Abstract
The aim of this study was to evaluate the intensity of oxidative stress by measuring the concentrations of lipid peroxidation products (LPO) in fetal membrane, umbilical cord, and placenta samples obtained from women with multiple pregnancies. Additionally, the effectiveness of protection against oxidative stress was assessed by measuring the activity of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase (GR). Due to the role of iron (Fe), copper (Cu), and zinc (Zn) as cofactors for antioxidant enzymes, the concentrations of these elements were also analyzed in the studied afterbirths. The obtained data were compared with newborn parameters, selected environmental factors, and the health status of women during pregnancy to determine the relationship between oxidative stress and the health of women and their offspring during pregnancy. The study involved women (n = 22) with multiple pregnancies and their newborns (n = 45). The Fe, Zn, and Cu levels in the placenta, umbilical cord, and fetal membrane were determined using inductively coupled plasma atomic emission spectroscopy (ICP-OES) using an ICAP 7400 Duo system. Commercial assays were used to determine SOD, GPx, GR, CAT, and LPO activity levels. The determinations were made spectrophotometrically. The present study also investigated the relationships between trace element concentrations in fetal membrane, placenta, and umbilical cord samples and various maternal and infant parameters in women. Notably, a strong positive correlation was observed between Cu and Zn concentrations in the fetal membrane (p = 0.66) and between Zn and Fe concentrations in the placenta (p = 0.61). The fetal membrane Zn concentration exhibited a negative correlation with shoulder width (p = −0.35), while the placenta Cu concentration was positively correlated with placenta weight (p = 0.46) and shoulder width (p = 0.36). The umbilical cord Cu level was positively correlated with head circumference (p = 0.36) and birth weight (p = 0.35), while the placenta Fe concentration was positively correlated with placenta weight (p = 0.33). Furthermore, correlations were determined between the parameters of antioxidative stress (GPx, GR, CAT, SOD) and oxidative stress (LPO) and the parameters of infants and maternal characteristics. A negative correlation was observed between Fe and LPO product concentrations in the fetal membrane (p = −0.50) and placenta (p = −0.58), while the Cu concentration positively correlated with SOD activity in the umbilical cord (p = 0.55). Given that multiple pregnancies are associated with various complications, such as preterm birth, gestational hypertension, gestational diabetes, and placental and umbilical cord abnormalities, research in this area is crucial for preventing obstetric failures. Our results could serve as comparative data for future studies. However, we advise caution when interpreting our results, despite achieving statistical significance.
1. Introduction
Pregnancy is a period of rapid growth and cell differentiation. Both the mother and the fetus’ bodies are sensitive to changes in the supply of nutrients, including trace elements [1]. Therefore, it is important to ensure an optimal amount of these elements. Iron (Fe), zinc (Zn), and copper (Cu) are elements that serve as cofactors for many physiological processes related to the body’s homeostasis [2,3]. These elements also affect the development of organs and tissues as well as proper fetal weight gain [4,5,6], reducing the risks of preeclampsia [7,8,9] and preterm birth [10]. The role of Fe, Zn, and Cu during pregnancy, as well as the characteristics of the interactions between these elements, were described in a previous study by this team [11].
During pregnancy, women experience changes in their immune system that lead to the occurrence of inflammatory states characterized by the activation of macrophages and microphages, which produce high levels of reactive oxygen species (ROS) such as superoxide (O2−•), hydroxyl radicals (OH−•), and hydrogen peroxide (H2O2). The placenta is the main source of ROS during pregnancy [12], and the metabolism of ROS plays a crucial role in pregnancy progression, with increased levels of ROS observed in maternal blood [13]. However, the elevated ROS levels during pregnancy are counterbalanced by an increase in antioxidant synthesis [14]. An optimal level of ROS is necessary for many physiological processes, such as proliferation, defense reactions, signal transduction, and gene expression [15].
Oxidative stress (OS) arises due to an imbalance between ROS production and the body’s antioxidant defense mechanisms. OS in the placenta can lead to oxidative damage that can have far-reaching effects on the growth of the placenta and fetus. Complications during pregnancy such as miscarriage, preeclampsia, fetal growth restriction, and preterm birth have been linked to OS. Excessive ROS production can result in uncontrolled lipid peroxidation (LPO), which can cause disrupted membrane integrity and alterations in cellular metabolism. While LPO typically occurs at a low rate in all tissues and cells under normal physiological conditions, OS can lead to an increase in LPO.
Proteins such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione reductase (GR) play a crucial role in protecting the body from oxidative stress. Of these, GPX and CAT are the main enzymes involved in neutralizing H2O2 [16]. The activity of SOD, CAT, and GPX has been observed to decrease in the placenta of women with preeclampsia [17,18,19], and lower CAT activity has been linked to premature births [20]. Optimal levels of SOD in the placenta protect the fetus from the adverse effects of oxygen by decreasing the concentration of lipid peroxides [21].
Trace elements, such as Fe, Zn, and Cu, also play a critical role in protecting against oxidative stress, with their availability being essential for enzymatic activity [22,23,24]. These elements can act as cofactors of enzymes involved in controlling free radicals in the body and are vital for antioxidant capacity [16]. Cu and Zn are structural components of transcription factors and SOD, and they act as signaling molecules [16]. Changes in Cu levels in the body can affect antioxidant systems and may play a role in pregnancy complications [16]. A deficiency of this micronutrient during pregnancy can lead to the development of oxidative stress, which may result in impaired fetal growth [25]. On the other hand, it has been shown that complications in the first trimester of pregnancy are more frequent in women with higher serum Cu levels than in those with lower levels. An inadequate Cu nutritional status leads to impaired antioxidant mechanisms, while an excessive Cu concentration promotes the production of reactive oxygen and nitrogen species [26]. A severe Zn deficiency in pregnant women can lead to fetal loss, fetal growth restriction, and low implantation rates [27]. Zn is a powerful antiradical and anti-inflammatory agent. Its ions form chelates with the sulfhydryl groups of proteins, shielding them from pro-oxidative processes. Zinc provides protection to cellular membranes from peroxidation by displacing Cu and Fe from their membrane-binding sites [16]. Zn is involved in the synthesis of antioxidant enzymes and acts as an enzymatic catalyst, playing a vital role in the metabolism of lipids, carbohydrates, and proteins. Along with Cu, Zn acts as a co-factor of Cu/Zn-SOD, which gets suppressed under Zn-deficient conditions [28]. Iron, like Cu, changes its oxidation state, leading to the production of reactive oxygen species, and is a component of SOD. Both Fe deficiency and excess during pregnancy are detrimental to fetal development [29]. The research has indicated that excess Fe saturation during the prenatal period may elevate the risks of miscarriage, premature birth, low birth weight, and being small for gestational age (SGA), with OS being one of the likely mechanisms behind these abnormalities. In pregnant women without Fe deficiency anemia (IDA), prophylactic Fe supplementation has been observed to trigger oxidative stress and limit the organism’s antioxidant capacity [30,31].
Limited data are available on the parameters of OS and antioxidant defense in afterbirths from women with multiple pregnancies [32], who have higher risks of mortality and morbidity [33]. Multiple pregnancies are associated with abnormalities in the placenta, which can lead to intrauterine growth restriction and abnormalities in the umbilical cord [34]. Therefore, the objective of this study was to assess the intensity of oxidative stress by quantifying the concentrations of lipid peroxidation products in fetal membrane, umbilical cord, and placenta samples collected from women with multiple pregnancies. Additionally, the effectiveness of protection against OS was evaluated by measuring the activity of antioxidant enzymes, namely SOD, CAT, GPX, and GR. In view of the role of Fe, Cu, and Zn as cofactors for antioxidant enzymes, the concentrations of these elements were also analyzed in the studied afterbirths. The obtained data were compared with the newborn parameters, selected environmental parameters, and maternal health during pregnancy.
2. Materials and Methods
2.1. Ethics Statement
The study was conducted between 2015 and 2021, following the approval of the Biometric Committee of the Pomeranian Medical University in Szczecin (KB-0012/76/14 from 13 October 2014). The patients provided written informed consent to participate and were informed of their right to withdraw their consent at any point during the study. The research was conducted in accordance with the principles of the Declaration of Helsinki.
2.2. Study Population
The study enrolled 22 European women with multiple pregnancies and their newborns (n = 45) at the Obstetrics and Gynecology Clinic of the Independent Public Clinical Hospital No. 2 of the Pomeranian Medical University in Szczecin, northwest Poland. The multiple pregnancies included 21 twin pregnancies, including diamniotic (n = 14), monochorionic diamniotic (n = 5), and monochorionic monoamniotic (n = 2) twin pregnancies, and 1 triple pregnancy (dichorionic triamniotic triplets), all of which were terminated by cesarean section. The study’s inclusion criteria were multiple pregnancies and newborns without perinatal illness, and the sample was randomly selected. All women were healthy and had no risk factors that could affect the neonatal parameters, with those having hypertension and diabetes excluded. Infants with anemia, chromosomal abnormalities, or birth defects were also excluded. Table 1 and Table 2 present the newborns’ characteristics.

Table 1.
Maternal and neonatal characteristics (AM, arithmetic mean; SD, standard deviation; Med, median; n, number of participants; BMI, body mass index).

Table 2.
Smoothed centiles for birthweight and birth length of the boys (n = 54) and girls (n = 65) (Fenton Growth Chart).
At birth, standard anthropometric procedures were used to measure the birth weight, length, and head circumference. The gestational age was determined by the crown–rump length measured in the first trimester using ultrasonography and calculated based on the first day of the last menstrual period using Naegele’s rule. The mothers’ anthropometric and biological characteristics, including their age, weight, and a morphological blood analysis, as well as the infants’ shoulder width, weight, length, head circumference, gestational age, and sex, were obtained from medical records. Additionally, data on the weight of the placenta and length of the umbilical cord were also collected from medical records.
The socio-demographic information, smoking history prior to pregnancy (n = 6), and obstetric and gynecological histories, including parity (number of previous deliveries), were collected through general questionnaires. During periodic medical interviews, the majority of women (n = 19) reported taking Prenatal DUO©, a daily iron (II) fumarate supplement with a 30 mg dose. The body mass index (BMI) was calculated according to a women’s weight in kilograms divided by the square of height in meters [35]; only 18 women were in the range of 18.5–24.9. Unfortunately, data regarding maternal dietary habits during pregnancy were not available, although there were no indications of nutritional impairment or malnutrition among the participants.
Immediately following delivery, placentas (n = 38), umbilical cords (n = 45), and fetal membranes (n = 42) were collected, weighed, measured, and stored at −80 °C until the study group had been assembled.
2.3. Determination of Metals in Afterbirths
The samples were first restored to room temperature and then dried for three weeks at 105 °C to determine their water content (gravimetric method), following the protocols of Kalisinska et al. [36] and Lanocha et al. [37]. The dried samples were then ground into a fine powder using a porcelain mortar and subsequently mineralized [38]. The levels of Fe, Cu, and Zn were analyzed through inductively coupled plasma atomic emission spectroscopy (ICP-OES) using an ICAP 7400 Duo instrument from Thermo Scientific (Waltham, MA, USA), with the wavelengths (nm) of Fe = 238.204, Zn = 213.856, and Cu = 224.700 being utilized. In order to validate the analytical techniques, samples of Bovine Muscle NIST-SRM 8414 reference material (National Institute of Standards and Technology, Gaithersburg, MD, USA) were also assayed. The concentrations of metals in the reference material provided by the manufacturers and our own determinations are shown in Table 3. The Fe, Cu, and Zn concentrations in the placenta, umbilical cord, and fetal membrane are presented in mg/kg−1 dry weight (dw).

Table 3.
An analysis of the reference material Bovine Muscle NIST-SRM 8414.
2.4. Determination of Oxidative Stress in Afterbirths
The tissue fragments were ground with a mortar and pestle, and pulverized and frozen samples were placed into a tube containing 500 µL of an appropriate buffer, following the protocol of a commercial enzyme assay kit, while being maintained at 4 °C. The material was vortexed and homogenized using a knife homogenizer. The resulting extract mixtures were centrifuged (3000× g for 10 min, at 4 °C) to obtain the supernatant, which was used for enzyme quantification.
The following reagent kits were used to determine the antioxidant enzyme activity: a Superoxide Dismutase Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), Catalase Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), Glutathione Peroxidase Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), Glutathione Reductase Assay Kit (Cayman Chemical, Ann Arbor, MI, USA), and Lipid Peroxidation (LPO) Assay Kit (G-bioscience, St Louis, MO, USA). The determinations were made spectrophotometrically in accordance with the protocols provided by the manufacturers and are presented in U/mg protein−1.
2.5. Statistical Analysis
A statistical analysis was performed using Statistica v13.0 (Stat Soft). A Shapiro–Wilk analysis was performed to test the normal distribution of the data. Due to the non-normal distribution of data, the Spearman rank correlation was used to analyze the relationship between the variables, determining the value of the coefficient and the level of statistical significance (rho, ρ). In order to assess differences between the parameters, a Kruskal–Wallis ANOVA followed by Mann–Whitney-U tests were used. The significance level was p < 0.05.
3. Results
The mean water constituents in the placenta, fetal membrane, and umbilical cord equaled approximately 83%, 85%, and 88%, respectively. In the scientific literature, metal concentrations are given in terms of the dry (mg kg−1 dw) or wet weight (mg kg−1 ww) of tissues and organs. In order to make it possible to compare our own results with the literature data expressed in mg kg−1 ww, aconversion factor of 1.5 was used in this study, since on average the afterbirths contained at least 85% water.
The concentrations of Fe, Zn, and Cu in the placenta, umbilical cord, and fetal membrane are presented in Table 4. The highest concentration of Fe (417.52 mg/kg−1 dw) was found in the placenta, while the lowest was found in the umbilical cord (200.17 mg/kg−1 dw). The zinc concentration was highest in the placenta (50.21 mg/kg−1 dw) and lowest in the fetal membrane (36.91 mg/kg−1 dw). In contrast, the highest concentration of Cu was found in the fetal membrane (7.31 mg/kg−1 dw), while the lowest was found in the placenta (4.47 mg/kg−1 dw). Accordingly, the Fe, Cu, and Zn concentrations differed significantly between the umbilical cord, placenta, and fetal membrane (Figure 1, Figure 2 and Figure 3).

Table 4.
Concentrations of iron (Fe), zinc (Zn), and copper (Cu) in the placenta, umbilical cord, and fetal membrane in groups for oxidative stress (mg/kg−1; dry mass, dw) (AM, arithmetic mean; Med, median; Max, maximum; Min, minimum; SD, standard deviation).
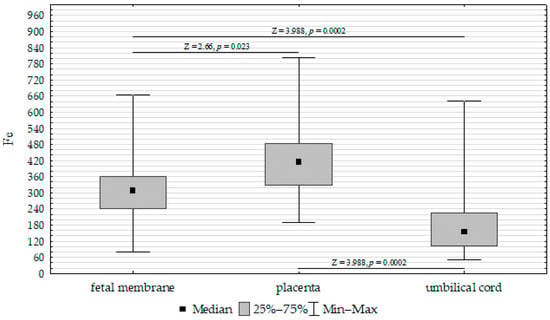
Figure 1.
Kruskal–Wallis test results for differences in iron (Fe) concentrations between the placenta, fetal membrane, and umbilical cord.
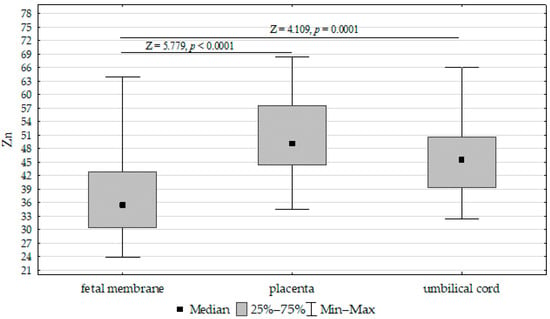
Figure 2.
Kruskal–Wallis test results for differences between zinc (Zn) concentrations in the placenta, fetal membrane, and umbilical cord.
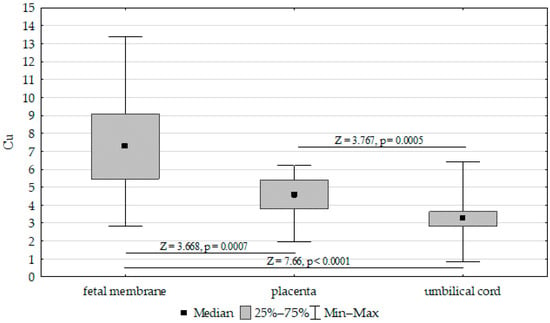
Figure 3.
Kruskal–Wallis test results for differences in cooper (Cu) concentrations between the placenta, fetal membrane, and umbilical cord.
In the study, we determined correlations between Fe, Cu, and Zn concentrations in the placenta, umbilical cord, and fetal membrane and the parameters of the infants, maternal characteristics, and gestational age. In the studied tissues from women of Poland, we found strong positive correlations between Cu and Zn concentrations in the fetal membrane (p = 0.66) and Zn and Fe concentrations in the placenta (p = 0.61). We found a correlation between the fetal membrane Zn concentration and shoulder width (p = −0.35); between the placenta Cu concentration and (i) placenta weight (p = 0.46) and (ii) shoulder width (p = 0.36); between the umbilical cord Cu level and (i) head circumference (p = 0.36) and (ii) birth weight (p = 0.35); and between the placenta Fe concentration and placenta weight (p = 0.33).
Furthermore, we compared the Fe, Cu, and Zn concentrations in the placenta, umbilical cord, and fetal membrane and the parameters of the infants, gestational age, maternal characteristics, supplementation, and cigarette smoking before pregnancy. The Mann–Whitney U test showed the following:
- Upregulation of placenta Zn in non-smoking women compared to those smoking before pregnancy (Figure 4);
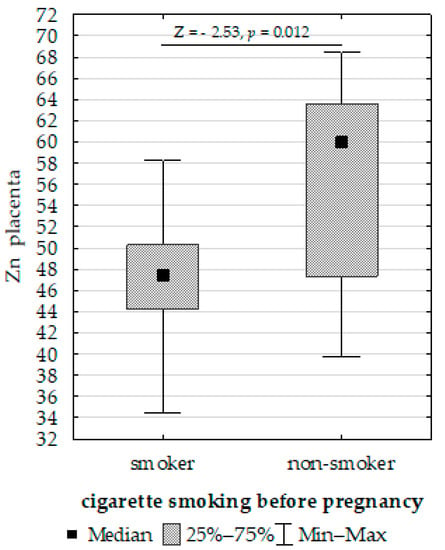 Figure 4. Mann–Whitney U test results for differences in placenta zinc (Zn) between non-smoking women and those smoking before pregnancy.
Figure 4. Mann–Whitney U test results for differences in placenta zinc (Zn) between non-smoking women and those smoking before pregnancy. - Upregulation of placenta Fe in newborns with >3 or <97 centiles for length compared to <3 or >97 (Figure 5);
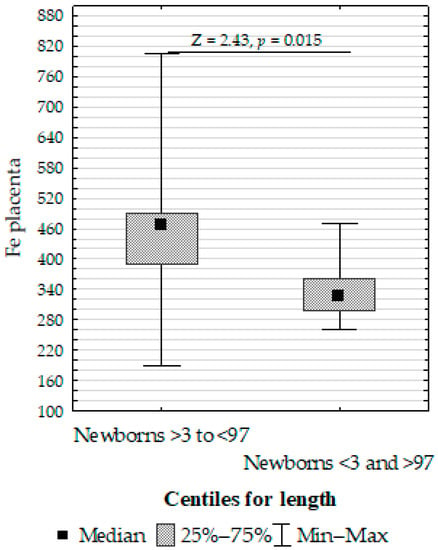 Figure 5. Mann–Whitney U test results for the differences in placenta iron (Fe) between the >3 to <97 centiles and <3 and >97 centiles groups.
Figure 5. Mann–Whitney U test results for the differences in placenta iron (Fe) between the >3 to <97 centiles and <3 and >97 centiles groups. - Upregulation of placenta Cu at ≥37 weeks of gestation compared to <37 weeks of gestation (Figure 6).
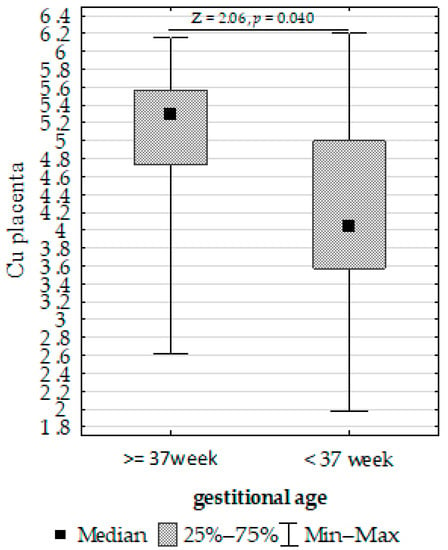 Figure 6. Mann–Whitney U test results for differences in placenta copper (Cu) depending on gestational age.
Figure 6. Mann–Whitney U test results for differences in placenta copper (Cu) depending on gestational age.
In addition, the concentrations of LPO and the activities of antioxidant enzymes (SOD, CAT, GPX, GR) were analyzed in fetal membranes, umbilical cords, and placentas obtained from women with multiple pregnancies, and the results are presented in Table 5. In the studied afterbirths, the activity of antioxidant enzymes (as measured by the AM assay) was observed to decrease in the following order:
- For GPx: fetal membrane > placenta > umbilical cord;
- For GR: placenta > umbilical cord > fetal membrane;
- For CAT and SOD: umbilical cord > fetal membrane > placenta.

Table 5.
The concentrations of antioxidants and pro-oxidants in the placenta, umbilical cord, and fetal membrane (U/mg protein−1) (AM, arithmetic mean; Med, median; Max, maximum; Min, minimum; SD, standard deviation; GPx, glutathione peroxidase; GR, glutathione reductase; CAT, catalase; SOD, superoxide dismutase; LPO, lipid peroxidation product levels).
Table 5.
The concentrations of antioxidants and pro-oxidants in the placenta, umbilical cord, and fetal membrane (U/mg protein−1) (AM, arithmetic mean; Med, median; Max, maximum; Min, minimum; SD, standard deviation; GPx, glutathione peroxidase; GR, glutathione reductase; CAT, catalase; SOD, superoxide dismutase; LPO, lipid peroxidation product levels).
| Placenta | Umbilical Cord | Fetal Membrane | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AM ± SD | Med. | Range | AM ± SD | Med. | Range | AM ± SD | Med | Range | |
| Total (n = 22) = 21 twins + 1 triplets Parameters antioxidative stress | |||||||||
| SOD | 0.04 ± 0.03 | 0.04 | 0.01–0.14 | 0.09 ± 0.07 | 0.07 | 0.01–0.29 | 0.06 ± 0.07 | 0.04 | 0.01–0.30 |
| CAT | 1 ± 1 | 0.69 | 0.17–8.26 | 3 ± 2 | 2.90 | 0.76–8.34 | 2 ± 2 | 1.46 | 0.27–8.83 |
| GPx | 5 ± 6 | 3.14 | 0.10–36.07 | 4 ± 4 | 3.82 | 0.30–18.31 | 6 ± 6 | 4.49 | 0.33–25.64 |
| GR | 6 ± 3 | 5.24 | 0.56–14.26 | 4 ± 3 | 3.52 | 0.14–13.24 | 4 ± 3 | 3.40 | 0.18–16.98 |
| SOD/GPx ratio | 0.03 ± 0.07 | 0.01 | 0.01–0.39 | 0.04 ± 0.05 | 0.02 | 0.01–0.26 | 0.02 ± 0.02 | 0.01 | 0.01–0.11 |
| SOD/CAT ratio | 0.07 ± 0.08 | 0.04 | 0.01–0.31 | 0.06 ± 0.17 | 0.03 | 0.01–1.1 | 0.03 ± 0.02 | 0.03 | 0.01–0.11 |
| Parameters oxidative stress | |||||||||
| LPO | 2 ± 1 | 2.26 | 0.04–4.19 | 2 ± 1 | 1.41 | 0.04–6.09 | 2 ± 1 | 1.42 | 0.11–4.67 |
The highest concentration of LPO was found in the placenta, while lower concentrations were observed in the umbilical cord and fetal membrane.
To assess whether the antioxidant defense mechanism is sufficient in the afterbirths, we investigated the ratios of SOD/GPx and SOD/CAT (Table 5). The highest SOD/GPx ratio was observed in the umbilical cord, while the SOD/CAT ratios were similar in all of the investigated tissues. We observed significant differences in SOD/GPx and SOD/CAT ratios between some of the parameters analyzed, as follows: SOD/GPx and GPx in the fetal membrane (p = −0.67); SOD/GPx and SOD in the fetal membrane (p = 0.64); SOD/GPx and GPx in the placenta (p = −0.85); SOD/GPx and SOD in the placenta (p = 0.39); the placenta SOD/GPx and Cu in the umbilical cord (p = 0.35); SOD/GPx and GPx in the umbilical cord (p = −0.55); SOD/GPx and SOD in the umbilical cord (p = 0.60); the fetal membrane SOD/CAT and GPx in the umbilical cord (p = 0.32); SOD/CAT and CAT in the fetal membrane (p = −0.45); SOD/CAT and SOD in the fetal membrane (p = 0.70); SOD/CAT and GR in the placenta (p = −0.37); SOD/CAT and CAT in the placenta (p = −0.70); SOD/CAT and SOD in the placenta (p = 0.41); placenta SOD/CAT and SOD in the umbilical cord (p = −0.55); the umbilical cord SOD/CAT and placenta GPx (p = −0.36); the umbilical cord SOD/CAT and placenta CAT (p = 0.47); SOD/CAT and SOD in the umbilical cord (p = 0.82); SOD/CAT and Zn in the umbilical cord (p = 0.36); SOD/CAT and Cu in the umbilical cord (p = 0.52).
Additionally, we determined correlations between parameters of antioxidative stress (GPx, GR, CAT, SOD) and oxidative stress (LPO) and the parameters of the infants and maternal characteristics (Table 6). We found negative correlations between Fe and LPO products concentrations in the fetal membrane (p = −0.50) and placenta (p = −0.58) and the Cu concentration and SOD activity in the umbilical cord (p = 0.55) (Table 6).

Table 6.
Spearman’s coefficients between Fe, Zn, and Cu concentrations in the placenta, umbilical cord, and fetal membrane and balance of pro-oxidant/antioxidant and anthropometric parameters of the infants.
Furthermore, we determined the correlations between antioxidative defense (SOD, CAT, GPx, GR) and oxidative stress (LPO) in the placenta, umbilical cord, and fetal membrane, and the characteristics of the infants and BMI (Table 6).
We compared SOD, CAT, GPx, GR, and LPO in the placenta, umbilical cord, and fetal membrane and the parameters of the infants, gestational age, maternal characteristics, supplementation, and cigarette smoking before pregnancy. The Mann–Whitney U test showed the following:
- Decreased umbilical cord GPx activity in women who did not take supplementation compared to those who did (Figure 7);
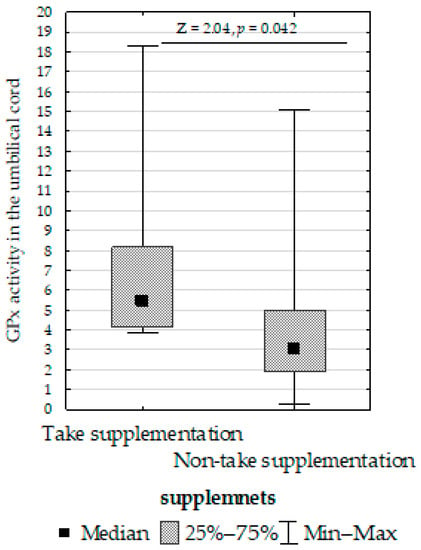 Figure 7. Mann–Whitney U test results for differences in glutathione peroxidase (GPx) activity between women taking supplementation and those not taking supplementation.
Figure 7. Mann–Whitney U test results for differences in glutathione peroxidase (GPx) activity between women taking supplementation and those not taking supplementation. - Increased umbilical cord CAT activity in infants with normal birth weight compared to those with low birth weight (Figure 8);
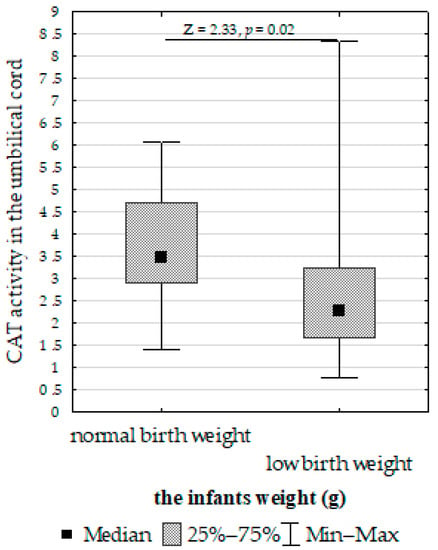 Figure 8. Mann–Whitney U test results for differences in catalase (CAT) activity between infants with normal and low birth weights.
Figure 8. Mann–Whitney U test results for differences in catalase (CAT) activity between infants with normal and low birth weights. - Increased placenta SOD activity concentration in non-smoking women compared to those who smoked before pregnancy (Figure 9);
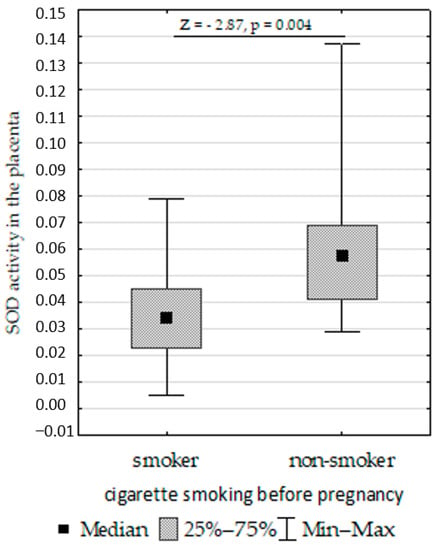 Figure 9. Mann–Whitney U test results for differences in superoxide dismutase (SOD).
Figure 9. Mann–Whitney U test results for differences in superoxide dismutase (SOD). - Decreased placenta GR activity in women with >18.5 to <25 BMI compared to women with <18.5 and >25 BMI (Figure 10).
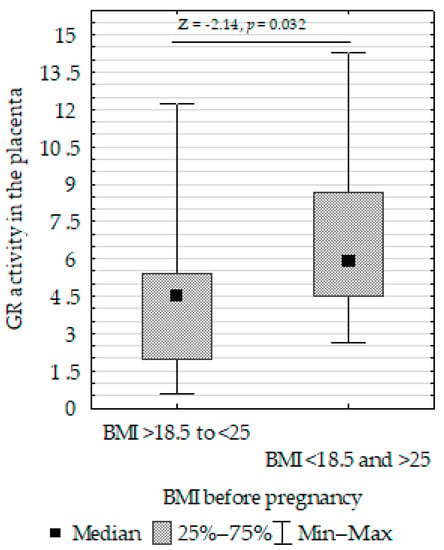 Figure 10. Mann–Whitney U test results for the differences in placenta GR activity between non-smoking women and those smoking before pregnancy activity between the >18.5 to <25 BMI and <18.5 and >25 BMI groups.
Figure 10. Mann–Whitney U test results for the differences in placenta GR activity between non-smoking women and those smoking before pregnancy activity between the >18.5 to <25 BMI and <18.5 and >25 BMI groups.
4. Discussion
4.1. Trace Element Concentrations in Afterbirth
Essential trace elements such as Fe, Cu, and Zn play a critical role in the normal progression of pregnancy and act as cofactors for antioxidant enzymes involved in enzymatic defense mechanisms against oxidative stress. However, there is currently a lack of research on the levels of these elements in the placenta, umbilical cord, and fetal membrane of women with multiple pregnancies. In this study, the concentrations of these elements were compared to those found in afterbirths obtained from women with singleton pregnancies.
4.2. Iron Concentration in Afterbirth
In this study, the mean Fe concentrations were found to be 417.5 mg/kg−1 dw in the placenta, 200.17 mg/kg−1 dw in the umbilical cord, and 317.10 mg/kg−1 dw in the fetal membrane as obtained from women with multiple pregnancies. Comparing these results with our previous research on Fe concentrations in afterbirths obtained from women with singleton pregnancies from the same study area, higher Fe concentrations were found in the placenta (640.73 mg/kg−1 dw), umbilical cord (640.73 mg/kg−1 dw), and fetal membrane (567.29 mg/kg−1 dw) [39]. However, Barad et al. [40] reported lower Fe concentrations in the placenta obtained from teenage women with singleton pregnancies (71.1 mg/kg−1 ww; 106.7 mg/kg−1 dw after conversion) than in women aged 20–46 years with multiple pregnancies (84.6 and 78.6 mg/kg−1 ww; 126.9 and 117.9 mg/kg−1 dw after conversion, respectively, for twin and triplet placentas). De Angelis et al. [41] reported that the Fe concentration in the placenta was higher in women with multiple pregnancies (26.05 μg/g−1 dw) than in those with singleton pregnancies (17.99 μg/g−1 dw), although this difference was not statistically significant. These differences may have been due to differences between the study groups.
Mbofung et al. [42] demonstrated higher Fe concentration in the placenta of female newborns than in those of male newborns. Reddy et al. [43] reported that the Fe concentration in the placenta of women from urban areas was higher than that of women from rural areas. Additionally, Irwinda et al. [44] found that the Fe concentration in the placenta of premature newborns was lower than that of full-term newborns.
In the present study, it was found that the concentration of Fe in the placenta had an impact on its weight, as confirmed by the analyses of Hindmarsh et al. [45] in women with singleton pregnancies. Godfrey et al. [46] demonstrated that Fe deficiency is associated with a larger placental weight, which may have significant implications for the infant. Levario-Carrillo et al. [47] confirmed a tendency for increased placental weight in women with iron deficiency anemia (IDA); however, there was no significant effect on the weight or growth of the newborn. On the other hand, other studies have shown that IDA during pregnancy can lead to low birth weight [48,49,50].
In the present study, no association was found between Fe supplementation and neonatal morphometric parameters. However, Shi et al. [51], in their study of women and newborns from singleton pregnancies, reported that Fe supplementation during pregnancy had an effect on increasing the birth weight of newborns, particularly in women with anemia. However, it is important that the supplementation is individually tailored, as the excessive intake of Fe may have negative effects on fetal development. Hwang et al. [52] demonstrated that increased maternal Fe intake can lead to fetal growth restriction. On the other hand, Preziosi et al. [53] found that the mean newborn length and Apgar score were higher in the group of pregnant women receiving Fe-containing supplements than in the group receiving a placebo. The relationship between Fe supplementation and higher birth weight has been confirmed, indicating that Fe supplementation may have an impact on neonatal morphometric parameters [54,55].
4.3. Zinc Concentration in Afterbirth
In the present study, the average concentrations of Zn in the placenta, umbilical cord, and fetal membrane obtained from women with multiple pregnancies from northwestern Poland were 50.21, 45.55, and 36.91 mg/kg−1 dw, respectively. Much higher concentrations of Zn in the placenta (66.90 mg/kg−1 dw), umbilical cord (54.65 mg/kg−1 dw), and fetal membrane (62.79 mg/kg−1 dw) were found in women with singleton pregnancies from the same study area [39]. De Angelis et al. [41] found that the Zn concentration in the placenta was higher in women with multiple pregnancies (5.9 μg/g−1 dw) than in those with singleton pregnancies (2.6 μg/g−1 dw), but the difference was not statistically significant. Mazurek et al. [56] reported much lower Zn concentrations in the placenta (ranging from 3.3 to 11.5 mg/kg−1 ww; ranging from 5.0 to 17.3 mg/kg−1 dw after conversion) obtained from women with singleton pregnancies in Poland. Kantola et al. [57] found higher concentrations of Zn in the placenta obtained from non-smoking women with singleton pregnancies from Finland, Estonia, and St. Petersburg (77.14 mg/kg−1 dw) than in the present study.
The present study showed differences in the placental concentrations of Zn between women who smoked and did not smoke before pregnancy. Kantola et al. [47] observed that the Zn concentration in the placenta of women who smoked during pregnancy was 20% higher than that of non-smoking women in a study involving women with singleton pregnancies from various regions in Europe. In contrast, Kutlu et al. [58] found that the concentration of Zn in the placenta of smoking women was lower than that of non-smoking women during pregnancy. These results suggest that changes in Zn concentration caused by nicotine may be due to the vasoconstriction of utero placental blood vessels, thereby disrupting the transport of nutrients, including macro- and micronutrients. Additionally, nicotine may contribute to the destruction of the epithelial fibers of the placenta responsible for the transport of these elements [59].
It has been observed that primary Zn deficiency may lead to fetal growth retardation, confirming the important role of Zn in normal prenatal child development [60]. Bermúdez et al. [61] found no association between anthropometric parameters and Zn levels in either umbilical or maternal blood. Kot et al. [62] found a negative correlation between the maternal blood Zn levels and newborn chest circumference, as well as between umbilical blood Zn levels and newborn head circumference. In the present study, a correlation was found only between the Zn concentration in the fetal membrane and the child’s shoulder width. A cohort study showed that Zn deficiency in pregnant women increases the risk of low birth weight (LBW) in newborns [63]. Simmer and Thompson [64] observed a relationship between a low maternal plasma Zn concentration and decreased small-for-gestational-age newborn ratio, while Lira et al. [65] demonstrated that Zn supplementation slightly increased birth weight. Sorouri et al. [66] found no significant impact on Zn sulfate daily supplementation (15 mg) from the 16th week of gestation until delivery in a randomized controlled trial.
4.4. Cooper Concentration in Afterbirth
In the present study, the mean Cu concentrations in the placenta, umbilical cord, and fetal membrane obtained from women with multiple pregnancies from northwestern Poland were 4.47, 3.21, and 7.26 mg/kg−1 dw, respectively. Much higher concentrations of Cu in the placenta (6.01 mg/kg−1 dw), umbilical cord (4.32 mg/kg−1 dw), and fetal membrane (8.91 mg/kg−1 dw) were found in women with singleton pregnancies from the same study area [39]. Much lower concentrations of Cu in the placenta (from 0.05 to 3.91 μg/g−1 ww; from 0.08 to 5.9 ug/g−1 dw after conversion) were reported by Mazurek et al. [56]. Higher concentrations of Cu in the placenta were found by Reddy et al. [43] in pregnant women from rural (78.4 μg/g−1 dw) and urban (61.4 μg/g−1 dw) areas, as well as in term newborns and preterm infants [44].
In addition, in the presented study, it was found that the concentration of Cu in the placenta significantly correlates with the placenta weight and shoulder width, and the concentration of Cu in the umbilical cord correlates with the head circumference. Kot et al. [39] showed a negative correlation between the concentration of Cu in fetal membranes collected from women with singleton pregnancies and the birth weight of newborns. Furthermore, Kot et al. [39] found a negative correlation between the concentration of Cu in umbilical cord blood collected from women with singleton pregnancies and newborn parameters (head circumference, chest circumference, and Apgar score in the first minute). Mbofung and Subbarau [42] demonstrated a positive correlation between the concentration of Cu in the placenta collected from women with singleton pregnancies and newborn birth weight. Kantola et al. [57] found a negative correlation between the Cu concentration in the placenta and the newborn birth weight, which was stronger in the group of smoking women than non-smoking women.
4.5. The Relationships between Essential Elements
The metabolism of Fe and Cu is interrelated, and during pregnancy, both a deficiency of Cu and an excess of Fe can affect each other [67]. In women, it has been found that Fe and Cu may competitively interact with each other at the stage of intestinal absorption, and Fe supplementation may decrease Cu levels in the body [68]. It has been observed that in cases of Fe deficiency in the placenta, the level of Cu increases to a higher level than in other tissues [69].
In the present study, a correlation was observed between the Fe concentration in the placenta and Zn and Cu concentrations in the placenta, and between the Fe level in the fetal membrane and Zn concentration in the fetal membrane. Additionally, a positive correlation was found between the Zn and Fe concentrations in the placenta and between the Zn concentration in the placenta and Cu levels in the placenta and umbilical cord, as well as between the Zn concentrations in the fetal membrane and umbilical cord and the Cu concentrations in these structures. Kinnamon [70] noted that Cu and Zn in the diet induce a competitive mechanism within the fetal and placental structures. Kantola et al. [57] observed a negative correlation between the Cu concentration in the placenta and the birth weight of the newborn.
Deficiencies of both Zn and Cu are well described in preterm infants and pregnant women [71], but not in the case of structures such as the placenta, fetal membrane, and umbilical cord from women with multiple pregnancies. It can be inferred that the antagonistic effects of Zn and Cu, Zn and Fe, and Cu and Fe may also occur at the level of maternal–fetal and fetal–placental, fetal membrane, and umbilical cord interactions.
4.6. Oxidative Stress and Fe, Cu, and Zn Concentrations
The human body naturally produces free radicals [72], but their uncontrolled production can cause lipid peroxidation and damage to cell membranes [73]. To prevent excessive OS, the body uses enzymatic and non-enzymatic antioxidants to scavenge free radicals and limit their concentrations [74].
During pregnancy, increases in lipid peroxidation and oxidative stress may pose a potential threat to the developing fetus. To counteract these adverse effects, changes are made to the fetal ROS defense system. However, this adaptive mechanism may not always provide adequate protection against harm [75]. Multiple pregnancies entail an even greater risk of oxidative stress and reduced antioxidant capacity, potentially linking oxidative stress to pregnancy complications [76]. For instance, Minghetti et al. [32] noted a correlation between oxidative stress and lower birth weight in newborns.
In the present study, the mean concentrations of GPx in the placenta, umbilical cord, and fetal membrane were 5.19, 4.45, and 6.37 U/mg protein−1, respectively. Mistry et al. [77] found that the GPx activity was lower in the placental tissues of women with preeclampsia than in normotensive women. Similarly, Biri et al. [78] observed that the GPx activity was lower in pregnant women with preeclampsia than in healthy women.
The mean concentrations of GR in the placenta, umbilical cord, and fetal membrane were 5.55, 4.05, and 3.85 U/mg protein−1, respectively. Das et al. [79] did not find significant differences in GR activity in the placentas of women with preeclampsia and healthy pregnant women.
The mean concentrations of CAT in the placenta, umbilical cord, and fetal membrane were 1.17, 3.21, and 2.28 U/mg protein−1, respectively. Ferreira et al. [80] found that the CAT activity was lower in women with preeclampsia than in healthy pregnant women.
The SOD activity levels in the placenta, umbilical cord, and fetal membrane were 0.04, 0.09, and 0.06 U/mg protein−1, respectively. Biri et al. [78] reported that the SOD activity in pregnant women with preeclampsia was similar to that of healthy pregnant women. Ferreira et al. [80] found that the SOD activity was higher in women with preeclampsia than in healthy pregnant women.
In the present study, it was observed that LPO was highest in the placenta (2.26 U/mg protein−1), followed by the umbilical cord (1.66 U/mg protein−1) and fetal membrane (1.65 U/mg protein−1). Similarly, Ohel et al. [81] reported that LPO was significantly higher in fetal membranes compared to the placenta. Furthermore, the study revealed that the concentrations of Fe in the placenta and fetal membrane affect the level of oxidative stress by modulating the antioxidant parameters in the placenta (GPx, GR) and umbilical cord (GPx).
Several researchers have confirmed the positive effect of Fe on antioxidant defense parameters [82,83,84]. Bozkaya et al. [85] demonstrated that enzymatic antioxidant markers were reduced in women with iron-deficiency anemia (IDA). The deficiency of Fe in women with IDA may intensify OS, causing changes in the function of the heart. Isler et al. [86] found that Fe had a positive effect on the activity of SOD and GPx. Researchers have noted that the activity of SOD is lower in patients with IDA and that Fe supplementation in patients with IDA restores optimal antioxidant activity. Excess Fe predisposes individuals to increased LPO activity [87,88]. Lachili et al. [30] demonstrated that pharmacological doses of Fe administered to pregnant women without anemia caused uncontrolled lipid peroxidation. Similarly, Rajendran et al. [89] found that Fe supplementation in non-anemic pregnant women resulted in increased oxidative stress and inflammation. The present study confirmed the relationship between the concentration of Fe in the placenta samples obtained from women with multiple pregnancies and LPO activity.
Metalloproteins (CAT and SOD) act as antioxidants by enzymatically detoxifying reactive oxygen species, including O2, H2O2, and -OOH. Cu/Zn superoxide dismutase, which is especially demanding for its proper function and dependent on its cofactors (Cu/Zn), was observed to be correlated with placental Cu and Zn concentrations and umbilical cord Cu and Zn levels in the present study. Additionally, a correlation was observed between the fetal membrane Cu level and umbilical cord Cu concentration with the fetal membrane CAT and umbilical cord Cu level with placenta CAT. The maintenance of pregnancy is significantly influenced by appropriate Cu/Zn SOD activity, according to several studies [90,91,92]. Cu/Zn SOD activity has been found to decrease in the placenta of women with gestational diabetes [93] and preeclampsia [94], indicating a possible link between Cu/Zn SOD activity and pregnancy complications. However, conflicting findings have been reported by Araújo Brito et al. [95], who suggest that SOD may not be a useful marker for preeclampsia, as the activity significantly increased in both groups (women with and without preeclampsia).
Oxidative stress can affect fetal anthropometric parameters, particularly birth weight [96,97]. In this study, SOD activity in the placenta was found to correlate with the birth weight and head circumference. Additionally, SOD activity in the umbilical cord correlated with the birth weight, length, and shoulder width. Saker et al. [98] demonstrated increased SOD activity in cases of intrauterine growth restriction (IUGR). Similarly, Biri et al. [78] found increased SOD activity in newborns with IUGR and suggested that the administration of antioxidants could be useful in the prevention or treatment of IUGR.
CAT activity affects newborn morphometric parameters [99]. In the present study, higher CAT activity was found in the group of newborns with normal weight compared to the group with low weight. Additionally, fetal membrane CAT activity correlated with head circumference and birth weight. Ordóñez-Díaz et al. [100] associated extra uterine growth restriction with an impaired antioxidant defense status. Similarly, Karowicz-Bilinska et al. [101] and Luo et al. [102] demonstrated that IUGR is associated with greater placental ROS and oxidative injury. Ashin et al. [96] noted that the severity of growth restriction strongly correlates with oxidative stress markers. In the present study, a correlation was also found between LPO activity in the umbilical cord and newborn length.
In the present study, we found that the SOD activity in the placenta was higher in pregnant women with multiple pregnancies who smoke. Similarly, Pizent et al. [103] found higher SOD activity in the placentas of smoking women compared to non-smokers, while the GPx activity was similar in both groups. Ermis et al. [104] found an increase in GPx activity in the serum of smoking women and their infants. Napierała et al. [105] demonstrated that the GPx, CAT, and SOD activity levels in the serum and milk of smoking women were higher than in non-smokers and those passively exposed to tobacco smoke.
4.7. Limitations
In this study, the participants represented only a small proportion of pregnant women in northwestern Poland. Measurements of key iron transport proteins, including transferrin receptor (TfR) and ferroportin (FPN), were not performed. Additionally, hepcidin, a hormone responsible for iron transport to the placenta, was not measured. Notably, hepcidin has been linked to inflammation, which could potentially lead to iron deficiency anemia (IDA). Furthermore, two trans-membrane copper proteins, high-affinity copper uptake protein 1 (CTR1) and DMT1, and zinc transporters Zrt-/Irt-like protein (ZIP) zinc transporters and ZnTs (zinc transporters) were not determined in this study. The levels of albumin and alpha-2-macroglobulin, which commonly form complexes with zinc, were not analyzed. Metallothioneins (MT), which play a vital role in cellular zinc transport, were also not determined.
Additionally, comparable data from twin gestation were not available in the existing scientific literature. Therefore, we included articles reporting umbilical cord blood measurements of Fe, Cu, and Zn. External factors such as diet, environmental pollutants, and the quality of life of pregnant women were not analyzed in this study. It is important for future research to consider the effects of hypoxia on the body’s antioxidant balance. During pregnancy, hypoxia can have negative consequences on fetal development and increase the risk of metabolic and cardiovascular complications. Specifically, hypoxia can lead to the formation of ROS by influencing the mitochondria of the placenta and uterus, which can cause oxidative stress [106,107]. Moreover, we did not consider the placental weight, position, perfusion, and function (determined by Doppler and biochemical tests such as PLGF), which could have a significant impact on fetal development.
However, a strength of this study is its focus on the concentrations of Fe, Cu, and Zn in the placentas of women with twin pregnancies, where specific research is lacking. Additionally, we compared these elements with the morphometric parameters of newborns. The measurement of selected oxidative stress parameters also adds unique significance to the study and provides valuable information about the occurrence of oxidative stress in multiple pregnancies.
5. Conclusions
The present study contributes novel information regarding the concentrations of iron, zinc, and copper, as well as the concentrations of lipid peroxidation products and the activities of antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase) in the placenta, umbilical cord, and fetal membrane of women with multiple pregnancies. These data can be utilized as comparative data in future studies, although our results should be interpreted with caution despite statistical significance being achieved.
Author Contributions
K.G.: literature search and review, manuscript draft preparation, writing manuscript, conceptualization, formal analysis, investigation, resources and preparation of manuscript revision, formal analysis. P.K. (Patrycja Kapczuk): methodology. D.K.S.: methodology. P.K. (Patrycja Kupnicka): validation of methodology. J.L.-K.: participation in writing the manuscript. S.K.K.: participation in writing the manuscript. N.Ł.-A.: participation in writing the manuscript. D.C.: participation in writing the manuscript. D.I.K.-B.: writing the manuscript, preparation of manuscript revision, supervision, and final acceptance of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The Pomeranian Medical University in Szczecin provided financial support (WFB-431-02/S/12/2023).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Biometric Committee of the Pomeranian Medical University in Szczecin (KB-0012/76/14 from 13 October 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient (s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This study was supported by the statutory budget of the Department of Biology and Medical Parasitology, Pomeranian Medical University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farias, P.M.; Marcelino, G.; Santana, L.F.; de Almeida, E.B.; Guimarães, R.d.C.A.; Pott, A.; Hiane, P.A.; Freitas, K.dC. Minerals in Pregnancy and Their Impact on Child Growth and Development. Molecules 2020, 25, 5630. [Google Scholar] [CrossRef] [PubMed]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The Role of Zinc, Copper, Manganese and Iron in Neurodegenerative Diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Savarino, G.; Corsello, A.; Corsello, G. Macronutrient Balance and Micronutrient Amounts through Growth and Development. Ital. J. Pediatr. 2021, 47, 109. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K. Iron Deficiency in Pregnancy. Am. J. Obstet. Gynecol. 2020, 223, 516. [Google Scholar] [CrossRef]
- Ozdemir, U.; Gulturk, S.; Aker, A.; Guvenal, T.; Imir, G.; Erselcan, T. Correlation between Birth Weight, Leptin, Zinc and Copper Levels in Maternal and Cord Blood. J. Physiol. Biochem. 2007, 63, 121–128. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.-F.; Hao, J.-H.; Chen, Y.-H.; Su, P.-Y.; Wang, Y.; Yu, Z.; Fu, L.; Xu, Y.-Y.; Zhang, C.; et al. Maternal Zinc Deficiency during Pregnancy Elevates the Risks of Fetal Growth Restriction: A Population-Based Birth Cohort Study. Sci. Rep. 2015, 5, 11262. [Google Scholar] [CrossRef] [PubMed]
- Ferdousi, S.; Akhtar, S.; Begum, S. Copper and Zinc Status in Patients with Preeclampsia in Bangladesh. Mymensingh Med. J. 2015, 24, 780–786. [Google Scholar] [PubMed]
- Onyegbule, A.O.; Onah, C.C.; Iheukwumere, B.C.; Udo, J.N.; Atuegbu, C.C.; Nosakhare, N.O. Serum Copper and Zinc Levels in Preeclamptic Nigerian Women. Niger. Med. J. 2016, 57, 182. [Google Scholar] [CrossRef]
- Detlefs, S.E.; Jochum, M.D.; Salmanian, B.; McKinney, J.R.; Aagaard, K.M. The Impact of Response to Iron Therapy on Maternal and Neonatal Outcomes among Pregnant Women with Anemia. Am. J. Obstet. Gynecol. 2022, 4, 100569. [Google Scholar] [CrossRef]
- Kiilholma, P.; Grönroos, M.; Erkkola, R.; Pakarinen, P.; Näntö, V. The Role of Calcium, Copper, Iron and Zinc in Preterm Delivery and Premature Rupture of Fetal Membranes. Gynecol. Obstet. Investig. 1984, 17, 194–201. [Google Scholar] [CrossRef]
- Grzeszczak, K.; Kwiatkowski, S.; Kosik-Bogacka, D. The Role of Fe, Zn, and Cu in Pregnancy. Biomolecules 2020, 10, 1176. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Jauniaux, E. Oxidative Stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Dhingra, R.; Arya, D.S.; Kalaivani, M.; Bhatla, N.; Kumar, R. Role of Oxidative Stress Markers and Antioxidants in the Placenta of Preeclamptic Patients. J. Obstet. Gynaecol. Res. 2010, 36, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Wołonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace Elements as an Activator of Antioxidant Enzymes. Postep. Hig. Med. Dosw. 2016, 70, 1483–1498. [Google Scholar] [CrossRef]
- Roland, L.; Beauchemin, D.; Acteau, G.; Fradette, C.; St-Pierre, I.; Bilodeau, J.F. Effects of Labor on Placental Expression of Superoxide Dismutases in Preeclampsia. Placenta 2010, 31, 392–400. [Google Scholar] [CrossRef]
- Wiktor, H.; Kankofer, M. Catalase Activity in Normal and Preeclamptic Placentas. Ginekol Pol. 2001, 72, 1228–1232. [Google Scholar]
- Dsouza, V.; Rani, A.; Patil, V.; Pisal, H.; Randhir, K.; Mehendale, S.; Wagh, G.; Gupte, S.; Joshi, S. Increased Oxidative Stress from Early Pregnancy in Women Who Develop Preeclampsia. Clin. Exp. Hypertens. 2016, 38, 225–232. [Google Scholar] [CrossRef]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Myatt, L.; Eis, A.L.W.; Brockman, D.E.; Kossenjans, W.; Greer, I.A.; Lyall, F. Differential Localization of Superoxide Dismutase Isoforms in Placental Villous Tissue of Normotensive, Pre-Eclamptic, and Intrauterine Growth- Restricted Pregnancies. J. Histochem. Cytochem. 1997, 45, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Turnlund, J.R.; Keyes, W.R.; Peiffer, G.L.; Scott, K.C. Copper Absorption, Excretion, and Retention by Young Men Consuming Low Dietary Copper Determined by Using the Stable Isotope 65Cu. Am. J. Clin. Nutr. 1998, 67, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.; Cruz, K.; Morais, J.; Beserra, J.; Severo, J.; de Oliveira, A. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants Mediate Both Iron Homeostasis and Oxidative Stress. Nutrients 2017, 9, 671. [Google Scholar] [CrossRef]
- Khayat, S.; Fanaei, H.; Ghanbarzehi, A. Minerals in Pregnancy and Lactation: A Review Article. J. Clin. Diagn. Res. 2017, 11, QE01–QE05. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Scherr, R.E.; Lanoue, L.; Keen, C.L. Influence of copper on early development: Prenatal and postnatal considerations. Biofactors 2010, 36, 136–152. [Google Scholar] [CrossRef]
- Wilson, R.L.; Grieger, J.A.; Bianco-Miotto, T.; Roberts, C.T. Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy Complications: A Systematic Review. Nutrients 2016, 8, 641. [Google Scholar] [CrossRef]
- Li, H.T.; Jiao, M.; Chen, J.; Liang, Y. Roles of zinc and copper in modulating the oxidative refolding of bovine copper, zinc superoxide dismutase. Acta Biochim. Biophys. Sin. 2010, 42, 183–194. [Google Scholar] [CrossRef]
- Rao, R.; Georgieff, M.K. Iron therapy for preterm infants. Clin. Perinatol. 2009, 36, 27–42. [Google Scholar] [CrossRef]
- Lachili, B.; Hininger, I.; Faure, H.; Arnaud, J.; Richard, M.J.; Favier, A.; Roussel, A.M. Increased lipid peroxidation in pregnant women after iron and vitamin C supplementation. Biol. Trace Elem. Res. 2001, 83, 103–110. [Google Scholar] [CrossRef]
- Rak, K.; Łoźna, K.; Styczyńska, M.; Bobak, Ł.; Bronkowska, M. Oxidative Stress at Birth Is Associated with the Concentration of Iron and Copper in Maternal Serum. Nutrients 2021, 13, 1491. [Google Scholar] [CrossRef]
- Minghetti, L.; Suppiej, A.; Greco, A.; Franzoi, M.; Pascoli, I.; Zanardo, V. Oxidative stress in twin neonates is influenced by birth weight and weight discordance. Clin. Biochem. 2011, 44, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Capdevila, E.; Botet, F.; Verd, S.; Ginovart, G.; Moliner, E.; Nicolàs, M.; Rios, J.; SEN1500 Network. Morbidity and mortality of very low birth weight multiples compared with singletons. J. Matern. -Fetal Neonatal Med. 2019, 32, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Hubinont, C.; Lewi, L.; Bernard, P.; Marbaix, E.; Debiève, F.; Jauniaux, E. Anomalies of the placenta and umbilical cord in twin gestations. Am. J. Obstet. Gynecol. 2015, 213 (Suppl. S4), S91–S102. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body mass index: Obesity, bmi, and health: A critical review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Kalisińska, E.; Salicki, W.; Mysłek, P.; Kavetska, K.M.; Jackowski, A. Using the Mallard to biomonitor heavy metal contamination of wetlands in north-western Poland. Sci. Total Environ. 2004, 320, 145–161. [Google Scholar] [CrossRef]
- Lanocha, N.; Kalisinska, E.; Kosik-Bogacka, D.; Budis, H.; Sokolowski, S.; Bohatyrewicz, A. Concentrations of trace elements in bones of the hip joint from patients after hip replacement surgery. J. Trace Elem. Med. Biol. 2012, 26, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, K.; Kapczuk, P.; Kupnicka, P.; Cecerska-Heryć, E.; Kwiatkowski, S.; Chlubek, D.; Kosik-Bogacka, D. Calcium, Potassium, Sodium, and Magnesium Concentrations in the Placenta, Umbilical Cord, and Fetal Membrane from Women with Multiple Pregnancies. Life 2023, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Malinowski, W.; Szymański, S.; Mularczyk, M.; Tomska, N.; Rotter, I. Interactions between 14 Elements in the Human Placenta, Fetal Membrane and Umbilical Cord. Int. J. Environ. Res. Public Health 2019, 16, 1615. [Google Scholar] [CrossRef]
- Barad, A.; Guillet, R.; Pressman, E.K.; Katzman, P.J.; Miller, R.K.; Darrah, T.H.; O’Brien, K.O. Placental Iron Content Is Lower than Previously Estimated and Is Associated with Maternal Iron Status in Women at Greater Risk of Gestational Iron Deficiency and Anemia. J. Nutr. 2022, 152, 737–746. [Google Scholar] [CrossRef]
- de Angelis, P.; Miller, R.K.; Darrah, T.H.; Katzman, P.J.; Pressman, E.K.; Kent, T.R.; O’Brien, K.O. Elemental Content of the Placenta: A Comparison between Two High-Risk Obstetrical Populations, Adult Women Carrying Multiples and Adolescents Carrying Singletons. Environ. Res. 2017, 158, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Mbofung, C.M.F.; Subbarau, V.V. Trace Element (Zinc, Copper, Iron and Magnesium) Concentrations in Human Placenta and Their Relationship to Birth Weight of Babies. Nutr. Res. 1990, 10, 359–366. [Google Scholar] [CrossRef]
- Reddy, Y.S.; Aparna, Y.; Ramalaksmi, B.A.; Dinesh Kumar, B. Lead and Trace Element Levels in Placenta, Maternal and Cord Blood: A Cross-Sectional Pilot Study. J. Obstet. Gynaecol. Res. 2014, 40, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Irwinda, R.; Wibowo, N.; Putri, A.S. The Concentration of Micronutrients and Heavy Metals in Maternal Serum, Placenta, and Cord Blood: A Cross-Sectional Study in Preterm Birth. J. Pregnancy 2019, 2019, 5062365. [Google Scholar] [CrossRef] [PubMed]
- Hindmarsh, P.C.; Geary, M.P.P.; Rodeck, C.H.; Jackson, M.R.; Kingdom, J.C.P. Effect of Early Maternal Iron Stores Placental Weight and Structure. Lancet 2000, 356, 719–723. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Redman, C.W.G.; Barker, D.J.P.; Osmond, C. The Effect of Maternal Anaemia and Iron Deficiency on the Ratio of Fetal Weight to Placental Weight. Br. J. Obstet. Gynaecol. 1991, 98, 886–891. [Google Scholar] [CrossRef]
- Levario-Carrillo, M.; Hernandez, M.; Vasquez, M.; Chavez, D.; Sanchez, C.; Corral, M. Effects of Iron-Deficiency Anemia on Placenta and Birth Weight. Ginecol. Obs. Mex. 2003, 71, 75–81. [Google Scholar]
- Figueiredo, A.C.M.G.; Gomes-Filho, I.S.; Silva, R.B.; Pereira, P.P.S.; Da Mata, F.A.F.; Lyrio, A.O.; Souza, E.S.; Cruz, S.S.; Pereira, M.G. Maternal Anemia and Low Birth Weight: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 601. [Google Scholar] [CrossRef]
- Col Madendag, I.; Eraslan Sahin, M.; Madendag, Y.; Sahin, E.; Demir, M.B.; Acmaz, B.; Acmaz, G.; Muderris, I.I. The Effect of Iron Deficiency Anemia Early in the Third Trimester on Small for Gestational Age and Birth Weight: A Retrospective Cohort Study on Iron Deficiency Anemia and Fetal Weight. Biomed. Res. Int. 2019, 2019, 7613868. [Google Scholar] [CrossRef]
- Rahmati, S.; Delpishe, A.; Azami, M.; Ahmadi, M.R.H.; Sayehmiri, K. Maternal Anemia during Pregnancy and Infant Low Birth Weight: A Systematic Review and Meta-Analysis. Int. J. Reprod. Biomed. 2017, 15, 125. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, Z.; Ma, L.; Zhang, B.; Dang, S.; Yan, H. Association between Maternal Iron Supplementation and Newborn Birth Weight: A Quantile Regression Analysis. Ital. J. Pediatr. 2021, 47, 133. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Lee, J.Y.; Kim, K.N.; Kim, H.; Ha, E.H.; Park, H.; Ha, M.; Kim, Y.; Hong, Y.C.; Chang, N. Maternal Iron Intake at Mid-Pregnancy Is Associated with Reduced Fetal Growth: Results from Mothers and Children’s Environmental Health (MOCEH) Study. Nutr. J. 2013, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, P.; Prual, A.; Galan, P.; Daouda, H.; Boureima, H.; Hercberg, S. Effect of Iron Supplementation on the Iron Status of Pregnant Women: Consequences for Newborns. Am. J. Clin. Nutr. 1997, 66, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, M.E.; Parvanta, I.; Ickes, L.; Yip, R.; Brittenham, G.M. Iron Supplementation during Pregnancy, Anemia, and Birth Weight: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2003, 78, 773–781. [Google Scholar] [CrossRef]
- Alwan, N.A.; Greenwood, D.C.; Simpson, N.A.B.; McArdle, H.J.; Godfrey, K.M.; Cade, J.E. Dietary Iron Intake during Early Pregnancy and Birth Outcomes in a Cohort of British Women. Hum. Reprod. 2011, 26, 911. [Google Scholar] [CrossRef]
- Mazurek, D.; Łoźna, K.; Bronkowska, M. The Concentration of Selected Elements in the Placenta According to Selected Sociodemographic Factors and Their Effect on Birth Mass and Birth Length of Newborns. J. Trace Elem. Med. Biol. 2020, 58, 126425. [Google Scholar] [CrossRef]
- Kantola, M.; Purkunen, R.; Kröger, P.; Tooming, A.; Juravskaja, J.; Pasanen, M.; Saarikoski, S.; Vartiainen, T. Accumulation of Cadmium, Zinc, and Copper in Maternal Blood and Developmental Placental Tissue: Differences between Finland, Estonia, and St. Petersburg. Environ. Res. 2000, 83, 54–66. [Google Scholar] [CrossRef]
- Kutlu, T.; Karagozler, A.A.; Gozukara, E.M. Relationship Among Placental Cadmium, Lead, Zinc, and Copper Levels in Smoking Pregnant Women. Biol. Trace Elem. Res. 2006, 114, 7–18. [Google Scholar] [CrossRef]
- Wickstrom, R. Effects of Nicotine During Pregnancy: Human and Experimental Evidence. Curr. Neuropharmacol. 2007, 5, 213. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef]
- Bermúdez, L.; García-Vicent, C.; López, J.; Torró, M.I.; Lurbe, E. Assessment of Ten Trace Elements in Umbilical Cord Blood and Maternal Blood: Association with Birth Weight. J. Transl. Med. 2015, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Kot, K.; Łanocha-Arendarczyk, N.; Kupnicka, P.; Szymański, S.; Malinowski, W.; Kalisińska, E.; Chlubek, D.; Kosik-Bogacka, D. Selected Metal Concentration in Maternal and Cord Blood. Int. J. Environ. Res. Public Health 2021, 18, 12407. [Google Scholar] [CrossRef] [PubMed]
- Mendes Garrido Abregg, F.; Caniffi, C.; Arranz, C.T.; Tomat, A.L. Impact of Zinc Deficiency During Prenatal and/or Postnatal Life on Cardiovascular and Metabolic Diseases: Experimental and Clinical Evidence. Adv. Nutr. 2022, 13, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Simmer, K.; Thompson, R.P.H. Maternal Zinc and Intrauterine Growth Retardation. Clin. Sci. 1985, 68, 395–399. [Google Scholar] [CrossRef]
- Lira, P.I.C.; Ashworth, A.; Morris, S.S. Effect of Zinc Supplementation on the Morbidity, Immune Function, and Growth of Low-Birth-Weight, Full-Term Infants in Northeast Brazil. Am. J. Clin. Nutr. 1998, 68, 418S–424S. [Google Scholar] [CrossRef]
- Zahiri Sorouri, Z.; Sadeghi, H.; Pourmarzi, D. The Effect of Zinc Supplementation on Pregnancy Outcome: A Randomized Controlled Trial. J. Matern. Neonatal Med. 2016, 29, 2194–2198. [Google Scholar] [CrossRef]
- Gambling, L.; McArdle, H.J. Iron, Copper and Fetal Development. Proc. Nutr. Soc. 2004, 63, 553–562. [Google Scholar] [CrossRef]
- Suliburska, J.; Skrypnik, K.; Chmurzyńska, A. Iron and Folic Acid Supplementation Affects Mineral Status in Female Rats with a Deficiency of These Micronutrients. Biol. Trace Elem. Res. 2021, 199, 3393. [Google Scholar] [CrossRef]
- Gambling, L.; Danzeisen, R.; Fosset, C.; Andersen, H.S.; Dunford, S.; Srai, S.K.S.; McArdle, H.J. Iron and Copper Interactions in Development and the Effect on Pregnancy Outcome. J. Nutr. 2003, 133, 1554S–1556S. [Google Scholar] [CrossRef]
- Kinnamon, K.E. Some Independent and Combined Effects of Copper, Molybdenum, and Zinc on the Placental Transfer of Zinc-65 in the Rat. J. Nutr. 1963, 81, 312–320. [Google Scholar] [CrossRef]
- Griffin, I.J.; Domellöf, M.; Bhatia, J.; Anderson, D.M.; Kler, N. Zinc and Copper Requirements in Preterm Infants: An Examination of the Current Literature. Early Hum. Dev. 2013, 89 (Suppl. S2), S29–S34. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Reiter, R.J.; Karbownik, M.; Tan, D.X.; Gitto, P.; Barberi, S.; Barberi, I. Causes of Oxidative Stress in the Pre- and Perinatal Period. Biol. Neonatol. 2002, 81, 146–157. [Google Scholar] [CrossRef]
- Jantsch, L.B.; de Lucca, L.; Dorneles, B.N.; Konopka, C.K.; Gonçalves, T.d.L. Evaluation of Oxidative Stress and δ-Aminolevulinate Dehydratase Activity in Twin Pregnancies. J. Matern. Fetal. Neonatal Med. 2020, 33, 3071–3076. [Google Scholar] [CrossRef]
- Mistry, H.D.; Kurlak, L.O.; Williams, P.J.; Ramsay, M.M.; Symonds, M.E.; Broughton Pipkin, F. Differential Expression and Distribution of Placental Glutathione Peroxidases 1, 3 and 4 in Normal and Preeclamptic Pregnancy. Placenta 2010, 31, 401–408. [Google Scholar] [CrossRef]
- Biri, A.; Bozkurt, N.; Gunaydin, G.; Korucuoglu, U.; Durak, I.; Kavutcu, M. Antioxidant Enzyme Activities and Lipid Peroxidation in Preeclampsia. Int. J. Gynecol. Obstet. 2007, 96, 196–197. [Google Scholar] [CrossRef]
- Das, B.; Saha-Roy, S.; Das Gupta, A.; Lahiri, T.K.; Das, H.N. Assessment of Placental Oxidative Stress in Pre-Eclampsia. J. Obstet. Gynecol. India 2012, 62, 39–42. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Fragoso, M.B.T.; Tenório, M.C.d.S.; da Paz Martins, A.S.; Borbely, A.U.; Moura, F.A.; Goulart, M.O.F.; de Oliveira, A.C.M. Biomarkers of Placental Redox Imbalance in Pregnancies with Preeclampsia and Consequent Perinatal Outcomes. Arch. Biochem. Biophys. 2020, 691, 108464. [Google Scholar] [CrossRef]
- Ohel, G.; Kisselevitz, R.; Margalioth, E.J.; Schenker, J.G. Ascorbate-Dependent Lipid Peroxidation in the Human Placenta and Fetal Membranes. Gynecol. Obstet. Investig. 1985, 19, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Layman, D.K.; Bell, R.R. Glutathione Peroxidase Activity in Iron-Deficient Rats. J. Nutr. 1981, 111, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Cao, G.; Oliveri, L.; Angerosa, M. Effects of Iron Deficiency Anemia and Its Treatment with Iron Polymaltose Complex in Pregnant Rats, Their Fetuses and Placentas: Oxidative Stress Markers and Pregnancy Outcome. Placenta 2012, 33, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Nuhu, F.; Seymour, A.M.; Bhandari, S. Impact of Intravenous Iron on Oxidative Stress and Mitochondrial Function in Experimental Chronic Kidney Disease. Antioxidants 2019, 8, 498. [Google Scholar] [CrossRef]
- Bozkaya, V.Ö.; Oskovi-Kaplan, Z.A.; Erel, O.; Keskin, L.H. Anemia in Pregnancy: It’s Effect on Oxidative Stress and Cardiac Parameters. J. Matern. Fetal. Neonatal Med. 2021, 34, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Isler, M.; Delibas, N.; Guclu, M.; Gultekin, F.; Sutcu, R.; Bahceci, M.; Kosar, A. Superoxide Dismutase and Glutathione Peroxidase in Erythrocytes of Patients with Iron Deficiency Anemia: Effects of Different Treatment Modalities. Croat. Med. J. 2002, 43, 16–19. [Google Scholar]
- Myatt, L.; Cui, X. Oxidative Stress in the Placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Lymperaki, E.; Tsikopoulos, A.; Makedou, K.; Paliogianni, E.; Kiriazi, L.; Charisi, C.; Vagdatli, E. Impact of Iron and Folic Acid Supplementation on Oxidative Stress during Pregnancy. J. Obstet. Trial 2015, 35, 803–806. [Google Scholar] [CrossRef]
- Rajendran, S.; Bobby, Z.; Habeebullah, S.; Elizabeth Jacob, S. Differences in the Response to Iron Supplementation on Oxidative Stress, Inflammation, and Hematological Parameters in Nonanemic and Anemic Pregnant Women. J. Matern. Neonatal Med. 2022, 35, 465–471. [Google Scholar] [CrossRef]
- Ghneim, H.K.; Al-Sheikh, Y.A.; Alshebly, M.M.; Aboul-Soud, M.A.M. Superoxide Dismutase Activity and Gene Expression Levels in Saudi Women with Recurrent Miscarriage. Mol. Med. Rep. 2016, 13, 2606. [Google Scholar] [CrossRef]
- Sugino, N.; Takiguchi, S.; Kashida, S.; Karube, A.; Nakamura, Y.; Kato, H. Superoxide Dismutase Expression in the Human Corpus Luteum during the Menstrual Cycle and in Early Pregnancy. Mol. Hum. Reprod. 2000, 6, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Ota, K.; Shirasawa, T.; Shimizu, T. Copper/Zinc Superoxide Dismutase Insufficiency Impairs Progesterone Secretion and Fertility in Female Mice. Biol. Reprod. 2012, 86, 16–17. [Google Scholar] [CrossRef]
- White, V.; Capobianco, E.; Higa, R.; Martínez, N.; Sosa, M.; Pustovrh, M.C.; Jawerbaum, A. Increased Nitration and Diminished Activity of Copper/Zinc Superoxide Dismutase in Placentas from Diabetic Rats. Free Radic. Res. 2010, 44, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Bakacak, M.; Kılınç, M.; Serin, S.; Ercan, Ö.; Köstü, B.; Avcı, F.; Kıran, H.; Kıran, G. Changes in Copper, Zinc, and Malondialdehyde Levels and Superoxide Dismutase Activities in Pre-Eclamptic Pregnancies. Med. Sci. Monit. 2015, 21, 2414. [Google Scholar] [CrossRef]
- Araújo Brito, J.; do Nascimento Marreiro, D.; Moita Neto, J.M.; e Silva, D.M.C.; de Sousa Almondes, K.G.; de Deus Valadares Neto, J.; do Nascimento Nogueira, N. Enzyme Activity of Superoxide Dismutase and Zincemia in Women with Preeclampsia. Nutr. Hosp. 2013, 28, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M.; Kido, T.; Kyono, Y.; Yoshida, A.; Suga, S.; Nakasone, R.; Abe, S.; Tanimura, K.; Nozu, K.; Fujioka, K. Correlation between Severity of Fetal Growth Restriction and Oxidative Stress in Severe Small-for-Gestational-Age Infants. Int. J. Environ. Res. Public Health 2021, 18, 10726. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Contreras-Chova, F.; Muñoz, S.; Araujo-Nepomuceno, E.; Bonillo, A.; Molina-Carballo, A.; Muñoz-Hoyos, A. Fluidity and Oxidative Stress in Erythrocytes from Very Low Birth Weight Infants during Their First 7 Days of Life. Free Radic. Res. 2007, 41, 1035–1040. [Google Scholar] [CrossRef]
- Saker, M.; Soulimane Mokhtari, N.; Merzouk, S.A.; Merzouk, H.; Belarbi, B.; Narce, M. Oxidant and Antioxidant Status in Mothers and Their Newborns According to Birthweight. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 95–99. [Google Scholar] [CrossRef]
- Guo, G.; Zhou, T.; Ren, F.; Sun, J.; Deng, D.; Huang, X.; Wassie, T.; Qazi, I.H.; Wu, X. Effect of Maternal Catalase Supplementation on Reproductive Performance, Antioxidant Activity and Mineral Transport in Sows and Piglets. Animals 2022, 12, 828. [Google Scholar] [CrossRef]
- Ordóñez-Díaz, M.D.; Gil-Campos, M.; Flores-Rojas, K.; Muñoz-Villanueva, M.C.; Mesa, M.D.; de la Torre-Aguilar, M.J.; Gil, Á.; Pérez-Navero, J.L. Impaired Antioxidant Defence Status Is Associated with Metabolic-Inflammatory Risk Factors in Preterm Children With Extrauterine Growth Restriction: The BIORICA Cohort Study. Front. Nutr. 2021, 8, 1140. [Google Scholar] [CrossRef]
- Karowicz-Bilinska, A.; Kȩdziora-Kornatowska, K.; Bartosz, G. Indices of Oxidative Stress in Pregnancy with Fetal Growth Restriction. Free Radic. Res. 2007, 41, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Luo, W.; Li, S.; Zhao, S.; Sho, T.; Xu, X.; Zhang, J.; Xu, W.; Xu, J. Reactive Oxygen Species Mediated Placental Oxidative Stress, Mitochondrial Content, and Cell Cycle Progression through Mitogen-Activated Protein Kinases in Intrauterine Growth Restricted Pigs. Reprod. Biol. 2018, 18, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Pizent, A.; Lazarus, M.; Kovačić, J.; Lovaković, B.T.; Karačonji, I.B.; Semren, T.Ž.; Sekovanić, A.; Orct, T.; Branović-čakanić, K.; Brajenović, N.; et al. Cigarette Smoking during Pregnancy: Effects on Antioxidant Enzymes, Metallothionein and Trace Elements in Mother-Newborn Pairs. Biomolecules 2020, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Ermis, B.; Ors, R.; Yildrim, A.; Tastekin, A.; Kardas, F.; Akcay, F. Influence of Smoking on Maternal and Neonatal Serum Malondialdehyde, Superoxide Dismutase, and Glutathione Peroxidase Levels. Ann. Clin. Lab. Sci. 2004, 34, 405–409. [Google Scholar]
- Napierala, M.; Merritt, T.A.; Miechowicz, I.; Mielnik, K.; Mazela, J.; Florek, E. The Effect of Maternal Tobacco Smoking and Second-Hand Tobacco Smoke Exposure on Human Milk Oxidant-Antioxidant Status. Environ. Res. 2019, 170, 110–121. [Google Scholar] [CrossRef]
- Nafie, K.; Hasan, A.; Alshakhrit, W.K.; Ismail, A.; Abbadi, O. Pathological features of early pregnancy disorders in women living at high altitude in KSA. J. Taibah Univ. Med. Sci. 2022, 18, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Zhang, L. Hypoxia and Mitochondrial Dysfunction in Pregnancy Complications. Antioxidants 2021, 10, 405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).