ASC Transporters Mediate D-Serine Transport into Astrocytes Adjacent to Synapses in the Mouse Brain
Abstract
1. Introduction
2. Materials and Methods
3. Results
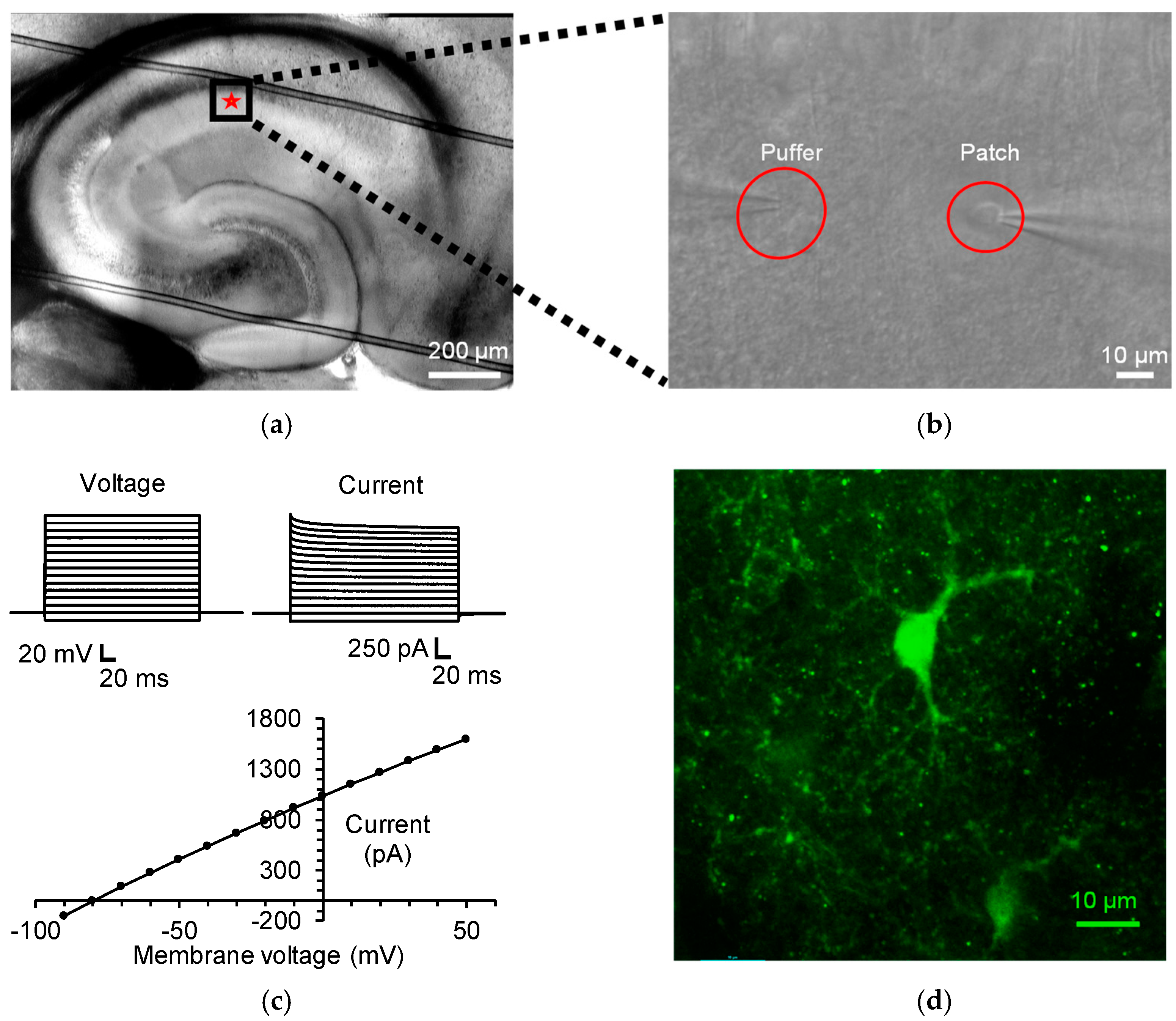
3.1. Identification of Stratum Radiatum Astrocytes
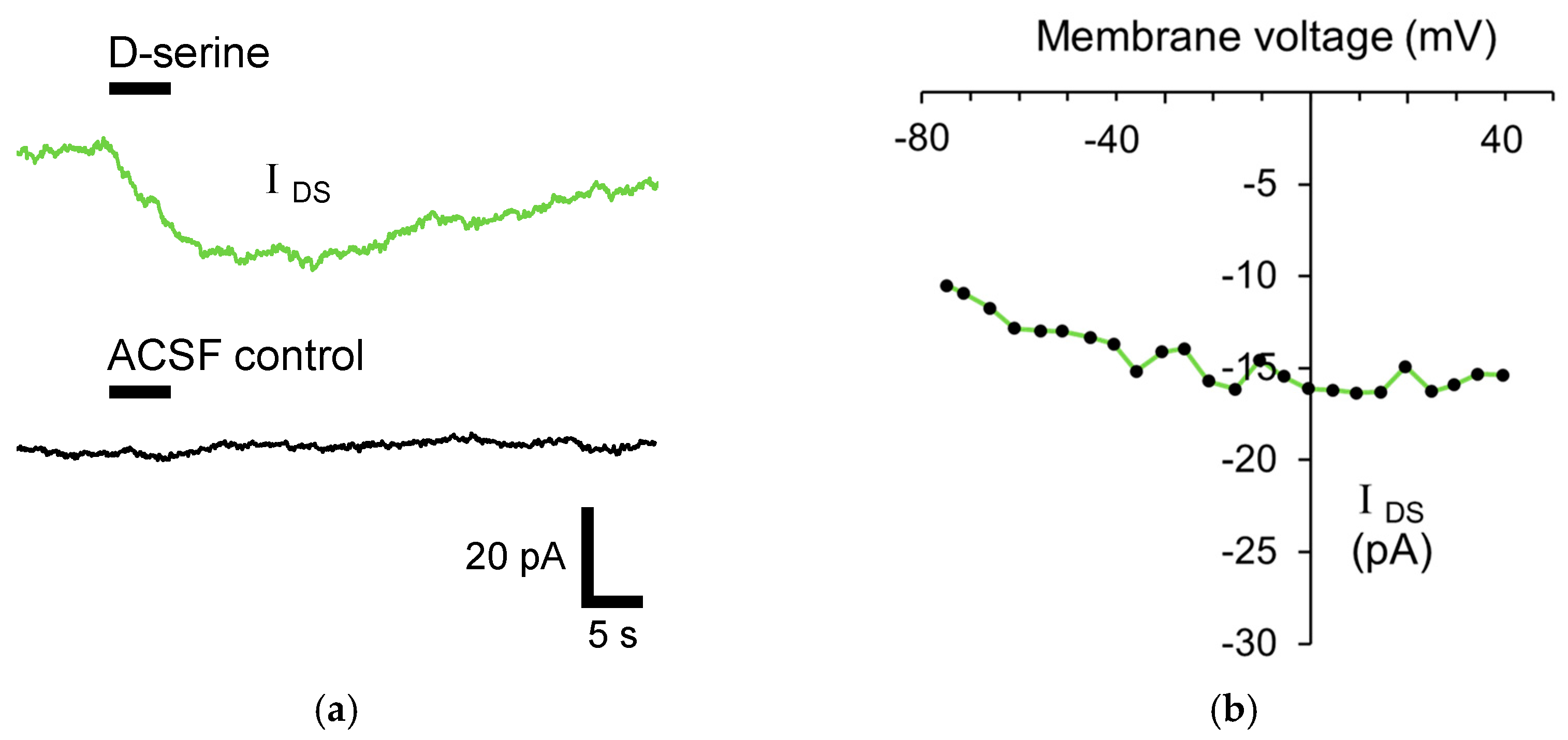
3.2. D-Serine Uptake into Stratum Radiatum Astrocytes
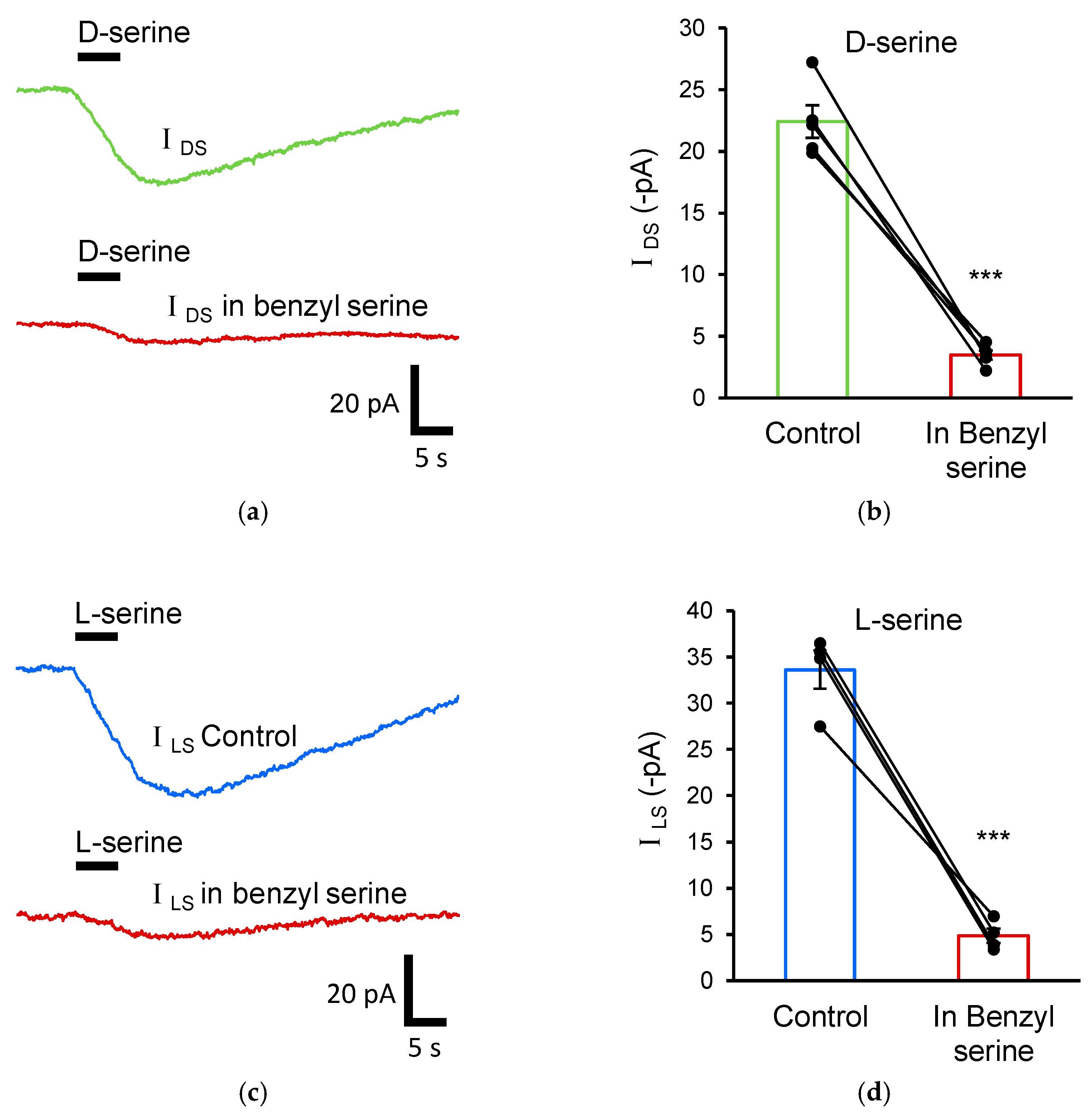
3.3. Astrocytic D-Serine Transport Is Mediated by ASC Transporters
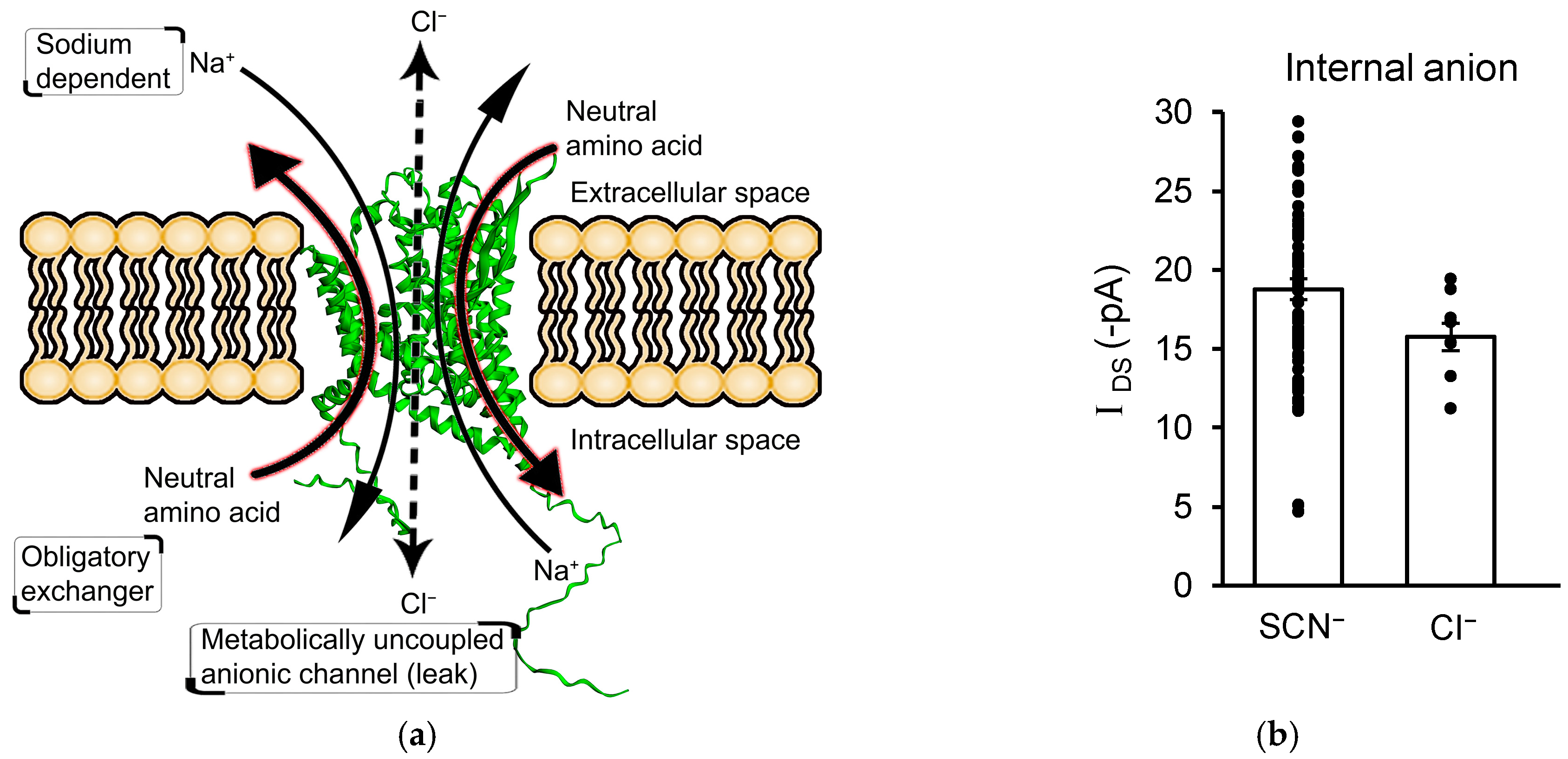
3.4. IDS Is Not Mediated by an Uncoupled Anion Channel Current
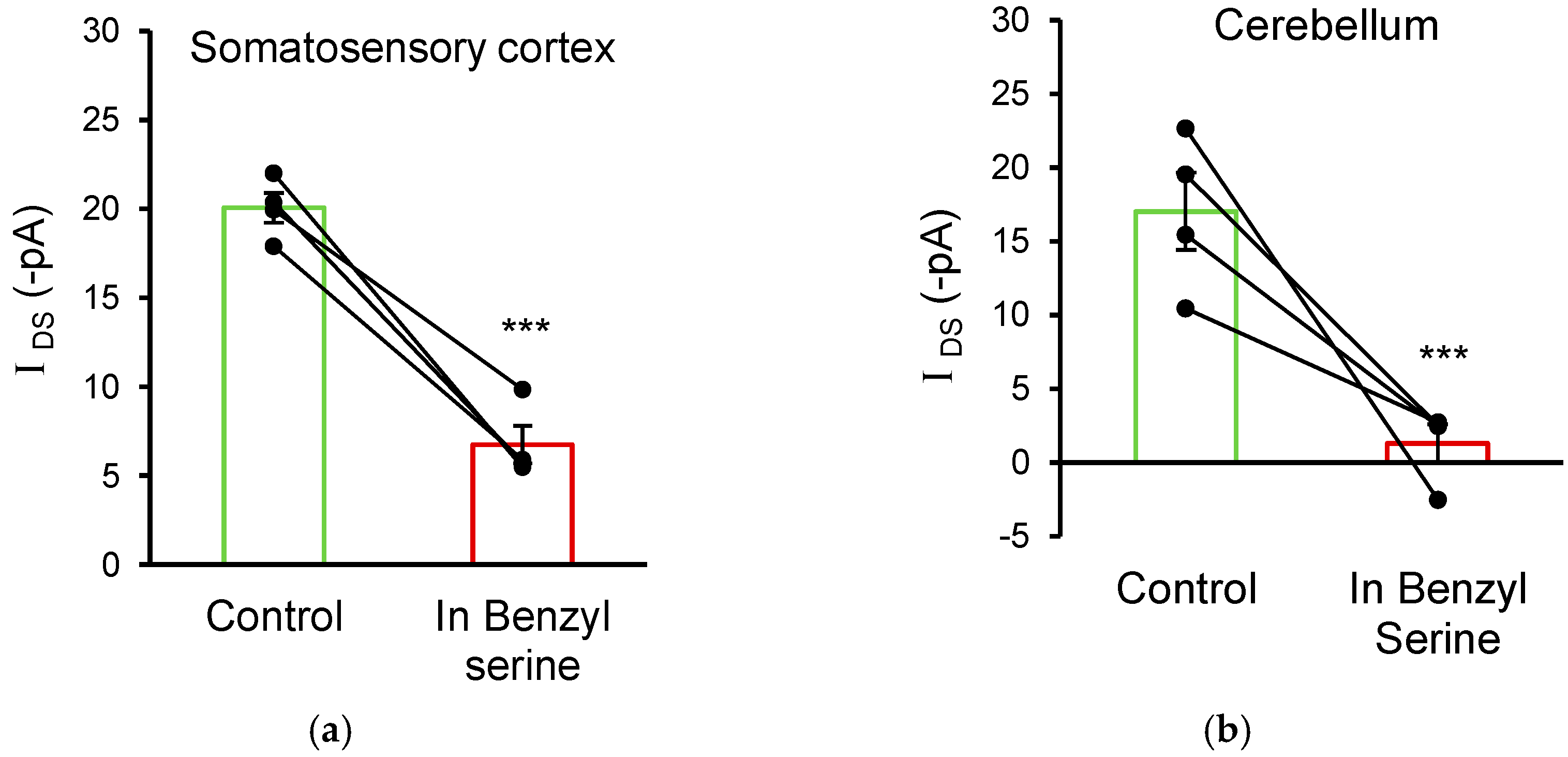
3.5. ASC Transporters Also Mediate D-Serine Uptake in Cortical and Cerebellar Glial Cells
4. Discussion
4.1. Astrocytic D-Serine Uptake Is Mediated by ASCT1 Transporters
4.2. D-Serine Produces Coupled Transport Currents in Astrocytes
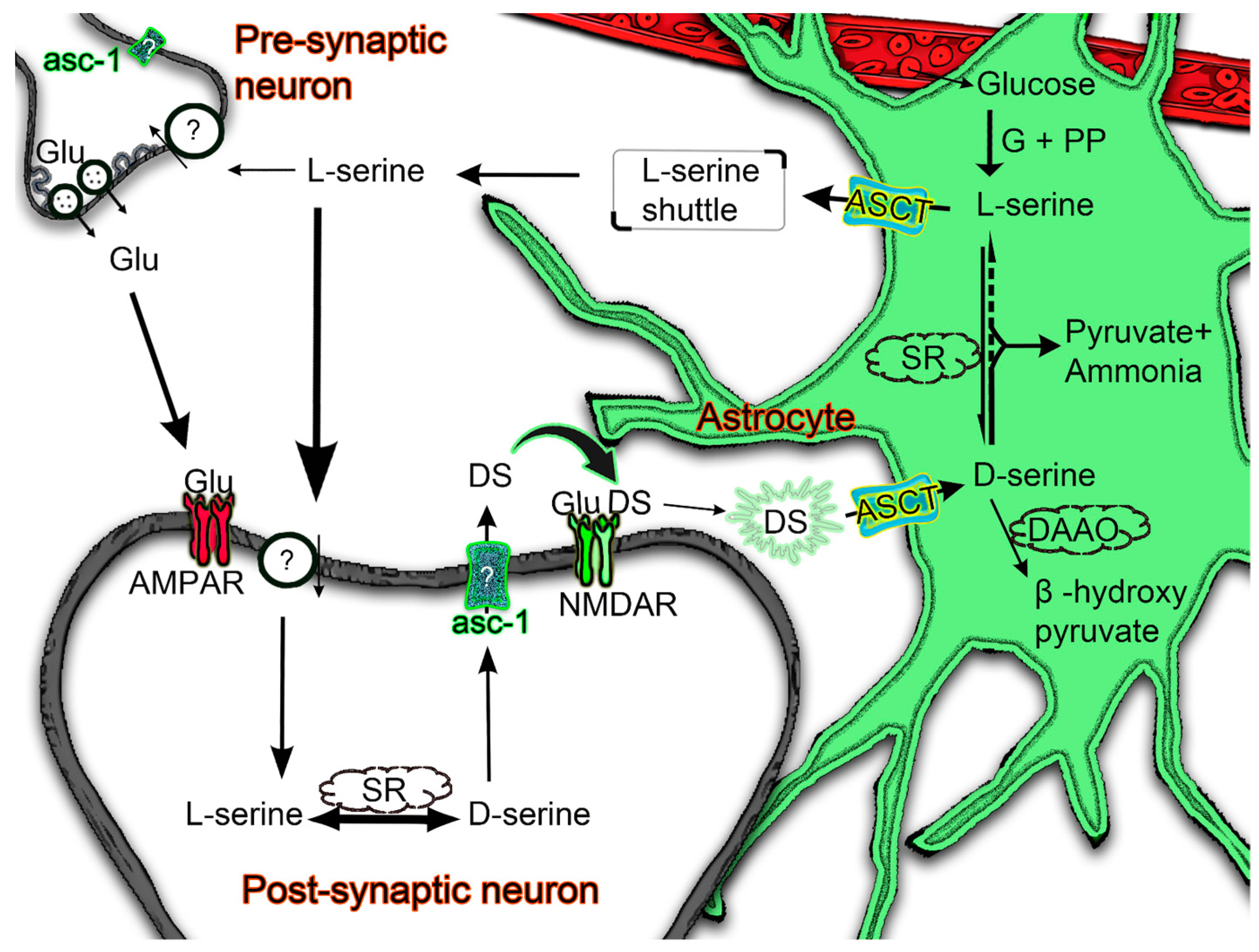
4.3. Physiological Implications of ASC Transporters and Enzymes in D-Serine Regulation
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashimoto, A.; Nishikawa, T.; Hayashi, T.; Fujii, N.; Harada, K.; Oka, T.; Takahashi, K. The presence of free D-serine in rat brain. FEBS Lett. 1992, 296, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Wolosker, H.; Blackshaw, S.; Snyder, S.H. Serine racemase: A glial enzyme synthesizing D-Serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc. Natl. Acad. Sci. USA 1999, 96, 13409–13414. [Google Scholar] [CrossRef] [PubMed]
- Kleckner, N.W.; Dingledine, R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 1988, 241, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, G.L.; Bliss, T.V.P. NMDA receptors—Their role in long-term potentiation. Trends Neurosci. 1987, 10, 288–293. [Google Scholar] [CrossRef]
- Kemp, J.A.; Leeson, P.D. The glycine site of the NMDA receptor--five years on. Trends Pharm. Sci. 1993, 14, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.W.; Ascher, P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 1987, 325, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.J.; Brady, R.O., Jr.; Molliver, M.E.; Snyder, S.H. D-Serine as a neuromodulator: Regional and developmental localizations in Rat Brain Glia resemble NMDA receptors. J. Neurosci. 1997, 17, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Kuryatov, A.; Laube, B.; Betz, H.; Kuhse, J. Mutational analysis of the glycine-binding site of the NMDA receptor: Structural similarity with bacterial amino acid-binding proteins. Neuron 1994, 12, 1291–1300. [Google Scholar] [CrossRef]
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in NMDA receptors. Nature 2005, 438, 185–192. [Google Scholar] [CrossRef]
- Papouin, T.; Ladepeche, L.; Ruel, J.; Sacchi, S.; Labasque, M.; Hanini, M.; Groc, L.; Pollegioni, L.; Mothet, J.P.; Oliet, S.H. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 2012, 150, 633–646. [Google Scholar] [CrossRef]
- Le Bail, M.; Martineau, M.; Sacchi, S.; Yatsenko, N.; Radzishevsky, I.; Conrod, S.; Ait Ouares, K.; Wolosker, H.; Pollegioni, L.; Billard, J.-M.; et al. Identity of the NMDA receptor coagonist is synapse specific and developmentally regulated in the hippocampus. Proc. Natl. Acad. Sci. USA 2015, 112, E204–E213. [Google Scholar] [CrossRef] [PubMed]
- Mothet, J.-P.; Parent, A.T.; Wolosker, H.; Brady, R.O.; Linden, D.J.; Ferris, C.D.; Rogawski, M.A.; Snyder, S.H. D-Serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 4926–4931. [Google Scholar] [CrossRef] [PubMed]
- Panatier, A.; Theodosis, D.T.; Mothet, J.-P.; Touquet, B.; Pollegioni, L.; Poulain, D.A.; Oliet, S.H.R. Glia-Derived D-Serine Controls NMDA Receptor Activity and Synaptic Memory. Cell 2006, 125, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Henneberger, C.; Papouin, T.; Oliet, S.H.R.; Rusakov, D.A. Long-term potentiation depends on release of D-Serine from astrocytes. Nature 2010, 463, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Miya, K.; Inoue, R.; Takata, Y.; Abe, M.; Natsume, R.; Sakimura, K.; Hongou, K.; Miyawaki, T.; Mori, H. Serine racemase is predominantly localized in neurons in mouse brain. J. Comp. Neurol. 2008, 510, 641–654. [Google Scholar] [CrossRef]
- Balu, D.T.; Takagi, S.; Puhl, M.D.; Benneyworth, M.A.; Coyle, J.T. D-Serine and Serine Racemase are Localized to Neurons in the Adult Mouse and Human Forebrain. Cell. Mol. Neurobiol. 2014, 34, 419–435. [Google Scholar] [CrossRef]
- Yamasaki, M.; Yamada, K.; Furuya, S.; Mitoma, J.; Hirabayashi, Y.; Watanabe, M. 3-Phosphoglycerate dehydrogenase, a key enzyme for l-serine biosynthesis, is preferentially expressed in the radial glia/astrocyte lineage and olfactory ensheathing glia in the mouse brain. J. Neurosci. 2001, 21, 7691–7704. [Google Scholar] [CrossRef]
- Neame, S.; Safory, H.; Radzishevsky, I.; Touitou, A.; Marchesani, F.; Marchetti, M.; Kellner, S.; Berlin, S.; Foltyn, V.; Engelender, S.; et al. The NMDA receptor activation by d-serine and glycine is controlled by an astrocytic Phgdh-dependent serine shuttle. Proc. Natl. Acad. Sci. USA 2019, 116, 201909458. [Google Scholar] [CrossRef]
- Wolosker, H.; Balu, D.T.; Coyle, J.T. The Rise and Fall of the d-Serine-Mediated Gliotransmission Hypothesis. Trends Neurosci. 2016, 39, 712–721. [Google Scholar] [CrossRef]
- Rosenberg, D.; Artoul, S.; Segal, A.C.; Kolodney, G.; Radzishevsky, I.; Dikopoltsev, E.; Foltyn, V.N.; Inoue, R.; Mori, H.; Billard, J.-M.; et al. Neuronal D-Serine and Glycine Release Via the Asc-1 Transporter Regulates NMDA Receptor-Dependent Synaptic Activity. J. Neurosci. 2013, 33, 3533–3544. [Google Scholar] [CrossRef]
- Sason, H.; Billard, J.M.; Smith, G.P.; Safory, H.; Neame, S.; Kaplan, E.; Rosenberg, D.; Zubedat, S.; Foltyn, V.N.; Christoffersen, C.T.; et al. Asc-1 Transporter Regulation of Synaptic Activity via the Tonic Release of D-Serine in the Forebrain. Cereb. Cortex 2017, 27, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Folorunso, O.O.; Barragan, E.V.; Berciu, C.; Harvey, T.L.; Coyle, J.T.; Balu, D.T.; Gray, J.A. Postsynaptic Serine Racemase Regulates NMDA Receptor Function. J. Neurosci. 2020, 40, 9564–9575. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Takahashi, K.; Nishikawa, T. Uptake of D- and L-serine in C6 glioma cells. Neurosci. Lett. 1997, 239, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.S.; Reis, M.; Panizzutti, R.; De Miranda, J.; Wolosker, H. Glial transport of the neuromodulator D-serine. Brain Res. 2002, 929, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, Y.; Segawa, H.; Kim, J.Y.; Chairoungdua, A.; Kim, D.K.; Matsuo, H.; Cha, S.H.; Endou, H.; Kanai, Y. Identification and characterization of a Na(+)-independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral D- and L-amino acids. J. Biol. Chem. 2000, 275, 9690–9698. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.J.; Chaudhry, F.A.; Gray, A.T.; Edwards, R.H. Amino acid transport system A resembles system N in sequence but differs in mechanism. Proc. Natl. Acad. Sci. USA 2000, 97, 7715–7720. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, T.; Huang, W.; Nakanishi, T.; Bridges, C.C.; Smith, S.B.; Prasad, P.D.; Ganapathy, M.E.; Ganapathy, V. Transport of D-serine via the amino acid transporter ATB(0,+) expressed in the colon. Biochem. Biophys. Res. Commun. 2002, 291, 291–295. [Google Scholar] [CrossRef]
- Foster, A.C.; Farnsworth, J.; Lind, G.E.; Li, Y.-X.; Yang, J.-Y.; Dang, V.; Penjwini, M.; Viswanath, V.; Staubli, U.; Kavanaugh, M.P. D-Serine Is a Substrate for Neutral Amino Acid Transporters ASCT1/SLC1A4 and ASCT2/SLC1A5, and Is Transported by Both Subtypes in Rat Hippocampal Astrocyte Cultures. PLoS ONE 2016, 11, e0156551. [Google Scholar] [CrossRef]
- Bodner, O.; Radzishevsky, I.; Foltyn, V.N.; Touitou, A.; Valenta, A.C.; Rangel, I.F.; Panizzutti, R.; Kennedy, R.T.; Billard, J.M.; Wolosker, H. D-Serine Signaling and NMDAR-Mediated Synaptic Plasticity Are Regulated by System A-Type of Glutamine/D-Serine Dual Transporters. J. Neurosci. 2020, 40, 6489–6502. [Google Scholar] [CrossRef]
- Ehmsen, J.T.; Liu, Y.; Wang, Y.; Paladugu, N.; Johnson, A.E.; Rothstein, J.D.; du Lac, S.; Mattson, M.P.; Höke, A. The astrocytic transporter SLC7A10 (Asc-1) mediates glycinergic inhibition of spinal cord motor neurons. Sci. Rep. 2016, 6, 35592. [Google Scholar] [CrossRef]
- Duelli, R.; Enerson, B.E.; Gerhart, D.Z.; Drewes, L.R. Expression of Large Amino Acid Transporter LAT1 in Rat Brain Endothelium. J. Cereb. Blood Flow Metab. 2000, 20, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, I.J.; Hwang, S.; Kook, J.H.; Lee, M.-C.; Shin, B.A.; Bae, C.S.; Yoon, J.H.; Ahn, S.G.; Kim, S.A.; et al. System l-amino acid transporters are differently expressed in rat astrocyte and C6 glioma cells. Neurosci. Res. 2004, 50, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.D.; Derazi, S.; Kilberg, M.S.; Anderson, K.J. Ontogeny and localization of the neutral amino acid transporter ASCT1 in rat brain. Dev. Brain Res. 2001, 130, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Shimizu, H.; Koike, T.; Furuya, S.; Watanabe, M. Neutral Amino Acid Transporter ASCT1 Is Preferentially Expressed in Ser-Synthetic/Storing Glial Cells in the Mouse Brain with Transient Expression in Developing Capillaries. J. Neurosci. 2003, 23, 550–560. [Google Scholar] [CrossRef]
- Melone, M.; Quagliano, F.; Barbaresi, P.; Varoqui, H.; Erickson, J.D.; Conti, F. Localization of the glutamine transporter SNAT1 in rat cerebral cortex and neighboring structures, with a note on its localization in human cortex. Cereb. Cortex 2004, 14, 562–574. [Google Scholar] [CrossRef] [PubMed]
- González-González, I.M.; Cubelos, B.; Giménez, C.; Zafra, F. Immunohistochemical localization of the amino acid transporter SNAT2 in the rat brain. Neuroscience 2005, 130, 61–73. [Google Scholar] [CrossRef]
- Broeer, A.; Brookes, N.; Ganapathy, V.; Dimmer, K.S.; Wagner, C.A.; Lang, F.; Bröer, S. The Astroglial ASCT2 Amino Acid Transporter as a Mediator of Glutamine Efflux. J. Neurochem. 1999, 73, 2184–2194. [Google Scholar]
- Yamamoto, T.; Nishizaki, I.; Furuya, S.; Hirabayashi, Y.; Takahashi, K.; Okuyama, S.; Yamamoto, H. Characterization of rapid and high-affinity uptake of L-serine in neurons and astrocytes in primary culture. FEBS Lett. 2003, 548, 69–73. [Google Scholar] [CrossRef]
- Dolińska, M.; Zabłocka, B.; Sonnewald, U.; Albrecht, J. Glutamine uptake and expression of mRNA’s of glutamine transporting proteins in mouse cerebellar and cerebral cortical astrocytes and neurons. Neurochem. Int. 2004, 44, 75–81. [Google Scholar] [CrossRef]
- Gliddon, C.M.; Shao, Z.; LeMaistre, J.L.; Anderson, C.M. Cellular distribution of the neutral amino acid transporter subtype ASCT2 in mouse brain. J. Neurochem. 2009, 108, 372–383. [Google Scholar] [CrossRef]
- Zerangue, N.; Kavanaugh, M.P. ASCT-1 Is a Neutral Amino Acid Exchanger with Chloride Channel Activity. J. Biol. Chem. 1996, 271, 27991–27994. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.A.; Schmitz, D.; Reimer, R.J.; Larsson, P.; Gray, A.T.; Nicoll, R.; Kavanaugh, M.; Edwards, R.H. Glutamine Uptake by Neurons: Interaction of Protons with System A Transporters. J. Neurosci. 2002, 22, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. Linear and Nonlinear Mixed Effects Models. Available online: https://CRAN.R-project.org/package=nlme (accessed on 9 April 2023).
- Grewer, C.; Grabsch, E. New inhibitors for the neutral amino acid transporter ASCT2 reveal its Na+-dependent anion leak. J. Physiol. 2004, 557, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.N.; Oxender, D.L.; Liang, M.; Vatz, K.A. The Use of N-Methylation to Direct the Route of Mediated Transport of Amino Acids. J. Biol. Chem. 1965, 240, 3609–3616. [Google Scholar] [CrossRef]
- Ishiwata, S.; Ogata, S.; Umino, A.; Shiraku, H.; Ohashi, Y.; Kajii, Y.; Nishikawa, T. Increasing effects of S-methyl-L-cysteine on the extracellular D-serine concentrations in the rat medial frontal cortex. Amino Acids 2013, 44, 1391–1395. [Google Scholar] [CrossRef]
- Kanai, Y.; Segawa, H.; Miyamoto, K.-I.; Uchino, H.; Takeda, E.; Endou, H. Expression Cloning and Characterization of a Transporter for Large Neutral Amino Acids Activated by the Heavy Chain of 4F2 Antigen (CD98). J. Biol. Chem. 1998, 273, 23629–23632. [Google Scholar] [CrossRef]
- Pinilla-Tenas, J.; Barber, A.; Lostao, M.P. Transport of proline and hydroxyproline by the neutral amino-acid exchanger ASCT1. J. Membr. Biol. 2003, 195, 27–32. [Google Scholar] [CrossRef]
- Scalise, M.; Pochini, L.; Panni, S.; Pingitore, P.; Hedfalk, K.; Indiveri, C. Transport mechanism and regulatory properties of the human amino acid transporter ASCT2 (SLC1A5). Amino Acids 2014, 46, 2463–2475. [Google Scholar] [CrossRef]
- Mazza, T.; Scalise, M.; Pappacoda, G.; Pochini, L.; Indiveri, C. The involvement of sodium in the function of the human amino acid transporter ASCT2. FEBS Lett. 2021, 595, 3030–3041. [Google Scholar] [CrossRef]
- Wang, J.; Dong, Y.; Grewer, C. Functional and Kinetic Comparison of Alanine Cysteine Serine Transporters ASCT1 and ASCT2. Biomolecules 2022, 12, 113. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2021, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.R.; Islam, S.A.; Sternberg, M.J.E. EzMol: A Web Server Wizard for the Rapid Visualization and Image Production of Protein and Nucleic Acid Structures. J. Mol. Biol. 2018, 430, 2244–2248. [Google Scholar] [CrossRef]
- Kaplan, E.; Zubedat, S.; Radzishevsky, I.; Valenta, A.C.; Rechnitz, O.; Sason, H.; Sajrawi, C.; Bodner, O.; Konno, K.; Esaki, K.; et al. ASCT1 (Slc1a4) transporter is a physiologic regulator of brain d-serine and neurodevelopment. Proc. Natl. Acad. Sci. USA 2018, 115, 9628–9633. [Google Scholar] [CrossRef] [PubMed]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Papouin, T.; Dunphy, J.; Tolman, M.; Foley, J.C.; Haydon, P.G. Astrocytic control of synaptic function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160154. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.S.; Choi, D.H.; Lee, H.N.; Kim, D.W.; Chung, C.K.; Cho, S.S. Expression of l-Serine Biosynthetic Enzyme 3-Phosphoglycerate Dehydrogenase (Phgdh) and Neutral Amino Acid Transporter ASCT1 Following an Excitotoxic Lesion in the Mouse Hippocampus. Neurochem. Res. 2009, 34, 827–834. [Google Scholar] [CrossRef]
- Mackenzie, B.; Schafer, M.K.; Erickson, J.D.; Hediger, M.A.; Weihe, E.; Varoqui, H. Functional properties and cellular distribution of the system A glutamine transporter SNAT1 support specialized roles in central neurons. J. Biol. Chem. 2003, 278, 23720–23730. [Google Scholar] [CrossRef]
- Helboe, L.; Egebjerg, J.; Møller, M.; Thomsen, C. Distribution and pharmacology of alanine–serine–cysteine transporter 1 (asc-1) in rodent brain. Eur. J. Neurosci. 2003, 18, 2227–2238. [Google Scholar] [CrossRef]
- Matsuo, H.; Kanai, Y.; Tokunaga, M.; Nakata, T.; Chairoungdua, A.; Ishimine, H.; Tsukada, S.; Ooigawa, H.; Nawashiro, H.; Kobayashi, Y.; et al. High affinity d- and l-serine transporter Asc-1: Cloning and dendritic localization in the rat cerebral and cerebellar cortices. Neurosci. Lett. 2004, 358, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.P.; Dougherty, J.D.; Heiman, M.; Schmidt, E.F.; Stevens, T.R.; Ma, G.; Bupp, S.; Shrestha, P.; Shah, R.D.; Doughty, M.L.; et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 2008, 135, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Sakimura, K.; Nakao, K.; Yoshikawa, M.; Suzuki, M.; Kimura, H. A novel Na(+)-Independent alanine-serine-cysteine transporter 1 inhibitor inhibits both influx and efflux of D-Serine. J. Neurosci. Res. 2016, 94, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Clémençon, B.; Simonin, A.; Leuenberger, M.; Lochner, M.; Weisstanner, M.; Hediger, M.A. The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol. Asp. Med. 2013, 34, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Fairman, W.A.; Vandenberg, R.J.; Arriza, J.L.; Kavanaugh, M.P.; Amara, S.G. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature 1995, 375, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Billups, B.; Rossi, D.; Attwell, D. Anion Conductance Behavior of the Glutamate Uptake Carrier in Salamander Retinal Glial Cells. J. Neurosci. 1996, 16, 6722–6731. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Diamond, J.M. Anion selectivity in biological systems. Physiol. Rev. 1977, 57, 109–156. [Google Scholar] [CrossRef]
- Fahlke, C.; Kortzak, D.; Machtens, J.P. Molecular physiology of EAAT anion channels. Pflug. Arch. Eur. J. Physiol. 2016, 468, 491–502. [Google Scholar] [CrossRef]
- Fossat, P.; Turpin, F.R.; Sacchi, S.; Dulong, J.; Shi, T.; Rivet, J.M.; Sweedler, J.V.; Pollegioni, L.; Millan, M.J.; Oliet, S.H.; et al. Glial D-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb. Cortex 2012, 22, 595–606. [Google Scholar] [CrossRef]
- Mothet, J.P.; Pollegioni, L.; Ouanounou, G.; Martineau, M.; Fossier, P.; Baux, G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl. Acad. Sci. USA 2005, 102, 5606–5611. [Google Scholar] [CrossRef]
- Martineau, M.; Galli, T.; Baux, G.; Mothet, J.P. Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. Glia 2008, 56, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Sacchi, S. Metabolism of the neuromodulator D-serine. Cell. Mol. Life Sci. 2010, 67, 2387–2404. [Google Scholar] [CrossRef] [PubMed]
- Horiike, K.; Tojo, H.; Arai, R.; Yamano, T.; Nozaki, M.; Maeda, T. Localization of D-amino acid oxidase in Bergmann glial cells and astrocytes of rat cerebellum. Brain Res. Bull. 1987, 19, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Horiike, K.; Tojo, H.; Arai, R.; Nozaki, M.; Maeda, T. d-Amino-acid oxidase is confined to the lower brain stem and cerebellum in rat brain: Regional differentiation of astrocytes. Brain Res. 1994, 652, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Nardacci, R.; Cimini, A.; Cer, M.P. Immunocytochemical localization of d-amino acid oxidase in rat brain. J. Neurocytol. 1999, 28, 169–185. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.; Panizzutti, R.; Foltyn, V.N.; Wolosker, H. Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonist D-serine. Proc. Natl. Acad. Sci. USA 2002, 99, 14542–14547. [Google Scholar] [CrossRef] [PubMed]
- Foltyn, V.N.; Bendikov, I.; De Miranda, J.; Panizzutti, R.; Dumin, E.; Shleper, M.; Li, P.; Toney, M.D.; Kartvelishvily, E.; Wolosker, H. Serine Racemase Modulates Intracellular D-Serine Levels through an α,β-Elimination Activity. J. Biol. Chem. 2005, 280, 1754–1763. [Google Scholar] [CrossRef]
- Xia, M.; Liu, Y.; Figueroa, D.J.; Chiu, C.S.; Wei, N.; Lawlor, A.M.; Lu, P.; Sur, C.; Koblan, K.S.; Connolly, T.M. Characterization and localization of a human serine racemase. Brain Res. Mol. Brain Res. 2004, 125, 96–104. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Takayasu, N.; Hashimoto, A.; Sato, Y.; Tamaki, R.; Tsukamoto, H.; Kobayashi, H.; Noda, S. The serine racemase mRNA is predominantly expressed in rat brain neurons. Arch. Histol. Cytol. 2007, 70, 127–134. [Google Scholar] [CrossRef]
- Ding, X.; Ma, N.; Nagahama, M.; Yamada, K.; Semba, R. Localization of D-serine and serine racemase in neurons and neuroglias in mouse brain. Neurol. Sci. 2011, 32, 263–267. [Google Scholar] [CrossRef]
- Ehmsen, J.T.; Ma, T.M.; Sason, H.; Rosenberg, D.; Ogo, T.; Furuya, S.; Snyder, S.H.; Wolosker, H. D-serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J. Neurosci. 2013, 33, 12464–12469. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, P.; Pochini, L.; Scalise, M.; Galluccio, M.; Hedfalk, K.; Indiveri, C. Large scale production of the active human ASCT2 (SLC1A5) transporter in Pichia pastoris--functional and kinetic asymmetry revealed in proteoliposomes. Biochim. Biophys. Acta 2013, 1828, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Gauthier-Coles, G.; Fairweather, S.J.; Bröer, A.; Bröer, S. Do Amino Acid Antiporters Have Asymmetric Substrate Specificity? Biomolecules 2023, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Bading, H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010, 11, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.J.; Tapanes, S.A.; Loris, Z.B.; Balu, D.T.; Sick, T.J.; Coyle, J.T.; Liebl, D.J. Enhanced astrocytic d-serine underlies synaptic damage after traumatic brain injury. J. Clin. Investig. 2017, 127, 3114–3125. [Google Scholar] [CrossRef] [PubMed]
- Tapanes, S.A.; Arizanovska, D.; Díaz, M.M.; Folorunso, O.O.; Harvey, T.; Brown, S.E.; Radzishevsky, I.; Close, L.N.; Jagid, J.R.; Graciolli Cordeiro, J.; et al. Inhibition of glial D-serine release rescues synaptic damage after brain injury. Glia 2022, 70, 1133–1152. [Google Scholar] [CrossRef]
- Balu, D.T.; Pantazopoulos, H.; Huang, C.C.Y.; Muszynski, K.; Harvey, T.L.; Uno, Y.; Rorabaugh, J.M.; Galloway, C.R.; Botz-Zapp, C.; Berretta, S.; et al. Neurotoxic astrocytes express the d-serine synthesizing enzyme, serine racemase, in Alzheimer’s disease. Neurobiol. Dis. 2019, 130, 104511. [Google Scholar] [CrossRef]
- Folorunso, O.O.; Harvey, T.L.; Brown, S.E.; Chelini, G.; Berretta, S.; Balu, D.T. The D-serine biosynthetic enzyme serine racemase is expressed by reactive astrocytes in the amygdala of human and a mouse model of Alzheimer’s disease. Neurosci. Lett. 2023, 792, 136958. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, J.; Zhang, H.; Barger, S.W. Serine Racemase Expression Differentiates Aging from Alzheimer’s Brain. Curr. Alzheimer. Res. 2022, 19, 494–502. [Google Scholar] [CrossRef]
- Orzylowski, M.; Fujiwara, E.; Mousseau, D.D.; Baker, G.B. An Overview of the Involvement of D-Serine in Cognitive Impairment in Normal Aging and Dementia. Front. Psychiatry 2021, 12, 754032. [Google Scholar] [CrossRef]
- Piubelli, L.; Pollegioni, L.; Rabattoni, V.; Mauri, M.; Princiotta Cariddi, L.; Versino, M.; Sacchi, S. Serum D-serine levels are altered in early phases of Alzheimer’s disease: Towards a precocious biomarker. Transl. Psychiatry 2021, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Cui, E.H.; Ji, C.M.; Zhang, X.B.; Wang, Z.A.; Sun, Y.Z.; Xu, J.C.; Zhai, X.F.; Chen, Z.J.; Li, J.; et al. Specific knockdown of hippocampal astroglial EphB2 improves synaptic function via inhibition of D-serine secretion in APP/PS1 mice. Am. J. Transl. Res. 2019, 11, 1073–1083. [Google Scholar] [PubMed]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Miraucourt, L.S.; Peirs, C.; Dallel, R.; Voisin, D.L. Glycine inhibitory dysfunction turns touch into pain through astrocyte-derived D-serine. Pain 2011, 152, 1340–1348. [Google Scholar] [CrossRef]
- Lefèvre, Y.; Amadio, A.; Vincent, P.; Descheemaeker, A.; Oliet, S.H.; Dallel, R.; Voisin, D.L. Neuropathic pain depends upon D-serine co-activation of spinal NMDA receptors in rats. Neurosci. Lett. 2015, 603, 42–47. [Google Scholar] [CrossRef]
- Javitt, D.C.; Kantrowitz, J.T. The glutamate/N-methyl-d-aspartate receptor (NMDAR) model of schizophrenia at 35: On the path from syndrome to disease. Schizophr. Res. 2022, 242, 56–61. [Google Scholar] [CrossRef]
- Basu, A.C.; Tsai, G.E.; Ma, C.L.; Ehmsen, J.T.; Mustafa, A.K.; Han, L.; Jiang, Z.I.; Benneyworth, M.A.; Froimowitz, M.P.; Lange, N.; et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry 2009, 14, 719–727. [Google Scholar] [CrossRef]
- Labrie, V.; Fukumura, R.; Rastogi, A.; Fick, L.J.; Wang, W.; Boutros, P.C.; Kennedy, J.L.; Semeralul, M.O.; Lee, F.H.; Baker, G.B.; et al. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum. Mol. Genet. 2009, 18, 3227–3243. [Google Scholar] [CrossRef]
- Matveeva, T.M.; Pisansky, M.T.; Young, A.; Miller, R.F.; Gewirtz, J.C. Sociality deficits in serine racemase knockout mice. Brain Behav. 2019, 9, e01383. [Google Scholar] [CrossRef] [PubMed]
- Jami, S.A.; Cameron, S.; Wong, J.M.; Daly, E.R.; McAllister, A.K.; Gray, J.A. Increased excitation-inhibition balance and loss of GABAergic synapses in the serine racemase knockout model of NMDA receptor hypofunction. J. Neurophysiol. 2021, 126, 11–27. [Google Scholar] [CrossRef]
- Morita, Y.; Ujike, H.; Tanaka, Y.; Otani, K.; Kishimoto, M.; Morio, A.; Kotaka, T.; Okahisa, Y.; Matsushita, M.; Morikawa, A.; et al. A genetic variant of the serine racemase gene is associated with schizophrenia. Biol. Psychiatry 2007, 61, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- de Bartolomeis, A.; Vellucci, L.; Austin, M.C.; De Simone, G.; Barone, A. Rational and Translational Implications of D-Amino Acids for Treatment-Resistant Schizophrenia: From Neurobiology to the Clinics. Biomolecules 2022, 12, 909. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Llenos, I.C.; Dulay, J.R.; Verma, N.; Sabunciyan, S.; Yolken, R.H. Changes in region- and cell type-specific expression patterns of neutral amino acid transporter 1 (ASCT-1) in the anterior cingulate cortex and hippocampus in schizophrenia, bipolar disorder and major depression. J. Neural Transm. 2007, 114, 261–271. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnan, K.S.; Billups, B. ASC Transporters Mediate D-Serine Transport into Astrocytes Adjacent to Synapses in the Mouse Brain. Biomolecules 2023, 13, 819. https://doi.org/10.3390/biom13050819
Krishnan KS, Billups B. ASC Transporters Mediate D-Serine Transport into Astrocytes Adjacent to Synapses in the Mouse Brain. Biomolecules. 2023; 13(5):819. https://doi.org/10.3390/biom13050819
Chicago/Turabian StyleKrishnan, Karthik Subramanian, and Brian Billups. 2023. "ASC Transporters Mediate D-Serine Transport into Astrocytes Adjacent to Synapses in the Mouse Brain" Biomolecules 13, no. 5: 819. https://doi.org/10.3390/biom13050819
APA StyleKrishnan, K. S., & Billups, B. (2023). ASC Transporters Mediate D-Serine Transport into Astrocytes Adjacent to Synapses in the Mouse Brain. Biomolecules, 13(5), 819. https://doi.org/10.3390/biom13050819







