Platelet Serotonin (5-HT) Concentration, Platelet Monoamine Oxidase B (MAO-B) Activity and HTR2A, HTR2C, and MAOB Gene Polymorphisms in Asthma
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Blood Collection
2.3. Determination of Platelet 5-HT Concentration
2.4. Determination of Platelet MAO-B Activity
2.5. DNA Extraction and Genotyping
2.6. Statistical Analysis
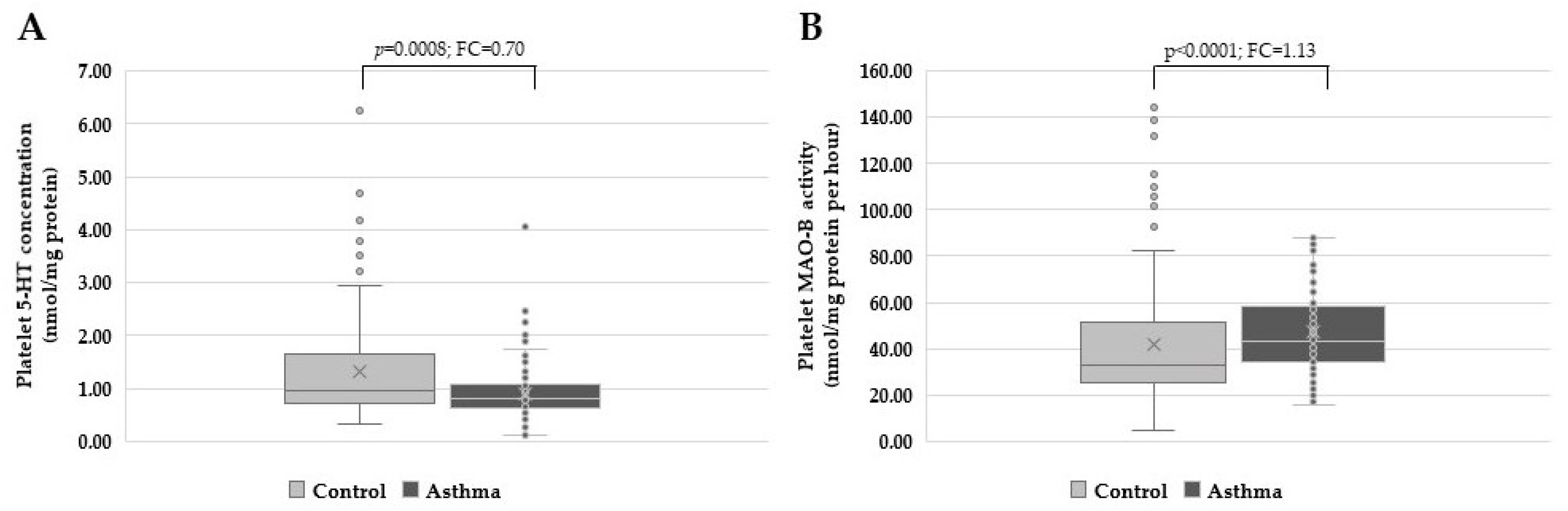
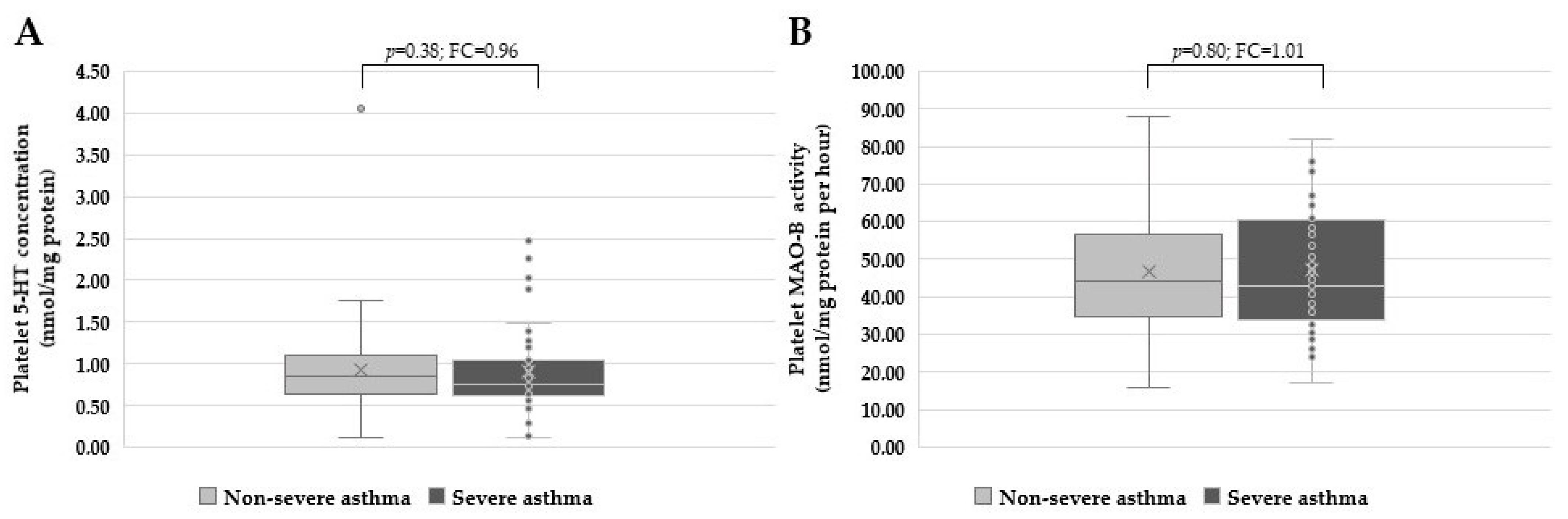
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention Global Strategy for Asthma Management and Prevention. Available online: https://ginasthma.org/archived-reports/ (accessed on 7 December 2022).
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Tran, L.; Sharrad, K.; Kopsaftis, Z.; Stallman, H.M.; Tai, A.; Spurrier, N.; Esterman, A.; Carson-Chahhoud, K. Pharmacological Interventions for the Treatment of Psychological Distress in Patients with Asthma: A Systematic Review and Meta-Analysis. J. Asthma 2021, 58, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Braido, F. Failure in Asthma Control: Reasons and Consequences. Scientifica 2013, 2013, 549252. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.W.; Ghushchyan, V.H.; Slejko, J.F.; Belozeroff, V.; Globe, D.R.; Lin, S.-L. The Burden of Adult Asthma in the United States: Evidence from the Medical Expenditure Panel Survey. J. Allergy Clin. Immunol. 2011, 127, 363–369. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.J.; Brightling, C. Pathogenesis of Asthma: Implications for Precision Medicine. Clin. Sci. 2017, 131, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, I.; Matera, M.G. 5-HT Modifiers as a Potential Treatment of Asthma. Trends Pharmacol. Sci. 2000, 21, 13–16. [Google Scholar] [CrossRef]
- Wu, H.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a Neurotransmitter: The Role of Serotonin in Inflammation and Immunity. Pharmacol. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Roumier, A.; Béchade, C.; Maroteaux, L. Serotonin and the Immune System. In Serotonin, The Mediator That Spans Evolution; Academic Press: Boston, MA, USA, 2019; pp. 181–196. [Google Scholar] [CrossRef]
- Müller, T.; Dürk, T.; Blumenthal, B.; Grimm, M.; Cicko, S.; Panther, E.; Sorichter, S.; Herouy, Y.; Di Virgilio, F.; Ferrari, D.; et al. 5-Hydroxytryptamine Modulates Migration, Cytokine and Chemokine Release and T-Cell Priming Capacity of Dendritic Cells in Vitro and in Vivo. PLoS ONE 2009, 4, e6453. [Google Scholar] [CrossRef]
- Arreola, R.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Velasco-Velázquez, M.A.; Garces-Alvarez, M.E.; Hurtado-Alvarado, G.; Quintero-Fabian, S.; Pavon, L. Immunomodulatory Effects Mediated by Serotonin. J. Immunol. Res. 2015, 2015, 354957. [Google Scholar] [CrossRef]
- Lechin, F.; Van Der Dijs, B.; Orozco, B.; Lechin, M.; Lechin, A.E. Increased Levels of Free Serotonin in Plasma of Symptomatic Asthmatic Patients. Ann. Allergy Asthma Immunol. 1996, 77, 245–253. [Google Scholar] [CrossRef]
- Dürk, T.; Duerschmied, D.; Müller, T.; Grimm, M.; Reuter, S.; Vieira, R.P.; Ayata, K.; Cicko, S.; Sorichter, S.; Walther, D.J.; et al. Production of Serotonin by Tryptophan Hydroxylase 1 and Release via Platelets Contribute to Allergic Airway Inflammation. Am. J. Respir. Crit. Care Med. 2013, 187, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Lechin, F.; Van der Dijs, B.; Lechin, A.E. Severe Asthma and Plasma Serotonin. Allergy Eur. J. Allergy Clin. Immunol. 2002, 57, 258–259. [Google Scholar] [CrossRef]
- Brand, T.; Anderson, G.M. The Measurement of Platelet-Poor Plasma Serotonin: A Systematic Review of Prior Reports and Recommendations for Improved Analysis. Clin. Chem. 2011, 57, 1376–1386. [Google Scholar] [CrossRef]
- Ostadkarampour, M.; Putnins, E.E. Monoamine Oxidase Inhibitors: A Review of Their Anti-Inflammatory Therapeutic Potential and Mechanisms of Action. Front. Pharmacol. 2021, 12, 676239. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Pitchford, S.; Page, C. Role of Platelets in Allergic Airway Inflammation. J. Allergy Clin. Immunol. 2015, 135, 1416–1423. [Google Scholar] [CrossRef]
- Brenner, B.; Harney, J.T.; Ahmed, B.A.; Jeffus, B.C.; Unal, R.; Mehta, J.L.; Kilic, F. Plasma Serotonin Levels and the Platelet Serotonin Transporter. J. Neurochem. 2007, 102, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Mercado, C.P.; Kilic, F. Molecular Mechanisms of SERT in Platelets: Regulation of Plasma Serotonin Levels. Mol. Interv. 2010, 10, 231–241. [Google Scholar] [CrossRef]
- Malmgren, R.; Olsson, P.; Tornling, G.; Unge, G. Acetylsalicyclic Asthma and Migraine—A Defect in Serotonin (5-HT) Uptake in Platelets. Thromb. Res. 1978, 13, 1137–1139. [Google Scholar] [CrossRef]
- Malmgren, R.; Grubbströum, J.; Olsson, P.; Theorell, H.; Tornling, G.; Unge, G. Defective Serotonin (5-HT) Transport Mechanism in Platelets from Patients with Endogenous and Allergic Asthma. Allergy 1982, 37, 29–39. [Google Scholar] [CrossRef]
- Matkar, N.M.; Rupwate, R.U.; Desai, N.K.; Kamat, S.R. Comparative Study of Platelet Histamine and Serotonin with Their Corresponding Plasma Oxidases in Asthmatics with Normals. J. Assoc. Physicians India 1999, 47, 878–882. [Google Scholar]
- Ramsay, R.R. Monoamine Oxidases: The Biochemistry of the Proteins as Targets in Medicinal Chemistry and Drug Discovery. Curr. Top. Med. Chem. 2012, 12, 2189–2209. [Google Scholar] [CrossRef]
- Prah, A.; Purg, M.; Stare, J.; Vianello, R.; Mavri, J. How Monoamine Oxidase A Decomposes Serotonin: An Empirical Valence Bond Simulation of the Reactive Step. J. Phys. Chem. B 2020, 124, 8259–8265. [Google Scholar] [CrossRef] [PubMed]
- Young, W.F.J.; Laws, E.R.J.; Sharbrough, F.W.; Weinshilboum, R.M. Human Monoamine Oxidase. Lack of Brain and Platelet Correlation. Arch. Gen. Psychiatry 1986, 43, 604–609. [Google Scholar] [CrossRef]
- Bortolato, M.; Shih, J.C. Behavioral Outcomes of Monoamine Oxidase Deficiency: Preclinical and Clinical Evidence, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 100, ISBN 9780123864673. [Google Scholar]
- Cases, O.; Seif, I.; Grimsby, J.; Gaspar, P.; Chen, K.; Pournin, S.; Müller, U.; Aguet, M.; Babinet, C.; Shih, J.C. Aggressive Behavior and Altered Amounts of Brain Serotonin and Norepinephrine in Mice Lacking MAOA. Science 1995, 268, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Holschneider, D.P.; Wu, W.; Rebrini, I.; Shih, J.C. A Spontaneous Point Mutation Produces Monoamine Oxidase A/B Knock-out Mice with Greatly Elevated Monoamines and Anxiety-like Behavior. J. Biol. Chem. 2004, 279, 39645–39652. [Google Scholar] [CrossRef]
- Lenders, J.W.; Eisenhofer, G.; Abeling, N.G.; Berger, W.; Murphy, D.L.; Konings, C.H.; Wagemakers, L.M.; Kopin, I.J.; Karoum, F.; van Gennip, A.H.; et al. Specific Genetic Deficiencies of the A and B Isoenzymes of Monoamine Oxidase Are Characterized by Distinct Neurochemical and Clinical Phenotypes. J. Clin. Investig. 1996, 97, 1010–1019. [Google Scholar] [CrossRef]
- Hsu, M.C.; Shih, J.C. Photoaffinity Labeling of Human Placental Monoamine Oxidase-A by 4-Fluoro-3-Nitrophenyl Azide. Mol. Pharmacol. 1988, 33, 237–241. [Google Scholar]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine Oxidase: From Genes to Behavior. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef]
- Umbarkar, P.; Singh, S.; Arkat, S.; Bodhankar, S.L.; Lohidasan, S.; Sitasawad, S.L. Monoamine Oxidase-A Is an Important Source of Oxidative Stress and Promotes Cardiac Dysfunction, Apoptosis, and Fibrosis in Diabetic Cardiomyopathy. Free Radic. Biol. Med. 2015, 87, 263–273. [Google Scholar] [CrossRef]
- Cho, Y.S.; Moon, H.-B. The Role of Oxidative Stress in the Pathogenesis of Asthma. Allergy. Asthma Immunol. Res. 2010, 2, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.L.; Oreland, L.; Reynolds, C.; McClearn, G.E. Importance of Genetic Effects for Monoamine Oxidase Activity in Thrombocytes in Twins Reared Apart and Twins Reared Together. Psychiatry Res. 1993, 46, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Pagani, F.; Baralle, F.E. Genomic Variants in Exons and Introns: Identifying the Splicing Spoilers. Nat. Rev. Genet. 2004, 5, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.Q.; Morimoto, K.; Shirakawa, T.; Hopkin, J.M.; Hashimoto, T.; Furuyama, J.; Kawai, M.; Sasaki, S.; Enomoto, T.; Yoshikawa, K.; et al. Association between Serotonin Type 2 Receptor (HTR2) and Bronchial Asthma in Humans. J. Med. Genet. 1996, 33, 525. [Google Scholar] [CrossRef]
- Flanagan, T.W.; Sebastian, M.N.; Battaglia, D.M.; Foster, T.P.; Cormier, S.A.; Nichols, C.D. 5-HT2 Receptor Activation Alleviates Airway Inflammation and Structural Remodeling in a Chronic Mouse Asthma Model. Life Sci. 2019, 236, 116790. [Google Scholar] [CrossRef] [PubMed]
- Koochak, S.E.; Deilami, G.D.; Ahangari, F.; Ahangari, G. Evaluation of Serotonin Receptor (5HT2RA) Gene Expression Changes in Peripheral Blood Mononuclear Cells of Asthma Allergic Patients. Glob. J. Pathol. Microbiol. 2018, 6, 8–14. [Google Scholar]
- Hilaire, G.; Voituron, N.; Menuet, C.; Ichiyama, R.M.; Subramanian, H.H.; Dutschmann, M. The Role of Serotonin in Respiratory Function and Dysfunction. Respir. Physiol. Neurobiol. 2010, 174, 76–88. [Google Scholar] [CrossRef]
- Kim, T.-H.; An, S.-H.; Cha, J.-Y.; Shin, E.-K.; Lee, J.-Y.; Yoon, S.-H.; Lee, Y.-M.; Uh, S.-T.; Park, S.-W.; Park, J.-S.; et al. Association of 5-Hydroxytryptamine (Serotonin) Receptor 4 (5-HTR4) Gene Polymorphisms with Asthma. Respirology 2011, 16, 630–638. [Google Scholar] [CrossRef]
- Bayer, H.; Müller, T.; Myrtek, D.; Sorichter, S.; Ziegenhagen, M.; Norgauer, J.; Zissel, G.; Idzko, M. Serotoninergic Receptors on Human Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2007, 36, 85–93. [Google Scholar] [CrossRef]
- Kang, B.N.; Ha, S.G.; Bahaie, N.S.; Hosseinkhani, M.R.; Ge, X.N.; Blumenthal, M.N.; Rao, S.P.; Sriramarao, P. Regulation of Serotonin-Induced Trafficking and Migration of Eosinophils. PLoS ONE 2013, 8, e54840. [Google Scholar] [CrossRef]
- Sheikhha, H.; Emadi, S.; Jorbozedar, S.; Deilami, G.; Ahangari, G. Investigation of Gene Expression Pattern of 5HTR2a and MAO-A in PBMCs of Individuals Who Had Been Exposed to Air Pollution in Highly Polluted Area. Recent Pat. Inflamm. Allergy Drug Discov. 2014, 8, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, G.; Koochak, S.E.; Amirabad, L.M.; Deilami, G.D. Investigation of 5-HT2A Gene Expression in PBMCs of Patients with Allergic Asthma. Inflamm. Allergy -Drug Targets 2015, 14, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Nau, F.J.; Miller, J.; Saravia, J.; Ahlert, T.; Yu, B.; Happel, K.I.; Cormier, S.A.; Nichols, C.D. Serotonin 5-HT₂ Receptor Activation Prevents Allergic Asthma in a Mouse Model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L191–L198. [Google Scholar] [CrossRef] [PubMed]
- MacHaffie, R.A.; Menbroker, L.R.; Mahler, D.J.; Barak, A.J. Studies in Allergy. II. Serum Serotonin Levels in Nonallergic, Pretreatment, and Posttreatment Allergic Human Beings and in Normal and Sensitized Guinea Pigs. J. Allergy 1960, 31, 106–110. [Google Scholar] [CrossRef]
- Cook, E.H.J.; Fletcher, K.E.; Wainwright, M.; Marks, N.; Yan, S.Y.; Leventhal, B.L. Primary Structure of the Human Platelet Serotonin 5-HT2A Receptor: Identify with Frontal Cortex Serotonin 5-HT2A Receptor. J. Neurochem. 1994, 63, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Serebruany, V.L.; El Mouelhi, M.; Pfannkuche, H.-J.; Rose, K.; Marro, M.; Angiolillo, D.J. Investigations on 5-HT₄ Receptor Expression and Effects of Tegaserod on Human Platelet Aggregation in Vitro. Am. J. Ther. 2010, 17, 543–552. [Google Scholar] [CrossRef]
- Massot, O.; Rousselle, J.C.; Fillion, M.P.; Januel, D.; Plantefol, M.; Fillion, G. 5-HT1B Receptors: A Novel Target for Lithium. Possible Involvement in Mood Disorders. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1999, 21, 530–541. [Google Scholar] [CrossRef]
- Stratz, C.; Trenk, D.; Bhatia, H.S.; Valina, C.; Neumann, F.-J.; Fiebich, B.L. Identification of 5-HT3 Receptors on Human Platelets: Increased Surface Immunoreactivity after Activation with Adenosine Diphosphate (ADP) and Thrombin Receptor-Activating Peptide (TRAP). Thromb. Haemost. 2008, 99, 784–786. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Wang, D.; Man, S.C.; Ng, R.; McAlonan, G.M.; Wong, H.K.; Wong, W.; Lee, J.; Tan, Q.-R. Platelet 5-HT1A Receptor Correlates with Major Depressive Disorder in Drug-Free Patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 53, 74–79. [Google Scholar] [CrossRef]
- Sreter, K.B.; Popovic-Grle, S.; Lampalo, M.; Konjevod, M.; Tudor, L.; Perkovic, M.N.; Jukic, I.; Bingulac-Popovic, J.; Stanic, H.S.; Markeljevic, J.; et al. Plasma Brain-Derived Neurotrophic Factor (BDNF) Concentration and Bdnf/Trkb Gene Polymorphisms in Croatian Adults with Asthma. J. Pers. Med. 2020, 10, 189. [Google Scholar] [CrossRef]
- Eiringhaus, K.; Renz, H.; Matricardi, P.; Skevaki, C. Component-Resolved Diagnosis in Allergic Rhinitis and Asthma. J. Appl. Lab. Med. 2019, 3, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Buhl, R.; Humbert, M.; Bjermer, L.; Chanez, P.; Heaney, L.G.; Pavord, I.; Quirce, S.; Virchow, J.C.; Holgate, S.; Djukanovic, R.; et al. Severe Eosinophilic Asthma: A Roadmap to Consensus. Eur. Respir. J. 2017, 49, 1700634. [Google Scholar] [CrossRef] [PubMed]
- Pavord, I.; Bahmer, T.; Braido, F.; Cosío, B.G.; Humbert, M.; Idzko, M.; Adamek, L. Severe T2-High Asthma in the Biologics Era: European Experts’ Opinion. Eur. Respir. Rev. 2019, 28, 190054. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma Phenotypes: The Evolution from Clinical to Molecular Approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P.G.; Modrek, B.; Choy, D.F.; Jia, G.; Abbas, A.R.; Ellwanger, A.; Arron, J.R.; Koth, L.L.; Fahy, J.V. T-Helper Type 2-Driven Inflammation Defines Major Subphenotypes of Asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, A.M.; Chipps, B.E.; Holguin, F.; Woodruff, P.G. T2-"Low" Asthma: Overview and Management Strategies. J. Allergy Clin. Immunol. Pract. 2020, 8, 452–463. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, K.S. Samter’s Triad: State of the Art. Clin. Exp. Otorhinolaryngol. 2018, 11, 71–80. [Google Scholar] [CrossRef]
- Svob Strac, D.; Nedic Erjavec, G.; Nikolac Perkovic, M.; Nenadic-Sviglin, K.; Konjevod, M.; Grubor, M.; Pivac, N. The Association between HTR1B Gene Rs13212041 Polymorphism and Onset of Alcohol Abuse. Neuropsychiatr. Dis. Treat. 2019, 15, 339–347. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Krajl, M. A Rapid Microfluorimetric Determination of Monoamine Oxidase. Biochem. Pharmacol. 1965, 14, 1684–1685. [Google Scholar] [CrossRef]
- Svob Strac, D.; Kovacic Petrovic, Z.; Nikolac Perkovic, M.; Umolac, D.; Nedic Erjavec, G.; Pivac, N. Platelet Monoamine Oxidase Type B, MAOB Intron 13 and MAOA-UVNTR Polymorphism and Symptoms of Post-Traumatic Stress Disorder. Stress 2016, 19, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A Simple Salting out Procedure for Extracting DNA from Human Nucleated Cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and Visualization of LD and Haplotype Maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Flachaire, E.; Beney, C.; Berthier, A.; Salandre, J.; Quincy, C.; Renaud, B. Determination of Reference Values for Serotonin Concentration in Platelets of Healthy Newborns, Children, Adults, and Elderly Subjects by HPLC with Electrochemical Detection. Clin. Chem. 1990, 36, 2117–2120. [Google Scholar] [CrossRef]
- Kumar, A.M.; Weiss, S.; Fernandez, J.B.; Cruess, D.; Eisdorfer, C. Peripheral Serotonin Levels in Women: Role of Aging and Ethnicity. Gerontology 1998, 44, 211–216. [Google Scholar] [CrossRef]
- Sagud, M.; Nikolac Perkovic, M.; Vuksan-Cusa, B.; Maravic, A.; Svob Strac, D.; Mihaljevic Peles, A.; Zivkovic, M.; Kusevic, Z.; Pivac, N. A Prospective, Longitudinal Study of Platelet Serotonin and Plasma Brain-Derived Neurotrophic Factor Concentrations in Major Depression: Effects of Vortioxetine Treatment. Psychopharmacology 2016, 233, 3259–3267. [Google Scholar] [CrossRef]
- Nenadic-Sviglin, K.; Nedic, G.; Nikolac, M.; Kozaric-Kovacic, D.; Stipcevic, T.; Muck Seler, D.; Pivac, N. Suicide Attempt, Smoking, Comorbid Depression, and Platelet Serotonin in Alcohol Dependence. Alcohol 2011, 45, 209–216. [Google Scholar] [CrossRef]
- Pivac, N.; Mück-Seler, D.; Mustapić, M.; Nenadić-Sviglin, K.; Kozarić-Kovacić, D. Platelet Serotonin Concentration in Alcoholic Subjects. Life Sci. 2004, 76, 521–531. [Google Scholar] [CrossRef]
- Padmavathi, P.; Reddy, V.D.; Swarnalatha, K.; Hymavathi, R.; Varadacharyulu, N.C. Influence of Altered Hormonal Status on Platelet 5-HT and MAO-B Activity in Cigarette Smokers. Indian J. Clin. Biochem. 2015, 30, 204–209. [Google Scholar] [CrossRef]
- Nedic Erjavec, G.; Bektic Hodzic, J.; Repovecki, S.; Nikolac Perkovic, M.; Uzun, S.; Kozumplik, O.; Tudor, L.; Mimica, N.; Svob Strac, D.; Pivac, N. Alcohol-Related Phenotypes and Platelet Serotonin Concentration. Alcohol 2021, 97, 41–49. [Google Scholar] [CrossRef]
- Bridge, T.P.; Soldo, B.J.; Phelps, B.H.; Wise, C.D.; Francak, M.J.; Wyatt, R.J. Platelet Monoamine Oxidase Activity: Demographic Characteristics Contribute to Enzyme Activity Variability. J. Gerontol. 1985, 40, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.; Pierucci, F.; Parvez, H.; Senatori, O. Monoamine Oxidase Expression during Development and Aging. Neurotoxicology 2004, 25, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Wiberg, A.; Oreland, L.; Marcusson, J.; Winblad, B. The Effect of Age on the Activity and Molecular Properties of Human Brain Monoamine Oxidase. J. Neural Transm. 1980, 49, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Konradi, C.; Mack-Burkhardt, F.; Riederer, P.; Heinsen, H.; Beckmann, H. Ontogenesis of Monoamine Oxidase-A and -B in the Human Brain Frontal Cortex. Brain Res. 1989, 499, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Volchegorskii, I.A.; Shemyakov, S.E.; Turygin, V.V.; Malinovskaya, N.V. Comparative Analysis of Age-Related Changes in Activities of Monoamine Oxidase-B and Antioxidant Defense Enzymes in Various Structures of Human Brain. Bull. Exp. Biol. Med. 2001, 132, 760–762. [Google Scholar] [CrossRef]
- Anthenelli, R.M.; Tipp, J.; Li, T.K.; Magnes, L.; Schuckit, M.A.; Rice, J.; Daw, W.; Nurnberger, J.I.J. Platelet Monoamine Oxidase Activity in Subgroups of Alcoholics and Controls: Results from the Collaborative Study on the Genetics of Alcoholism. Alcohol. Clin. Exp. Res. 1998, 22, 598–604. [Google Scholar] [CrossRef]
- Pivac, N.; Knezevic, J.; Mustapic, M.; Dezeljin, M.; Muck-Seler, D.; Kozaric-Kovacic, D.; Balija, M.; Matijevic, T.; Pavelic, J. The Lack of Association between Monoamine Oxidase (MAO) Intron 13 Polymorphism and Platelet MAO-B Activity among Men. Life Sci. 2006, 79, 45–49. [Google Scholar] [CrossRef]
- Coccini, T.; Randine, G.; Castoldi, A.F.; Balloni, L.; Baiardi, P.; Manzo, L. Lymphocyte Muscarinic Receptors and Platelet Monoamine Oxidase-B as Biomarkers of CNS Function: Effects of Age and Gender in Healthy Humans. Environ. Toxicol. Pharmacol. 2005, 19, 715–720. [Google Scholar] [CrossRef]
- Snell, L.D.; Glanz, J.; Tabakoff, B. Relationships between Effects of Smoking, Gender, and Alcohol Dependence on Platelet Monoamine Oxidase-B: Activity, Affinity Labeling, and Protein Measurements. Alcohol. Clin. Exp. Res. 2002, 26, 1105–1113. [Google Scholar] [CrossRef]
- Berlin, I.; Anthenelli, R.M. Monoamine Oxidases and Tobacco Smoking. Int. J. Neuropsychopharmacol. 2001, 4, 33–42. [Google Scholar] [CrossRef]
- Fowler, J.S.; Logan, J.; Wang, G.-J.; Volkow, N.D. Monoamine Oxidase and Cigarette Smoking. Neurotoxicology 2003, 24, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Nikolac Perkovic, M.; Svob Strac, D.; Nedic Erjavec, G.; Uzun, S.; Podobnik, J.; Kozumplik, O.; Vlatkovic, S.; Pivac, N. Monoamine Oxidase and Agitation in Psychiatric Patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 69, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Yiallouros, P.K.; Lamnisos, D.; Kolokotroni, O.; Moustaki, M.; Middleton, N. Associations of Body Fat Percent and Body Mass Index with Childhood Asthma by Age and Gender. Obesity 2013, 21, E474–E482. [Google Scholar] [CrossRef]
- Fenger, R.V.; Gonzalez-Quintela, A.; Vidal, C.; Husemoen, L.-L.; Skaaby, T.; Thuesen, B.H.; Aadahl, M.; Madsen, F.; Linneberg, A. The Longitudinal Relationship of Changes of Adiposity to Changes in Pulmonary Function and Risk of Asthma in a General Adult Population. BMC Pulm. Med. 2014, 14, 208. [Google Scholar] [CrossRef]
- Shore, S.A.; Fredberg, J.J. Obesity, Smooth Muscle, and Airway Hyperresponsiveness. J. Allergy Clin. Immunol. 2005, 115, 925–927. [Google Scholar] [CrossRef]
- Dixon, A.E.; Peters, U. The Effect of Obesity on Lung Function. Expert Rev. Respir. Med. 2018, 12, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Llorente, M.A.; Romero, R.; Chueca, N.; Martinez-Cañavate, A.; Gomez-Llorente, C. Obesity and Asthma: A Missing Link. Int. J. Mol. Sci. 2017, 18, 1490. [Google Scholar] [CrossRef]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and Asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef]
- Mohanan, S.; Tapp, H.; McWilliams, A.; Dulin, M. Obesity and Asthma: Pathophysiology and Implications for Diagnosis and Management in Primary Care. Exp. Biol. Med. 2014, 239, 1531–1540. [Google Scholar] [CrossRef]
- McLoughlin, R.F.; McDonald, V.M. The Management of Extrapulmonary Comorbidities and Treatable Traits; Obesity, Physical Inactivity, Anxiety, and Depression, in Adults with Asthma. Front. Allergy 2021, 2, 735030. [Google Scholar] [CrossRef]
- Holguin, F.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Erzurum, S.C.; Fitzpatrick, A.M.; Gaston, B.; Israel, E.; Jarjour, N.N.; et al. Obesity and Asthma: An Association Modified by Age of Asthma Onset. J. Allergy Clin. Immunol. 2011, 127, 1486–1493.e2. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Bunting, B.P.; Carr, E.; Strain, J.J.; Stewart-Knox, B.J. Obesity, Whole Blood Serotonin and Sex Differences in Healthy Volunteers. Obes. Facts 2012, 5, 399–407. [Google Scholar] [CrossRef]
- Ritze, Y.; Schollenberger, A.; Hamze Sinno, M.; Bühler, N.; Böhle, M.; Bárdos, G.; Sauer, H.; Mack, I.; Enck, P.; Zipfel, S.; et al. Gastric Ghrelin, GOAT, Leptin, and LeptinR Expression as Well as Peripheral Serotonin Are Dysregulated in Humans with Obesity. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2016, 28, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Binetti, J.; Bertran, L.; Riesco, D.; Aguilar, C.; Martínez, S.; Sabench, F.; Porras, J.A.; Camaron, J.; Del Castillo, D.; Richart, C.; et al. Deregulated Serotonin Pathway in Women with Morbid Obesity and NAFLD. Life 2020, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Young, R.L.; Lumsden, A.L.; Martin, A.M.; Schober, G.; Pezos, N.; Thazhath, S.S.; Isaacs, N.J.; Cvijanovic, N.; Sun, E.W.L.; Wu, T.; et al. Augmented Capacity for Peripheral Serotonin Release in Human Obesity. Int. J. Obes. 2018, 42, 1880–1889. [Google Scholar] [CrossRef]
- Ehrlich, S.; Franke, L.; Schott, R.; Salbach-Andrae, H.; Pfeiffer, E.; Lehmkuhl, U.; Uebelhack, R. Platelet Monoamine Oxidase Activity in Underweight and Weight-Recovered Females with Anorexia Nervosa. Pharmacopsychiatry 2008, 41, 226–231. [Google Scholar] [CrossRef]
- Malmgren, R.; Olsson, P.; Tornling, G.; Unge, G. The 5-Hydroxytryptamine Take-up Mechanism in Normal Platelets and Platelets from Migraine and Asthmatic Patients. Thromb. Res. 1980, 18, 733–741. [Google Scholar] [CrossRef]
- Jakubauskiene, E.; Janaviciute, V.; Peciuliene, I.; Söderkvist, P.; Kanopka, A. G/A Polymorphism in Intronic Sequence Affects the Processing of MAO-B Gene in Patients with Parkinson Disease. FEBS Lett. 2012, 586, 3698–3704. [Google Scholar] [CrossRef]
- Balciuniene, J.; Emilsson, L.; Oreland, L.; Pettersson, U.; Jazin, E. Investigation of the Functional Effect of Monoamine Oxidase Polymorphisms in Human Brain. Hum. Genet. 2002, 110, 1–7. [Google Scholar] [CrossRef]
- Garpenstrand, H.; Ekblom, J.; Forslund, K.; Rylander, G.; Oreland, L. Platelet Monoamine Oxidase Activity Is Related to MAOB Intron 13 Genotype. J. Neural Transm. 2000, 107, 523–530. [Google Scholar] [CrossRef]
- Jansson, M.; McCarthy, S.; Sullivan, P.F.; Dickman, P.; Andersson, B.; Oreland, L.; Schalling, M.; Pedersen, N.L. MAOA Haplotypes Associated with Thrombocyte-MAO Activity. BMC Genet. 2005, 6, 46. [Google Scholar] [CrossRef]
- Girmen, A.S.; Baenziger, J.; Hotamisligil, G.S.; Konradi, C.; Shalish, C.; Sullivan, J.L.; Breakefield, X.O. Relationship between Platelet Monoamine Oxidase B Activity and Alleles at the MAOB Locus. J. Neurochem. 1992, 59, 2063–2066. [Google Scholar] [CrossRef] [PubMed]
- Nedic Erjavec, G.; Nenadic Sviglin, K.; Nikolac Perkovic, M.; Muck-Seler, D.; Jovanovic, T.; Pivac, N. Association of Gene Polymorphisms Encoding Dopaminergic System Components and Platelet MAO-B Activity with Alcohol Dependence and Alcohol Dependence-Related Phenotypes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 321–327. [Google Scholar] [CrossRef]
- Pivac, N.; Knezevic, J.; Kozaric-Kovacic, D.; Dezeljin, M.; Mustapic, M.; Rak, D.; Matijevic, T.; Pavelic, J.; Muck-Seler, D. Monoamine Oxidase (MAO) Intron 13 Polymorphism and Platelet MAO-B Activity in Combat-Related Posttraumatic Stress Disorder. J. Affect. Disord. 2007, 103, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Bakulin, M.P.; Ioffe, E.I. Contents of biologically active substances (histamine and serotonin) in patients with bronchial asthma. Ter. Arkh. 1979, 51, 45–49. [Google Scholar] [PubMed]

| SNP | Platelet 5-HT Concentration (nmol/mg Protein) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HTR2A/C | Genotypes | Carriers | Carriers | |||||||
| rs6314 | AA | AG | GG | A | GG | G | AA | |||
| Control subjects | 1.52 0.93; - | 1.04 0.75; 1.70 | 0.91 0.69; 1.54 | p = 0.28 | 1.04 0.78; 1.80 | 0.91 0.69; 1.54 | p = 0.14; U = 1257.0 | 0.96 0.71; 1.63 | 1.52 0.93; - | p = 0.42; U = 75.0 |
| Asthma patients | 0.79 0.79; 0.7 | 0.88 0.69; 1.19 | 0.79 0.59; 1.07 | p = 0.41 | 0.85 0.69; 1.18 | 0.79 0.59; 1.07 | p = 0.19; U = 1079.0 | 0.82 0.62; 1.09 | 0.79 0.79; 0.79 | p = 0.97; U = 57.0 |
| rs6313 | AA | AG | GG | A | GG | G | AA | |||
| Control subjects | 0.94 0.73; 1.6 | 1.01 0.74; 1.54 | 0.92 0.70; 1.66 | p = 0.97 | 0.98 0.74; 1.61 | 0.92 0.70; 1.66 | p = 0.84; U = 1522.0 | 0.98 0.71; 1.61 | 0.94 0.73; 1.68 | p = 0.85; U = 1179.0 |
| Asthma patients | 0.81 0.62; 1.1 | 0.75 0.61; 1.09 | 0.89 0.65; 1.09 | p = 0.39 | 0.76 0.62; 1.09 | 0.89 0.65; 1.09 | p = 0.22; U = 1487.0 | 0.82 0.62; 1.09 | 0.81 0.62; 1.15 | p = 0.90; U = 1022.0 |
| rs3813929 | CC | CT | TT | C | TT | T | CC | |||
| Control subjects | 1.03 0.74; 1.66 | 0.86 0.66; 1.22 | 0.92 0.78; 1.53 | p = 0.54 | 0.98 0.71; 1.66 | 0.92 0.78; 1.53 | p = 1.00; U = 742.0 | 0.90 0.70; 1.28 | 1.03 0.74; 1.66 | p = 0.40; U = 1182.0 |
| Asthma patients | 0.82 0.55; 1.09 | 0.76 0.64; 1.12 | 0.76 0.56; 0.97 | p = 0.83 | 0.82 0.62; 1.09 | 0.76 0.56; 0.97 | p = 0.60; U = 299.0 | 0.76 0.64; 1.10 | 0.82 0.55; 1.09 | p = 0.97; U = 1110.0 |
| rs518147 | CC | CG | GG | C | GG | G | CC | |||
| Control subjects | 1.04 0.80; 2.05 | 0.86 0.68; 1.13 | 0.99 0.65; 1.60 | p = 0.20 | 0.94 0.73; 1.67 | 0.99 0.65; 1.60 | p = 0.70; U = 1720.0 | 0.94 0.68; 1.55 | 1.04 0.80; 2.05 | p = 0.12; U = 1220.0 |
| Asthma patients | 0.82 0.59; 1.08 | 0.76 0.66; 1.11 | 0.79 0.58; 1.09 | p = 0.78 | 0.82 0.64; 1.09 | 0.79 0.58; 1.09 | p = 0.50; U = 1661.0 | 0.78 0.61; 1.09 | 0.82 0.59; 1.08 | p = 0.74; U = 1500.0 |
| MAOB | Platelet MAO-B activity (nmol/mg of protein per hour) | |||||||||
| rs1799836 | CC | CT | TT | T | CC | C | TT | |||
| Control subjects | 36.72 25.80; 59.67 | 40.94 31.52; 52.86 | 28.08 22.58; 41.41 | p = 0.013 | 32.23 25.70; 51.08 | 36.72 25.80; 59.67 | p = 0.40; U = 1532.0 | 38.85 28.61; 58.44 | 28.08 22.58; 41.41 | p = 0.008 U = 1256.0 |
| Asthma patients | 41.45 31.22; 54.13 | 46.42 35.21; 65.73 | 41.45 34.66; 56.16 | p = 0.11 | 43.76 35.26; 61.56 | 41.45 31.22; 54.13 | p = 0.14; U = 1257.0 | 44.72 34.07; 58.94 | 41.45 34.66; 56.16 | p = 0.59; U = 1602.5 |
| rs6651806 | AA | AC | CC | A | CC | C | AA | |||
| Control subjects | 29.81 25.07; 51.12 | 41.92 31.52; 61.61 | 30.24 21.77; 49.29 | p = 0.03 | 34.13 25.73; 51.31 | 30.24 21.77; 49.29 | p = 0.55; U = 1129.0 | 36.88 28.99; 58.69 | 29.81 25.07; 51.12 | p = 0.10; U = 1438.0 |
| Asthma patients | 41.63 34.01; 59.69 | 45.36 35.36; 61.99 | 47.40 31.41; 56.31 | p = 0.85 | 42.62 35.08; 59.90 | 47.40 31.41; 56.31 | p = 0.69; U = 1114.0 | 45.76 33.72; 57.76 | 41.63 34.01; 59.69 | p = 0.93; U = 1708.5 |
| Clinical Parameters | Platelet 5-HT Concentration (nmol/mg Protein) | Platelet MAO-B Activity (nmol/mg of Protein Per Hour) | |
|---|---|---|---|
| Total Serum IgE (IU/mL) | p = 0.52; r = 0.06 | p = 0.66; r = 0.04 | Spearman correlation |
| Blood eosinophils (×109/L) | p = 0.63; r = 0.04 | p = 0.39; r = 0.08 | |
| Blood neutrophils (×109/L) | p = 0.52; r = 0.06 | p = 0.02; r = 0.21 | |
| FENO (ppb) | p = 0.99; r = −0.001 | p = 0.70; r = −0.04 | |
| FEV1 (% of predicted value) | p = 0.92; r = 0.01 | p = 0.60; r = 0.05 | |
| FVC (% of predicted value) | p = 0.28; r = 0.10 | p = 0.21; r = 0.11 | |
| PEF (% of predicted value) | p = 0.20; r = −0.12 | p = 0.73; r = −0.03 | |
| DLCO (%) | p = 0.20; r = −0.12 | p = 0.78; r = −0.03 | |
| Duration of disease (years) | p = 0.99; r = −0.001 | p = 0.63; r = 0.04 | |
| Comorbidities (N) | p = 0.88; r = −0.01 | p = 0.61; r = 0.05 | |
| Penicillin allergy | p = 0.28; U = 1018.0 | p = 0.86; U = 1159.0 | Mann-Whitney test |
| Nutritive allergy | p = 0.69; U = 602.0 | p = 0.43; U = 556.0 | |
| Animal dander/feather allergy | p = 0.71; U = 866.5 | p = 0.08; U = 678.0 | |
| Dust allergy | p = 0.66; U = 1676.0 | p = 0.12; U = 1469.0 | |
| Pollen allergy | p = 0.30; U = 1578.0 | p = 0.02; U = 1345.0 | |
| Fungal/mould allergy | p = 0.34; U = 401.50 | p = 0.86; U = 481.5 | |
| Early onset of asthma (age < 12 years) | p = 0.99; U = 1039.0 | p = 0.90; U = 1021.0 | |
| History of pneumonia | p = 0.77; U = 997.5 | p = 0.57; U = 957.5 | |
| Emergency intervention (ever) | p = 0.82; U = 1341.0 | p = 0.55; U = 1280.0 | |
| Hospitalization for asthma (ever) | p = 0.16; U = 1529.0 | p = 0.73; U = 1732.0 | |
| Nasal polyps | p = 0.38; U = 1104.0 | p = 0.62; U = 1164.0 | |
| Aspirin sensitivity | p = 0.27; U = 472.5 | p = 0.90; U = 580.0 | |
| Allergen specific immunotherapy | p = 0.89; U = 770.5 | p = 0.71; U = 740.0 | |
| Oral corticosteroid therapy | p = 0.59; U = 1262.0 | p = 0.09; U = 1067.0 | |
| Biological therapy | p = 0.08; U = 751.5 | p = 0.33; U = 860.5 |
| Asthma Phenotypes | Platelet 5-HT Concentration (nmol/mg Protein) | Platelet MAO-B Activity (nmol/mg of Protein Per Hour) | ||
|---|---|---|---|---|
| T2-high (N = 94) T2-low (N = 26) | 0.79 (0.62; 1.09) | p = 0.73; U = 1168.00 FC = 0.95 | 42.98 (35.30; 58.48) | p = 0.60; U = 1140.00 FC = 0.96 |
| 0.91 (0.60; 1.09) | 44.27 (31.81; 58.98) | |||
| Non-allergic (N = 42) Allergic (N = 78) | 0.85 (0.62; 1.18) | p = 0.77; U = 1585.00 FC = 1.02 | 37.79 (31.67; 57.17) | p = 0.047; U = 1278.00 FC = 1.13 |
| 0.77 (0.62; 1.08) | 44.87 (36.73; 58.60) | |||
| Non-eosinophilic (N = 73) Eosinophilic (N = 47) | 0.81 (0.62; 1.05) | p = 0.39; U = 1554.00 FC = 1.09 | 41.91 (33.63; 59.14) | p = 0.57; U = 1609.00 FC = 1.03 |
| 0.86 (0.64; 1.19) | 44.61 (35.32; 57.49) | |||
| Non-AERD (N = 111) AERD (N = 9) | 0.81 (0.62; 1.08) | p = 0.42; U = 413.50 FC = 1.05 | 43.15 (34.07; 58.60) | p = 0.71; U = 457.00 FC = 0.95 |
| 0.82 (0.69; 1.31) | 36.50 (33.93; 57.21) |
| Findings | Sample Type/Method | Study |
|---|---|---|
| ↓ 5-HT platelet concentration and ↑ MAO-B platelet activity in asthma patients no association of HTR2A, HTR2C and MAOB polymorphisms with asthma | human platelets/spectrofluorometry human DNA samples/Real-Time PCR | this study |
| 5-HT2 activation decreases airway hyperresponsiveness | BALB/c mice (BALF)/PCR, multiplex assays | [38] |
| ↑ HTR2A gene expression in asthma patients vs. control group | human mononuclear cells/Real-Time PCR | [39] |
| ↑ 5HTR2A gene expression in asthma patients vs. control group | human PBMCs/Real-Time PCR | [45] |
| 5-HT2 receptor activation has anti-inflammatory effects | BALB/c mice (BALF)/Real-Time PCR, ELISA | [46] |
| ↑ 5HTR2A gene expression in allergic asthma patients vs. control group no changes in MAO-A expression | human PBMCs/Real-Time PCR | [44] |
| ↑ 5-HT levels in BALF of asthma patients ↓ 5-HT in the serum of asthma patients | mouse model and human samples (BALF, cell supernatant, plasma, serum)/enzyme immunoassay | [14] |
| ↑ frequency of HTR4 alleles (+142828G>A and +122769G>A) in asthma patients ↑ frequency of haplotype 1 in block 2 ↓ frequency of haplotype 4 in block 3 | human DNA samples/Real-Time PCR | [41] |
| ↑ MAO plasma activity and ↓ levels of platelet 5-HT and histamine in asthma patients | human plasma and platelets | [23] |
| ↑ free 5-HT levels in symptomatic asthma patients vs. asymptomatic patients | human plasma/HPLC-ECD | [13,15] |
| no association between HTR2 variants and bronchial asthma | human DNA samples/MspI restriction polymorphism | [37] |
| altered active 5-HT-transport in asthma patients vs. control group ↑ 5-HT plasma levels | human plasma (PPP/PRP)/14C- 5-HT uptake | [22,100] |
| ↑ 5-HT during an asthma attack | human blood/fluorometry | [108] |
| ↓ 5-HT uptake in acetylsalicylic acid-induced asthmatic patients vs. control group | human plasma (PPP/PRP)/14C- 5-HT uptake | [21] |
| ↑ 5-HT serum levels in allergic subjects vs. control group | human and guinea pigs serum/spectrofluorometry | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konjevod, M.; Sreter, K.B.; Popovic-Grle, S.; Lampalo, M.; Tudor, L.; Jukic, I.; Nedic Erjavec, G.; Bingulac-Popovic, J.; Safic Stanic, H.; Nikolac Perkovic, M.; et al. Platelet Serotonin (5-HT) Concentration, Platelet Monoamine Oxidase B (MAO-B) Activity and HTR2A, HTR2C, and MAOB Gene Polymorphisms in Asthma. Biomolecules 2023, 13, 800. https://doi.org/10.3390/biom13050800
Konjevod M, Sreter KB, Popovic-Grle S, Lampalo M, Tudor L, Jukic I, Nedic Erjavec G, Bingulac-Popovic J, Safic Stanic H, Nikolac Perkovic M, et al. Platelet Serotonin (5-HT) Concentration, Platelet Monoamine Oxidase B (MAO-B) Activity and HTR2A, HTR2C, and MAOB Gene Polymorphisms in Asthma. Biomolecules. 2023; 13(5):800. https://doi.org/10.3390/biom13050800
Chicago/Turabian StyleKonjevod, Marcela, Katherina B. Sreter, Sanja Popovic-Grle, Marina Lampalo, Lucija Tudor, Irena Jukic, Gordana Nedic Erjavec, Jasna Bingulac-Popovic, Hana Safic Stanic, Matea Nikolac Perkovic, and et al. 2023. "Platelet Serotonin (5-HT) Concentration, Platelet Monoamine Oxidase B (MAO-B) Activity and HTR2A, HTR2C, and MAOB Gene Polymorphisms in Asthma" Biomolecules 13, no. 5: 800. https://doi.org/10.3390/biom13050800
APA StyleKonjevod, M., Sreter, K. B., Popovic-Grle, S., Lampalo, M., Tudor, L., Jukic, I., Nedic Erjavec, G., Bingulac-Popovic, J., Safic Stanic, H., Nikolac Perkovic, M., Markeljevic, J., Samarzija, M., Pivac, N., & Svob Strac, D. (2023). Platelet Serotonin (5-HT) Concentration, Platelet Monoamine Oxidase B (MAO-B) Activity and HTR2A, HTR2C, and MAOB Gene Polymorphisms in Asthma. Biomolecules, 13(5), 800. https://doi.org/10.3390/biom13050800







