Selenium and Selenoproteins in Health
Abstract
1. Introduction
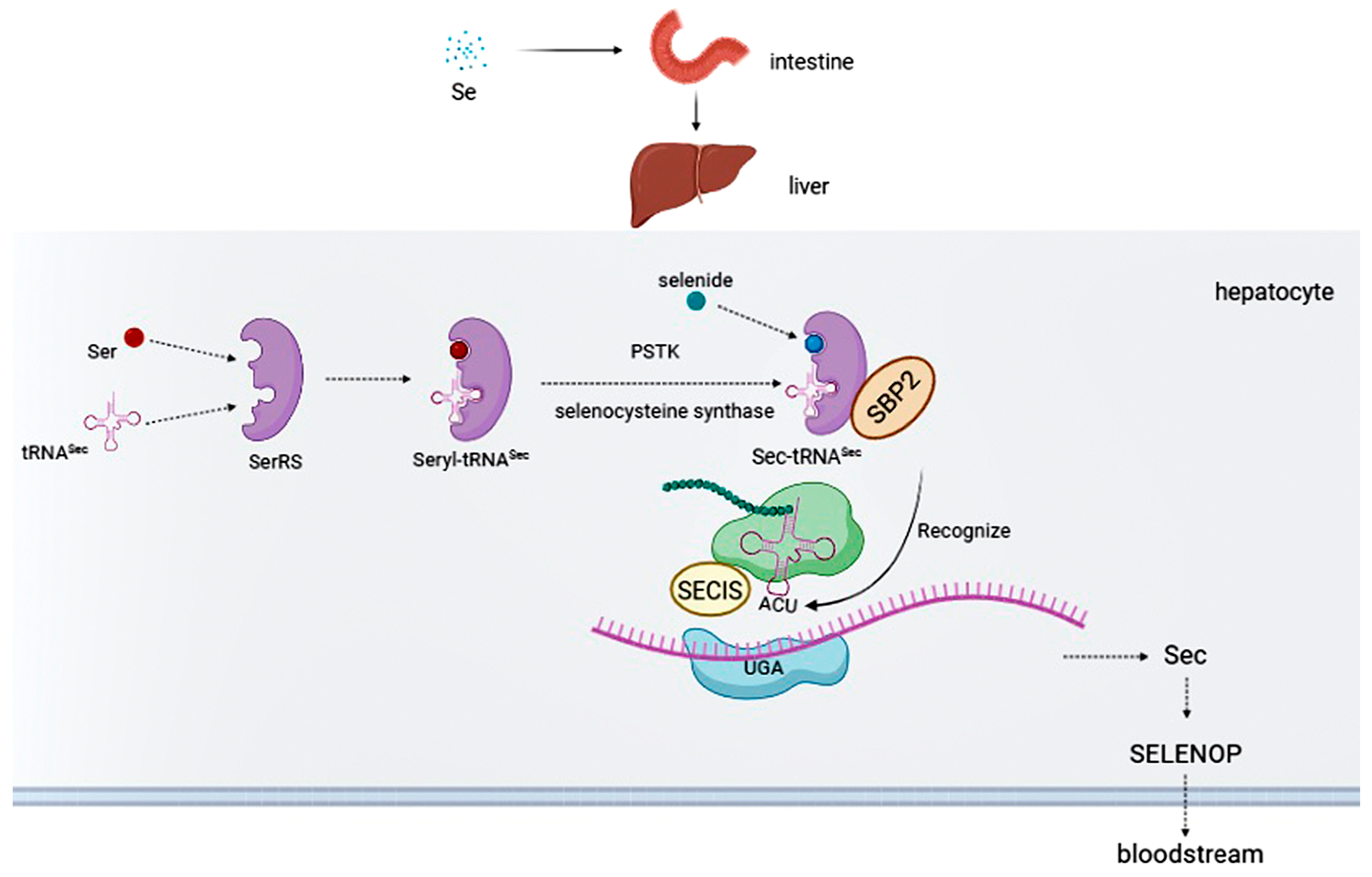
2. Selenium Intake
3. Selenoproteins
4. Health Effects of Selenium and Selenoproteins
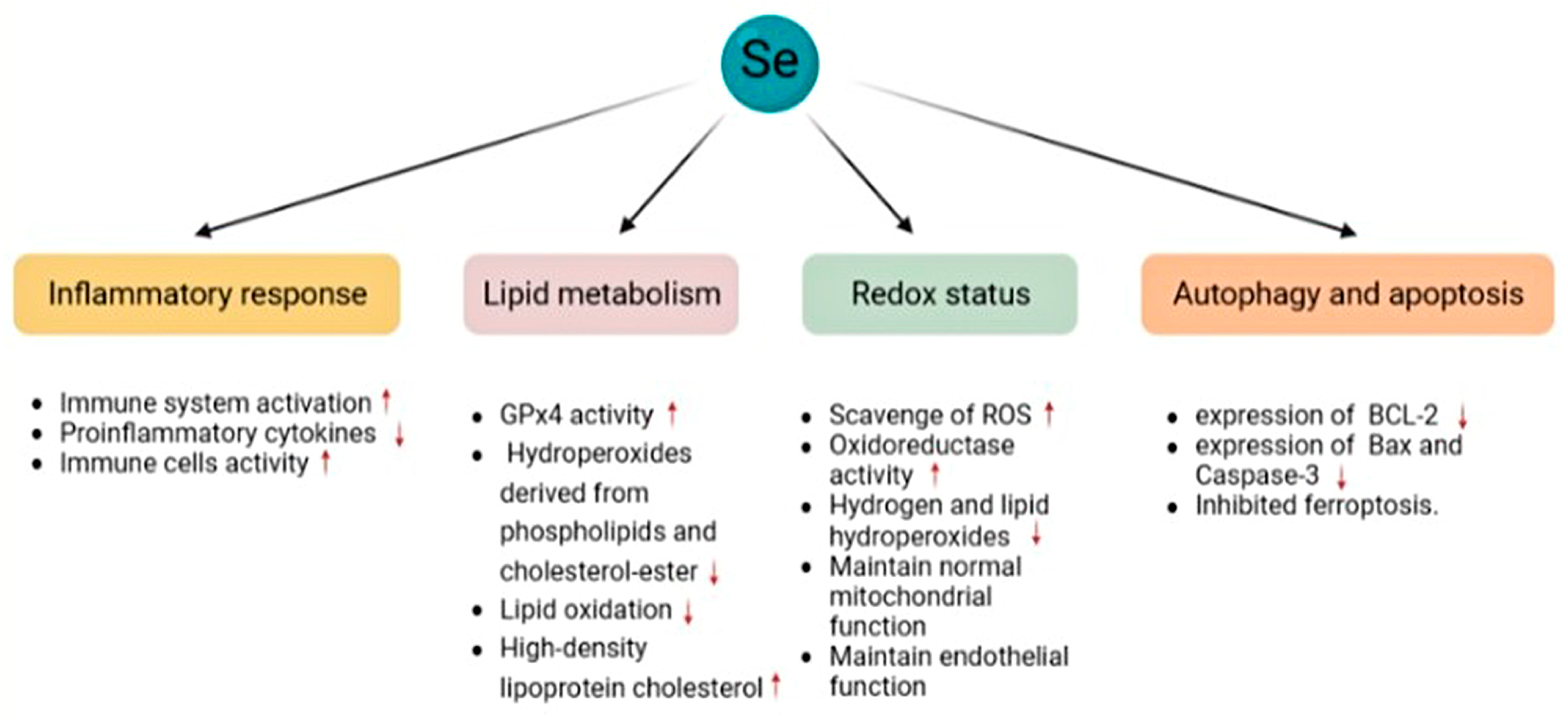
4.1. Oxidative Stress
4.2. Immune System
4.2.1. Innate Immunity
4.2.2. Adaptive Immunity
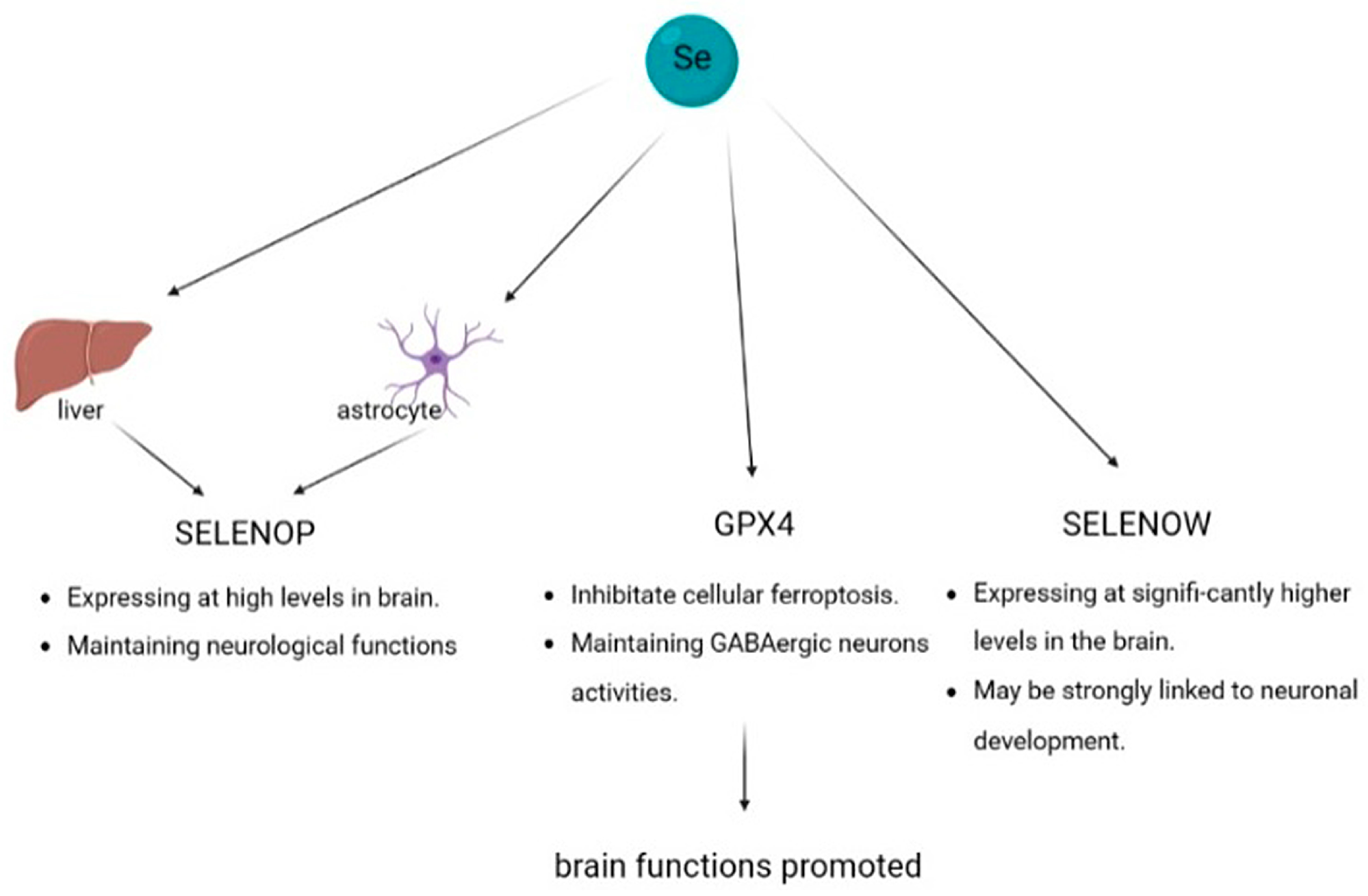
4.3. Brain Function
4.3.1. Selenoprotein P
4.3.2. Glutathione Peroxidase 4
4.3.3. Selenoprotein W
4.4. Cardiovascular System
4.5. Cancer
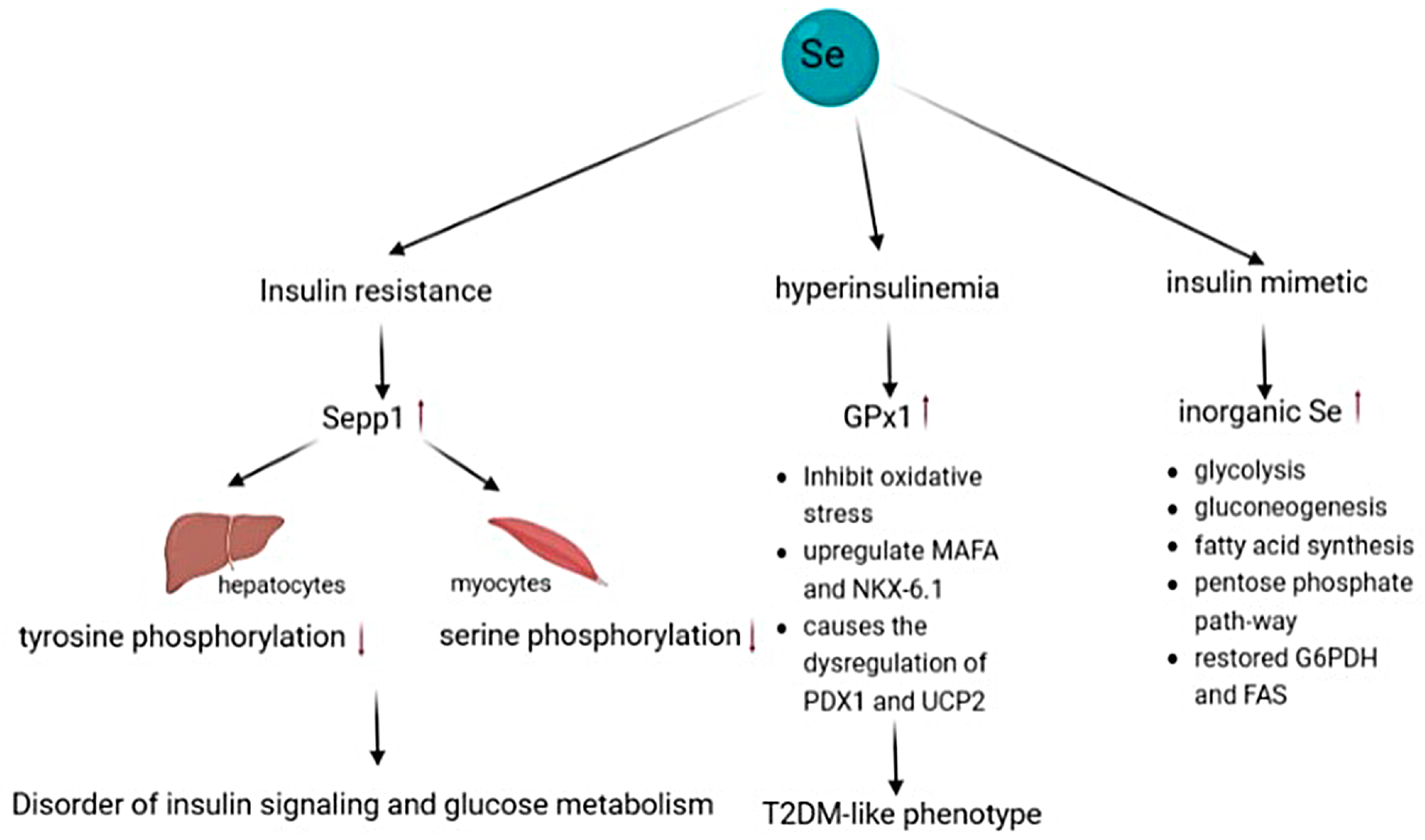
4.6. Type 2 Diabetes
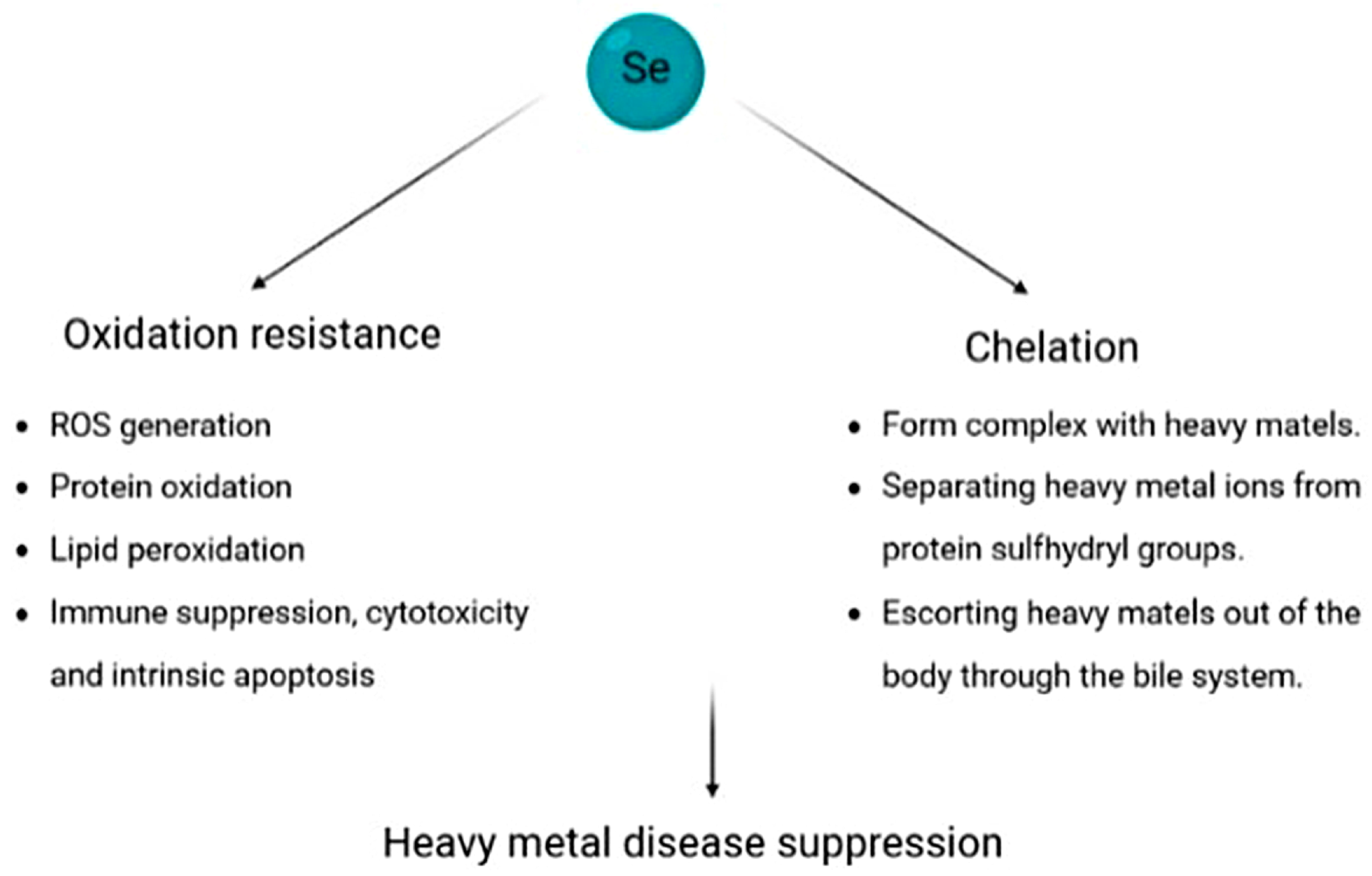
4.7. Heavy Metal-Based Illness
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mertz, W. The Essential Trace Elements. Science 1981, 213, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Foltz, C.M. Factor 3 activity of selenium compounds. J. Biol. Chem. 1958, 233, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, T.C. Selenocysteine. Annu. Rev. Biochem. 1996, 65, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.; Holmgren, A. The thioredoxin system in cancer. Semin. Cancer Biol. 2006, 16, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Lee, J.; Jung, J.; Carlson, B.A.; Chang, M.J.; Chang, C.B.; Kang, S.-B.; Lee, B.C.; Gladyshev, V.N.; Hatfield, D.L.; et al. Selenophosphate synthetase 1 deficiency exacerbates osteoarthritis by dysregulating redox homeostasis. Nat. Commun. 2022, 13, 779. [Google Scholar] [CrossRef]
- Xie, M.; Sun, X.; Li, P.; Shen, X.; Fang, Y. Selenium in cereals: Insight into species of the element from total amount. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2914–2940. [Google Scholar] [CrossRef]
- Alcântara, D.B.; Dionísio, A.P.; Artur, A.G.; Silveira, B.K.; Lopes, A.F.; Guedes, J.A.; Luz, L.R.; Nascimento, R.F.; Lopes, G.S.; Hermsdorff, H.H.; et al. Selenium in Brazil nuts: An overview of agronomical aspects, recent trends in analytical chemistry, and health outcomes. Food Chem. 2022, 372, 131207. [Google Scholar] [CrossRef]
- Yang, H.; Yang, X.; Ning, Z.; Kwon, S.Y.; Li, M.-L.; Tack, F.M.; Kwon, E.E.; Rinklebe, J.; Yin, R. The beneficial and hazardous effects of selenium on the health of the soil-plant-human system: An overview. J. Hazard. Mater. 2022, 422, 126876. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Selenium in plant foods: Speciation analysis, bioavailability, and factors affecting composition. Crit. Rev. Food Sci. Nutr. 2021, 61, 1340–1352. [Google Scholar] [CrossRef]
- Finley, J.W. Bioavailability of selenium from foods. Nutr. Rev. 2006, 64, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Ip, C.; Hayes, C.; Budnick, R.M.; Ganther, H.E. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Res. 1991, 51, 595–600. [Google Scholar]
- Klimaszewska, M.; Górska, S.; Dawidowski, M.; Podsadni, P.; Turło, J. Biosynthesis of Se-methyl-seleno-l-cysteine in Basidiomycetes fungus Lentinula edodes (Berk.) Pegler. Springerplus 2016, 5, 733. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E. Regulation of Selenium Metabolism and Transport. Annu. Rev. Nutr. 2015, 35, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Adadi, P.; Barakova, N.V.; Muravyov, K.Y.; Krivoshapkina, E.F. Designing selenium functional foods and beverages: A review. Food Res. Int. 2019, 120, 708–725. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.D.; Pipkin, F.B.; Redman, C.W.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Leiter, O.; Zhuo, Z.; Rust, R.; Wasielewska, J.M.; Grönnert, L.; Kowal, S.; Overall, R.W.; Adusumilli, V.S.; Blackmore, D.G.; Southon, A.; et al. Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging. Cell Metab. 2022, 34, 408–423. [Google Scholar] [CrossRef]
- Gharipour, M.; Ouguerram, K.; Nazih, E.; Salehi, M.; Behmanesh, M.; Roohafza, H.; Hosseini, S.M.; Nezafati, P.; Dianatkhah, M.; Gharipour, A.; et al. Effects of selenium supplementation on expression of SEPP1 in mRNA and protein levels in subjects with and without metabolic syndrome suffering from coronary artery disease: Selenegene study a double-blind randomized controlled trial. J. Cell Biochem. 2018, 119, 8282–8289. [Google Scholar] [CrossRef]
- Hill, K.E.; Wu, S.; Motley, A.K.; Stevenson, T.D.; Winfrey, V.P.; Capecchi, M.R.; Atkins, J.; Burk, R.F. Production of selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis. J. Biol. Chem. 2012, 287, 40414–40424. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Zhang, Y.; Romero, H.; Salinas, G.; Gladyshev, V.N. Dynamic evolution of selenocysteine utilization in bacteria: A balance between selenoprotein loss and evolution of selenocysteine from redox active cysteine residues. Genome. Biol. 2006, 7, R94. [Google Scholar] [CrossRef] [PubMed]
- Castellano, S.; Andrés, A.M.; Bosch, E.; Bayes, M.; Guigó, R.; Clark, A.G. Low exchangeability of selenocysteine, the 21st amino acid, in vertebrate proteins. Mol. Biol. Evol. 2009, 26, 2031–2040. [Google Scholar] [CrossRef]
- Shu, N.; Cheng, Q.; Arnér, E.S.; Davies, M.J. Inhibition and crosslinking of the selenoprotein thioredoxin reductase-1 by p-benzoquinone. Redox Biol. 2020, 28, 101335. [Google Scholar] [CrossRef]
- Zeida, A.; Trujillo, M.; Ferrer-Sueta, G.; Denicola, A.; Estrin, D.A.; Radi, R. Catalysis of Peroxide Reduction by Fast Reacting Protein Thiols. Chem. Rev. 2019, 119, 10829–10855. [Google Scholar] [CrossRef] [PubMed]
- Mobli, M.; Morgenstern, D.; King, G.F.; Alewood, P.F.; Muttenthaler, M. Site-specific pK(a) determination of selenocysteine residues in selenovasopressin by using 77Se NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 2011, 50, 11952–11955. [Google Scholar] [CrossRef] [PubMed]
- Mousa, R.; Dardashti, R.N.; Metanis, N. Selenium and Selenocysteine in Protein Chemistry. Angew. Chem. Int. Ed. Engl. 2017, 56, 15818–15827. [Google Scholar] [CrossRef]
- Sheppard, K.; Yuan, J.; Hohn, M.J.; Jester, B.; Devine, K.M.; Söll, D. From one amino acid to another: tRNA-dependent amino acid biosynthesis. Nucleic Acids. Res. 2008, 36, 1813–1825. [Google Scholar] [CrossRef]
- Arnér, E.S. Common modifications of selenocysteine in selenoproteins. Essays Biochem. 2020, 64, 45–53. [Google Scholar] [CrossRef]
- Commans, S.; Böck, A. Selenocysteine inserting tRNAs: An overview. FEMS Microbiol. Rev. 1999, 23, 335–351. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Y.; Tian, Q.; Jia, Q.; Gao, Y.; Zhang, Q.; Zhou, C.; Xie, W. SerRS-tRNASec complex structures reveal mechanism of the first step in selenocysteine biosynthesis. Nucleic Acids. Res. 2015, 43, 10534–10545. [Google Scholar]
- Fischer, N.; Neumann, P.; Bock, L.V.; Maracci, C.; Wang, Z.; Paleskava, A.; Konevega, A.L.; Schröder, G.F.; Grubmüller, H.; Ficner, R.; et al. The pathway to GTPase activation of elongation factor SelB on the ribosome. Nature 2016, 540, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, E.; Agostini, M.; Mitchell, C.; Schoenmakers, N.; Papp, L.; Rajanayagam, O.; Padidela, R.; Ceron-Gutierrez, L.; Doffinger, R.; Prevosto, C.; et al. Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J. Clin. Investig. 2010, 120, 4220–4235. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, A.M.; Liao, X.-H.; Abdullah, M.S.Y.; Lado-Abeal, J.; Majed, F.A.; Moeller, L.; Boran, G.; Schomburg, L.; Weiss, R.E.; Refetoff, S. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat. Genet. 2005, 37, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Chavatte, L.; Ii, B.A.B.; Driscoll, D.M. Ribosomal protein L30 is a component of the UGA-selenocysteine recoding machinery in eukaryotes. Nat. Struct. Mol. Biol. 2005, 12, 408–416. [Google Scholar] [CrossRef]
- Papp, L.V.; Wang, J.; Kennedy, D.; Boucher, D.; Zhang, Y.; Gladyshev, V.N.; Singh, R.N.; Khanna, K.K. Functional characterization of alternatively spliced human SECISBP2 transcript variants. Nucleic Acids. Res. 2008, 36, 7192–7206. [Google Scholar] [CrossRef]
- Peeler, J.C.; Weerapana, E. Chemical Biology Approaches to Interrogate the Selenoproteome. Acc. Chem. Res. 2019, 52, 2832–2840. [Google Scholar] [CrossRef]
- Stadtman, T.C. Selenium biochemistry. Annu. Rev. Biochem. 1990, 59, 111–127. [Google Scholar] [CrossRef]
- Spallholz, J.E.; Boylan, L.M.; Larsen, H.S. Advances in understanding selenium’s role in the immune system. Ann. N. Y. Acad. Sci. 1990, 587, 123–139. [Google Scholar] [CrossRef]
- Allan, C.B.; Lacourciere, G.M.; Stadtman, T.C. Responsiveness of selenoproteins to dietary selenium. Annu. Rev. Nutr. 1999, 19, 1–16. [Google Scholar] [CrossRef]
- Schomburg, L.; Köhrle, J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol. Nutr. Food Res. 2008, 52, 1235–1246. [Google Scholar] [CrossRef]
- Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Burk, R.F. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J. Biol. Chem. 2008, 283, 6854–6860. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.E.; Jowett, J.B.M.; Elliott, K.S.; Gao, Y.; Gluschenko, K.; Wang, J.; Azim, D.M.A.; Cai, G.; Mahaney, M.C.; Comuzzie, A.G.; et al. Genetic variation in selenoprotein S influences inflammatory response. Nat. Genet. 2005, 37, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.A.; Hoffmann, P.R. The human selenoproteome: Recent insights into functions and regulation. Cell Mol. Life. Sci. 2009, 66, 2457–2478. [Google Scholar] [CrossRef] [PubMed]
- Beilstein, M.; Vendeland, S.; Barofsky, E.; Jensen, O.; Whanger, P. Selenoprotein W of rat muscle binds glutathione and an unknown small molecular weight moiety. J. Inorg. Biochem. 1996, 61, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Muntoni, F.; Brockington, M.; Godfrey, C.; Ackroyd, M.; Robb, S.; Manzur, A.; Kinali, M.; Mercuri, E.; Kaluarachchi, M.; Feng, L.; et al. Muscular dystrophies due to defective glycosylation of dystroglycan. Acta Myol. 2007, 26, 129–135. [Google Scholar] [PubMed]
- Zhao, Y.; Wang, H.; Zhou, J.; Shao, Q. Glutathione Peroxidase GPX1 and its Dichotomous Roles in Cancer. Cancers 2022, 14, 2560. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, Y.; Fei, Y.; Zhang, R.; Han, Y.; Lu, S. GPx1 knockdown suppresses chondrogenic differentiation of ATDC5 cells through induction of reductive stress. Acta Biochim. Biophys. Sin. 2017, 49, 110–118. [Google Scholar] [CrossRef]
- Kafeel, S.; Hashim, Z.; Fawwad, A.; Nawab, S.N. Predisposition of SOD1, GPX1, CAT genetic variants and their haplotypes in cataractogenesis of type 2 diabetes mellitus in Pakistan. Acta Diabetol. 2022, 59, 623–632. [Google Scholar] [CrossRef]
- Johnson, L.A.; Phillips, J.A.; Mauer, C.; Edwards, M.; Balldin, V.H.; Hall, J.R.; Barber, R.; Conger, T.L.; Ho, E.J.; O’bryant, S.E. The impact of GPX1 on the association of groundwater selenium and depression: A Project FRONTIER study. BMC. Psychiatry 2013, 13, 7. [Google Scholar] [CrossRef]
- Pei, J.; Fu, W.; Yang, L.; Zhang, Z.; Liu, Y. Oxidative stress is involved in the pathogenesis of Keshan disease (an endemic dilated cardiomyopathy) in China. Oxid. Med. Cell Longev. 2013, 2013, 474203. [Google Scholar] [CrossRef]
- Orhan, H.; Marol, S.; Hepşen, I.F.; Şahin, G. Effects of some probable antioxidants on selenite-induced cataract formation and oxidative stress-related parameters in rats. Toxicology 1999, 139, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, Y.; Wang, C.; Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Kipp, A.P. Physiological functions of GPx2 and its role in inflammation-triggered carcinogenesis. Ann. N. Y. Acad. Sci. 2012, 1259, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Esworthy, R.S.; Yang, L.; Frankel, P.H.; Chu, F.-F. Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. J. Nutr. 2005, 135, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Worley, B.L.; Phaëton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qin, Y.; Cheng, Z.; Cheng, X.; Wang, R.; Luo, X.; Zhao, Y.; Zhang, D.; Li, G. Gpx3 and Egr1 Are Involved in Regulating the Differentiation Fate of Cardiac Fibroblasts under Pressure Overload. Oxid. Med. Cell Longev. 2022, 2022, 3235250. [Google Scholar] [CrossRef]
- Covington, T.A.; Pilz, P.M.; Mulhern, R.M.; Ngoy, S.; Loscalzo, A.; Liu, J.; Fisch, S.; Grune, J. GPx3 deficiency exacerbates maladaptive right ventricular remodeling in experimental pulmonary artery banding. Am. J. Physiol. Lung. Cell Mol. Physiol. 2023, 324, L550–L556. [Google Scholar] [CrossRef]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Contribution of ferroptosis and GPX4’s dual functions to osteoarthritis progression. EBioMedicine 2022, 76, 103847. [Google Scholar]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, S.; Liu, X.; Deng, F.; Wang, P.; Yang, L.; Hu, L.; Huang, K.; He, J. PRMT4 promotes ferroptosis to aggravate doxorubicin-induced cardiomyopathy via inhibition of the Nrf2/GPX4 pathway. Cell Death Differ. 2022, 29, 1982–1995. [Google Scholar] [CrossRef]
- Zhang, J.; Bi, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wei, S.; Lv, Y.; et al. Involvement of GPX4 in irisin’s protection against ischemia reperfusion-induced acute kidney injury. J. Cell Physiol. 2021, 236, 931–945. [Google Scholar] [CrossRef]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; Barros, P.d.L.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef] [PubMed]
- Shema, R.; Kulicke, R.; Cowley, G.S.; Stein, R.; Root, D.E.; Heiman, M. Synthetic lethal screening in the mammalian central nervous system identifies Gpx6 as a modulator of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 268–272. [Google Scholar] [CrossRef]
- Lin, W.; Tang, Y.; Zhang, M.; Liang, B.; Wang, M.; Zha, L.; Yu, Z. Integrated Bioinformatic Analysis Reveals TXNRD1 as a Novel Biomarker and Potential Therapeutic Target in Idiopathic Pulmonary Arterial Hypertension. Front. Med. 2022, 9, 894584. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Meng, W.; Zeng, X.; Zhao, H.; Liu, W.; Zhang, T. TXNRD1 Is an Unfavorable Prognostic Factor for Patients with Hepatocellular Carcinoma. Biomed. Res. Int. 2017, 2017, 4698167. [Google Scholar] [CrossRef]
- Liang, J.; Wang, S.; Hu, J.; Hong, X.; Zhu, M.; Liu, X.; Alswadeh, M.; Mo, F.; Dai, M. Targeted inhibition of TXNRD1 prevents cartilage extracellular matrix degeneration by activating Nrf2 pathway in osteoarthritis. Biochem. Biophys. Res. Commun. 2022, 635, 267–276. [Google Scholar] [CrossRef]
- Kudin, A.P.; Baron, G.; Zsurka, G.; Hampel, K.G.; Elger, C.E.; Grote, A.; Weber, Y.; Lerche, H.; Thiele, H.; Nürnberg, P.; et al. Homozygous mutation in TXNRD1 is associated with genetic generalized epilepsy. Free Radic. Biol. Med. 2017, 106, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.N.C.; ANZRAG Consortium; Loomis, S.J.; Kang, J.H.; Allingham, R.R.; Gharahkhani, P.; Khor, C.C.; Burdon, K.P.; Aschard, H.; Chasman, D.I.; et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat. Genet. 2016, 48, 189–194. [Google Scholar] [CrossRef]
- Sibbing, D.; Pfeufer, A.; Perisic, T.; Mannes, A.M.; Fritz-Wolf, K.; Unwin, S.; Sinner, M.F.; Gieger, C.; Gloeckner, C.J.; Wichmann, H.-E.; et al. Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy. Eur. Heart J. 2011, 32, 1121–1133. [Google Scholar] [CrossRef]
- Kariž, S.; Mankoč, S.; Petrovič, D. Association of thioredoxin reductase 2 (TXNRD2) gene polymorphisms with myocardial infarction in Slovene patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2015, 108, 323–328. [Google Scholar] [CrossRef]
- Gencheva, R.; Arnér, E.S.J. Thioredoxin Reductase Inhibition for Cancer Therapy. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Aboobakar, I.F.; Kinzy, T.G.; Zhao, Y.; Fan, B.; Pasquale, L.R.; Qassim, A.; Kolovos, A.; Schmidt, J.M.; Craig, J.E.; Bailey, J.N.C.; et al. Mitochondrial TXNRD2 and ME3 genetic risk scores are associated with specific primary open-angle glaucoma phenotypes. Ophthalmology 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Turanov, A.A.; Mariotti, M.; Hwang, J.Y.; Lee, S.-G.; Paulo, J.A.; Yim, S.H.; Gygi, S.P.; Chung, J.-J.; Gladyshev, V.N. Selenoprotein TXNRD3 supports male fertility via the redox regulation of spermatogenesis. J. Biol. Chem. 2022, 298, 102183. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Du, P.; Zhu, Y.; Zhang, X.; Cai, J.; Zhang, Z. Thioredoxin reductase 3 suppression promotes colitis and carcinogenesis via activating pyroptosis and necrosis. Cell Mol. Life Sci. 2022, 79, 106. [Google Scholar] [CrossRef]
- França, M.M.; German, A.; Fernandes, G.W.; Liao, X.-H.; Bianco, A.C.; Refetoff, S.; Dumitrescu, A.M. Dumitrescu. Human Type 1 Iodothyronine Deiodinase (DIO1) Mutations Cause Abnormal Thyroid Hormone Metabolism. Thyroid 2021, 31, 202–207. [Google Scholar] [CrossRef]
- Bruinstroop, E.; Zhou, J.; Tripathi, M.; Yau, W.W.; Boelen, A.; Singh, B.K.; Yen, P.M. Early induction of hepatic deiodinase type 1 inhibits hepatosteatosis during NAFLD progression. Mol. Metab. 2021, 53, 101266. [Google Scholar] [CrossRef]
- Poplawski, P.; Rybicka, B.; Boguslawska, J.; Rodzik, K.; Visser, T.J.; Nauman, A.; Piekielko-Witkowska, A. Induction of type 1 iodothyronine deiodinase expression inhibits proliferation and migration of renal cancer cells. Mol. Cell Endocrinol. 2017, 442, 58–67. [Google Scholar] [CrossRef]
- Bomer, N.; Hollander, W.D.; Ramos, Y.F.M.; Bos, S.D.; van der Breggen, R.; Lakenberg, N.; Pepers, B.A.; van Eeden, A.E.; Darvishan, A.; Tobi, E.W.; et al. Underlying molecular mechanisms of DIO2 susceptibility in symptomatic osteoarthritis. Ann. Rheum. Dis. 2015, 74, 1571–1579. [Google Scholar] [CrossRef]
- Bradley, D.; Liu, J.; Blaszczak, A.; Wright, V.; Jalilvand, A.; Needleman, B.; Noria, S.; Renton, D.; Hsueh, W. Adipocyte DIO2 Expression Increases in Human Obesity but Is Not Related to Systemic Insulin Sensitivity. J. Diabetes Res. 2018, 2018, 2464652. [Google Scholar] [CrossRef]
- Guo, T.-W.; Zhang, F.-C.; Yang, M.-S.; Gao, X.-C.; Bian, L.; Duan, S.-W.; Zheng, Z.-J.; Gao, J.-J.; Wang, H.; Li, R.-L.; et al. Positive association of the DIO2 (deiodinase type 2) gene with mental retardation in the iodine-deficient areas of China. J. Med. Genet. 2004, 41, 585–590. [Google Scholar] [CrossRef]
- Meulenbelt, I.; Bos, S.D.; Chapman, K.; van der Breggen, R.; Houwing-Duistermaat, J.J.; Kremer, D.; Kloppenburg, M.; Carr, A.; Tsezou, A.; González, A.; et al. Meta-analyses of genes modulating intracellular T3 bio-availability reveal a possible role for the DIO3 gene in osteoarthritis susceptibility. Ann. Rheum. Dis. 2011, 70, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Brent, G.A. Thyroid hormone and the brain: Mechanisms of action in development and role in protection and promotion of recovery after brain injury. Pharmacol. Ther. 2018, 186, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Vidart, J.; Axelrud, L.; Braun, A.C.; Marschner, R.A.; Wajner, S.M. Relationship among Low T3 Levels, Type 3 Deiodinase, Oxidative Stress, and Mortality in Sepsis and Septic Shock: Defining Patient Outcomes. Int. J. Mol. Sci. 2023, 24, 3935. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, H.; Meng, F.; Sun, X.; Feng, X.; Chen, J.; Li, L.; Liu, J. Methionine Sulfoxide Reductase B1 Regulates Hepatocellular Carcinoma Cell Proliferation and Invasion via the Mitogen-Activated Protein Kinase Pathway and Epithelial-Mesenchymal Transition. Oxid. Med. Cell Longev. 2018, 2018, 5287971. [Google Scholar] [CrossRef]
- Lee, H.-J.; Park, J.S.; Yoo, H.J.; Lee, H.M.; Lee, B.C.; Kim, J.H. The Selenoprotein MsrB1 Instructs Dendritic Cells to Induce T-Helper 1 Immune Responses. Antioxidants 2020, 9, 1021. [Google Scholar] [CrossRef]
- Carlisle, A.E.; Lee, N.; Matthew-Onabanjo, A.N.; Spears, M.E.; Park, S.J.; Youkana, D.; Doshi, M.B.; Peppers, A.; Li, R.; Joseph, A.B.; et al. Selenium detoxification is required for cancer-cell survival. Nat. Metab. 2020, 2, 603–611. [Google Scholar] [CrossRef]
- Flowers, B.; Bochnacka, O.; Poles, A.; Diamond, A.M.; Kastrati, I. Distinct Roles of SELENOF in Different Human Cancers. Biomolecules 2023, 13, 486. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Zhou, J.; Liu, H. Selenoprotein F Knockout Caused Glucose Metabolism Disorder in Young Mice by Disrupting Redox Homeostasis. Antioxidants 2022, 11, 2105. [Google Scholar] [CrossRef]
- Bertz, M.; Kühn, K.; Koeberle, S.C.; Müller, M.F.; Hoelzer, D.; Thies, K.; Deubel, S.; Thierbach, R.; Kipp, A.P. Selenoprotein H controls cell cycle progression and proliferation of human colorectal cancer cells. Free Radic. Biol. Med. 2018, 127, 98–107. [Google Scholar] [CrossRef]
- Sarma, A.S.; Siddardha, B.; Pragna Lakshmi, T.; Ranganath, P.; Dalal, A. A novel homozygous synonymous splicing variant in SELENOI gene causes spastic paraplegia 81. J. Gene Med. 2023, 2023, e3501. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Song, G.L. Roles of Selenoproteins in Brain Function and the Potential Mechanism of Selenium in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 646518. [Google Scholar] [CrossRef] [PubMed]
- Abdurahman, A.; Li, Y.; Jia, S.-Z.; Xu, X.-W.; Lin, S.-J.; Ouyang, P.; He, Z.J.; Zhang, Z.-H.; Liu, Q.; Xu, Y.; et al. Knockdown of the SELENOK gene induces ferroptosis in cervical cancer cells. Metallomics 2023, 15, mfad019. [Google Scholar] [CrossRef] [PubMed]
- Rogachev, V.V.; Goltyaev, M.V.; Varlamova, E.G.; Turovsky, E.A. Turovsky. Molecular Mechanisms of the Cytotoxic Effect of Recombinant Selenoprotein SELENOM on Human Glioblastoma Cells. Int. J. Mol. Sci. 2023, 24, 6469. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Huang, J.; Yang, J.; Chen, X.; Zhang, H.; Zhu, Y.; Liu, Q.; Zhang, Z. The protective effect of selenoprotein M on non-alcoholic fatty liver disease: The role of the AMPKα1-MFN2 pathway and Parkin mitophagy. Cell Mol. Life Sci. 2022, 79, 354. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, C.; Ouyang, P.; Cai, Z.; Liu, X.; Abdurahman, A.; Peng, J.; Li, Y.; Zhang, Z.; Song, G.-L. SELENOM Knockout Induces Synaptic Deficits and Cognitive Dysfunction by Influencing Brain Glucose Metabolism. J. Agric. Food Chem. 2023, 71, 1607–1619. [Google Scholar] [CrossRef]
- Pozzer, D.; Varone, E.; Chernorudskiy, A.; Schiarea, S.; Missiroli, S.; Giorgi, C.; Pinton, P.; Canato, M.; Germinario, E.; Nogara, L.; et al. A maladaptive ER stress response triggers dysfunction in highly active muscles of mice with SELENON loss. Redox Biol. 2019, 20, 354–366. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Lv, H.-J.; Wu, Y.; Liu, S.; Deng, X.; Shi, B.; Fu, J. Comprehensive Analysis of Expression and Prognostic Value of Selenoprotein Genes in Thyroid Cancer. Genet. Test. Mol. Biomarkers 2022, 26, 159–173. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Q.; Song, T.; Wang, Z.; Song, L.; Yang, Y.; Shao, J.; Li, J.; Ni, Y.; Chao, N.; et al. The heterogeneous immune landscape between lung adenocarcinoma and squamous carcinoma revealed by single-cell RNA sequencing. Signal. Transduct. Target. Ther. 2022, 7, 289. [Google Scholar] [CrossRef]
- Short, S.P.; Pilat, J.M.; Barrett, C.W.; Reddy, V.K.; Haberman, Y.; Hendren, J.R.; Marsh, B.J.; Keating, C.E.; Motley, A.K.; Hill, K.E.; et al. Colonic Epithelial-Derived Selenoprotein P Is the Source for Antioxidant-Mediated Protection in Colitis-Associated Cancer. Gastroenterology 2021, 160, 1694–1708. [Google Scholar] [CrossRef]
- Ekoue, D.N.; Ansong, E.; Liu, L.; Macias, V.; Deaton, R.; Lacher, C.; Picklo, M.; Nonn, L.; Gann, P.H.; Kajdacsy-Balla, A.; et al. Correlations of SELENOF and SELENOP genotypes with serum selenium levels and prostate cancer. Prostate 2018, 78, 279–288. [Google Scholar] [CrossRef]
- Schweizer, U.; Wirth, E.K.; Klopstock, T.; Hölter, S.M.; Becker, L.; Moskovitz, J.; Grune, T.; Fuchs, H.; Gailus-Durner, V.; de Angelis, M.H.; et al. Seizures, ataxia and parvalbumin-expressing interneurons respond to selenium supply in Selenop-deficient mice. Redox Biol. 2022, 57, 102490. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Orho-Melander, M.; Struck, J.; Bergmann, A.; Melander, O. Selenoprotein-P Deficiency Predicts Cardiovascular Disease and Death. Nutrients 2019, 11, 1852. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.R.; Durães, C.; Ziros, P.G.; Pestana, A.; Esteves, C.; Neves, C.; Carvalho, D.; Bongiovanni, M.; Renaud, C.O.; Chartoumpekis, D.V.; et al. Interaction of Genetic Variations in NFE2L2 and SELENOS Modulates the Risk of Hashimoto’s Thyroiditis. Thyroid 2019, 29, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.; Tomczak, J.; Staniszewski, R.; Oszkinis, G. Associations and interactions between variants in selenoprotein genes, selenoprotein levels and the development of abdominal aortic aneurysm, peripheral arterial disease, and heart failure. PLoS ONE 2018, 13, e0203350. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Turovsky, E.A. The Role of Selenoproteins SELENOM and SELENOT in the Regulation of Apoptosis, ER Stress, and Calcium Homeostasis in the A-172 Human Glioblastoma Cell Line. Biology 2022, 11, 811. [Google Scholar] [CrossRef]
- Alsharif, I.; Boukhzar, L.; Lefranc, B.; Godefroy, D.; Aury-Landas, J.; Rego, J.D.; Rego, J.D.; Naudet, F.; Arabo, A.; Chagraoui, A.; et al. Cell-penetrating, antioxidant SELENOT mimetic protects dopaminergic neurons and ameliorates motor dysfunction in Parkinson’s disease animal models. Redox Biol. 2021, 40, 101839. [Google Scholar] [CrossRef]
- Rocca, C.; Pasqua, T.; Boukhzar, L.; Anouar, Y.; Angelone, T. Progress in the emerging role of selenoproteins in cardiovascular disease: Focus on endoplasmic reticulum-resident selenoproteins. Cell Mol. Life. Sci. 2019, 76, 3969–3985. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Kim, J.M.; Kim, M.Y.; Kim, J.-R.; Lee, H.-W.; Chung, Y.W.; Shin, H.-I.; Kim, T.; Park, E.-S.; et al. Selenoprotein W ensures physiological bone remodeling by preventing hyperactivity of osteoclasts. Nat. Commun. 2021, 12, 2258. [Google Scholar] [CrossRef]
- Liao, C.; Hardison, R.C.; Kennett, M.J.; Carlson, B.A.; Paulson, R.F.; Prabhu, K.S. Selenoproteins regulate stress erythroid progenitors and spleen microenvironment during stress erythropoiesis. Blood 2018, 131, 2568–2580. [Google Scholar] [CrossRef]
- Shengyu, C.; Yinhua, L.; Yuanhong, L.; Jinbo, Z.; Can, F.; Hao, X.; Changjiang, Z. Selenium alleviates heart remodeling through Sirt1/AKT/GSK-3β pathway. Int. Immunopharmacol. 2022, 111, 109158. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Wu, P.; Chu, Y.; Gui, S.; Zheng, Y.; Chen, X. Dietary Selenium Alleviated Mouse Liver Oxidative Stress and NAFLD Induced by Obesity by Regulating the KEAP1/NRF2 Pathway. Antioxidants 2022, 11, 349. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Zhang, J.; Song, M.; Shao, B.; Han, Y.; Yang, X.; Li, Y. The Protective Effect of Selenium on T-2-Induced Nephrotoxicity Is Related to the Inhibition of ROS-Mediated Apoptosis in Mice Kidney. Biol. Trace Elem. Res. 2022, 200, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Marcocci, C.; Leo, M.; Altea, M.A. Oxidative stress in graves’ disease. Eur. Thyroid. J. 2012, 1, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, T.; Riccio, M.; Zambonin, L.; Vinceti, M.; De Pol, A.; Hakim, G. Low levels of selenium compounds are selectively toxic for a human neuron cell line through ROS/RNS increase and apoptotic process activation. Neurotoxicology 2011, 32, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Tosatto, S.C.; Bosello, V.; Fogolari, F.; Mauri, P.; Roveri, A.; Toppo, S.; Flohé, L.; Ursini, F.; Maiorino, M. The catalytic site of glutathione peroxidases. Antioxid. Redox Signal. 2008, 10, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Masuda, R.; Kimura, R.; Karasaki, T.; Sase, S.; Goto, K. Modeling the Catalytic Cycle of Glutathione Peroxidase by Nuclear Magnetic Resonance Spectroscopic Analysis of Selenocysteine Selenenic Acids. J. Am. Chem. Soc. 2021, 143, 6345–6350. [Google Scholar] [CrossRef]
- Chu, F.; Doroshow, J.; Esworthy, R. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J. Biol. Chem. 1993, 268, 2571–2576. [Google Scholar] [CrossRef]
- Wingler, K.; Böcher, M.; Flohé, L.; Kollmus, H.; Brigelius-Flohé, R. mRNA stability and selenocysteine insertion sequence efficiency rank gastrointestinal glutathione peroxidase high in the hierarchy of selenoproteins. Eur. J. Biochem. 1999, 259, 149–157. [Google Scholar] [CrossRef]
- Björnstedt, M.; Xue, J.; Huang, W.; Akesson, B.; Holmgren, A. The thioredoxin and glutaredoxin systems are efficient electron donors to human plasma glutathione peroxidase. J. Biol. Chem. 1994, 269, 29382–29384. [Google Scholar] [CrossRef]
- Schwarz, M.; Löser, A.; Cheng, Q.; Wichmann-Costaganna, M.; Schädel, P.; Werz, O.; Arnér, E.S.; Kipp, A.P. Side-by-side comparison of recombinant human glutathione peroxidases identifies overlapping substrate specificities for soluble hydroperoxides. Redox Biol. 2023, 59, 102593. [Google Scholar] [CrossRef]
- Chi, Q.; Zhang, Q.; Lu, Y.; Zhang, Y.; Xu, S.; Li, S. Roles of selenoprotein S in reactive oxygen species-dependent neutrophil extracellular trap formation induced by selenium-deficient arteritis. Redox Biol. 2021, 44, 102003. [Google Scholar] [CrossRef]
- Serfass, R.E.; Ganther, H.E. Defective microbicidal activity in glutathione peroxidase-deficient neutrophils of selenium-deficient rats. Nature 1975, 255, 640–641. [Google Scholar] [CrossRef] [PubMed]
- Pagmantidis, V.; Méplan, C.; van Schothorst, E.M.; Keijer, J.; Hesketh, J.E. Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. Am. J. Clin. Nutr. 2008, 87, 181–189. [Google Scholar] [CrossRef]
- Pan, S.; Li, T.; Tan, Y.; Xu, H. Selenium-containing nanoparticles synergistically enhance Pemetrexed&NK cell-based chemoimmunotherapy. Biomaterials 2022, 280, 121321. [Google Scholar] [PubMed]
- Gong, D.; Sun, K.; Yin, K.; Wang, X. Selenium mitigates the inhibitory effect of TBBPA on NETs release by regulating ROS/MAPK pathways-induced carp neutrophil apoptosis and necroptosis. Fish Shellfish Immunol. 2023, 132, 108501. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-J.; Mao, X.-T.; Li, Y.-Y.; Liu, D.-D.; Fan, K.-Q.; Liu, R.-B.; Wu, T.-T.; Wang, H.-L.; Zhang, Y.; Yang, B.; et al. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn’s disease. Immunity 2021, 54, 1728–1744.e7. [Google Scholar] [CrossRef]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biol. Trace Elem. Res. 1994, 41, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Enqvist, M.; Nilsonne, G.; Hammarfjord, O.; Wallin, R.P.; Björkström, N.K.; Björnstedt, M.; Hjerpe, A.; Ljunggren, H.G.; Dobra, K.; Malmberg, K.J.; et al. Selenite induces posttranscriptional blockade of HLA-E expression and sensitizes tumor cells to CD94/NKG2A-positive NK cells. J. Immunol. 2011, 187, 3546–3554. [Google Scholar] [CrossRef]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium and human immune cell functions. I. Effect on lymphocyte proliferation and interleukin 2 receptor expression. Biol. Trace Elem. Res. 1994, 41, 103–114. [Google Scholar] [CrossRef]
- Nelson, S.M.; Lei, X.; Prabhu, K.S. Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. J. Nutr. 2011, 141, 1754–1761. [Google Scholar] [CrossRef]
- de Toledo, J.; Fraga-Silva, T.F.; Borim, P.A.; de Oliveira, L.R.; Oliveira, E.D.; Périco, L.L.; Hiruma-Lima, C.A.; de Souza, A.A.L.; de Oliveira, C.A.F.; Padilha, P.M.; et al. Organic Selenium Reaches the Central Nervous System and Downmodulates Local Inflammation: A Complementary Therapy for Multiple Sclerosis? Front. Immunol. 2020, 11, 571844. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef] [PubMed]
- Boyne, R.; Arthur, J.R. The response of selenium-deficient mice to Candida albicans infection. J. Nutr. 1986, 116, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L. The etiologies, pathophysiology, and alternative/complementary treatment of asthma. Altern. Med. Rev. 2001, 6, 20–47. [Google Scholar]
- Gholami, M.; Zendedel, A.; Khayat, Z.K.; Ghanad, K.; Nazari, A.; Pirhadi, A. Selenium effect on ischemia-reperfusion injury of gastrocnemius muscle in adult rats. Biol. Trace Elem. Res. 2015, 164, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zendehdel, A.; Gharibi, Z.; Anbari, K.; Abbaszadeh, A.; Khayat, Z.K.; Khorramabadi, R.M.; Soleymaninejad, M.; Gholami, M. Ameliorate Peripheral Nerve Ischemic-Reperfusion Injury via Decreased TNF-α. Biol. Trace Elem. Res. 2017, 176, 328–337. [Google Scholar] [CrossRef]
- Safaralizadeh, R.; Nourizadeh, M.; Zare, A.; Kardar, G.A.; Pourpak, Z. Influence of selenium on mast cell mediator release. Biol. Trace Elem. Res. 2013, 154, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Chow, C. Induction of eosinophilic enteritis and eosinophilia in rats by vitamin E and selenium deficiency. Exp. Mol. Pathol. 1988, 48, 182–192. [Google Scholar] [CrossRef]
- Weitzel, F.; Wendel, A. Selenoenzymes regulate the activity of leukocyte 5-lipoxygenase via the peroxide tone. J. Biol. Chem. 1993, 268, 6288–6292. [Google Scholar] [CrossRef]
- Dalla Puppa, L.; Savaskan, N.E.; Bräuer, A.U.; Behne, D.; Kyriakopoulos, A. The role of selenite on microglial migration. Ann. N. Y. Acad. Sci. 2007, 1096, 179–183. [Google Scholar] [CrossRef]
- Branco, V.; Coppo, L.; Aschner, M.; Carvalho, C. N-Acetylcysteine or Sodium Selenite Prevent the p38-Mediated Production of Proinflammatory Cytokines by Microglia during Exposure to Mercury (II). Toxics 2022, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Shrimali, R.K.; Irons, R.D.; Carlson, B.A.; Sano, Y.; Gladyshev, V.N.; Park, J.M.; Hatfield, D.L. Hatfield. Selenoproteins mediate T cell immunity through an antioxidant mechanism. J. Biol. Chem. 2008, 283, 20181–20185. [Google Scholar] [CrossRef] [PubMed]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T cell activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, M.S.; Davis, M.M.; Garcia, K.C. Deconstructing the form and function of the TCR/CD3 complex. Immunity 2006, 24, 133–139. [Google Scholar] [CrossRef]
- Yue, T.; Zhan, X.; Zhang, D.; Jain, R.; Wang, K.-W.; Choi, J.H.; Misawa, T.; Su, L.; Quan, J.; Hildebrand, S.; et al. SLFN2 protection of tRNAs from stress-induced cleavage is essential for T cell-mediated immunity. Science 2021, 372, eaba4220. [Google Scholar] [CrossRef]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.-R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef]
- Roy, M.; Kiremidjian-Schumacher, L.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Selenium supplementation enhances the expression of interleukin 2 receptor subunits and internalization of interleukin 2. Proc. Soc. Exp. Biol. Med. 1993, 202, 295–301. [Google Scholar] [CrossRef]
- Thikkurissy, S.; Pavone, A.; Rega, A.; Bae, R.; Roy, M.; Wishe, H.I.; Kiremidjian-Schumacher, L. Effect of interleukin-2 and selenium on the growth of squamous cell carcinoma cells. Otolaryngol. Head. Neck. Surg. 2001, 124, 142–149. [Google Scholar] [CrossRef]
- Verma, S.; Hoffmann, F.W.; Kumar, M.; Huang, Z.; Roe, K.; Nguyen-Wu, E.; Hashimoto, A.S.; Hoffmann, P.R. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J. Immunol. 2011, 186, 2127–2137. [Google Scholar] [CrossRef]
- Ren, X.; Wang, S.; Zhang, C.; Hu, X.; Zhou, L.; Li, Y.; Xu, L. Selenium ameliorates cadmium-induced mouse leydig TM3 cell apoptosis via inhibiting the ROS/JNK/c-jun signaling pathway. Ecotoxicol. Environ. Saf. 2020, 192, 110266. [Google Scholar] [CrossRef]
- Dehghani, M.; Shokrgozar, N.; Ramzi, M.; Kalani, M.; Golmoghaddam, H.; Arandi, N. The impact of selenium on regulatory T cell frequency and immune checkpoint receptor expression in patients with diffuse large B cell lymphoma (DLBCL). Cancer Immunol. Immunother. 2021, 70, 2961–2969. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M. Promotion of interleukin-2 activity as a strategy for ‘rejuvenating’ geriatric immune function. Med. Hypotheses 1997, 48, 47–54. [Google Scholar] [CrossRef]
- Peretz, A.A.; Nève, J.; Desmedt, J.; Duchateau, J.; Dramaix, M.; Famaey, J.P. Lymphocyte response is enhanced by supplementation of elderly subjects with selenium-enriched yeast. Am. J. Clin. Nutr. 1991, 53, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.W.; Hashimoto, A.C.; Shafer, L.A.; Dow, S.; Berry, M.J.; Hoffmann, P.R. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J. Nutr. 2010, 140, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yang, T.; Wang, W.; Xu, S. Effect of selenium antagonist lead-induced damage on Th1/Th2 imbalance in the peripheral blood lymphocytes of chickens. Ecotoxicol. Environ. Saf. 2019, 175, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Th1 and Th2 in human diseases. Clin. Immunol. Immunopathol. 1996, 80, 225–235. [Google Scholar] [CrossRef]
- Hawkes, W.C.; Hwang, A.; Alkan, Z. The effect of selenium supplementation on DTH skin responses in healthy North American men. J. Trace Elem. Med. Biol. 2009, 23, 272–280. [Google Scholar] [CrossRef]
- Hasselmark, L.; Malmgren, R.; Zetterström, O.; Unge, G. Selenium supplementation in intrinsic asthma. Allergy 1993, 48, 30–36. [Google Scholar]
- Yao, Y.; Chen, Z.; Zhang, H.; Chen, C.; Zeng, M.; Yunis, J.; Wei, Y.; Wan, Y.; Wang, N.; Zhou, M.; et al. Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat. Immunol. 2021, 22, 1127–1139. [Google Scholar] [CrossRef]
- Werz, O.; Steinhilber, D. Selenium-dependent peroxidases suppress 5-lipoxygenase activity in B-lymphocytes and immature myeloid cells. The presence of peroxidase-insensitive 5-lipoxygenase activity in differentiated myeloid cells. Eur. J. Biochem. 1996, 242, 90–97. [Google Scholar] [CrossRef]
- Vega, L.; Rodríguez-Sosa, M.; García-Montalvo, E.A.; Del Razo, L.M.; Elizondo, G. Non-optimal levels of dietary selenomethionine alter splenocyte response and modify oxidative stress markers in female mice. Food Chem. Toxicol. 2007, 45, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-H.; Holmström, A.; Li, X.; Wu, R.T.Y.; Zeng, H.; Xiao, Z. Effect of dietary selenium and cancer cell xenograft on peripheral T and B lymphocytes in adult nude mice. Biol. Trace Elem. Res. 2012, 146, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jin, Y.; Hall, K.S.; Liang, C.; Unverzagt, F.W.; Ji, R.; Murrell, J.R.; Cao, J.; Shen, J.; Ma, F.; et al. Selenium level and cognitive function in rural elderly Chinese. Am. J. Epidemiol. 2007, 165, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Roberts, B.R.; Malpas, C.B.; Vivash, L.; Genc, S.; Saling, M.M.; Desmond, P.; Steward, C.; Hicks, R.J.; Callahan, J.; et al. Supranutritional Sodium Selenate Supplementation Delivers Selenium to the Central Nervous System: Results from a Randomized Controlled Pilot Trial in Alzheimer’s Disease. Neurotherapeutics 2019, 16, 192–202. [Google Scholar] [CrossRef]
- Corcoran, N.M.; Martin, D.; Hutter-Paier, B.; Windisch, M.; Nguyen, T.; Nheu, L.; Sundstrom, L.E.; Costello, A.J.; Hovens, C.M. Sodium selenate specifically activates PP2A phosphatase, dephosphorylates tau and reverses memory deficits in an Alzheimer’s disease model. J. Clin. Neurosci. 2010, 17, 1025–1033. [Google Scholar] [CrossRef]
- van Eersel, J.; Ke, Y.D.; Liu, X.; Delerue, F.; Kril, J.J.; Götz, J.; Ittner, L.M. Sodium selenate mitigates tau pathology, neurodegeneration, and functional deficits in Alzheimer’s disease models. Proc. Natl. Acad. Sci. USA 2010, 107, 13888–13893. [Google Scholar] [CrossRef]
- Sun, C.; Du, Z.; Liu, X.; Yang, Y.; Zhou, S.; Li, C.; Cao, X.; Zhao, Q.; Wong, K.; Chen, W.; et al. Selenium Forms and Dosages Determined Their Biological Actions in Mouse Models of Parkinson’s Disease. Nutrients 2022, 15, 11. [Google Scholar] [CrossRef]
- Sophiabadi, M.; Rastgoo, N.; Haghdoost-Yazdi, H. Dopaminergic Neuronal Death in Substantia Nigra Associates with Serum Levels of Total Bilirubin, Selenium, and Zinc: Evidences from 6-Hydroxydopamine Animal Model of Parkinson’s Disease. Biol. Trace Elem. Res. 2022, 200, 4058–4067. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Molz, P.; Dallemole, D.R.; dos Santos, A.P.; Müller, T.E.; Cappelletti, L.; da Silva, M.G.; Franke, S.I.R.; Prá, D.; Henriques, J.A.P. Selenium reduces bradykinesia and DNA damage in a rat model of Parkinson’s disease. Nutrition 2015, 31, 359–365. [Google Scholar] [CrossRef]
- Tawfik, K.M.; Moustafa, Y.M.; El-Azab, M.F. Neuroprotective mechanisms of sildenafil and selenium in PTZ-kindling model: Implications in epilepsy. Eur. J. Pharmacol. 2018, 833, 131–144. [Google Scholar] [CrossRef]
- Nazıroglu, M. Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem. Res. 2009, 34, 2181–2191. [Google Scholar] [CrossRef]
- Schibler, U. Selenium cysteine and epileptic seizures. Nat. Rev. Mol. Cell Biol. 2018, 19, 753. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E.; Motley, A.K.; Winfrey, V.P.; Kurokawa, S.; Mitchell, S.L.; Zhang, W. Selenoprotein P and apolipoprotein E receptor-2 interact at the blood-brain barrier and also within the brain to maintain an essential selenium pool that protects against neurodegeneration. FASEB J. 2014, 28, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E.; Olson, G.E.; Weeber, E.J.; Motley, A.K.; Winfrey, V.P.; Austin, L.M. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J. Neurosci. 2007, 27, 6207–6211. [Google Scholar] [CrossRef]
- Schweizer, U.; Streckfuss, F.; Pelt, P.; Carlson, B.A.; Hatfield, D.L.; Köhrle, J.; Schomburg, L. Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply. Biochem. J. 2005, 386, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L.; Schweizer, U.; Holtmann, B.; Flohé, L.; Sendtner, M.; Köhrle, J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem. J. 2003, 370, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Sasuclark, A.R.; Khadka, V.S.; Pitts, M.W. Cell-Type Specific Analysis of Selenium-Related Genes in Brain. Antioxidants 2019, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Scharpf, M.; Schweizer, U.; Arzberger, T.; Roggendorf, W.; Schomburg, L.; Köhrle, J. Neuronal and ependymal expression of selenoprotein P in the human brain. J. Neural. Transm. 2007, 114, 877–884. [Google Scholar] [CrossRef]
- Caito, S.W.; Milatovic, D.; Hill, K.E.; Aschner, M.; Burk, R.F.; Valentine, W.M. Progression of neurodegeneration and morphologic changes in the brains of juvenile mice with selenoprotein P deleted. Brain Res. 2011, 1398, 1–12. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.P.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e21. [Google Scholar] [CrossRef] [PubMed]
- Yant, L.J.; Ran, Q.; Rao, L.; Van Remmen, H.; Shibatani, T.; Belter, J.G.; Motta, L.; Richardson, A.; Prolla, T.A. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic. Biol. Med. 2003, 34, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-E.; Chen, L.; Na, R.; Liu, Y.; Rios, C.; Van Remmen, H.; Richardson, A.; Ran, Q. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free Radic. Biol. Med. 2012, 52, 1820–1827. [Google Scholar] [CrossRef]
- Wirth, E.K.; Conrad, M.; Winterer, J.; Wozny, C.; Carlson, B.A.; Roth, S.; Schmitz, D.; Bornkamm, G.W.; Coppola, V.; Tessarollo, L.; et al. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 2010, 24, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Y.; Zhang, Y.; Kuang, X.; Liu, Y.; Guo, M.; Ma, L.; Zhang, D.; Li, Q. Iron Metabolism and Ferroptosis in Epilepsy. Front. Neurosci. 2020, 14, 601193. [Google Scholar] [CrossRef] [PubMed]
- Kahn-Kirby, A.H.; Amagata, A.; Maeder, C.I.; Mei, J.J.; Sideris, S.; Kosaka, Y.; Hinman, A.; Malone, S.A.; Bruegger, J.J.; Wang, L.; et al. Targeting ferroptosis: A novel therapeutic strategy for the treatment of mitochondrial disease-related epilepsy. PLoS ONE 2019, 14, e0214250. [Google Scholar] [CrossRef]
- Yoo, M.H.; Gu, X.; Xu, X.M.; Kim, J.Y.; Carlson, B.A.; Patterson, A.D.; Cai, H.; Gladyshev, V.N.; Hatfield, D.L. Delineating the role of glutathione peroxidase 4 in protecting cells against lipid hydroperoxide damage and in Alzheimer’s disease. Antioxid. Redox Signal 2010, 12, 819–827. [Google Scholar] [CrossRef]
- Bellinger, F.P.; Bellinger, M.T.; Seale, L.A.; Takemoto, A.S.; Raman, A.V.; Miki, T.; Manning-Boğ, A.B.; Berry, M.J.; White, L.R.; Ross, G.W. Glutathione Peroxidase 4 is associated with Neuromelanin in Substantia Nigra and Dystrophic Axons in Putamen of Parkinson’s brain. Mol. Neurodegener. 2011, 6, 8. [Google Scholar] [CrossRef]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Chung, Y.W.; Jeong, D.; Noh, O.J.; Park, Y.H.; Kang, S.I.; Lee, M.G.; Lee, T.-H.; Bin Yim, M.; Kim, I.Y. Antioxidative role of selenoprotein W in oxidant-induced mouse embryonic neuronal cell death. Mol. Cells 2009, 27, 609–613. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Schweizer, U.; Savaskan, N.E.; Hua, D.; Kipnis, J.; Hatfield, D.L.; Gladyshev, V.N. Comparative analysis of selenocysteine machinery and selenoproteome gene expression in mouse brain identifies neurons as key functional sites of selenium in mammals. J. Biol. Chem. 2008, 283, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. Keshan disease, selenium deficiency, and the selenoproteome. N. Engl. J. Med. 2014, 370, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Han, S.; Zhang, Y.; Zou, Y.; Su, S.; Zhou, H.; Zhang, X.; Liang, H.; Hou, J.; et al. A Spatial Ecological Study on Serum Selenium and Keshan Disease in Heilongjiang Province, China. Biol. Trace Elem. Res. 2021, 199, 3253–3261. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.A.; Noiva, R.; Wells, I.C. Selenium concentrations in plasma of patients with arteriographically defined coronary atherosclerosis. Clin. Chem. 1984, 30, 1171–1173. [Google Scholar] [CrossRef]
- Krohn, R.M.; Lemaire, M.; Silva, L.F.N.; Lemarié, C.; Bolt, A.; Mann, K.K.; Smits, J. High-selenium lentil diet protects against arsenic-induced atherosclerosis in a mouse model. J. Nutr. Biochem. 2016, 27, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, D.C.; Behr, S.R. Vitamin E combined with selenium inhibits atherosclerosis in hypercholesterolemic rabbits independently of effects on plasma cholesterol concentrations. Circ. Res. 1998, 83, 366–377. [Google Scholar] [CrossRef]
- Zhang, Y.; Cartland, S.P.; Henriquez, R.; Patel, S.; Gammelgaard, B.; Flouda, K.; Hawkins, C.L.; Rayner, B.S. Selenomethionine supplementation reduces lesion burden, improves vessel function and modulates the inflammatory response within the setting of atherosclerosis. Redox Biol. 2020, 29, 101409. [Google Scholar] [CrossRef]
- Luoma, P.V.; Sotaniemi, E.A.; Korpela, H.; Kumpulainen, J. Serum selenium, glutathione peroxidase activity and high-density lipoprotein cholesterol—Effect of selenium supplementation. Res. Commun. Chem. Pathol. Pharmacol. 1984, 46, 469–472. [Google Scholar]
- Maiorino, M.; Conrad, M.; Ursini, F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid. Redox Signal 2018, 29, 61–74. [Google Scholar] [CrossRef]
- Leitinger, N. Cholesteryl ester oxidation products in atherosclerosis. Mol. Aspects. Med. 2003, 24, 239–250. [Google Scholar] [CrossRef]
- Hussein, O.; Rosenblat, M.; Refael, G.; Aviram, M. Dietary selenium increases cellular glutathione peroxidase activity and reduces the enhanced susceptibility to lipid peroxidation of plasma and low-density lipoprotein in kidney transplant recipients. Transplantation 1997, 63, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Toivanen, J.L. Effects of selenium, vitamin E and vitamin C on human prostacyclin and thromboxane synthesis in vitro. Prostaglandins. Leukot. Med. 1987, 26, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Luoma, P.V.; Stengård, J.; Korpela, H.; Rautio, A.; Sotaniemi, E.A.; Suvanto, E.; Marniemi, J. Lipid peroxides, glutathione peroxidase, high density lipoprotein subfractions and apolipoproteins in young adults. J. Intern. Med. 1990, 227, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N. Engl. J. Med. 2003, 349, 1605–1613. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Streicher, K.L.; Mullarky, I.K.; Gandy, J.C.; Trigona, W.; Corl, C.M. Selenium inhibits 15-hydroperoxyoctadecadienoic acid-induced intracellular adhesion molecule expression in aortic endothelial cells. Free Radic. Biol. Med. 2008, 44, 34–43. [Google Scholar] [CrossRef]
- Gao, J.; Tian, X.; Yan, X.; Wang, Y.; Wei, J.; Wang, X.; Yan, X.; Song, G. Selenium Exerts Protective Effects Against Fluoride-Induced Apoptosis and Oxidative Stress and Altered the Expression of Bcl-2/Caspase Family. Biol. Trace Elem. Res. 2021, 199, 682–692. [Google Scholar] [CrossRef]
- Han, Q.; Wang, A.; Fu, Q.; Zhou, S.; Bao, J.; Xing, H. Protective role of selenium on ammonia-mediated nephrotoxicity via PI3K/AKT/mTOR pathway: Crosstalk between autophagy and cytokine release. Ecotoxicol. Environ. Saf. 2022, 242, 113918. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Selenium and outcome in heart failure. Eur. J. Heart Fail 2020, 22, 1415–1423. [Google Scholar]
- Fleming, C.R.; Lie, J.; McCall, J.; O’Brien, J.; Baillie, E.E.; Thistle, J.L. Selenium deficiency and fatal cardiomyopathy in a patient on home parenteral nutrition. Gastroenterology 1982, 83, 689–693. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.-I.; Josse, R.; Vieth, R.; Blanco Mejia, S.; Viguiliouk, E.; Nishi, S.; Sahye-Pudaruth, S.; et al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J. Am. Coll. Cardiol. 2018, 71, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Virtamo, J.; Valkeila, E.; Alfthan, G.; Punsar, S.; Huttunen, J.K.; Karvonen, M.J. Serum selenium and the risk of coronary heart disease and stroke. Am. J. Epidemiol. 1985, 122, 276–282. [Google Scholar] [CrossRef]
- Salvini, S.; Hennekens, C.H.; Morris, J.; Willett, W.C.; Stampfer, M.J. Plasma levels of the antioxidant selenium and risk of myocardial infarction among U.S. physicians. Am. J. Cardiol. 1995, 76, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Eagle, K.; Jiang, Y.; Shi, X.; Li, M.; Obholzer, N.P.; Hu, T.; Perez, M.W.; Koren, J.V.; Kitano, A.; Yi, J.S.; et al. An oncogenic enhancer encodes selective selenium dependency in AML. Cell Stem. Cell 2022, 29, 386–399.e7. [Google Scholar] [CrossRef]
- Sundström, H.; Korpela, H.; Viinikka, L.; Kauppila, A. Serum selenium and glutathione peroxidase, and plasma lipid peroxides in uterine, ovarian or vulvar cancer, and their responses to antioxidants in patients with ovarian cancer. Cancer Lett. 1984, 24, 1–10. [Google Scholar] [CrossRef]
- Schwartz, M.K. Role of trace elements in cancer. Cancer Res. 1975, 35, 3481–3487. [Google Scholar]
- Willett, W.; Morris, J.S.; Pressel, S.; Taylor, J.; Polk, B.F.; Stampfer, M.; Rosner, B.; Schneider, K.; Hames, C. Prediagnostic serum selenium and risk of cancer. Lancet 1983, 2, 130–134. [Google Scholar] [CrossRef]
- Lotan, Y.; Goodman, P.J.; Youssef, R.F.; Svatek, R.S.; Shariat, S.F.; Tangen, C.M.; Thompson, I.M.; Klein, E.A. Evaluation of vitamin E and selenium supplementation for the prevention of bladder cancer in SWOG coordinated SELECT. J. Urol. 2012, 187, 2005–2010. [Google Scholar] [CrossRef]
- Reid, M.E.; Duffield-Lillico, A.J.; Garland, L.; Turnbull, B.W.; Clark, L.C.; Marshall, J.R. Selenium supplementation and lung cancer incidence: An update of the nutritional prevention of cancer trial. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1285–1291. [Google Scholar] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Zhu, Y.J.; Li, W.G. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol. Trace Elem. Res. 1997, 56, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Flowers, B.; Poles, A.; Kastrati, I. Selenium and breast cancer—An update of clinical and epidemiological data. Arch. Biochem. Biophys. 2022, 732, 109465. [Google Scholar] [CrossRef]
- Clark, L.C.; Dalkin, B.; Krongrad, A.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Witherington, R.; Herlong, J.H.; Janosko, E.; Carpenter, D.; et al. Decreased incidence of prostate cancer with selenium supplementation: Results of a double-blind cancer prevention trial. Br. J. Urol. 1998, 81, 730–734. [Google Scholar] [CrossRef]
- Mukhtar, M.; Ashfield, N.; Vodickova, L.; Vymetalkova, V.; Levy, M.; Liska, V.; Bruha, J.; Bendova, P.; O’sullivan, J.; Doherty, G.; et al. The Associations of Selenoprotein Genetic Variants with the Risks of Colorectal Adenoma and Colorectal Cancer: Case-Control Studies in Irish and Czech Populations. Nutrients. 2022, 14, 2718. [Google Scholar] [CrossRef]
- Efendić, S.; Luft, R.; Wajngot, A. Aspects of the pathogenesis of type 2 diabetes. Endocr. Rev. 1984, 5, 395–410. [Google Scholar] [CrossRef]
- Stranges, S.; Marshall, J.R.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; Reid, M.E. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Rothman, K.J. Selenium exposure and the risk of type 2 diabetes: A systematic review and meta-analysis. Eur. J. Epidemiol. 2018, 33, 789–810. [Google Scholar] [CrossRef]
- Misu, H.; Takamura, T.; Takayama, H.; Hayashi, H.; Matsuzawa-Nagata, N.; Kurita, S.; Ishikura, K.; Ando, H.; Takeshita, Y.; Ota, T.; et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010, 12, 483–495. [Google Scholar] [CrossRef]
- Misu, H.; Takayama, H.; Saito, Y.; Mita, Y.; Kikuchi, A.; Ishii, K.-A.; Chikamoto, K.; Kanamori, T.; Tajima, N.; Lan, F.; et al. Deficiency of the hepatokine selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat. Med. 2017, 23, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Harmon, J.S.; Bogdani, M.; Parazzoli, S.D.; Mak, S.S.; Oseid, E.A.; Berghmans, M.; Leboeuf, R.C.; Robertson, R.P. beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology 2009, 150, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dai, C.; Guo, M.; Taylor, B.; Harmon, J.S.; Sander, M.; Robertson, R.P.; Powers, A.C.; Stein, R. Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Invest. 2013, 123, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Vatamaniuk, M.Z.; Wang, S.K.; Roneker, C.A.; Simmons, R.A.; Lei, X.G. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia 2008, 51, 1515–1524. [Google Scholar] [CrossRef]
- Erbayraktar, Z.; Yılmaz, O.; Artmann, A.T.; Cehreli, R.; Coker, C. Effects of selenium supplementation on antioxidant defense and glucose homeostasis in experimental diabetes mellitus. Biol. Trace Elem. Res. 2007, 118, 217–226. [Google Scholar] [CrossRef]
- Ayaz, M.; Can, B.; Ozdemir, S.; Turan, B. Protective effect of selenium treatment on diabetes-induced myocardial structural alterations. Biol. Trace Elem. Res. 2002, 89, 215–226. [Google Scholar] [CrossRef]
- Ezaki, O. The insulin-like effects of selenate in rat adipocytes. J. Biol. Chem. 1990, 265, 1124–1128. [Google Scholar] [CrossRef]
- McNeill, J.H.; Delgatty, H.L.; Battell, M.L. Insulinlike effects of sodium selenate in streptozocin-induced diabetic rats. Diabetes 1991, 40, 1675–1678. [Google Scholar] [CrossRef]
- Ghosh, R.; Mukherjee, B.; Chatterjee, M. A novel effect of selenium on streptozotocin-induced diabetic mice. Diabetes Res. 1994, 25, 165–171. [Google Scholar]
- Magnuson, M.A.; Andreone, T.L.; Printz, R.L.; Koch, S.; Granner, D.K. Rat glucokinase gene: Structure and regulation by insulin. Proc. Natl. Acad. Sci. USA 1989, 86, 4838–4842. [Google Scholar] [CrossRef]
- Lv, Y.; Xie, L.; Dong, C.; Yang, R.; Long, T.; Yang, H.; Chen, L.; Zhang, L.; Chen, X.; Luo, X.; et al. Co-exposure of serum calcium, selenium and vanadium is nonlinearly associated with increased risk of type 2 diabetes mellitus in a Chinese population. Chemosphere 2021, 263, 128021. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Yang, N.; Li, X. Advances in Understanding How Heavy Metal Pollution Triggers Gastric Cancer. Biomed. Res. Int. 2016, 2016, 7825432. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Alexander, J.; Alehagen, U.; Tinkov, A.; Skalny, A.; Larsson, A.; Crisponi, G.; Nurchi, V.M. The Aging Kidney-As Influenced by Heavy Metal Exposure and Selenium Supplementation. Biomolecules 2021, 11, 1078. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Mahmood, R. Mercury chloride toxicity in human erythrocytes: Enhanced generation of ROS and RNS, hemoglobin oxidation, impaired antioxidant power, and inhibition of plasma membrane redox system. Environ. Sci. Pollut. Res. Int. 2019, 26, 5645–5657. [Google Scholar] [CrossRef]
- Kim, S.H.; Sharma, R.P. Mercury-induced apoptosis and necrosis in murine macrophages: Role of calcium-induced reactive oxygen species and p38 mitogen-activated protein kinase signaling. Toxicol. Appl. Pharmacol. 2004, 196, 47–57. [Google Scholar] [CrossRef]
- Casalino, E.; Sblano, C.; Landriscina, C. Enzyme activity alteration by cadmium administration to rats: The possibility of iron involvement in lipid peroxidation. Arch. Biochem. Biophys. 1997, 346, 171–179. [Google Scholar] [CrossRef]
- Cao, X.; Fu, M.; Bi, R.; Zheng, X.; Fu, B.; Tian, S.; Liu, C.; Li, Q.; Liu, J. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 2021, 263, 128346. [Google Scholar] [CrossRef]
- Chayapong, J.; Madhyastha, H.; Madhyastha, R.; Nurrahmah, Q.I.; Nakajima, Y.; Choijookhuu, N.; Hishikawa, Y.; Maruyama, M. Arsenic trioxide induces ROS activity and DNA damage, leading to G0/G1 extension in skin fibroblasts through the ATM-ATR-associated Chk pathway. Environ. Sci. Pollut. Res. Int. 2017, 24, 5316–5325. [Google Scholar] [CrossRef]
- Bramanti, E.; Onor, M.; Colombaioni, L. Neurotoxicity Induced by Low Thallium Doses in Living Hippocampal Neurons: Evidence of Early Onset Mitochondrial Dysfunction and Correlation with Ethanol Production. ACS. Chem. Neurosci. 2019, 10, 451–459. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, X.; Yu, J.; Xie, J.; Li, C.; Liu, D.; Tang, C.; Wang, C. Lead-induced oxidative damage in rats/mice: A meta-analysis. J. Trace Elem. Med. Biol. 2020, 58, 126443. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, J.Y.; Kim, J.; Lee, J.-H.; Hahn, J.-S.; Gu, M.B.; Yoon, J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef]
- Chitta, K.R.; Figueroa, J.A.L.; Caruso, J.A.; Merino, E.J. Selenium mediated arsenic toxicity modifies cytotoxicity, reactive oxygen species and phosphorylated proteins. Metallomics 2013, 5, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Saikiran, G.; Mitra, P.; Sharma, S.; Kumar, P.K.; Sharma, P. Selenium, oxidative stress and inflammatory markers in handicraft workers occupationally exposed to lead. Arch. Environ. Occup. Health 2022, 77, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Ganther, H.E. Interactions of vitamin E and selenium with mercury and silver. Ann. N. Y. Acad. Sci. 1980, 355, 212–226. [Google Scholar] [CrossRef]
- Sun, H.-J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.-P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef]
- Schöpfer, J.; Drasch, G.; Schrauzer, G.N. Selenium and cadmium levels and ratios in prostates, livers, and kidneys of nonsmokers and smokers. Biol. Trace Elem. Res. 2010, 134, 180–187. [Google Scholar] [CrossRef]
- Kazi, T.G.; Kolachi, N.F.; Afridi, H.I.; Kazi, N.G.; Sirajuddin; Naeemullah; Arain, S.S. Effects of mineral supplementation on liver cirrhotic/cancer male patients. Biol. Trace Elem. Res. 2012, 150, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-M.; Lin, Y.-C.; Huang, Y.-L.; Shiue, H.-S.; Pu, Y.-S.; Huang, C.-Y.; Chung, C.-J. Effect of plasma selenium, red blood cell cadmium, total urinary arsenic levels, and eGFR on renal cell carcinoma. Sci. Total Environ. 2021, 750, 141547. [Google Scholar] [CrossRef]

| Function | Health Effects | |
|---|---|---|
| GPX1 | Antioxidant activity; reduce cellular H2O2. | Cancers [46]; chondrogenic differentiation [47]; T2DM [48]; depression [49]; Keshan disease [50]; cataracts [51]; macular degeneration [52]. |
| GPX2 | Antioxidant activity, protect the mucosa of the gastrointestinal tract and various endothelial cells from oxidative damage. | Cancers [53]; intestinal inflammation [54]. |
| GPX3 | Reduce lipid hydro peroxides and H2O2. | Cancers [55]; myocardial fibrosis [56]; ventricular remodeling [57]. |
| GPX4 | Antioxidant activity; decrease phosphatidylcholine hydroperoxide; suppress cellular ferroptosis. | Osteoarthritis [58]; cancers [59]; cardiomyopathy [60]; ischemia-reperfusion injury [61]; brain function [62]. |
| GPX6 | Not known. | Huntington’s disease [63]. |
| TXNRD1 | Antioxidant activity; regenerate thioredoxin; suppress cell ferroptosis. | Idiopathic pulmonary arterial hypertension [64] hepatocellular carcinoma [65]; osteoarthritis [66]; genetic generalized epilepsy [67]; Keshan disease [50]. |
| TXNRD2 | Regenerate thioredoxin; regulate cell proliferation and apoptosis. | Primary open-angle glaucoma [68]; CVDs [69,70]; cancers [71]; glaucoma [72]. |
| TXNRD3 | Antioxidant activity; suppress pyroptosis. | Male reproduction [73]; colitis and carcinogenesis [74]. |
| DIO1 | Activate T3. | Thyroid hormone metabolism [75]; inhibit hepatosteatosis [76]; renal cancer [77]. |
| DIO2 | Activate T3. | Osteoarthritis [78]; obesity [79]; mental retardation [80]. |
| DIO3 | Inactivate T3. | Osteoarthritis [81]; brain development [82]; sepsis and septic shock [83]. |
| MSRB1 | Antioxidant activity; anti-inflammatory effect; regulate immune responses. | Hepatocellular carcinoma [84]; inflammatory response [85]. |
| SEPHS2 | Sec synthesis. | Cancers [86]. |
| SELENOF | Immunomodulation; regulate glycogenolysis and lipogenesis; participate in vitamin A metabolism. | Cancers [87]; glucose metabolism disorder [88]. |
| SELENOH | Regulate cell cycle progression and proliferation. | Colorectal cancer [89]. |
| SELENOI | Critical enzyme in the central nervous system; T cell activation; neural development; plasmalogen maintenance. | Hereditary spastic paraplegia 81 [90]. |
| SELENOK | Oxidation resistance; Ca2+ flux regulation; immune regulation; apoptosis regulation; suppress cellular ferroptosis. | AD [91]; cervical cancer [92]. |
| SELENOM | Glucose metabolism; Ca2+ flux regulation; apoptosis regulation. | Glioblastoma [93]; non-alcoholic fatty liver disease [94]; synaptic deficits and cognitive dysfunction [95]. |
| SELENON | Muscle development; calcium haemostasis. | Myopathies [96]. |
| SELENOO | Not known. | Thyroid cancer [97]. |
| SELENOP | Antioxidant activity; maintain neuronal activity; transport selenium to tissues; regulate pancreatic β cell function. | Cancers [98,99,100]; seizures and ataxia [101]; CVDs [102]. |
| SELENOS | Regulate inflammation; induce ER stress apoptosis; immune regulation. | Hashimoto’s thyroiditis [103]; CVDs [104]. |
| SELENOT | Promote nerve regeneration; Ca2+ flux regulation; apoptosis regulation; maintain endoplasmic reticulum homeostasis; regulate glucose and lipid metabolism. | Glioblastoma [105]; AD [106]; CVDs [107]. |
| SELENOV | Regulate glucose and lipid metabolism; prevent endoplasmic reticulum stress and oxidative injury; maintain male reproduction. | Not known. |
| SELENOW | Oxidation resistance; regulate bone metabolism; support erythroblast development; muscle development. | Osteoporosis [108]; anemia [109]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. https://doi.org/10.3390/biom13050799
Zhang F, Li X, Wei Y. Selenium and Selenoproteins in Health. Biomolecules. 2023; 13(5):799. https://doi.org/10.3390/biom13050799
Chicago/Turabian StyleZhang, Fan, Xuelian Li, and Yumiao Wei. 2023. "Selenium and Selenoproteins in Health" Biomolecules 13, no. 5: 799. https://doi.org/10.3390/biom13050799
APA StyleZhang, F., Li, X., & Wei, Y. (2023). Selenium and Selenoproteins in Health. Biomolecules, 13(5), 799. https://doi.org/10.3390/biom13050799






