization and processing of wheat germ and maize industrial by-products. Ind. Crops Prod. 2013, 42, 292–298. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kunitz, M. Crystalline soybean trypsin inhibitor. II. General properties. J. Gen. Physiol. 1947, 30, 291–310. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Kokowsky, N.; Cohen, W. The preparation and properties of two new chromogenic substrates for trypsin. Arch. Biochem. Biophys. 1961, 95, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Valaitis, A.P. Gypsy moth midgut proteinases: Purification and characterization of luminal trypsin, elastase and the brush border membrane leucine aminopeptidase. Insect Biochem. Mol. Biol. 1995, 25, 139–149. [Google Scholar] [CrossRef]
- Bernfeld, P. Amylases, alpha and beta. In Methods in Enzymology; Colowick, P.C., Kaplan, O.N., Eds.; Academic Press: New York, NY, USA, 1955; Volume 1, pp. 1949–1958. [Google Scholar] [CrossRef]
- Doane, W.W. Quantification of amylases in Drosophila separated by acrylamide gel electrophoresis. J. Exp. Zool. 1967, 164, 363–377. [Google Scholar] [CrossRef]
- Lazarević, J.; Perić-Mataruga, V.; Leković, S.; Nenadović, V. The properties of a-amylase from the midgut of Lymantria dispar larvae. In The Gypsy Moth Outbreaks in Serbia; Adamović, Ž., Ed.; Entomological Society of Serbia: Belgrade, Serbia, 1998; pp. 95–114. [Google Scholar]
- Baker, J.E. Properties of glycosidases from the maize weevil, Sitophilus zeamais. Insect Biochem. 1991, 21, 615–621. [Google Scholar] [CrossRef]
- Arreguin-Espinosa, R.; Arreguin, G.; Gonsales, C. Purification andproperties of a lipase from Cephaloilea presignis (Coleoptera, Chrysomelidae). Biotechol. Appl. Biochem. 2000, 31, 239–244. [Google Scholar] [CrossRef]
- Mrdaković, M.; Lazarević, J.; Perić-Mataruga, V.; Ilijin, L.; Vlahović, M. Partial characterization of a lipase from gypsy moth (Lymantria dispar L.) larval midgut. Folia Biol. 2008, 56, 103–110. [Google Scholar] [CrossRef]
- Barbosa, P.; Krischik, V.A. Influence of alkaloids on feeding preference of eastern deciduous forest trees by the gypsy moth Lymantria dispar. Am. Nat. 1987, 130, 53–69. [Google Scholar] [CrossRef]
- Kinney, K.K.; Lindroth, R.L.; Jung, S.M.; Nordheim, E.V. Effects of CO2 and NO3− availability on deciduous trees: Phytochemistry and insect performance. Ecology 1997, 78, 215–230. [Google Scholar]
- Barbehenn, R.V.; Kapila, M.; Kileen, S.; Nusbaum, C.P. Acquiring nutrients from tree leaves: Effects of leaf maturity and development type on a generalist caterpillar. Oecologia 2017, 184, 59–73. [Google Scholar] [CrossRef]
- Rossiter, M.; Schultz, J.C.; Baldwin, I.T. Relationships among defoliation, red oak phenolics, and gypsy moth growth and reproduction. Ecology 1988, 69, 267–277. [Google Scholar] [CrossRef]
- Osier, T.L.; Lindroth, R.L. Effects of genotype, nutrient availability, and defoliation on aspen phytochemistry and insect performance. J. Chem. Ecol. 2001, 27, 1289–1313. [Google Scholar] [CrossRef]
- Ruhnke, H.; Schädler, M.; Klotz, S.; Matthies, D.; Brandl, R. Variability in leaf traits, insect herbivory and herbivore performance within and among individuals of four broad-leaved tree species. Basic. Appl. Ecol. 2009, 10, 726–736. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Niewiadomski, J.; Pecci, C.; Salminen, J.P. Physiological benefits of feeding in the spring by Lymantria dispar caterpillars on red oak and sugar maple leaves: Nutrition versus oxidative stress. Chemoecology 2013, 23, 59–70. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Haugberg, N.; Kochmanski, J.; Menachem, B.; Miller, C. Physiological factors affecting the rapid decrease in protein assimilation efficiency by a caterpillar on newly-mature tree leaves. Physiol. Entomol. 2014, 39, 69–79. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Haugberg, N.; Kochmanski, J.; Menachem, B. Effects of leaf maturity and wind stress on the nutrition of the generalist caterpillar Lymantria dispar feeding on poplar. Physiol. Entomol. 2015, 40, 212–222. [Google Scholar] [CrossRef]
- Inoue, M.N.; Suzuki-Ohno, Y.; Haga, Y.; Aarai, H.; Sano, T.; Martemyanov, V.V.; Kunimi, Y. Population dynamics and geographical distribution of the gypsy moth, Lymantria dispar, in Japan. Forest Ecol. Manag. 2019, 434, 154–164. [Google Scholar] [CrossRef]
- Williams, R.S.; Norby, R.J.; Lincoln, D.E. Effects of elevated CO2 and temperature-grown red and sugar maple on gypsy moth performance. Glob. Chang. Biol. 2000, 6, 685–695. [Google Scholar] [CrossRef]
- Williams, R.S.; Lincoln, D.E.; Norby, R.J. Development of gypsy moth larvae feeding on red maple saplings at elevated CO2 and temperature. Oecologia 2003, 137, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.M.; Faske, T.M.; Banahene, N.; Grim, D.; Agosta, S.J.; Parry, D.; Tobin, P.C.; Johnson, D.M.; Grayson, K.L. Variation in growth and developmental responses to supraoptimal temperatures near latitudinal range limits of gypsy moth Lymantria dispar (L.), an expanding invasive species. Physiol. Entomol. 2017, 42, 181–190. [Google Scholar] [CrossRef]
- Hunter, A.F.; Lechowicz, M.J. Foliage quality changes during canopy development of some northern hardwood trees. Oecologia 1992, 89, 316–323. [Google Scholar] [CrossRef]
- Foster, J.R.; Townsend, P.A.; Mladenoff, D.J. Mapping asynchrony between gypsy moth egg-hatch and forest leaf-out: Putting the phenological window hypothesis in a spatial context. For. Ecol. Manag. 2013, 287, 67–76. [Google Scholar] [CrossRef]
- Martemyanov, V.V.; Pavlushin, S.V.; Dubovskiy, I.M.; Yushkova, Y.V.; Morosov, S.V.; Chernyak, E.I.; Efimov, V.M.; Ruuhola, T.; Glupov, V.V. Asynchrony between host plant and insects-defoliator within a tritrophic system: The role of herbivore innate immunity. PLoS ONE 2015, 10, e0130988. [Google Scholar] [CrossRef]
- Martemyanov, V.V.; Belousova, I.A.; Pavlushin, S.V.; Dubovskiy, I.M.; Ershov, N.I.; Alikina, T.Y.; Kabilov, M.R.; Glupov, V.V. Phenological asynchrony between host plant and gypsy moth reduces insect gut microbiota and susceptibility to Bacillus thuringiensis. Ecol. Evol. 2016, 6, 7298–7310. [Google Scholar] [CrossRef] [PubMed]
- Tobin, P.C.; Gray, D.R.; Liebhold, A.M. Supraoptimal temperatures influence the range dynamics of a non-native insect. Divers. Distrib. 2014, 20, 813–823. [Google Scholar] [CrossRef]
- Ponomarev, V.I.; Klobukov, G.I.; Napalkova, V.V.; Akhanaev, Y.B.; Pavlushin, S.V.; Yakimova, M.E.; Subbotina, A.O.; Picq, S.; Cusson, M.; Martemyanov, V.V. Phenological features of the spongy moth, Lymantria dispar (L.) (Lepidoptera: Erebidae), in the northernmost portions of its Eurasian range. Insects 2023, 14, 276. [Google Scholar] [CrossRef]
- Melin, M.; Viiri, H.; Tikkanen, O.P.; Elfving, R.; Neuvonen, S. From a rare inhabitant into a potential pest–status of the nun moth in Finland based on pheromone trapping. Silva Fenn. 2020, 54, 10262. [Google Scholar] [CrossRef]
- Keena, M.A.; Richards, J.Y. Comparison of survival and development of gypsy moth Lymantria dispar L.(Lepidoptera: Erebidae) populations from different geographic areas on North American conifers. Insects 2020, 11, 260. [Google Scholar] [CrossRef]
- Jahant-Miller, C.; Miller, R.; Parry, D. Size-dependent flight capacity and propensity in a range-expanding invasive insect. Insect Sci. 2022, 29, 879–888. [Google Scholar] [CrossRef]
- Soukhovolsky, V.; Tarasova, O.; Pavlushin, S.; Osokina, E.; Akhanaev, Y.; Kovalev, A.; Martemyanov, V. Economics of a feeding budget: A case of diversity of host plants for Lymantria dispar L.(Lepidoptera) feeding on leaves and needles. Diversity 2023, 15, 102. [Google Scholar] [CrossRef]
- Knapp, R.; Casey, T.M. Thermal ecology, behavior, and growth of gypsy moth and eastern tent caterpillars. Ecology 1986, 67, 598–608. [Google Scholar] [CrossRef]
- Keena, M.A.; Shi, J. Effects of temperature on first instar Lymantria (Lepidoptera: Erebidae) survival and development with and without food. Environ. Entomol. 2019, 48, 655–666. [Google Scholar] [CrossRef]
- Gibbs, P.M. Evidence of Local Adaptation to Climate in an Invasive Ectotherm: A Study on the Eurasian Gypsy Moth (Lymantria dispar) in North America. M.S. Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2020. [Google Scholar]
- Maksimović, M. Experimental Researches on the Influence of Temperature upon the Development and the Dynamics of Population of the Gypsy Moth (Liparis dispar L.); Biological Institute of N. R. Serbia: Belgrade, Serbia, 1958; p. 115. [Google Scholar]
- Logan, J.A.; Casagrande, R.A.; Liebhold, A.M. Modeling environment for simulation of gypsy moth (Lepidoptera: Lymantriidae) larval phenology. Environ. Entomol. 1991, 20, 1516–1525. [Google Scholar] [CrossRef]
- Karimi-Malati, A.; Fathipour, Y.; Talebi, A.A. Development response of Spodoptera exigua to eight constant temperatures: Linear and nonlinear modeling. J. Asia-Pac. Entomol. 2014, 17, 349–354. [Google Scholar] [CrossRef]
- Limbu, S.; Keena, M.; Chen, F.; Cook, G.; Nadel, H.; Hoover, K. Effects of temperature on development of Lymantria dispar asiatica and Lymantria dispar japonica (Lepidoptera: Erebidae). Environ. Entomol. 2017, 46, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Tammaru, T.; Ruohomäki, K.; Montola, M. Crowding-induced plasticity in Epirrita autumnata (Lepidoptera: Geometridae): Weak evidence of specific modifications in reaction norms. Oikos 2000, 90, 171–181. [Google Scholar] [CrossRef]
- Zhang, B.; Helen, H.S.; Wang, J.J.; Huai, L.I.U. Performance and enzyme activity of beet armyworm Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae) under various nutritional conditions. Agric. Sci. China 2011, 10, 737–746. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Schafellner, C. Gypsy moth feeding in the canopy of a CO2-enriched mature forest. Glob. Chang. Biol. 2004, 10, 1899–1908. [Google Scholar] [CrossRef]
- Couture, J.J.; Meehan, T.D.; Lindroth, R.L. Atmospheric change alters foliar quality of host trees and performance of two outbreak insect species. Oecologia 2012, 168, 863–876. [Google Scholar] [CrossRef]
- Keating, S.T.; Yendol, W.G.; Schultz, J.C. Relationship between susceptibility of gypsy moth larvae (Lepidoptera: Lymantriidae) to a baculovirus and host plant foliage constituents. Environ. Entomol. 1988, 17, 952–958. [Google Scholar] [CrossRef]
- Stockhoff, B.A. Protein intake by gypsy moth larvae on homogeneous and heterogeneous diets. Physiol. Entomol. 1993, 18, 409–419. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Knister, J.; Marsik, F.; Jahant-Miller, C.; Nham, W. Nutrients are assimilated efficiently by Lymantria dispar caterpillars from the mature leaves of trees in the Salicaceae. Physiol. Entomol. 2015, 40, 72–81. [Google Scholar] [CrossRef]
- Lee, K.P.; Jang, T.; Ravzanaadii, N.; Rho, M.S. Macronutrient balance modulates the temperature-size rule in an ectotherm. Am. Nat. 2015, 186, 212–222. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Biochemistry and molecular biology of digestion. In Insect Molecular Biology and Biochemistry, 1st ed.; Gilbert, L.I., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 365–418. [Google Scholar]
- Zeng, J.; Shi, Z.; Shi, J.; Guo, J.; Zhang, G.; Zhang, J. Ambient temperature-mediated enzymic activities and intestinal microflora in Lymantria dispar larvae. Arch. Insect Biochem. Physiol. 2019, 102, e21597. [Google Scholar] [CrossRef] [PubMed]
- Weidlich, S.; Huster, J.; Hoffmann, K.H.; Woodring, J. Environmental control of trypsin secretion in the midgut of the two-spotted field cricket, Gryllus bimaculatus. J. Insect Physiol. 2012, 58, 1477–1484. [Google Scholar] [CrossRef]
- Weidlich, S. The Regulation of Digestive Enzyme Release in the Two-Spotted Field Cricket Gryllus bimaculatus (de Geer): Effects of Endogenous and Environmental Factors. Doctoral Dissertation, Universitaet Bayreuth, Bayreuth, Germany, 2013. [Google Scholar]
- Akbar, S.M.; Pavani, T.; Nagaraja, T.; Sharma, H.C. Influence of CO2 and temperature on metabolism and development of Helicoverpa armigera (Noctuidae: Lepidoptera). Environ. Entomol. 2016, 45, 229–236. [Google Scholar] [CrossRef]
- Ilijin, L.; Grčić, A.; Mrdaković, M.; Vlahović, M.; Todorović, D.; Filipović, A.; Matić, D.; Perić Mataruga, V. The effects of temperature stress and population origin on the thermal sensitivity of Lymantria dispar L. (Lepidoptera: Erebidae) larvae. Sci. Rep. 2022, 12, 21858. [Google Scholar] [CrossRef]
- Karasov, W.H.; Douglas, A.E. Comparative digestive physiology. Compr. Physiol. 2013, 3, 741. [Google Scholar]
- Lazarević, J.; Janković-Tomanić, M. Dietary and phylogenetic correlates of digestive trypsin activity in insect pests. Entomol. Exp. Appl. 2015, 157, 123–151. [Google Scholar] [CrossRef]
- Huang, J.H.; Jing, X.; Douglas, A.E. The multi-tasking gut epithelium of insects. Insect Biochem. Mol. Biol. 2015, 67, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.; Bruno, D.; Brilli, M.; Gianfranceschi, N.; Tian, L.; Tettamanti, G.; Caccia, S.; Casartelli, M. Black soldier fly larvae adapt to different food substrates through morphological and functional responses of the midgut. Int. J. Mol. Sci. 2020, 21, 4955. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, J.M.; Perić-Mataruga, V.D. Nutritive stress effects on growth and digestive physiology of Lymantria dispar larvae. Jugosl. Med. Biohemija 2003, 22, 53–59. [Google Scholar] [CrossRef]
- Milanović, S.; Miletić, Z.; Marković, Č.; Šešlija Jovanović, D.; Trailović, Z.; Jankovský, L.; Lazarević, J. Suitability of turkey oak, european beech, and hornbeam to gypsy moth feeding. Forests 2022, 13, 1006. [Google Scholar] [CrossRef]
- Christeller, J.T.; Poulton, J.; Markwick, N.M.; Simpson, R.M. The effect of diet on the expression of lipase genes in the midgut of the light brown apple moth (Epiphyas postvittana Walker; Tortricidae). Insect Mol. Biol. 2010, 19, 9–25. [Google Scholar] [CrossRef]
- Lwalaba, D.; Hoffmann, K.H.; Woodring, J. Control of the release of digestive enzymes in the larvae of the fall armyworm, Spodoptera frugiperda. Arch. Insect Biochem. Physiol. 2010, 73, 14–29. [Google Scholar] [CrossRef]
- Fujimoto, J.; Kanou, C.; Eguchi, Y.; Matsuo, Y. Adaptation to a starch environment and regulation of alpha-amylase in Drosophila. Biochem. Gen. 1999, 37, 53–62. [Google Scholar] [CrossRef]
- Zinke, I.; Schütz, C.S.; Katzenberger, J.D.; Bauer, M.; Pankratz, M.J. Nutrient control of gene expression in Drosophila: Microarray analysis of starvation and sugar-dependent response. EMBO J. 2002, 21, 6162–6173. [Google Scholar] [CrossRef] [PubMed]
- Wool, D.; Namir, Z.; Bergerson, O. Dietary regulation of amylase activity levels in flour beetles (Coleoptera: Tenebrionidae): (Tribolium). Ann. Entomol. Soc. Am. 1986, 79, 407–413. [Google Scholar] [CrossRef]
- Karimi-Pormehr, M.S.; Borzoui, E.; Naseri, B.; Dastjerdi, H.R.; Mansouri, S.M. Two-sex life table analysis and digestive physiology of Sitotroga cerealella (Olivier) (Lepidoptera: Gelechiidae) on different barley cultivars. J. Stored Prod. Res. 2018, 75, 64–71. [Google Scholar] [CrossRef]
- Nemati-Kalkhoran, M.; Razmjou, J.; Borzoui, E.; Naseri, B. Comparison of life table parameters and digestive physiology of Rhyzopertha dominica (Coleoptera: Bostrichidae) fed on various barley cultivars. J. Insect Sci. 2018, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.D.; Geng, Y.S.; Hu, T.Y.; Li, W.X.; Liang, Y.Y.; Hao, D.J. Comparing the performance of Hyphantria cunea (Lepidoptera: Arctiidae) on artificial and natural diets: Feasibility of mass-rearing on artificial diets. J. Econom. Entomol. 2023, 116, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Broadway, R.M.; Duffey, S.S. The effect of dietary protein on the growth and digestive physiology of larval Heliothis zea and Spodoptera exigua. J. Insect Physiol. 1986, 32, 673–680. [Google Scholar] [CrossRef]
- Kamin-Belsky, N.; Wool, D. Dietary modification of digestive physiology in larvae of almond moth (Lepidoptera: Phyticidae). J. Econom. Entomol. 1991, 84, 768–775. [Google Scholar] [CrossRef]
- Mrdaković, M.; Stojković, B.; Perić-Mataruga, V.; Ilijin, L.; Vlahović, M.; Lazarević, J. Adaptive phenotypic plasticity of gypsy moth digestive enzymes. Open. Life Sci. 2014, 9, 309–319. [Google Scholar] [CrossRef]
- Bezzar-Bendjazia, R.; Kilani-Morakchi, S.; Maroua, F.; Aribi, N. Azadirachtin induced larval avoidance and antifeeding by disruption of food intake and digestive enzymes in Drosophila melanogaster (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2017, 143, 135–140. [Google Scholar] [CrossRef]

| Ingredient | HpHc | HpLc | LpHc | LpLc |
|---|---|---|---|---|
| Agar | 8.34 | 8.34 | 8.34 | 8.34 |
| Wheat germ | 27.58 | 27.58 | 27.58 | 27.58 |
| Casein | 15.26 | 15.26 | 0 | 0 |
| Potato starch | 16.99 | 2.00 | 16.99 | 2.00 |
| Alpha-cellulose | 20.71 | 35.70 | 35.97 | 50.96 |
| Wesson salt mix | 4.45 | 4.45 | 4.45 | 4.45 |
| Sorbic acid | 1.11 | 1.11 | 1.11 | 1.11 |
| Vanderzant vitamin mix | 5.56 | 5.56 | 5.56 | 5.56 |
| Within Groups Loadings | Standardized Coefficients | |||
|---|---|---|---|---|
| Variable | Root 1 | Root 2 | Root 1 | Root 2 |
| Development duration | −0.978 | −0.210 | −0.953 | −0.327 |
| Larval mass | +0.324 | −0.946 | +0.211 | −0.985 |
| Within Groups Loadings | Standardized Coefficients | |||||
|---|---|---|---|---|---|---|
| Variable | Root 1 | Root 2 | Root 3 | Root 1 | Root 2 | Root 3 |
| PA | +0.080 | −0.132 | +0.391 | +0.496 | −1.055 | −0.276 |
| TRY | +0.015 | −0.040 | +0.567 | −0.200 | −0.469 | +1.391 |
| ELA | +0.131 | +0.181 | +0.155 | +0.382 | +0.988 | −0.770 |
| LAP | −0.777 | −0.002 | +0.056 | −1.156 | −0.116 | −0.286 |
| AMY | −0.082 | +0.517 | +0.318 | +0.171 | +0.905 | +0.158 |
| α-GLUC | −0.184 | +0.136 | +0.694 | −0.197 | +0.372 | +0.581 |
| LIP | −0.027 | −0.284 | +0.028 | +0.264 | −0.510 | +0.011 |
| Response Variable | Predictor 1 | β ± SE | t | p | Adjusted R2, Whole Model |
|---|---|---|---|---|---|
| Development | PA | −0.759 ± 0.150 | −5.06 | 0.002 | 0.950 |
| duration | TRY | +0.286 ± 0.114 | 2.51 | 0.046 | p < 0.001 |
| LAP | +0.345 ± 0.121 | 2.85 | 0.029 | ||
| α-GLUC | +0.283 ± 0.114 | 2.48 | 0.048 | ||
| LIP | +0.894 ± 0.122 | 7.31 | <0.001 | ||
| Body mass | LAP | −0.443 ± 0.049 | −9.07 | <0.001 | 0.977 |
| AMY | +0.414 ± 0.071 | 5.82 | <0.001 | p < 0.001 | |
| LIP | −0.505 ± 0.072 | 7.01 | <0.001 | ||
| Growth rate | PA | +0.684 ± 0.146 | 4.69 | 0.003 | 0.935 |
| TRY | −0.312 ± 0.130 | −2.39 | 0.054 | p < 0.001 | |
| AMY | +0.317 ± 0.133 | 2.39 | 0.054 | ||
| α-GLUC | −0.501 ± 0.103 | −4.85 | 0.003 | ||
| LIP | −0.973 ± 0.135 | −7.23 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarević, J.; Milanović, S.; Šešlija Jovanović, D.; Janković-Tomanić, M. Temperature- and Diet-Induced Plasticity of Growth and Digestive Enzymes Activity in Spongy Moth Larvae. Biomolecules 2023, 13, 821. https://doi.org/10.3390/biom13050821
Lazarević J, Milanović S, Šešlija Jovanović D, Janković-Tomanić M. Temperature- and Diet-Induced Plasticity of Growth and Digestive Enzymes Activity in Spongy Moth Larvae. Biomolecules. 2023; 13(5):821. https://doi.org/10.3390/biom13050821
Chicago/Turabian StyleLazarević, Jelica, Slobodan Milanović, Darka Šešlija Jovanović, and Milena Janković-Tomanić. 2023. "Temperature- and Diet-Induced Plasticity of Growth and Digestive Enzymes Activity in Spongy Moth Larvae" Biomolecules 13, no. 5: 821. https://doi.org/10.3390/biom13050821
APA StyleLazarević, J., Milanović, S., Šešlija Jovanović, D., & Janković-Tomanić, M. (2023). Temperature- and Diet-Induced Plasticity of Growth and Digestive Enzymes Activity in Spongy Moth Larvae. Biomolecules, 13(5), 821. https://doi.org/10.3390/biom13050821






