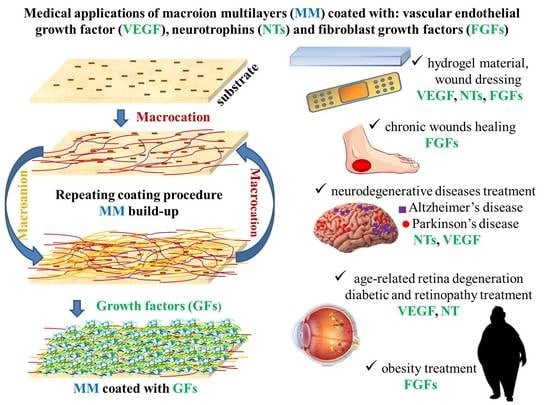
Biocompatible Macroion/Growth Factor Assemblies for Medical Applications
Abstract
1. Introduction
2. Macroions
2.1. Biocompatible Synthetic Macroions
2.1.1. PDADMAC
2.1.2. PAH and Its Derivatives
2.1.3. PAEs
2.1.4. BPEI
2.1.5. PAMAM Dendrimers
2.1.6. PAA
2.2. Polysaccharides as Examples of Biocompatible, Natural Macroions
2.2.1. CS
2.2.2. HA/Hyaluronan
2.2.3. Heparin
2.2.4. λ-Carrageenan
2.2.5. ChS
2.2.6. Protein-Polypeptide Nanoparticles
2.3. GFs
2.3.1. NTs
2.3.2. FGFs
2.3.3. VEGF
3. Macroion Layers and Macroion Complexes in Growth Factor Delivery
3.1. PAH-Based Assemblies
3.2. BPEI-Based Assemblies
3.3. PAMAM Dendrimer-Based Assemblies
3.4. PAE and PAA-Based Assemblies
3.5. CS-Based Assemblies
3.6. Heparin-Based Assemblies
3.7. ChS-Based Assemblies
3.8. Hydrogel-Based Polysaccharides Containing GFs
4. Conclusions
5. Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Definition |
| AFM | atomic force microscopy |
| ARG | arginine |
| ASOs | antisense oligonucleotides |
| BDNF | brain-derived neurotrophic factor |
| BMP-2 | bone morphogenetic protein-2 |
| BNCT | boron neutron capture therapy |
| bPEI | branched polyethyleneimine |
| CC | complex coacervates |
| CG | coarse-grained |
| ChS | chondroitin sulfate |
| CS | chitosan |
| CTGF | connective tissue growth factor |
| DA | acetylation degree |
| DP | dendronized polymer |
| ECM | extracellular matrix |
| EGF | epidermal growth factor |
| FA | folic acid |
| FGF | fibroblast growth factor |
| FGFR | fibroblast growth factor receptors |
| FR | folate receptor |
| GAGs | glycosaminoglycans |
| GF | growth factor |
| HA | hyaluronic acid |
| HDGF | hepatoma-derived growth factor |
| HGF | hepatocyte growth factor |
| HIV | human immunodeficiency virus |
| HMW | high molecular weight |
| hNPCs | human neural progenitor cells |
| HPAEs | hyperbranched poly(β-amino ester) |
| HPV | human papillomavirus |
| HSPGs | heparan sulphate proteoglycans |
| IEP | isoelectric point |
| IGF | insulin-like growth factor |
| IL-1 | interleukin-1 |
| IL-6 | interleukin-6 |
| IL-10 | interleukin-10 |
| IL-12 | interleukin-12 |
| IL-17 | interleukin-17 |
| LbL | layer-by-layer |
| LMW | low molecular weight |
| LMWH | low molecular mass heparin |
| lPEI | linear polyethyleneimine |
| MALS | multi-angle light scattering |
| MAPK | mitogen-activated protein kinase |
| MD | molecular dynamics |
| MM | macroion multilayers |
| NGF | nerve growth factor |
| NT | neurotrophin |
| NT3 | neurotrophin-3 |
| NT4/5 | neurotrophin–4/5 |
| PAA | poly(acrylic acid) |
| PAEs; PBAEs | poly(β-aminoesters) |
| PAH | poly(allylamine hydrochloride) |
| PAMAM dendrimers | polyamidoamine dendrimers |
| PAMAM-COO | PAMAM dendrimers with carboxylic groups |
| PAMAM-NH2 | PAMAM dendrimers with primary amines |
| PDADMAC | poly(diallyldimethylammonium chloride) |
| PDGF | platelet-derived growth factor |
| PEG | polyethylene glycol |
| PI3K-AKT | phosphoinositide 3 kinase/AKT |
| PIGF | placental growth factor |
| pKa | negative base-10 logarithm of the acid dissociation constant |
| PLCγ | phospholipase C gamma |
| PLL | poly(L-lysine) |
| pro-NGF | precursor nerve growth factor |
| PSS | poly(sodium-4-styrenesulfonate) |
| QCM | quartz crystal microbalance |
| RES | reticuloendothelial system |
| rhBMP-2 | recombinant human bone morphogenetic protein-2 |
| SANS | small-angle neutron scattering |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SAXS | small-angle X-ray scattering method |
| STAT | signal transducer and activator of transcription |
| TNFR | tumor necrosis factor receptor |
| Trk | tropomyosin-related kinase |
| VEGF | vascular endothelial growth factor |
| VEGF-A; VPF | vascular permeability factor |
| Wnt | Wingless and interleukin-1 |
| XPS | X-ray photoelectron spectroscopy |
References
- Michna, A. Macroion Adsorption—Electrokinetic and Optical Methods. Adv. Colloid Interface Sci. 2017, 250, 95–131. [Google Scholar] [CrossRef] [PubMed]
- Lyklema, J. Fundamentals of Interface and Colloid Science, 1st ed.; Lyklema, J., Ed.; Academic Press: Cambridge, MA, USA, 2005; Volume V, ISBN 0-12-460530-3. [Google Scholar]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-Based Polyelectrolyte Multilayers. Curr. Opin. Colloid Interface Sci. 2010, 15, 417–426. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Michna, A.; Warszyński, P.; Wilk, K.A. Colloidal Characteristics and Functionality of Rationally Designed Esculin-Loaded Hydrogel Microcapsules. J. Colloid Interface Sci. 2018, 530, 444–458. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Yu, D.G.; Tran, C.H.; Wang, M.; Varyambath, A.; Kim, J.; Kim, I. Efficient Synthesis of Folate-Conjugated Hollow Polymeric Capsules for Accurate Drug Delivery to Cancer Cells. Biomacromolecules 2021, 22, 732–742. [Google Scholar] [CrossRef]
- Wang, D.; Gong, X.; Heeger, P.S.; Rininsland, F.; Bazan, G.C.; Heeger, A.J. Biosensors from Conjugated Polyelectrolyte Complexes. Proc. Natl. Acad. Sci. USA 2002, 99, 49–53. [Google Scholar] [CrossRef]
- Kang, B.; Opatz, T.; Landfester, K.; Wurm, F.R. Carbohydrate Nanocarriers in Biomedical Applications: Functionalization and Construction. Chem. Soc. Rev. 2015, 44, 8301–8325. [Google Scholar] [CrossRef]
- Anandhakumar, S.; Raichur, A.M. Polyelectrolyte/Silver Nanocomposite Multilayer Films as Multifunctional Thin Film Platforms for Remote Activated Protein and Drug Delivery. Acta Biomater. 2013, 9, 8864–8874. [Google Scholar] [CrossRef]
- Yang, M.; Choi, D.; Choi, M.; Hong, J. Nanoporous Multilayer Films for Controlled Antigen Protein Release. J. Ind. Eng. Chem. 2016, 33, 221–225. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, J.; Gao, C.; Shen, J. Incorporation of Basic Fibroblast Growth Factor by a Layer-by-Layer Assembly Technique to Produce Bioactive Substrates. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83, 285–292. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.L.; Rodriguez, N.M.; Shah, N.J.; Hammond, P.T. Characterization of Tunable FGF-2 Releasing Polyelectrolyte Multilayers. Biomacromolecules 2010, 11, 2053–2059. [Google Scholar] [CrossRef]
- Illergård, J.; Wågberg, L.; Ek, M. Bacterial-Growth Inhibiting Properties of Multilayers Formed with Modified Polyvinylamine. Colloids Surfaces B Biointerfaces 2011, 88, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Lytle, T.K.; Radhakrishna, M.; Madinya, J.J.; Vélez, J.; Sing, C.E.; Perry, S.L. Sequence and Entropy-Based Control of Complex Coacervates. Nat. Commun. 2017, 8, 1273. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex Coacervation: Principles, Mechanisms and Applications in Microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Chunder, A.; Sarkar, S.; Yu, Y.; Zhai, L. Fabrication of Ultrathin Polyelectrolyte Fibers and Their Controlled Release Properties. Colloids Surfaces B Biointerfaces 2007, 58, 172–179. [Google Scholar] [CrossRef]
- Köse, M.D.; Bayraktar, O.; Heinz, Ö.K. Application of Complex Coacervates in Controlled Delivery. In Design and Development of New Nanocarriers; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 475–507. ISBN 9780128136270. [Google Scholar]
- Xu, Y.; Mazzawi, M.; Chen, K.; Sun, L.; Dubin, P.L. Protein Purification by Polyelectrolyte Coacervation: Influence of Protein Charge Anisotropy on Selectivity. Biomacromolecules 2011, 12, 1512–1522. [Google Scholar] [CrossRef]
- Park, U.; Lee, M.S.; Jeon, J.; Lee, S.; Hwang, M.P.; Wang, Y.; Yang, H.S.; Kim, K. Coacervate-Mediated Exogenous Growth Factor Delivery for Scarless Skin Regeneration. Acta Biomater. 2019, 90, 179–191. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, B.; Lau, H.C.; Kim, A. Gelatin-Alginate Complexes for EGF Encapsulation: Effects of H-Bonding and Electrostatic Interactions. Pharmaceutics 2019, 11, 530. [Google Scholar] [CrossRef]
- Dong, D.; Cui, B. Comparison of Rheological Properties of Different Protein/Gum Arabic Complex Coacervates. J. Food Process Eng. 2019, 42, e13196. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Z.; Cai, P.; Jiang, T.; Li, Y.; Yuan, Y.; Li, R.; Khor, S.; Lu, Y.; Wang, J.; et al. Dual Delivery of BFGF- and NGF-Binding Coacervate Confers Neuroprotection by Promoting Neuronal Proliferation. Cell. Physiol. Biochem. 2018, 47, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Gohil, S.V.; Padmanabhan, A.; Deschamps, J.; Nair, L.S. 7-Chitosan-Based Scaffolds for Growth Factor Delivery. In Chitosan Based Biomaterials; Jennings, J.A., Bumgardner, J.D., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Volume 2, pp. 175–207. ISBN 9780081002285. [Google Scholar]
- Kim, S.H.; Turnbull, J.; Guimond, S. Extracellular Matrix and Cell Signalling: The Dynamic Cooperation of Integrin, Proteoglycan and Growth Factor Receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef]
- Caterson, B.; Melrose, J. Keratan Sulfate, a Complex Glycosaminoglycan with Unique Functional Capability. Glycobiology 2018, 28, 182–206. [Google Scholar] [CrossRef]
- Schwartz, N.B.; Domowicz, M.S. Proteoglycans in Brain Development and Pathogenesis. FEBS Lett. 2018, 592, 3791–3805. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Wysocki, A. Interactions between Extracellular Matrix and Growth Factors in Wound Healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth Factor Delivery-Based Tissue Engineering: General Approaches and a Review of Recent Developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef]
- Kirkpatrick, C.A.; Dimitroff, B.D.; Rawson, J.M.; Selleck, S.B. Spatial Regulation of Wingless Morphogen Distribution and Signaling by Dally-like Protein. Dev. Cell 2004, 7, 513–523. [Google Scholar] [CrossRef]
- Kreuger, J.; Perez, L.; Giraldez, A.J.; Cohen, S.M. Opposing Activities of Dally-like Glypican at High and Low Levels of Wingless Morphogen Activity. Dev. Cell 2004, 7, 503–512. [Google Scholar] [CrossRef]
- Patel, V.N.; Knox, S.M.; Likar, K.M.; Lathrop, C.A.; Hossain, R.; Eftekhari, S.; Whitelock, J.M.; Elkin, M.; Vlodavsky, I.; Hoffman, M.P. Heparanase Cleavage of Perlecan Heparan Sulfate Modulates FGF10 Activity during Ex Vivo Submandibular Gland Branching Morphogenesis. Development 2007, 134, 4177–4186. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel Biomaterial Strategies for Controlled Growth Factor Delivery for Biomedical Applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Wight, T.N. A Role for Proteoglycans in Vascular Disease. Matrix Biol. 2018, 71–72, 396–420. [Google Scholar] [CrossRef] [PubMed]
- Caon, I.; Bartolini, B.; Parnigoni, A.; Caravà, E.; Moretto, P.; Viola, M.; Karousou, E.; Vigetti, D.; Passi, A. Revisiting the Hallmarks of Cancer: The Role of Hyaluronan. Semin. Cancer Biol. 2020, 62, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Balaji, A.B.; Pakalapati, H.; Khalid, M.; Walvekar, R.; Siddiqui, H. Natural and Synthetic Biocompatible and Biodegradable Polymers. In Biodegradable and Biocompatible Polymer Composites: Processing, Properties and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 3–32. ISBN 9780081009703. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chemie-Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- ten Brummelhuis, N.; Schlaad, H. Stimuli-Responsive Star Polymers through Thiol-Yne Core Functionalization/Crosslinking of Block Copolymer Micelles. Polym. Chem. 2011, 2, 1180–1184. [Google Scholar] [CrossRef]
- Zednik, J.; Riva, R.; Lussis, P.; Jérôme, C.; Jérôme, R.; Lecomte, P. PH-Responsive Biodegradable Amphiphilic Networks. Polymer 2008, 49, 697–702. [Google Scholar] [CrossRef]
- Scranton, A.B.; Rangarajan, B.; Klier, J. Biomedical Applications of Polyelectrolytes. In Biopolymers II. Advances in Polymer Science; Peppas, N.A., Langer, R.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 3–54. [Google Scholar]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A Review on Synthesis, Properties and Applications of Natural Polymer Based Carrageenan Blends and Composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. Engl. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Jäger, M.; Schubert, S.; Ochrimenko, S.; Schubert, U.S.; Schubert, S. Branched and Linear Poly(Ethylene Imine)-Based Conjugates: Synthetic Modification, Characterization, and Application. Chem. Soc. Rev. 2012, 41, 4755–4767. [Google Scholar] [CrossRef]
- Bhatia, S. Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-41129-3. [Google Scholar]
- Wandrey, C.; Hernández-Barajas, J.; Hunkeler, D. Diallyldimethylammonium Chloride and Its Polymers. Radic. Polym. Polyelectrolytes 1999, 145, 123–183. [Google Scholar] [CrossRef]
- Essafi, W.; Spiteri, M.N.; Williams, C.; Boue, F. Hydrophobic Polyelectrolytes in Better Polar Solvent. Structure and Chain Conformation as Seen by SAXS and SANS. Macromolecules 2009, 42, 9568–9580. [Google Scholar] [CrossRef]
- Michna, A.; Adamczyk, Z.; Kubiak, K.; Jamroży, K. Formation of PDADMAC Monolayers Evaluated in Situ by QCM and Streaming Potential Measurements. J. Colloid Interface Sci. 2014, 428, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Morga, M.; Michna, A.; Adamczyk, Z. Formation and Stability of Polyelectrolyte/Polypeptide Monolayers Determined by Electrokinetic Measurements. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 529, 302–310. [Google Scholar] [CrossRef]
- Michna, A.; Adamczyk, Z.; Sofińska, K.; Matusik, K. Monolayers of Poly(Amido Amine) Dendrimers on Mica–In Situ Streaming Potential Measurements. J. Colloid Interface Sci. 2017, 485, 232–241. [Google Scholar] [CrossRef]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of Polysaccharide Particle-Based Functional Drug Delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef]
- Wen, Y.; Oh, J.K. Recent Strategies to Develop Polysaccharide-Based Nanomaterials for Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Boddohi, S.; Killingsworth, C.E.; Kipper, M.J. Polyelectrolyte Multilayer Assembly as a Function of PH and Ionic Strength Using the Polysaccharides Chitosan and Heparin. Biomacromolecules 2008, 9, 2021–2028. [Google Scholar] [CrossRef]
- Whistler, R.L. Solubility of Polysaccharides and Their Behavior in Solution. In Carbohydrates in Solution; Isbell, H.S., Ed.; ACS Publications: Washington, DC, USA, 1973; pp. 242–255. [Google Scholar]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.G.; Kim, I. Recent Advances in Poly(α-L-Glutamic Acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
- Fraser-Reid, B.O.; Tatsuta, K.; Thiem, J. Glycoscience. Chemistry and Chemical Biology, 2nd ed.; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-36154-1. [Google Scholar]
- Mori, Y.; Shingo, N.; Satoko, K.; Mitsuyuki, K.; Satoshi, S.; Takemi, M.; Masayuki, I. Preparation and Characterization of Low-Molecular-Weight Heparin / Protamine Nanoparticles (LMW-H/P NPs ) as FGF-2 Carrier. Int. J. Nanomed. 2010, 5, 147–155. [Google Scholar] [CrossRef]
- de Paz, J.L.; Noti, C.; Böhm, F.; Werner, S.; Seeberger, P.H. Potentiation of Fibroblast Growth Factor Activity by Synthetic Heparin Oligosaccharide Glycodendrimers. Chem. Biol. 2007, 14, 879–887. [Google Scholar] [CrossRef]
- Sun, C.; Liu, M.; Sun, P.; Yang, M.; Yates, E.A.; Guo, Z.; Fernig, D.G. Sulfated Polysaccharides Interact with Fibroblast Growth Factors and Protect from Denaturation. FEBS Open Bio 2019, 9, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Delair, T.; Brua, C.; Veron, L.; Pavirani, A.; Kolbe, H.V.J. Synthesis of Polyallylamine Derivatives and Their Use as Gene Transfer Vectors in Vitro. Bioconjug. Chem. 1999, 10, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.M.; Langer, R. Degradable Poly (β-Amino Esters ): Synthesis, Characterization, and Self-Assembly with Plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- Naji, A.; Netz, R.R. Attraction of Like-Charged Macroions in the Strong-Coupling Limit. Eur. Phys. J. E 2004, 13, 43–59. [Google Scholar] [CrossRef]
- Stelmakh, A.; Cai, W.; Baumketner, A. Attraction between Like-Charged Macroions Mediated by Specific Counterion Configurations. J. Phys. Chem. B 2019, 123, 9971–9983. [Google Scholar] [CrossRef]
- Simončič, M.; Hritz, J.; Lukšič, M. Biomolecular Complexation on the “Wrong Side”: A Case Study of the Influence of Salts and Sugars on the Interactions between Bovine Serum Albumin and Sodium Polystyrene Sulfonate. Biomacromolecules 2022, 23, 4412–4426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, M.; Hu, C.; Liu, B.; Hu, M.; Zhang, X.; Wang, W.; Zhou, Y.; Wang, H. Correlation between Gel-Forming Ability, Supramolecular Aggregates and Main-Chain Conformation of Dendronized Polymer Gelators. N. J. Chem. 2011, 35, 103–110. [Google Scholar] [CrossRef]
- Maurer, S.A.; Bedbrook, C.N.; Radke, C.J. Cellulase Adsorption and Reactivity on a Cellulose Surface from Flow Ellipsometry. Ind. Eng. Chem. Res. 2012, 51, 11389–11400. [Google Scholar] [CrossRef]
- Alonso, T.; Irigoyen, J.; Iturri, J.J.; Larena, I.L.; Moya, S.E. Study of the Multilayer Assembly and Complex Formation of Poly(Diallyldimethylammonium Chloride) (PDADMAC) and Poly(Acrylic Acid) (PAA) as a Function of PH. Soft Matter 2013, 9, 1920–1928. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, J.; Jiang, G.; Liu, L.; Liu, Y. Cross-Linked Poly(Vinyl Alcohol)/Poly (Diallyldimethylammonium Chloride) as Anion-Exchange Membrane for Fuel Cell Applications. J. Power Sources 2013, 240, 359–367. [Google Scholar] [CrossRef]
- dos Santos, R.L.O.; Gamarra, J.G.A.; Lincopan, N.; Petri, D.F.S.; Paula, C.R.; Coto, N.P.; Dias, R.B. Production of Medical Grade Silicone for Facial Prosthesis with Bactericidal Properties from the Inclusion of Poly (Diallyldimethylammonium Chloride): An in Vitro Study. Pesqui. Bras. Odontopediatria Clin. Integr. 2019, 19, e3962. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Jamroży, K.; Batys, P.; Michna, A. Influence of Ionic Strength on Poly(Diallyldimethylammonium Chloride) Macromolecule Conformations in Electrolyte Solutions. J. Colloid Interface Sci. 2014, 435, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Marcelo, G.; Tarazona, M.P.; Saiz, E. Conformational Properties of Poly(Diallyldimethyl Ammonium Chloride) (PDDA) Determined by Combination of Molecular Dynamics, Rotational Isomeric States and Monte Carlo Procedures. Polymer 2004, 45, 1321–1330. [Google Scholar] [CrossRef]
- Trzciński, S.; Vårum, K.M.; Staszewska, D.U.; Smidsrød, O.; Bohdanecký, M. Comparative Studies on Molecular Chain Parameters of Chitosans and Poly(Diallyldimethylammonium Chloride): The Stiffness B-Parameter and the Temperature Coefficient of Intrinsic Viscosity. Carbohydr. Polym. 2002, 48, 171–178. [Google Scholar] [CrossRef]
- Vaccaro, A.; Hierrezuelo, J.; Skarba, M.; Galletto, P.; Kleimann, J.; Borkovec, M. Structure of an Adsorbed Polyelectrolyte Monolayer on Oppositely Charged Colloidal Particles. Langmuir 2009, 25, 4864–4867. [Google Scholar] [CrossRef]
- Xie, F.; Nylander, T.; Piculell, L.; Utsel, S.; Wågberg, L.; Åkesson, T.; Forsman, J. Polyelectrolyte Adsorption on Solid Surfaces: Theoretical Predictions and Experimental Measurements. Langmuir 2013, 29, 12421–12431. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Zembala, M.; Warszynski, P.; Jachimska, B. Characterization of Polyelectrolyte Multilayers by the Streaming Potential Method. Langmuir 2004, 20, 10517–10525. [Google Scholar] [CrossRef]
- Kreke, M.R.; Badami, A.S.; Brady, J.B.; Michael Akers, R.; Goldstein, A.S. Modulation of Protein Adsorption and Cell Adhesion by Poly(Allylamine Hydrochloride) Heparin Films. Biomaterials 2005, 26, 2975–2981. [Google Scholar] [CrossRef]
- Porus, M.; Maroni, P.; Borkovec, M. Response of Adsorbed Polyelectrolyte Monolayers to Changes in Solution Composition. Langmuir 2012, 28, 17506–17516. [Google Scholar] [CrossRef]
- Dejeu, J.; Diziain, S.; Dange, C.; Membrey, F.; Charraut, D.; Foissy, A. Stability of Self-Assembled Polymer Films Investigated by Optical Laser Reflectometry. Langmuir 2008, 24, 3090–3098. [Google Scholar] [CrossRef]
- Morga, M.; Adamczyk, Z. Monolayers of Cationic Polyelectrolytes on Mica-Electrokinetic Studies. J. Colloid Interface Sci. 2013, 407, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Zhao, Z.; Wang, J.; Yang, C.; Chen, C.; Gao, L. Surface Engineering of Gold Nanoparticles for in Vitro SiRNA Delivery. Nanoscale 2012, 4, 5102–5109. [Google Scholar] [CrossRef] [PubMed]
- Shifeta, N.T.; Hamukwaya, S.L.; An, Q.; Hao, H.; Mashingaidze, M.M. Layer-by-Layer Fabrication of PAH/PAMAM/Nano-CaCO3 Composite Films and Characterization for Enhanced Biocompatibility. Int. J. Biomater. 2022, 2022, 6331465. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.C.; Zhai, L.; Cohen, R.E.; Rubner, M.F. Controlled Drug Release from Porous Polyelectrolyte Multilayers. Biomacromolecules 2006, 7, 357–364. [Google Scholar] [CrossRef]
- Wytrwal, M.; Leduc, C.; Sarna, M.; Goncalves, C.; Kepczynski, M.; Midoux, P.; Nowakowska, M.; Pichon, C. Gene Delivery Efficiency and Intracellular Trafficking of Novel Poly(Allylamine) Derivatives. Int. J. Pharm. 2015, 478, 921–929. [Google Scholar] [CrossRef]
- Ibie, C.O.; Thompson, C.J.; Knott, R. Synthesis, Characterisation and in Vitro Evaluation of Novel Thiolated Derivatives of Polyallylamine and Quaternised Polyallylamine. Colloid Polym. Sci. 2015, 293, 1737–1748. [Google Scholar] [CrossRef][Green Version]
- Ito, Y.; Chen, G.; Imanishi, Y. Micropatterned Immobilization of Epidermal Growth Factor To Regulate Cell Function. Bioconjug. Chem. 1998, 9, 277–282. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Keskin, D.; Shi, L. Poly(β-Amino Esters): Synthesis, Formulations, and Their Biomedical Applications. Adv. Healthc. Mater. 2019, 8, 1801359. [Google Scholar] [CrossRef]
- Zhou, D.; Pierucci, L.; Gao, Y.; O’Keeffe Ahern, J.; Huang, X.; Sigen, A.; Wang, W. Thermo- and PH-Responsive, Coacervate-Forming Hyperbranched Poly(β-Amino Ester)s for Selective Cell Binding. ACS Appl. Mater. Interfaces 2017, 9, 5793–5802. [Google Scholar] [CrossRef]
- Song, W.; Tang, Z.; Li, M.; Lv, S.; Yu, H.; Ma, L.; Zhuang, X.; Huang, Y.; Chen, X. Tunable PH-Sensitive Poly(β-Amino Ester)s Synthesized from Primary Amines and Diacrylates for Intracellular Drug Delivery. Macromol. Biosci. 2012, 12, 1375–1383. [Google Scholar] [CrossRef]
- Bechler, S.L.; Lynn, D.M. Characterization of Degradable Polyelectrolyte Multilayers Fabricated Using DNA and a Fluorescently-Labeled Poly(β-Amino Ester): Shedding Light on the Role of the Cationic Polymer in Promoting Surface-Mediated Gene Delivery. Biomacromolecules 2012, 13, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias-Zambrano, O.; Shrestha, T.B.; Pyle, M.; Montes-Gonzalez, M.; Troyer, D.L.; Bossmann, S.H. Development of a Gene Delivery System Composed of a Cell-Penetrating Peptide and a Nontoxic Polymer. ACS Appl. Bio Mater. 2020, 3, 7418–7427. [Google Scholar] [CrossRef] [PubMed]
- Routkevitch, D.; Sudhakar, D.; Conge, M.; Varanasi, M.; Tzeng, S.Y.; Wilson, D.R.; Green, J.J. Efficiency of Cytosolic Delivery with Poly(β-Amino Ester) Nanoparticles Is Dependent on the Effective PKa of the Polymer. ACS Biomater. Sci. Eng. 2020, 6, 3411–3421. [Google Scholar] [CrossRef]
- Tsai, P.F.; Chang, W.Y.; Hsiao, Y.C.; Li, K.J.; Shau, M. Da Synthesis and Characterization of Cationic Glycidyl-Based Poly(Aminoester)-Folic Acid Targeting Conjugates and Study on Gene Delivery. Molecules 2012, 17, 9056–9069. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, R.C.; Yi, W.J.; Zhao, Z.G. Biodegradable Poly(Amino Ester) with Aromatic Backbone as Efficient Nonviral Gene Delivery Vectors. Molecules 2017, 22, 566. [Google Scholar] [CrossRef]
- Li, Y.; He, Z.; Lyu, J.; Wang, X.; Qiu, B.; Lara-s, I.; Zhang, J.; Zeng, M.; Xu, Q.; Sigen, A.; et al. Hyperbranched Poly (β-Amino Ester)s (HPAEs ) Structure Optimisation for Enhanced Gene Delivery: Non-Ideal Termination Elimination. Nanomaterials 2022, 12, 3892. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, J.Y.; O’Keeffe Ahern, J.; Cutlar, L.; Zhou, D.; Lin, F.H.; Wang, W. Highly Branched Poly(β-Amino Esters) for Non-Viral Gene Delivery: High Transfection Efficiency and Low Toxicity Achieved by Increasing Molecular Weight. Biomacromolecules 2016, 17, 3640–3647. [Google Scholar] [CrossRef]
- Cutlar, L.; Zhou, D.; Gao, Y.; Zhao, T.; Greiser, U.; Wang, W.; Wang, W. Highly Branched Poly(β-Amino Esters): Synthesis and Application in Gene Delivery. Biomacromolecules 2015, 16, 2609–2617. [Google Scholar] [CrossRef]
- Kafil, V.; Omidi, Y. Cytotoxic Impacts of Linear and Branched Polyethylenimine Nanostructures in A431 Cells. BioImpacts 2011, 1, 23–30. [Google Scholar] [CrossRef]
- Koper, G.J.M.; Van Duijvenbode, R.C.; Stam, D.D.P.W.; Steuerle, U.; Borkovec, M. Synthesis and Protonation Behavior of Comblike Poly(Ethyleneimine). Macromolecules 2003, 36, 2500–2507. [Google Scholar] [CrossRef]
- Crea, F.; Crea, P.; De Robertis, A.; Sammartano, S. Thermodynamic Study for the Protonation of Branched Poly(Ethylenimine) in NaCl(Aq) and Its Dependence on Ionic Strength. J. Chem. Eng. Data 2007, 52, 279–285. [Google Scholar] [CrossRef]
- Griffiths, P.C.; Paul, A.; Stilbs, P.; Petterson, E. Charge on Poly(Ethylene Imine): Comparing Electrophoretic NMR Measurements and PH Titrations. Macromolecules 2005, 38, 3539–3542. [Google Scholar] [CrossRef]
- Pfau, A.; Schrepp, W.; Horn, D. Detection of a Single Molecule Adsorption Structure of Poly (Ethylenimine) Macromolecules by AFM. Langmuir 1999, 15, 3219–3225. [Google Scholar] [CrossRef]
- Saftics, A.; Agócs, E.; Fodor, B.; Patkó, D.; Petrik, P.; Kolari, K.; Aalto, T.; Fürjes, P.; Horvath, R.; Kurunczi, S. Investigation of Thin Polymer Layers for Biosensor Applications. Appl. Surf. Sci. 2013, 281, 66–72. [Google Scholar] [CrossRef]
- Godbey, W.T.; Barry, M.A.; Saggau, P.; Wu, K.K.; Mikos, A.G. Poly (Ethylenimine) -Mediated Transfection: A New Paradigm for Gene Delivery. Inc. J. Biomed. Mater. Res. 1999, 51, 321–328. [Google Scholar] [CrossRef]
- Bahadur, K.C.R.; Uluda, H. PEI and Its Derivatives for Gene Therapy. In Polymers and Nanomaterials for Gene Therapy; Narain, R., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 29–54. ISBN 9780081005200. [Google Scholar]
- de la Hoz, R.; Diban, N.; Berciano, M.T.; San Emeterio, C.; Urtiaga, A.; Lafarga, M.; Rodríguez-Rey, J.C.; Tapia, O. Coaxial Synthesis of PEI-Based Nanocarriers of Encapsulated RNA-Therapeutics to Specifically Target Muscle Cells. Biomolecules 2022, 12, 1012. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.; Joo, S.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, Applications, and Properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in Biomedical Applications-Reflections on the Field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in Drug Delivery and Targeting: Drug-Dendrimer Interactions and Toxicity Issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [CrossRef]
- Vu, M.T.; Bach, L.G.; Nguyen, D.C.; Ho, M.N.; Nguyen, N.H.; Tran, N.Q.; Nguyen, D.H.; Nguyen, C.K.; Thi, T.T.H. Modified Carboxyl-Terminated PAMAM Dendrimers as Great Cytocompatible Nano-Based Drug Delivery System. Int. J. Mol. Sci. 2019, 20, 2016. [Google Scholar] [CrossRef]
- Conti, D.S.; Brewer, D.; Grashik, J.; Avasarala, S.; Da Rocha, S.R.P. Poly(Amidoamine) Dendrimer Nanocarriers and Their Aerosol Formulations for SiRNA Delivery to the Lung Epithelium. Mol. Pharm. 2014, 11, 1808–1822. [Google Scholar] [CrossRef]
- Cakara, D.; Kleimann, J.; Borkovec, M. Microscopic Protonation Equilibria of Poly(Amidoamine) Dendrimers from Macroscopic Titrations. Macromolecules 2003, 36, 4201–4207. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Chang, H.; Cheng, Y. Surface-Engineered Dendrimers in Gene Delivery. Chem. Rev. 2015, 115, 5274–5300. [Google Scholar] [CrossRef] [PubMed]
- Nisato, G.; Ivkov, R.; Amis, E.J. Size Invariance of Polyelectrolyte Dendrimers. Macromolecules 2000, 33, 4172–4176. [Google Scholar] [CrossRef]
- Porcar, L.; Liu, Y.; Verduzco, R.; Hong, K.; Butler, P.D.; Magid, L.J.; Smith, G.S.; Chen, W.-R. Structural Investigation of PAMAM Dendrimers in Aqueous Solutions Using Small-Angle Neutron Scattering: Effect of Generation. J. Phys. Chem. B 2008, 112, 14772–14778. [Google Scholar] [CrossRef] [PubMed]
- Maiti, P.K. PAMAM Dendrimer: A PH Controlled Nanosponge. Can. J. Chem. 2017, 95, 991–998. [Google Scholar] [CrossRef]
- Welch, P.; Muthukumar, M. Tuning the Density Profile of Dendritic Polyelectrolytes. Macromolecules 1998, 31, 5892–5897. [Google Scholar] [CrossRef]
- Lee, I.; Athey, B.D.; Wetzel, A.W.; Meixner, W.; Baker, J.R. Structural Molecular Dynamics Studies on Polyamidoamine Dendrimers for a Therapeutic Application: Effects of PH and Generation. Macromolecules 2002, 35, 4510–4520. [Google Scholar] [CrossRef]
- Jachimska, B.; Tokarczyk, K. Combining Surface Plasmon Resonance and Quartz Crystal Microbalance to Determine Hydration of Dendrimer Monolayers. J. Phys. Chem. C 2016, 120, 19678–19685. [Google Scholar] [CrossRef]
- Tokarczyk, K.; Jachimska, B. Quantitative Interpretation of PAMAM Dendrimers Adsorption on Silica Surface. J. Colloid Interface Sci. 2017, 503, 86–94. [Google Scholar] [CrossRef]
- Mureşan, L.; Maroni, P.; Popa, I.; Porus, M.; Longtin, R.; Papastavrou, G.; Borkovec, M. Conformational Changes of Polyamidoamine (PAMAM) Dendrimers Adsorbed on Silica Substrates. Macromolecules 2011, 44, 5069–5071. [Google Scholar] [CrossRef]
- Porus, M.; Clerc, F.; Maroni, P.; Borkovec, M. Ion-Specific Responsiveness of Polyamidoamine (PAMAM) Dendrimers Adsorbed on Silica Substrates. Macromolecules 2012, 45, 3919–3927. [Google Scholar] [CrossRef]
- Wolski, P.; Panczyk, T. Conformational Properties of PAMAM Dendrimers Adsorbed on the Gold Surface Studied by Molecular Dynamics Simulation. J. Phys. Chem. C 2019, 123, 22603–22613. [Google Scholar] [CrossRef]
- Lin, S.T.; Maiti, P.K.; Goddard, W.A. Dynamics and Thermodynamics of Water in PAMAM Dendrimers at Subnanosecond Time Scales. J. Phys. Chem. B 2005, 109, 8663–8672. [Google Scholar] [CrossRef] [PubMed]
- Haensler, J.; Szoka, F.C. Polyamidoamine Cascade Polymers Mediate Efficient Transfection of Cells in Culture. Bioconjug. Chem. 1993, 4, 372–379. [Google Scholar] [CrossRef]
- Santos, J.L.; Oramas, E.; Pêgo, A.P.; Granja, P.L.; Tomás, H. Osteogenic Differentiation of Mesenchymal Stem Cells Using PAMAM Dendrimers as Gene Delivery Vectors. J. Control. Release 2009, 134, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.P.; Shukla, R.; Kotlyar, A.; Kukowska-Latallo, J.; Baker, J.R., Jr. Dendrimer-Based Tumor Cell Targeting of Fibroblast Growth Factor-1. Bioorg. Med. Chem. Lett. 2010, 20, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Igartúa, D.E.; Martinez, C.S.; Temprana, C.F.; Alonso, S.; del V. Prieto, M.J. PAMAM Dendrimers as a Carbamazepine Delivery System for Neurodegenerative Diseases: A Biophysical and Nanotoxicological Characterization. Int. J. Pharm. 2018, 544, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; An, S.; Choi, S.; Jung, H.S.; Chang, S.; Ko, J.H.; Hye, J.; Kim, T.K.; Soo, J.; Park, J.H.; et al. Effective Healing of Diabetic Skin Wounds by Using Nonviral Gene Therapy Based on Minicircle Vascular Endothelial Growth Factor DNA and a Cationic Dendrimer. J. Gene Med. 2012, 14, 272–278. [Google Scholar] [CrossRef]
- Navath, R.S.; Menjoge, A.R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Injectable PAMAM Dendrimer-PEG Hydrogels for the Treatment of Genital Infections: Formulation and in Vitro and in Vivo Evaluation. Mol. Pharm. 2011, 8, 1209–1223. [Google Scholar] [CrossRef]
- Lo, S.T.; Kumar, A.; Hsieh, J.T.; Sun, X. Dendrimer Nanoscaffolds for Potential Theranostics of Prostate Cancer with a Focus on Radiochemistry. Mol. Pharm. 2013, 10, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Patri, A.K.; Simak, J.; Hall, J.B.; Semberova, J.; Lacerda, S.H.D.P.; Mcneil, S.E. Nanoparticle Size and Surface Charge Determine Effects of PAMAM Dendrimers on Human Platelets in Vitro. Mol. Pharm. 2012, 9, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus, W.; Duncan, R. Dendrimers: Relationship between Structure and Biocompatibility in Vitro, and Preliminary Studies on the Biodistribution of 125I-Labelled Polyamidoamine Dendrimers in Vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Kurokawa, Y.; Win-Shwe, T.T.; Zeng, Q.; Hirano, S.; Zhang, Z.; Sone, H. Effects of PAMAM Dendrimers with Various Surface Functional Groups and Multiple Generations on Cytotoxicity and Neuronal Differentiation Using Human Neural Progenitor Cells. J. Toxicol. Sci. 2016, 41, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Gaballu, F.; Dehghan, G.; Ghaffari, M.; Yekta, R.; Abbaspour-Ravasjani, S.; Baradaran, B.; Dolatabadi, J.E.N.; Hamblin, M.R. PAMAM Dendrimers as Efficient Drug and Gene Delivery Nanosystems for Cancer Therapy. Appl. Mater. Today 2018, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Zhang, J.; Wang, H.; Lv, J.; Cheng, Y. A Combination of Guanidyl and Phenyl Groups on a Dendrimer Enables Efficient SiRNA and DNA Delivery. Biomacromolecules 2017, 18, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Navath, R.S.; Menjoge, A.R.; Balakrishnan, B.; Bellair, R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Inhibition of Bacterial Growth and Intramniotic Infection in a Guinea Pig Model of Chorioamnionitis Using PAMAM Dendrimers. Int. J. Pharm. 2010, 395, 298–308. [Google Scholar] [CrossRef]
- Ertürk, A.S.; Gürbüz, M.U.; Tülü, M.T. The Effect of PAMAM Dendrimer Concentration, Generation Size and Surface Functional Group on the Aqueous Solubility of Candesartan Cilexetil. Pharm. Dev. Technol. 2017, 22, 111–121. [Google Scholar] [CrossRef]
- Micali, N.; Scolaro, L.M.; Romeo, A.; Lombardo, D.; Lesieur, P.; Mallamace, F. Structural Properties of Methanol-Polyamidoamine Dendrimer Solutions. Phys. Rev. E 1998, 58, 6229–6235. [Google Scholar] [CrossRef]
- Lombardo, D. Liquid-like Ordering of Negatively Charged Poly (Amidoamine) (PAMAM) Dendrimers in Solution. Langmuir 2009, 25, 3271–3275. [Google Scholar] [CrossRef]
- Tsukruk, V.V.; Rinderspacher, F.; Bliznyuk, V.N. Self-Assembled Multilayer Films from Dendrimers. Langmuir 1997, 13, 2171–2176. [Google Scholar] [CrossRef]
- Ajikumar, P.K.; Ng, J.K.; Tang, Y.C.; Lee, J.Y.; Stephanopoulos, G.; Too, H.P. Carboxyl-Terminated Dendrimer-Coated Bioactive Interface for Protein Microarray: High-Sensitivity Detection of Antigen in Complex Biological Samples. Langmuir 2007, 23, 5670–5677. [Google Scholar] [CrossRef]
- Katzur, V.; Eichler, M.; Deigele, E.; Stage, C.; Karageorgiev, P.; Geis-Gerstorfer, J.; Schmalz, G.; Ruhl, S.; Rupp, F.; Müller, R. Surface-Immobilized PAMAM-Dendrimers Modified with Cationic or Anionic Terminal Functions: Physicochemical Surface Properties and Conformational Changes after Application of Liquid Interface Stress. J. Colloid Interface Sci. 2012, 366, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Schilrreff, P.; Mundiña-Weilenmann, C.; Romero, E.L.; Morilla, M.J. Selective Cytotoxicity of PAMAM G5 Core–PAMAM G2.5 Shell Tecto-Dendrimers on Melanoma Cells. Int. J. Nanomed. 2012, 7, 4121–4133. [Google Scholar] [CrossRef]
- Balastre, M.; Persello, J.; Foissy, A.; Argillier, J.-F. Binding and Ion-Exchange Analysis in the Process of Adsorption of Anionic Polyelectrolytes on Barium Sulfate. J. Colloid Interface Sci. 1999, 219, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Bratek, A.; Jachimska, B.; Jasiński, T.; Warszyński, P. Structure of Poly(Acrylic Acid) in Electrolyte Solutions Determined from Simulations and Viscosity Measurements. J. Phys. Chem. B 2006, 110, 22426–22435. [Google Scholar] [CrossRef]
- Batys, P.; Luukkonen, S.; Sammalkorpi, M. Ability of the Poisson-Boltzmann Equation to Capture Molecular Dynamics Predicted Ion Distribution around Polyelectrolytes. Phys. Chem. Chem. Phys. 2017, 19, 24583–24593. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Bratek, A.; Szelag, E.; Bastrzyk, A.; Michna, A.; Barbasz, J. Colloid Particle Deposition on Heterogeneous Surfaces Produced by Polyelectrolyte Adsorption. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 343, 111–117. [Google Scholar] [CrossRef]
- Whitty, E.G.; Maniego, A.R.; Bentwitch, S.A.; Guillaneuf, Y.; Jones, M.R.; Gaborieau, M.; Castignolles, P. Cellular Response to Linear and Branched Poly(Acrylic Acid). Macromol. Biosci. 2015, 15, 1724–1734. [Google Scholar] [CrossRef]
- Tanchak, O.M.; Yager, K.G.; Fritzsche, H.; Harroun, T.; Katsaras, J.; Barrett, C.J. Water Distribution in Multilayers of Weak Polyelectrolytes. Langmuir 2006, 22, 5137–5143. [Google Scholar] [CrossRef]
- Liufu, S.; Xiao, H.; Li, Y. Adsorption of Poly(Acrylic Acid) onto the Surface of Titanium Dioxide and the Colloidal Stability of Aqueous Suspension. J. Colloid Interface Sci. 2005, 281, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, S.; Wiśniewska, M.; Marczewski, A.W.; Pikus, S. Application of the SAXS Method and Viscometry for Determination of the Thickness of Adsorbed Polymer Layers at the ZrO2-Polymer Solution Interface. J. Colloid Interface Sci. 2003, 267, 1–8. [Google Scholar] [CrossRef]
- Yuan, W.; Lu, Z.; Li, C.M. Charged Drug Delivery by Ultrafast Exponentially Grown Weak Polyelectrolyte Multilayers: Amphoteric Properties, Ultrahigh Loading Capacity and PH-Responsiveness. J. Mater. Chem. 2012, 22, 9351–9357. [Google Scholar] [CrossRef]
- Psarra, E.; Foster, E.; König, U.; You, J.; Ueda, Y.; Eichhorn, K.J.; Müller, M.; Stamm, M.; Revzin, A.; Uhlmann, P. Growth Factor-Bearing Polymer Brushes-Versatile Bioactive Substrates Influencing Cell Response. Biomacromolecules 2015, 16, 3530–3542. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.N.; Dong, R.; Ober, C.K.; Baird, B.A. Cellular Responses to Patterned Poly(Acrylic Acid) Brushes. Langmuir 2011, 27, 7016–7023. [Google Scholar] [CrossRef]
- Joshi, R.V.; Nelson, C.E.; Poole, K.M.; Skala, M.C.; Duvall, C.L. Dual PH- and Temperature-Responsive Microparticles for Protein Delivery to Ischemic Tissues. Acta Biomater. 2013, 9, 6526–6534. [Google Scholar] [CrossRef]
- Soni, V.; Pandey, V.; Tiwari, R.; Asati, S.; Tekade, R.K. Design and Evaluation of Ophthalmic Delivery Formulations. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 473–538. ISBN 9780128179093. [Google Scholar]
- Polysaccharide Carriers for Drug Delivery, 1st ed.; Maiti, S., Jana, S., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2019; ISBN 9780081025536. [Google Scholar]
- Rodríguez, A.; Kleinbeck, K.; Mizenina, O.; Kizima, L.; Levendosky, K.; Jean-Pierre, N.; Villegas, G.; Ford, B.E.; Cooney, M.L.; Teleshova, N.; et al. In Vitro and in Vivo Evaluation of Two Carrageenan-Based Formulations to Prevent HPV Acquisition. Antivir. Res. 2014, 108, 88–93. [Google Scholar] [CrossRef]
- Harding, S.E.; Tombs, M.P.; Adams, G.G.; Paulsen, B.S.; Inngjerdingen, K.T.; Barsett, H. Marine Polysaccharides. In An Introduction to Polysaccharide Biotechnology; Harding, S.E., Tombs, M.P., Adams, G.G., Paulsen, B.S., Inngjerdingen, K.T., Barsett, H., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 153–192. ISBN 9781482246971. [Google Scholar]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Skovstrup, S.; Hansen, S.G.; Skrydstrup, T.; Schiøtt, B. Conformational Flexibility of Chitosan: A Molecular Modeling Study. Biomacromolecules 2010, 11, 3196–3207. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the Degree of Acetylation on Some Biological Properties of Chitosan Films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Tsereteli, L.; Grafmüller, A. An Accurate Coarse-Grained Model for Chitosan Polysaccharides in Aqueous Solution. PLoS ONE 2017, 12, e0180938. [Google Scholar] [CrossRef] [PubMed]
- Desbrieres, J. Viscosity of Semiflexible Chitosan Solutions: Influence of Concentration, Temperature, and Role of Intermolecular Interactions. Biomacromolecules 2002, 3, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Influence of Acetic Acid Concentration on the Solubilization of Chitosan. Polymer 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Solubilization of Chitosan in Strong Acid Medium. Int. J. Polym. Anal. Characr. 1999, 5, 267–276. [Google Scholar] [CrossRef]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. Macromolecular Conformation of Chitosan in Dilute Solution: A New Global Hydrodynamic Approach. Carbohydr. Polym. 2009, 76, 616–621. [Google Scholar] [CrossRef]
- Claesson, P.M.; Ninham, B.W. PH-Dependent Interactions between Adsorbed Chitosan Layers. Langmuir 1992, 8, 1406–1412. [Google Scholar] [CrossRef]
- Tiraferri, A.; Maroni, P.; Caro Rodríguez, D.; Borkovec, M. Mechanism of Chitosan Adsorption on Silica from Aqueous Solutions. Langmuir 2014, 30, 4980–4988. [Google Scholar] [CrossRef]
- Mun, S.; Decker, E.A.; McClements, D.J. Influence of Droplet Characteristics on the Formation of Oil-in-Water Emulsions Stabilized by Surfactant-Chitosan Layers. Langmuir 2005, 21, 6228–6234. [Google Scholar] [CrossRef] [PubMed]
- Pišlová, M.; Šubrt, M.; Polívková, M.; Kolářová, K. Deposition of Thin Metal Layers on Chitosan Films. Mater. Technol. 2018, 33, 845–853. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- de Sousa Victor, R.; da Cunha Santos, A.; de Sousa, B.V.; de Araújo Neves, G.; de Lima Santana, L.N.; Menezes, R.R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef] [PubMed]
- Divband, B.; Pouya, B.; Hassanpour, M.; Alipour, M.; Salehi, R.; Rahbarghazi, R.; Shahi, S.; Aghazadeh, Z.; Aghazadeh, M. Towards Induction of Angiogenesis in Dental Pulp Stem Cells Using Chitosan-Based Hydrogels Releasing Basic Fibroblast Growth Factor. Biomed Res. Int. 2022, 2022, 5401461. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Anjum, M.M.; Patel, K.K. Gemcitabine Cationic Polymeric Nanoparticles against Ovarian Cancer: Formulation, Characterization, and Targeted Drug Delivery. Drug Deliv. 2022, 29, 1060–1074. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic Acid (Hyaluronan): A Review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Matsuoka, S. Experimental Approaches to Hyaluronan Structure. Carbohydr. Res. 2005, 340, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Cowman, M.K.; Spagnoli, C.; Kudasheva, D.; Li, M.; Dyal, A.; Kanai, S.; Balazs, E.A. Extended, Relaxed, and Condensed Conformations of Hyaluronan Observed by Atomic Force Microscopy. Biophys. J. 2005, 88, 590–602. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.O.; Linardy, E.; Dreaden, E.C.; Zhdanov, V.P.; Hammond, P.T.; Cho, N.J. Adsorption of Hyaluronic Acid on Solid Supports: Role of PH and Surface Chemistry in Thin Film Self-Assembly. J. Colloid Interface Sci. 2015, 448, 197–207. [Google Scholar] [CrossRef]
- Ogueri, K.S.; Jafari, T.; Ivirico, J.L.E.; Laurencin, C.T. Polymeric Biomaterials for Scaffold-Based Bone Regenerative Engineerings. Regen. Eng. Transl. Med. 2019, 5, 128–154. [Google Scholar] [CrossRef]
- Phua, S.Z.F.; Yang, G.; Lim, W.Q.; Verma, A.; Chen, H.; Thanabalu, T.; Zhao, Y. Catalase-Integrated Hyaluronic Acid as Nanocarriers for Enhanced Photodynamic Therapy in Solid Tumor. ACS Nano 2019, 13, 4742–4751. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Yao, X.; Zhang, Y.; Wu, W.; Jiang, X. Hyaluronic Acid Nanogels with Enzyme-Sensitive Cross-Linking Group for Drug Delivery. J. Control. Release 2015, 205, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable Conductive Injectable Hydrogels as Novel Antibacterial, Anti-Oxidant Wound Dressings for Wound Healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Capila, I.-P.I.; Linhardt, R.J. Heparin-Protein Interactions. Angew. Chem. Int. Ed. 2002, 41, 390–412. [Google Scholar] [CrossRef]
- Mourier, P.; Anger, P.; Martinez, C.; Herman, F.; Viskov, C. Quantitative Compositional Analysis of Heparin Using Exhaustive Heparinase Digestion and Strong Anion Exchange Chromatography. Anal. Chem. Res. 2015, 3, 46–53. [Google Scholar] [CrossRef]
- Banik, N.; Yang, S.B.; Kang, T.B.; Lim, J.H.; Park, J. Heparin and Its Derivatives: Challenges and Advances in Therapeutic Biomolecules. Int. J. Mol. Sci. 2021, 22, 10524. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Moon, C.W.; Lee, Y.; Park, T.G. Intracellular Delivery of Heparin Complexed with Chitosan-g-Poly(Ethylene Glycol) for Inducing Apoptosis. Pharm. Res. 2009, 26, 93–100. [Google Scholar] [CrossRef]
- Barrantes, A.; Wengenroth, J.; Arnebrant, T.; Haugen, H.J. Poly-L-Lysine/Heparin Multilayer Coatings Prevent Blood Protein Adsorption. J. Colloid Interface Sci. 2017, 485, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef]
- Beni, S.; Limtiaco, J.F.K.; Larive, C.K. Analysis and Characterization of Heparin Impurities. Anal. Bioanal. Chem. 2011, 399, 527–539. [Google Scholar] [CrossRef]
- Hirsh, J.; Levine, M.N. Low Molecular Weight Heparins. Blood 1992, 79, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yamane, Y.; Saito, S.; Koizumi, T. Effects of Calcium and Magnesium on the Anticoagulant Action of Heparin. Chem. Pharm. Bull. 1983, 31, 3214–3221. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, G.; Finet, S.; Tatarenko, K.; Korneeva, E.; Ebel, C. Conformation of Heparin Studied with Macromolecular Hydrodynamic Methods and X-Ray Scattering. Eur. Biophys. J. 2003, 32, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Rossi, S.; Tamayo, M.; Pedraz, J.L.; Dominguez-Gil, A.; Caramella, C. On the Employment of λ-Carrageenan in a Matrix System. II. λ-Carrageenan and Hydroxypropylmethylcellulose Mixtures. J. Control. Release 1994, 30, 175–182. [Google Scholar] [CrossRef]
- Nakashima, H.; Kido, Y.; Kobayashi, N.; Motoki, Y.; Neushul, M.; Yamamoto, N. Purification and Characterization of an Avian Myeloblastosis and Human Immunodeficiency Virus Reverse Transcriptase Inhibitor, Sulfated Polysaccharides Extracted from Sea Algae. Antimicrob. Agents Chemother. 1987, 31, 1524–1528. [Google Scholar] [CrossRef]
- Pereira, L.; Critchley, A.T. The COVID 19 Novel Coronavirus Pandemic 2020: Seaweeds to the Rescue? Why Does Substantial, Supporting Research about the Antiviral Properties of Seaweed Polysaccharides Seem to Go Unrecognized by the Pharmaceutical Community in These Desperate Times? J. Appl. Phycol. 2020, 32, 1875–1877. [Google Scholar] [CrossRef]
- Briones, A.V.; Sato, T.; Bigol, U.G. Antibacterial Activity of Polyethylenimine/Carrageenan Multilayer against Pathogenic Bacteria. Adv. Chem. Eng. Sci. 2014, 4, 233–241. [Google Scholar] [CrossRef]
- Imeson, A.P. Carrageenan and Furcellaran. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 164–185. ISBN 9781845694142. [Google Scholar]
- Bono, A.; Anisuzzaman, S.M.; Ding, O.W. Effect of Process Conditions on the Gel Viscosity and Gel Strength of Semi-Refined Carrageenan (SRC) Produced from Seaweed (Kappaphycus Alvarezii). J. King Saud Univ.-Eng. Sci. 2014, 26, 3–9. [Google Scholar] [CrossRef]
- Gupta, M.N.; Raghava, S. Smart Systems Based on Polysaccharides. In Natural-Based Polymers for Biomedical Applications; Reis, R.L., Neves, N., Mano, J.F., Gomes, M.E., Marques, A.P., Azevedo, H.S., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 129–161. ISBN 9781845692643. [Google Scholar]
- Alba, K.; Kontogiorgos, V. Seaweed Polysaccharides (Agar, Alginate Carrageenan). In Reference Module in Food Science; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 240–250. [Google Scholar]
- Almutairi, F.M.; Adams, G.G.; Kök, M.S.; Lawson, C.J.; Gahler, R.; Wood, S.; Foster, T.J.; Rowe, A.J.; Harding, S.E. An Analytical Ultracentrifugation Based Study on the Conformation of Lambda Carrageenan in Aqueous Solution. Carbohydr. Polym. 2013, 97, 203–209. [Google Scholar] [CrossRef]
- Berth, G.; Vukovic, J.; Lechner, M.D. Physicochemical Characterization of Carrageenans—A Critical Reinvestigation. J. Appl. Polym. Sci. 2008, 110, 3508–3524. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In Vivo Antitumor and Immunomodulation Activities of Different Molecular Weight Lambda-Carrageenans from Chondrus Ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Nakata, R.; Miyazaki, T.; Morita, Y.; Ishida, E.; Iwatsuki, R.; Ohtsuki, C. Apatite Formation Abilities of Various Carrageenan Gels in Simulated Body Environment. J. Ceram. Soc. Jpn. 2010, 118, 487–490. [Google Scholar] [CrossRef][Green Version]
- Hoffman, R. Carrageenans Inhibit Growth-Factor Binding. Biochem. J. 1993, 289, 331–334. [Google Scholar] [CrossRef]
- Jafari, A.; Farahani, M.; Sedighi, M.; Rabiee, N.; Savoji, H. Carrageenans for Tissue Engineering and Regenerative Medicine Applications: A Review. Carbohydr. Polym. 2022, 281, 119045. [Google Scholar] [CrossRef] [PubMed]
- Thành, T.T.T.; Yuguchi, Y.; Mimura, M.; Yasunaga, H.; Takano, R.; Urakawa, H.; Kajiwara, K. Molecular Characteristics and Gelling Properties of the Carrageenan Family, 1: Preparation of Novel Carrageenans and Their Dilute Solution Properties. Macromol. Chem. Phys. 2002, 203, 15–23. [Google Scholar] [CrossRef]
- Slootmaekers, D.; Mandel, M.; Reynaers, H. Dynamic Light Scattering by κ- and λ-Carrageenan Solutions. Int. J. Biol. Macromol. 1991, 13, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zabik, M.E.; Aldrich, P.J. The Effect of Selected Anions of Potassium Salts on the Viscosities of Lambda-Carrageenan Dispersions. J. Food Sci. 1965, 30, 111–117. [Google Scholar] [CrossRef]
- Zabik, M.E.; Aldrich, P.J. The Effect of Cations on the Viscosity of Lambda-Carrageenan. J. Food Sci. 1967, 32, 91–97. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Silva, T.H.; Reis, R.L.; Mano, J.F. Nanocoatings Containing Sulfated Polysaccharides Prepared by Layer-by-Layer Assembly as Models to Study Cell-Material Interactions. J. Mater. Chem. B 2013, 1, 4406–4418. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Layer-by-Layer Assembled Cell Instructive Nanocoatings Containing Platelet Lysate. Biomaterials 2015, 48, 56–65. [Google Scholar] [CrossRef]
- Schoeler, B.; Delorme, N.; Doench, I.; Sukhorukov, G.B.; Fery, A.; Glinel, K. Polyelectrolyte Films Based on Polysaccharides of Different Conformations: Effects on Multilayer Structure and Mechanical Properties. Biomacromolecules 2006, 7, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Laufer, G.; Kirkland, C.; Cain, A.A.; Grunlan, J.C. Oxygen Barrier of Multilayer Thin Films Comprised of Polysaccharides and Clay. Carbohydr. Polym. 2013, 95, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Elizarova, I.S.; Luckham, P.F. Fabrication of Polyelectrolyte Multilayered Nano-Capsules Using a Continuous Layer-by-Layer Approach. J. Colloid Interface Sci. 2016, 470, 92–99. [Google Scholar] [CrossRef]
- Michna, A.; Maciejewska-Prończuk, J.; Pomorska, A.; Wasilewska, M.; Kilicer, T.; Witt, J.; Ozcan, O. Effect of the Anchoring Layer and Transport Type on the Adsorption Kinetics of Lambda Carrageenan. J. Phys. Chem. B 2021, 125, 7797–7808. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Meng, Y.; Li, J.; Chen, J.; Liu, Y.; Bai, X. Chondroitin Sulfate: Extraction, Purification, Microbial and Chemical Synthesis. J. Chem. Technol. Biotechnol. 2014, 89, 1445–1465. [Google Scholar] [CrossRef]
- Sharma, R.; Kuche, K.; Thakor, P.; Bhavana, V.; Srivastava, S.; Mehra, N.K.; Jain, S. Chondroitin Sulfate: Emerging Biomaterial for Biopharmaceutical Purpose and Tissue Engineering. Carbohydr. Polym. 2022, 286, 119305. [Google Scholar] [CrossRef]
- Vessella, G.; Vázquez, J.A.; Valcárcel, J.; Lagartera, L.; Monterrey, D.T.; Bastida, A.; García-junceda, E.; Bedini, E.; Fernández-mayoralas, A.; Revuelta, J. Deciphering Structural Determinants in Chondroitin Sulfate Binding to FGF-2: Paving the Way to Enhanced Predictability of Their Biological Functions. Polymers 2021, 13, 313. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Farran, A.; Montell, E.; Vergés, J.; Pelletier, J.P. Discrepancies in Composition and Biological Effects of Different Formulations of Chondroitin Sulfate. Molecules 2015, 20, 4277–4289. [Google Scholar] [CrossRef]
- Cordero-Arias, L.; Boccaccini, A.R. Electrophoretic Deposition of Chondroitin Sulfate-Chitosan/Bioactive Glass Composite Coatings with Multilayer Design. Surf. Coatings Technol. 2017, 315, 417–425. [Google Scholar] [CrossRef]
- Leite, Á.J.; Sher, P.; Mano, J.F. Chitosan/Chondroitin Sulfate Multilayers as Supports for Calcium Phosphate Biomineralization. Mater. Lett. 2014, 121, 62–65. [Google Scholar] [CrossRef]
- Malki, M.; Shapira, A.; Dvir, T. Chondroitin Sulfate-AuNRs Electroactive Scaffolds for on-Demand Release of Biofactors. J. Nanobiotechnol. 2022, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Assal, Y.; Mizuguchi, Y.; Mie, M.; Kobatake, E. Growth Factor Tethering to Protein Nanoparticles via Coiled-Coil Formation for Targeted Drug Delivery. Bioconjug. Chem. 2015, 26, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Kiani, M.; Farokhi, M.; Kundu, S.C.; Reis, R.L.; Gholami, M.; Bardania, H.; Dinarvand, R.; Geramifar, P.; Beiki, D.; et al. Targeted Delivery System Based on Gemcitabine-Loaded Silk Fibroin Nanoparticles for Lung Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 31600–31611. [Google Scholar] [CrossRef]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent Advancement of Gelatin Nanoparticles in Drug and Vaccine Delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Wang, H.; Zou, Q.; Boerman, O.C.; Nijhuis, A.W.G.; Jansen, J.A.; Li, Y.; Leeuwenburgh, S.C.G. Combined Delivery of BMP-2 and BFGF from Nanostructured Colloidal Gelatin Gels and Its Effect on Bone Regeneration in Vivo. J. Control. Release 2013, 166, 172–181. [Google Scholar] [CrossRef]
- Kundu, J.; Chung, Y., II; Kim, Y.H.; Tae, G.; Kundu, S.C. Silk Fibroin Nanoparticles for Cellular Uptake and Control Release. Int. J. Pharm. 2010, 388, 242–250. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, G.; Lin, X.; Chatzinikolaidou, M.; Jennissen, H.P.; Laub, M.; Uludaǧ, H. Polyethylenimine-Coated Albumin Nanoparticles for BMP-2 Delivery. Biotechnol. Prog. 2008, 24, 945–956. [Google Scholar] [CrossRef]
- Li, X.; Qiu, L.; Zhu, P.; Tao, X.; Imanaka, T.; Zhao, J.; Huang, Y.; Tu, Y.; Cao, X. Epidermal Growth Factor-Ferritin H-Chain Protein Nanoparticles for Tumor Active Targeting. Small 2012, 8, 2505–2514. [Google Scholar] [CrossRef]
- Mero, A.; Campisi, M. Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef]
- Liao, Y.H.; Jones, S.A.; Forbes, B.; Martin, G.P.; Brown, M.B. Hyaluronan: Pharmaceutical Characterization and Drug Delivery. Drug Deliv. J. Deliv. Target. Ther. Agents 2005, 12, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.E.; Lin, P.; Cowman, M.K. Self-Association of Hyaluronate Segments in Aqueous NaCl Solution. Arch. Biochem. Biophys. 1988, 265, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Babensee, J.E.; McIntire, L.V.; Mikos, A.G. Growth Factor Delivery for Tissue Engineering. Pharm. Res. 2000, 17, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Mina, M. Growth Factors: Biochemical Signals for Tissue Engineering. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 85–97. ISBN 9780123971579. [Google Scholar]
- Enomoto, H.; Kishima, Y.; Yoshida, K.; Nakamura, H. Hepatoma-Derived Growth Factor: Involvement in Liver Development and Regeneration. In Trends in Gastroenterology and Hepatology; Asakura, H., Aoyagi, Y., Nakazawa, S., Eds.; Springer: Tokyo, Japan, 2001; pp. 302–305. [Google Scholar]
- Ramazani, Y.; Knops, N.; Elmonem, M.A.; Nguyen, T.Q.; Arcolino, F.O.; van den Heuvel, L.; Levtchenko, E.; Kuypers, D.; Goldschmeding, R. Connective Tissue Growth Factor (CTGF) from Basics to Clinics. Matrix Biol. 2018, 68–69, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Medamana, J.; Clark, R.A.; Butler, J. Platelet-Derived Growth Factor in Heart Failure. In Heart Failure. Handbook of Experimental Pharmacology; Bauersachs, J., Butler, J., Sandner, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 355–369. ISBN 978-3-319-29806-1. [Google Scholar]
- Sim, G.C.; Radvanyi, L. The IL-2 Cytokine Family in Cancer Immunotherapy. Cytokine Growth Factor Rev. 2014, 25, 377–390. [Google Scholar] [CrossRef]
- Asiri, A.; Saidin, S.; Sani, M.H.; Al-Ashwal, R.H. Epidermal and Fibroblast Growth Factors Incorporated Polyvinyl Alcohol Electrospun Nanofibers as Biological Dressing Scaffold. Sci. Rep. 2021, 11, 5634. [Google Scholar] [CrossRef]
- Bhutada, S.S.; Sriram, M.; Katti, D.S. Sulfated Carboxymethylcellulose Conjugated Electrospun Fibers as a Growth Factor Presenting System for Tissue Engineering. Carbohydr. Polym. 2021, 268, 118256. [Google Scholar] [CrossRef]
- Miar, S.; Ong, J.L.; Bizios, R.; Guda, T. Electrically Stimulated Tunable Drug Delivery From Polypyrrole-Coated Polyvinylidene Fluoride. Front. Chem. 2021, 9, 599631. [Google Scholar] [CrossRef]
- Benbow, N.L.; Karpiniec, S.; Krasowska, M.; Beattie, D.A. Incorporation of FGF-2 into Pharmaceutical Grade Fucoidan/Chitosan Polyelectrolyte Multilayers. Mar. Drugs 2020, 18, 531. [Google Scholar] [CrossRef]
- Shah, N.J.; Macdonald, M.L.; Beben, Y.M.; Padera, R.F.; Samuel, R.E.; Hammond, P.T. Tunable Dual Growth Factor Delivery from Polyelectrolyte Multilayer Films. Biomaterials 2011, 32, 6183–6193. [Google Scholar] [CrossRef]
- Xue, Y.; Liang, H.; Yang, R.; Deng, K.; Tang, M.; Zhang, M. The Role of Pro- and Mature Neurotrophins in the Depression. Behav. Brain Res. 2021, 404, 113162. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.L.; Comaposada-Baró, R.; Vilar, M. Neurotrophins and Neurotrophin Receptors. In Hormonal Signaling in Biology and Medicine; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 83–106. ISBN 9780128138144. [Google Scholar]
- Machalińska, A.; Kawa, M.; Pius-Sadowska, E.; Stępniewski, J.; Nowak, W.; Rogińska, D.; Kaczyńska, K.; Baumert, B.; Wiszniewska, B.; Józkowicz, A.; et al. Long-Term Neuroprotective Effects of NT-4-Engineered Mesenchymal Stem Cells Injected Intravitreally in a Mouse Model of Acute Retinal Injury. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8292–8305. [Google Scholar] [CrossRef] [PubMed]
- Dąbkowska, M.; Adamczak, M.; Barbasz, J.; Cieśla, M.; Machaliński, B. Adsorption/Desorption Transition of Recombinant Human Neurotrophin 4: Physicochemical Characterization. Langmuir 2017, 33, 9548–9557. [Google Scholar] [CrossRef]
- Houlton, J.; Abumaria, N.; Hinkley, S.F.R.; Clarkson, A.N. Therapeutic Potential of Neurotrophins for Repair after Brain Injury: A Helping Hand from Biomaterials. Front. Genet. 2019, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Aarão, T.L.d.S.; de Sousa, J.R.; Falcão, A.S.C.; Falcão, L.F.M.; Quaresma, J.A.S. Nerve Growth Factor and Pathogenesis of Leprosy: Review and Update. Front. Immunol. 2018, 9, 939. [Google Scholar] [CrossRef]
- Nickl-Jockschat, T.; Michel, T.M. The Role of Neurotrophic Factors in Autism. Mol. Psychiatry 2011, 16, 478–490. [Google Scholar] [CrossRef]
- Bertrand, T.; Kothe, M.; Liu, J.; Dupuy, A.; Rak, A.; Berne, P.F.; Davis, S.; Gladysheva, T.; Valtre, C.; Crenne, J.Y.; et al. The Crystal Structures of TrkA and TrkB Suggest Key Regions for Achieving Selective Inhibition. J. Mol. Biol. 2012, 423, 439–453. [Google Scholar] [CrossRef]
- Skerratt, S.E.; Andrews, M.; Bagal, S.K.; Bilsland, J.; Brown, D.; Bungay, P.J.; Cole, S.; Gibson, K.R.; Jones, R.; Morao, I.; et al. The Discovery of a Potent, Selective, and Peripherally Restricted Pan-Trk Inhibitor (PF-06273340) for the Treatment of Pain. J. Med. Chem. 2016, 59, 10084–10099. [Google Scholar] [CrossRef]
- Vilar, M. Structural Characterization of the P75 Neurotrophin Receptor: A Stranger in the TNFR Superfamily. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 104, pp. 57–87. [Google Scholar]
- Liu, P.; Li, S.; Tang, L. Nerve Growth Factor: A Potential Therapeutic Target for Lung Diseases. Int. J. Mol. Sci. 2021, 22, 9112. [Google Scholar] [CrossRef]
- Wise, B.L.; Seidel, M.F.; Lane, N.E. The Evolution of Nerve Growth Factor Inhibition in Clinical Medicine. Nat. Rev. Rheumatol. 2021, 17, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.S.; Sundrani, D.P.; Joshi, S.R. Neurotrophins: Role in Placental Growth and Development. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 104, pp. 243–261. ISBN 9780128122631. [Google Scholar]
- Pearce, F.L.; Banks, B.E.C.; Banthorpe, D.V.; Berry, A.R.; Davies, H.F.S.; Vernon, C.A. The Isolation and Characterization of Nerve-Growth Factor from the Venom of Vipera Russelli. Eur. J. Biochem. 1972, 29, 417–425. [Google Scholar] [CrossRef]
- Barde, Y.A.; Edgar, D.; Thoenen, H. Purification of a New Neurotrophic Factor from Mammalian Brain. EMBO J. 1982, 1, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Brain-Derived Neurotrophic Factor and Its Clinical Implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Dąbkowska, M.; Łuczkowska, K.; Rogińska, D.; Sobuś, A.; Wasilewska, M.; Ulańczyk, Z.; Machaliński, B. Novel Design of (PEG-ylated ) PAMAM - Based Nanoparticles for Sustained Delivery of BDNF to Neurotoxin-Injured Differentiated Neuroblastoma Cells. J. Nanobiotechnol. 2020, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Travaglia, A.; Magrì, A.; Satriano, C.; La Mendola, D. Adsorption of NGF and BDNF Derived Peptides on Gold Surfaces. Phys. Chem. Chem. Phys. 2014, 16, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Belluscio, L.; Squinto, S.; Ip, N.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. Neurotrophin-3: A Neurotrophic Factor Related to NGF and BDNF. Science 1990, 247, 1446–1451. [Google Scholar] [CrossRef]
- Matatagui, D.; Bastida, Á.; Horrillo, M.C. Novel SH-SAW Biosensors for Ultra-Fast Recognition of Growth Factors. Biosensors 2022, 12, 17. [Google Scholar] [CrossRef]
- Dąbkowska, M.; Rogińska, D.; Kłos, P.; Sobuś, A.; Adamczak, M.; Litwińska, Z.; Machalińska, A.; Machaliński, B. Electrostatic Complex of Neurotrophin 4 with Dendrimer Nanoparticles: Controlled Release of Protein in Vitro and in Vivo. Int. J. Nanomed. 2019, 14, 6117–6131. [Google Scholar] [CrossRef]
- Oral, E.; Kirkan, T.S.; Yildirim, A.; Kotan, Z.; Cansever, Z.; Ozcan, H.; Aliyev, E.; Gulec, M. Serum Brain-Derived Neurotrophic Factor Differences between the Luteal and Follicular Phases in Premenstrual Dysphoric Disorder. Gen. Hosp. Psychiatry 2015, 37, 266–272. [Google Scholar] [CrossRef]
- Mizui, T.; Hattori, K.; Ishiwata, S.; Hidese, S.; Yoshida, S.; Kunugi, H.; Kojima, M. Cerebrospinal Fluid BDNF Pro-Peptide Levels in Major Depressive Disorder and Schizophrenia. J. Psychiatr. Res. 2019, 113, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, W.W.; Jiang, Z.; Feng, M.J. Advances in Treatment of Neurodegenerative Diseases: Perspectives for Combination of Stem Cells with Neurotrophic Factors. World J. Stem Cells 2020, 12, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Kotzbauer, P.T.; Holtzman, D.M. Expectations and Challenges in the Therapeutic Use of Neurotrophic Factors. Ann. Neurol. 2006, 59, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Curran, G.L. Permeability at the Blood-Brain and Blood-Nerve Barriers of the Neurotrophic Factors: NGF, CNTF, NT-3, BDNF. Mol. Brain Res. 1996, 36, 280–286. [Google Scholar] [CrossRef]
- Nutt, J.G.; Burchiel, K.J.; Comella, C.L.; Jankovic, J.; Lang, A.E.; Laws, E.R.J.; Lozano, A.; Penn, R.; Simpson, R.K.J.; Stacy, M.; et al. Randomized, Double-Blind Trial of Glial Cell Line-Derived Neurotrophic Factor (GDNF) in PD. Neurology 2003, 60, 69–73. [Google Scholar] [CrossRef]
- Oliveira, N.K.; Ferreira, R.N.; Nogueira, S.D.; Chiari, E.; Ribeiro, E.; Massara, P. Cardiac Autonomic Denervation and Expression of Neurotrophins (NGF and BDNF) and Their Receptors during Experimental Chagas Disease. Growth Factors 2017, 35, 161–170. [Google Scholar] [CrossRef]
- Sacchetti, M.; Lambiase, A.; Schmidl, D.; Schmetterer, L.; Ferrari, M.; Mantelli, F.; Allegretti, M.; Garhoefer, G. Effect of Recombinant Human Nerve Growth Factor Eye Drops in Patients with Dry Eye: A Phase IIa, Open Label, Multiple-Dose Study. Br. J. Ophthalmol. 2020, 104, 127–135. [Google Scholar] [CrossRef]
- Schäbitz, W.R.; Steigleder, T.; Cooper-Kuhn, C.M.; Schwab, S.; Sommer, C.; Schneider, A.; Kuhn, H.G. Intravenous Brain-Derived Neurotrophic Factor Enhances Poststroke Sensorimotor Recovery and Stimulates Neurogenesis. Stroke 2007, 38, 2165–2172. [Google Scholar] [CrossRef]
- Shibata, S.B.; Cortez, S.R.; Beyer, L.A.; Wiler, J.A.; Di Polo, A.; Pfingst, B.E.; Raphael, Y. Transgenic BDNF Induces Nerve Fiber Regrowth into the Auditory Epithelium in Deaf Cochleae. Exp. Neurol. 2010, 223, 464–472. [Google Scholar] [CrossRef]
- Stefani, A.; Pierantozzi, M.; Cardarelli, S.; Stefani, L.; Cerroni, R.; Conti, M.; Garasto, E.; Mercuri, N.B.; Marini, C.; Sucapane, P. Neurotrophins as Therapeutic Agents for Parkinson’s Disease; New Chances From Focused Ultrasound? Front. Neurosci. 2022, 16, 846681. [Google Scholar] [CrossRef]
- Taylor, S.J.; McDonald, J.W.; Sakiyama-Elbert, S.E. Controlled Release of Neurotrophin-3 from Fibrin Gels for Spinal Cord Injury. J. Control. Release 2004, 98, 281–294. [Google Scholar] [CrossRef]
- Itoh, N.; Ornitz, D.M. Fibroblast Growth Factors: From Molecular Evolution to Roles in Development, Metabolism and Disease. J. Biochem. 2011, 149, 121–130. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor Signaling Pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Dostálová, I.; Kaválková, P.; Haluzíková, D.; Lacinová, Z.; Mráz, M.; Papežová, H.; Haluzík, M. Plasma Concentrations of Fibroblast Growth Factors 19 and 21 in Patients with Anorexia Nervosa. J. Clin. Endocrinol. Metab. 2008, 93, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Tang, S.; Yuan, Y.; Liu, R.; Chen, Q. Roles of FGF8 Subfamily in Embryogenesis and Oral-maxillofacial Diseases (Review). Int. J. Oncol. 2019, 54, 797–806. [Google Scholar] [CrossRef]
- Watson, J.; Francavilla, C. Regulation of FGF10 Signaling in Development and Disease. Front. Genet. 2018, 9, 500. [Google Scholar] [CrossRef]
- Unger, E.F.; Goncalves, L.; Epstein, S.E.; Chew, E.Y.; Trapnell, C.B.; Cannon, R.O.; Quyyumi, A.A.; Loscalzo, F.; Stiber, J.A. Effects of a Single Intracoronary Injection of Basic Fibroblast Growth Factor in Stable Angina Pectoris. Am. J. Cardiol. 2000, 85, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Min, D.; Guo, G.; Liao, X.; Fu, Z. Experimental Study of Epidermal Growth Factor and Acidic Fibroblast Growth Factor in the Treatment of Diabetic Foot Wounds. Exp. Ther. Med. 2018, 15, 5365–5370. [Google Scholar] [CrossRef] [PubMed]
- Giralt, M.; Gavaldà-Navarro, A.; Villarroya, F. Fibroblast Growth Factor-21, Energy Balance and Obesity. Mol. Cell. Endocrinol. 2015, 418, 66–73. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Sadeghnia, H.R.; Hoseini, S.J.; Ghorbani, A.; Ghazavi, H.; Ghasemi, F.; Hosseinzadeh, H. Fibroblast Growth Factor 1 Gene-Transfected Adipose-Derived Mesenchymal Stem Cells Modulate Apoptosis and Inflammation in the Chronic Constriction Injury Model of Neuropathic Pain. Iran. J. Pharm. Res. 2020, 19, 151–159. [Google Scholar] [CrossRef]
- Chen, G.J.; Forough, R. Fibroblast Growth Factors, Fibroblast Growth Factor Receptors, Diseases, and Drugs. Recent Pat. Cardiovasc. Drug Discov. 2006, 1, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, T.J.; Hoppert, T.; Schneider, A.; Gemeinhardt, S.; Köcher, M.; Ibing, R.; Strupp, G. Induktion Der Myokardialen Neoangiogenese Durch Humane Wachstumsfaktoren (Induction of Myocardial Neoangiogenesis by Human Growth Factors. A New Therapeutic Option in Coronary Heart Disease). Herz 2000, 25, 589–599. [Google Scholar] [CrossRef]
- Huang, H.W.; Yang, C.M.; Yang, C.H. Fibroblast Growth Factor Type 1 Ameliorates High-glucose-induced Oxidative Stress and Neuroinflammation in Retinal Pigment Epithelial Cells and a Streptozotocin-induced Diabetic Rat Model. Int. J. Mol. Sci. 2021, 22, 7233. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.C.; Wang, H.Y.; Li, V.L.; Chen, Y.C.; Wang, A.N.; Wu, C.J.; Li, Y.; Zhao, G.; Lin, C.; et al. Polyelectrolyte Multilayer Composite Coating on 316 L Stainless Steel for Controlled Release of Dual Growth Factors Accelerating Restoration of Bone Defects. Mater. Sci. Eng. C 2021, 126, 112187. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Koh, W.G.; Lee, H.J. Effects of Basic Fibroblast Growth Factor Combined with an Injectable in Situ Crosslinked Hyaluronic Acid Hydrogel for a Dermal Filler. React. Funct. Polym. 2021, 164, 104933. [Google Scholar] [CrossRef]
- Hu, C.; Ayan, B.; Chiang, G.; Chan, A.H.P.; Rando, T.A.; Huang, N.F. Comparative Effects of Basic Fibroblast Growth Factor Delivery or Voluntary Exercise on Muscle Regeneration after Volumetric Muscle Loss. Bioengineering 2022, 9, 37. [Google Scholar] [CrossRef]
- Liu, K.; Lv, Z.; Huang, H.; Yu, S.; Xiao, L.; Li, X.; Li, G.; Liu, F. FGF3 from the Hypothalamus Regulates the Guidance of Thalamocortical Axons. Dev. Neurosci. 2021, 42, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Grines, C.L.; Watkins, M.W.; Dib, N.; Barbeau, G.; Moreadith, R.; Andrasfay, T.; Engler, R.L. Effects of Ad5FGF-4 in Patients With Angina. J. Am. Coll. Cardiol. 2007, 50, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Jung, N.; Kim, N.; Ha, J.C.; Park, J.H.; Han, K.; Chang, M.; Lee, J.; Kim, C.H. Effect of Cysteine-Free Human Fibroblast Growth Factor-5s Mutant (FGF5sC93S) on Hair Growth. Dermatol. Ther. 2020, 33, e14530. [Google Scholar] [CrossRef]
- Floss, T.; Arnold, H.; Braun, T. A Role for FGF-6 in Skeletal Muscle Regeneration. Genes Dev. 1997, 11, 2040–2051. [Google Scholar] [CrossRef]
- Xu, B.; Liu, C.; Zhang, H.; Zhang, R.; Tang, M.; Huang, Y.; Jin, L.; Xu, L.; Hu, C.; Jia, W. Skeletal Muscle–Targeted Delivery of Fgf6 Protects Mice from Diet-Induced Obesity and Insulin Resistance. JCI Insight 2021, 6, e149969. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.M.; Wang, W.P. Expression and Function of Fibroblast Growth Factor (FGF) 7 during Liver Regeneration. Cell. Physiol. Biochem. 2011, 27, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yoon, S.J.; Lee, D.H.; Pyun, Y.C.; Kim, W.Y.; Lee, J.H.; Khang, G.; Chun, H.J.; Yang, D.H. Preparation of Foam Dressings Based on Gelatin, Hyaluronic Acid, and Carboxymethyl Chitosan Containing Fibroblast Growth Factor-7 for Dermal Regeneration. Polymers 2021, 13, 3279. [Google Scholar] [CrossRef]
- Tsikandelova, R.; Mladenov, P.; Planchon, S.; Kalenderova, S.; Praskova, M.; Mihaylova, Z.; Stanimirov, P.; Mitev, V.; Renaut, J.; Ishkitiev, N. Proteome Response of Dental Pulp Cells to Exogenous FGF8. J. Proteom. 2018, 183, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, I.O.; Chen, H.M.; Cheng, P.H.; Chang, C.Y.; Tsai, S.J.; Chuang, J.I.; Wu, C.C.; Huang, B.M.; Sun, H.S.; Chen, C.M.; et al. FGF9 Induces Neurite Outgrowth upon ERK Signaling in Knock-in Striatal Huntington’s Disease Cells. Life Sci. 2021, 267, 118952. [Google Scholar] [CrossRef]
- Itoh, N. FGF10: A Multifunctional Mesenchymal-Epithelial Signaling Growth Factor in Development, Health, and Disease. Cytokine Growth Factor Rev. 2016, 28, 63–69. [Google Scholar] [CrossRef]
- Yeo, Y.; Yi, E.S.; Kim, J.; Jo, E.; Seo, S.; Kim, R.; Kim, K.L.; Sung, J.; Park, S.G.; Suh, W. Smooth Muscle Cell Remodeling in Pulmonary Arterial Hypertension. Hypertension 2020, 76, 1778–1786. [Google Scholar] [CrossRef]
- Yu, H.; Wang, H.; Qie, A.; Wang, J.; Liu, Y.; Gu, G.; Yang, J.; Zhang, H.; Pan, W.; Tian, Z.; et al. FGF13 Enhances Resistance to Platinum Drugs by Regulating HCTR1 and ATP7A via a Microtubule-Stabilizing Effect. Cancer Sci. 2021, 112, 4655–4668. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, J.; Wang, H.; Shan, B.; Yin, C.; Yu, H.; Zhang, X.; Dong, Z.; Yu, Y.; Zhao, R.; et al. Fibroblast Growth Factor 13 Stabilizes Microtubules to Promote Na+ Channel Function in Nociceptive DRG Neurons and Modulates Inflammatory Pain. J. Adv. Res. 2021, 31, 97–111. [Google Scholar] [CrossRef]
- Bosch, M.K.; Kanakamedala, A. Intracellular FGF14 (IFGF14) Is Required for Spontaneous and Evoked Firing in Cerebellar Purkinje Neurons and for Motor Coordination and Balance. J. Neurosci. 2015, 35, 6752–6769. [Google Scholar] [CrossRef]
- Yu, W.; Huang, X.; Tian, X.; Zhang, H.; He, L.; Wang, Y.; Nie, Y.; Hu, S.; Lin, Z.; Zhou, B.; et al. GATA4 Regulates Fgf16 to Promote Heart Repair after Injury. Development 2016, 143, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Sontag, D.P.; Wang, J.; Kardami, E.; Cattini, P.A. FGF-2 and FGF-16 Protect Isolated Perfused Mouse Hearts from Acute Doxorubicin-Induced Contractile Dysfunction. Cardiovasc. Toxicol. 2013, 13, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Scearce-Levie, K.; Roberson, E.D.; Gerstein, H.; Cholfin, J.A.; Mandiyan, V.S.; Shah, N.M.; Rubenstein, J.L.R.; Mucke, L. Abnormal Social Behaviors in Mice Lacking Fgf17. Genes Brain Behav. 2008, 7, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Mok, S.C.; Oliva, E.; Kim, S.; Mohapatra, G.; Birrer, M.J. FGF18 as a Prognostic and Therapeutic Biomarker in Ovarian Cancer. J. Clin. Investig. 2013, 123, 4435–4448. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Kanzaki, H.; Chiba, T.; Ao, J.; Kanayama, K.; Maruta, S.; Kusakabe, Y.; Saito, T.; Kobayashi, K.; Kiyono, S.; et al. Serum Fibroblast Growth Factor 19 Serves as a Potential Novel Biomarker for Hepatocellular Carcinoma. BMC Cancer 2019, 19, 1088. [Google Scholar] [CrossRef]
- Benoit, B.; Meugnier, E.; Castelli, M.; Chanon, S.; Vieille-Marchiset, A.; Durand, C.; Bendridi, N.; Pesenti, S.; Monternier, P.A.; Durieux, A.C.; et al. Fibroblast Growth Factor 19 Regulates Skeletal Muscle Mass and Ameliorates Muscle Wasting in Mice. Nat. Med. 2017, 23, 990–996. [Google Scholar] [CrossRef]
- Niu, J.; Xie, J.; Guo, K.; Zhang, X.; Xia, F.; Zhao, X.; Song, L.; Zhuge, D.; Li, X.; Zhao, Y.; et al. Efficient Treatment of Parkinson’s Disease Using Ultrasonography-Guided Rhfgf20 Proteoliposomes. Drug Deliv. 2018, 25, 1560–1569. [Google Scholar] [CrossRef]
- Woo, Y.C.; Xu, A.; Wang, Y.; Lam, K.S.L. Fibroblast Growth Factor 21 as an Emerging Metabolic Regulator: Clinical Perspectives. Clin. Endocrinol. 2013, 78, 489–496. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Bae, I.H.; Kim, M.; Huh, Y.; Choi, J.; Bae, S.; Choi, I.Y.; Kim, H.H.; Kim, D.K. Preparation, Characterization, and Pharmacological Study of a Novel Long-Acting FGF21 with a Potential Therapeutic Effect in Obesity. Biologicals 2021, 69, 49–58. [Google Scholar] [CrossRef]
- Zhu, S.; Ying, Y.; Wu, Q.; Ni, Z.; Huang, Z.; Cai, P.; Tu, Y.; Ying, W.; Ye, J.; Zhang, R.; et al. Alginate Self-Adhesive Hydrogel Combined with Dental Pulp Stem Cells and FGF21 Repairs Hemisection Spinal Cord Injury via Apoptosis and Autophagy Mechanisms. Chem. Eng. J. 2021, 426, 130827. [Google Scholar] [CrossRef]
- Li, S.; Jia, H.; Liu, Z.; Wang, N.; Guo, X.; Cao, M.; Fang, F.; Yang, J.; Li, J.; He, Q.; et al. Fibroblast Growth Factor-21 as a Novel Metabolic Factor for Regulating Thrombotic Homeostasis. Sci. Rep. 2022, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Yu, M.; Wei, H.; Yao, S.; Chen, S.Y.; Zhu, X.L.; Li, Y.F. Fibroblast Growth Factor 22 Is a Novel Modulator of Depression through Interleukin-1β. CNS Neurosci. Ther. 2017, 23, 907–916. [Google Scholar] [CrossRef]
- Stanczyk, M.; Chrul, S.; Wyka, K.; Tkaczyk, M. Serum Intact Fibroblast Growth Factor 23 in Healthy Paediatric Population. Open Med. 2021, 16, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Kourtidou, C.; Stangou, M.; Marinaki, S.; Tziomalos, K. Novel Cardiovascular Risk Factors in Patients with Diabetic Kidney Disease. Int. J. Mol. Sci. 2021, 22, 11196. [Google Scholar] [CrossRef]
- Ferreira, I.G.; Pucca, M.B.; Oliveira, I.S.d.; Cerni, F.A.; Jacob, B.d.C.d.S.; Arantes, E.C. Snake Venom Vascular Endothelial Growth Factors (SvVEGFs): Unravelling Their Molecular Structure, Functions, and Research Potential. Cytokine Growth Factor Rev. 2021, 60, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Merler, E.; Abernathy, C.; Williams, G. Isolation of a Tumor Factor Responsible for Angiogenesis. J. Exp. Med. 1971, 133, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Harris, R.; Miners, J.S.; Allen, S.; Love, S. VEGFR1 and VEGFR2 in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 741–752. [Google Scholar] [CrossRef]
- Collén, A.; Bergenhem, N.; Carlsson, L.; Chien, K.R.; Hoge, S.; Gan, L.M.; Fritsche-Danielson, R. VEGFA MRNA for Regenerative Treatment of Heart Failure. Nat. Rev. Drug Discov. 2022, 21, 79–80. [Google Scholar] [CrossRef]
- Blann, A.D.; Brown, J.E.; Heitmar, R. Angiogenesis, Metabolism, Endothelial and Platelet Markers in Diabetes and Cardiovascular Disease. Br. J. Biomed. Sci. 2022, 79, 10313. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Cui, H.; Shi, J.; Yuan, G.; Shi, S.; Hu, Y. The Role of the VEGF Family in Coronary Heart Disease. Front. Cardiovasc. Med. 2021, 8, 738325. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Gadila, S.K.G. Neuropathogenicity of Non-Viable Borrelia Burgdorferi Ex Vivo. Sci. Rep. 2022, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.M.; Tran, N.T.; Patel, S.S.; Hou, J.; Jhaveri, K.D.; Parikh, R.; Selamet, U.; Ghobry, L.; Wassef, O.; Barsoum, M.; et al. Thrombotic Microangiopathy and Acute Kidney Injury Induced After Intravitreal Injection of Vascular Endothelial Growth Factor Inhibitors VEGF Blockade-Related TMA After Intravitreal Use. Front. Med. 2020, 7, 579603. [Google Scholar] [CrossRef] [PubMed]
- Daien, V.; Eldem, B.M.; Talks, J.S.; Korobelnik, J.F.; Mitchell, P.; Finger, R.P.; Sakamoto, T.; Wong, T.Y.; Evuarherhe, O.; Carter, G.; et al. Real-World Data in Retinal Diseases Treated with Anti-Vascular Endothelial Growth Factor (Anti-VEGF) Therapy—A Systematic Approach to Identify and Characterize Data Sources. BMC Ophthalmol. 2019, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Koenig, G.; Charpiot, A.; Debry, C.; Voegel, J.C.; Lavalle, P.; Vautier, D. VEGF-Functionalized Polyelectrolyte Multilayers as Proangiogenic Prosthetic Coatings. Adv. Funct. Mater. 2008, 18, 1767–1775. [Google Scholar] [CrossRef]
- Vodouhê,, C.; Schmittbuhl, M.; Boulmedais, F.; Bagnard, D.; Vautier, D.; Schaaf, P.; Egles, C.; Voegel, J.C.; Ogier, J. Effect of Functionalization of Multilayered Polyelectrolyte Films on Motoneuron Growth. Biomater. 2005, 26, 545–554. [Google Scholar] [CrossRef]
- Zhang, C.-B.; Cao, H.-L.; Qian, L.; Tu, J.; Guo, X.; Liu, Z.; Dong, Z. Enhancement Effect of Ultrasound-Induced Microbubble Cavitation on Branched Polyethylenimine-Mediated VEGF(165) Transfection with Varied N/P Ratio. Ultrasound Med. Biol. 2013, 39, 161–171. [Google Scholar] [CrossRef]
- Won, Y.W.; McGinn, A.N.; Lee, M.; Nam, K.; Bull, D.A.; Kim, S.W. Post-Translational Regulation of a Hypoxia-Responsive VEGF Plasmid for the Treatment of Myocardial Ischemia. Biomaterials 2013, 34, 6229–6238. [Google Scholar] [CrossRef]
- Huang, M.; Vitharana, S.N.; Peek, L.J.; Coop, T.; Berkland, C. Polyelectrolyte Complexes Stabilize and Controllably Release Vascular Endothelial Growth Factor. Biomacromolecules 2007, 8, 1607–1614. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.; Kim, Y.J.; Lee, E.; Choi, J.S. Gene Delivery of PAMAM Dendrimer Conjugated with the Nuclear Localization Signal Peptide Originated from Fibroblast Growth Factor 3. Int. J. Pharm. 2014, 459, 10–18. [Google Scholar] [CrossRef]
- Emamian, M.; Abbaspour, A.; Shahani, T.; Biglari, A.; Sharafi, A. Non-Viral Suicide Gene Therapy: Cytosine Deaminase Gene Directed by VEGF Promoter and 5-Fluorocytosine as a Gene Directed Enzyme/Prodrug System in Breast Cancer Model. Drug Res. 2021, 71, 395–406. [Google Scholar] [CrossRef]
- Backer, M.V.; Gaynutdinov, T.I.; Patel, V.; Bandyopadhyaya, A.K.; Thirumamagal, B.T.S.; Tjarks, W.; Barth, R.F.; Claffey, K.; Backer, J.M. Vascular Endothelial Growth Factor Selectively Targets Boronated Dendrimers to Tumor Vasculature. Mol. Cancer Ther. 2005, 4, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Han, U.; Park, H.H.; Kim, Y.J.; Park, T.H.; Park, J.H.; Hong, J. Efficient Encapsulation and Sustained Release of Basic Fibroblast Growth Factor in Nanofilm: Extension of the Feeding Cycle of Human Induced Pluripotent Stem Cell Culture. ACS Appl. Mater. Interfaces 2017, 9, 25087–25097. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and Chitosan in Selected Biomedical Applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Mi, F.L.; Sung, H.W.; Kuo, P.L. Heparin-Functionalized Chitosan–Alginate Scaffolds for Controlled Release of Growth Factor. Int. J. Pharm. 2009, 376, 69–75. [Google Scholar] [CrossRef]
- Shi, S.; Cheng, X.; Wang, J.; Zhang, W.; Peng, L.; Zhang, Y. RhBMP-2 Microspheres-Loaded Chitosan/Collagen Scaffold Enhanced Osseointegration: An Experiment in Dog. J. Biomater. Appl. 2009, 23, 331–346. [Google Scholar] [CrossRef] [PubMed]
- De la Riva, B.; Sánchez, E.; Hernández, A.; Reyes, R.; Tamimi, F.; López-Cabarcos, E.; Delgado, A.; vora, C. Local Controlled Release of VEGF and PDGF from a Combined Brushite–Chitosan System Enhances Bone Regeneration. J. Control. Release 2010, 143, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef]
- Mao, Z.; Ma, L.; Zhou, J.; Gao, C.; Shen, J. Bioactive Thin Film of Acidic Fibroblast Growth Factor Fabricated by Layer-by-Layer Assembly. Bioconjug. Chem. 2005, 16, 1316–1322. [Google Scholar] [CrossRef]
- Kumorek, M.; Kubies, D.; Riedel, T. Protein Interactions with Quaternized Chitosan/Heparin Multilayers. Physiol. Res. 2016, 65, S253–S261. [Google Scholar] [CrossRef] [PubMed]
- Kumorek, M.; Kubies, D.; Filová, E.; Houska, M.; Kasoju, N.; Chánová, E.M.; Matějka, R.; Krýslová, M.; Bačáková, L.; Rypáček, F. Cellular Responses Modulated by FGF-2 Adsorbed on Albumin/Heparin Layer-by-Layer Assemblies. PLoS ONE 2015, 10, e0125484. [Google Scholar] [CrossRef] [PubMed]
- Almodóvar, J.; Bacon, S.; Gogolski, J.; Kisiday, J.D.; Kipper, M.J. Polysaccharide-Based Polyelectrolyte Multilayer Surface Coatings Can Enhance Mesenchymal Stem Cell Response to Adsorbed Growth Factors. Biomacromolecules 2010, 11, 2629–2639. [Google Scholar] [CrossRef]
- De Cock, L.J.; De Koker, S.; De Vos, F.; Vervaet, C.; Remon, J.P.; De Geest, B.G. Layer-by-Layer Incorporation of Growth Factors in Decellularized Aortic Heart Valve Leaflets. Biomacromolecules 2010, 11, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, A.M.; Frederik, C.; John, C. Biomimetic Surface Delivery of NGF and BDNF to Enhance Neurite Outgrowth. Biotechnol. Bioeng. 2020, 117, 3124–3135. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama-Elbert, S.E.; Hubbell, J.A. Controlled Release of Nerve Growth Factor from a Heparin-Containing Fibrin-Based Cell Ingrowth Matrix. J. Control. Release 2000, 69, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Tezcaner, A.; Hicks, D.; Boulmedais, F.; Sahel, J.; Schaaf, P.; Voegel, J.C.; Lavalle, P. Polyelectrolyte Multilayer Films as Substrates for Photoreceptor Cells. Biomacromolecules 2006, 7, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Y.; Zou, Y.; Huang, W.; Zhu, L.; Liu, F.; Wang, D.; Guo, K.; Hu, J.; Chen, J.; et al. Heparin-Poloxamer Hydrogel-Encapsulated RhFGF21 Enhances Wound Healing in Diabetic Mice. FASEB J. 2019, 33, 9858–9870. [Google Scholar] [CrossRef]
- Ishihara, M.; Obara, K.; Ishizuka, T.; Fujita, M.; Sato, M.; Masuoka, K.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Controlled Release of Fibroblast Growth Factors and Heparin from Photocrosslinked Chitosan Hydrogels and Subsequent Effect on in Vivo Vascularization. J. Biomed. Mater. Res. A 2003, 64, 551–559. [Google Scholar] [CrossRef]
- Obara, K.; Ishihara, M.; Ishizuka, T.; Fujita, M. Photocrosslinkable Chitosan Hydrogel Containing Fibroblast Growth Factor-2 Stimulates Wound Healing in Healing-Impaired Db / Db Mice. Biomaterials 2003, 24, 3437–3444. [Google Scholar] [CrossRef]
- Freudenberg, U.; Zieris, A.; Chwalek, K.; Tsurkan, M.V.; Maitz, M.F.; Atallah, P.; Levental, K.R.; Eming, S.A.; Werner, C. Heparin Desulfation Modulates VEGF Release and Angiogenesis in Diabetic Wounds. J. Control. Release 2015, 220, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Liu, Y.; Shu, X.Z.; Prestwich, G.D. Injectable Glycosaminoglycan Hydrogels for Controlled Release of Human Basic Fibroblast Growth Factor. Biomaterials 2005, 26, 6054–6067. [Google Scholar] [CrossRef] [PubMed]
- Ding, I.; Peterson, A.M. Half-Life Modeling of Basic Fibroblast Growth Factor Released from Growth Factor-Eluting Polyelectrolyte Multilayers. Sci. Rep. 2021, 11, 9808. [Google Scholar] [CrossRef]
- Jha, A.K.; Mathur, A.; Svedlund, F.L.; Ye, J.; Yeghiazarians, Y.; Healy, K.E. Molecular Weight and Concentration of Heparin in Hyaluronic Acid-Based Matrices Modulates Growth Factor Retention Kinetics and Stem Cell Fate. J. Control. Release 2015, 209, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Parajó, Y.; D’Angelo, I.; Welle, A.; Garcia-Fuentes, M.; Alonso, M.J. Hyaluronic Acid/Chitosan Nanoparticles as Delivery Vehicles for VEGF and PDGF-BB. Drug Deliv. 2010, 17, 596–604. [Google Scholar] [CrossRef]
- O’Dwyer, J.; Murphy, R.; González-Vázquez, A.; Kovarova, L.; Pravda, M.; Velebny, V.; Heise, A.; Duffy, G.P.; Cryan, S.A. Translational Studies on the Potential of a Vegf Nanoparticle-Loaded Hyaluronic Acid Hydrogel. Pharmaceutics 2021, 13, 779. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.S.; Ng, C.K.; Thompson, A.Y.; Poser, J.W.; Spiro, R.C. Hyaluronate-Heparin Conjugate Gels for the Delivery of Basic Fibroblast Growth Factor (FGF-2). J. Biomed. Mater. Res. 2002, 62, 128–135. [Google Scholar] [CrossRef]
- Ehsanipour, A.; Sathialingam, M.; Rad, L.M.; de Rutte, J.; Bierman, R.D.; Liang, J.; Xiao, W.; Carlo, D.D.; Seidlits, S.K. Injectable, Macroporous Scaffolds for Delivery of Therapeutic Genes to the Injured Spinal Cord Injectable, Macroporous Scaffolds for Delivery of Therapeutic Genes to the Injured Spinal Cord. APL Bioeng. 2021, 5, 016104. [Google Scholar] [CrossRef]

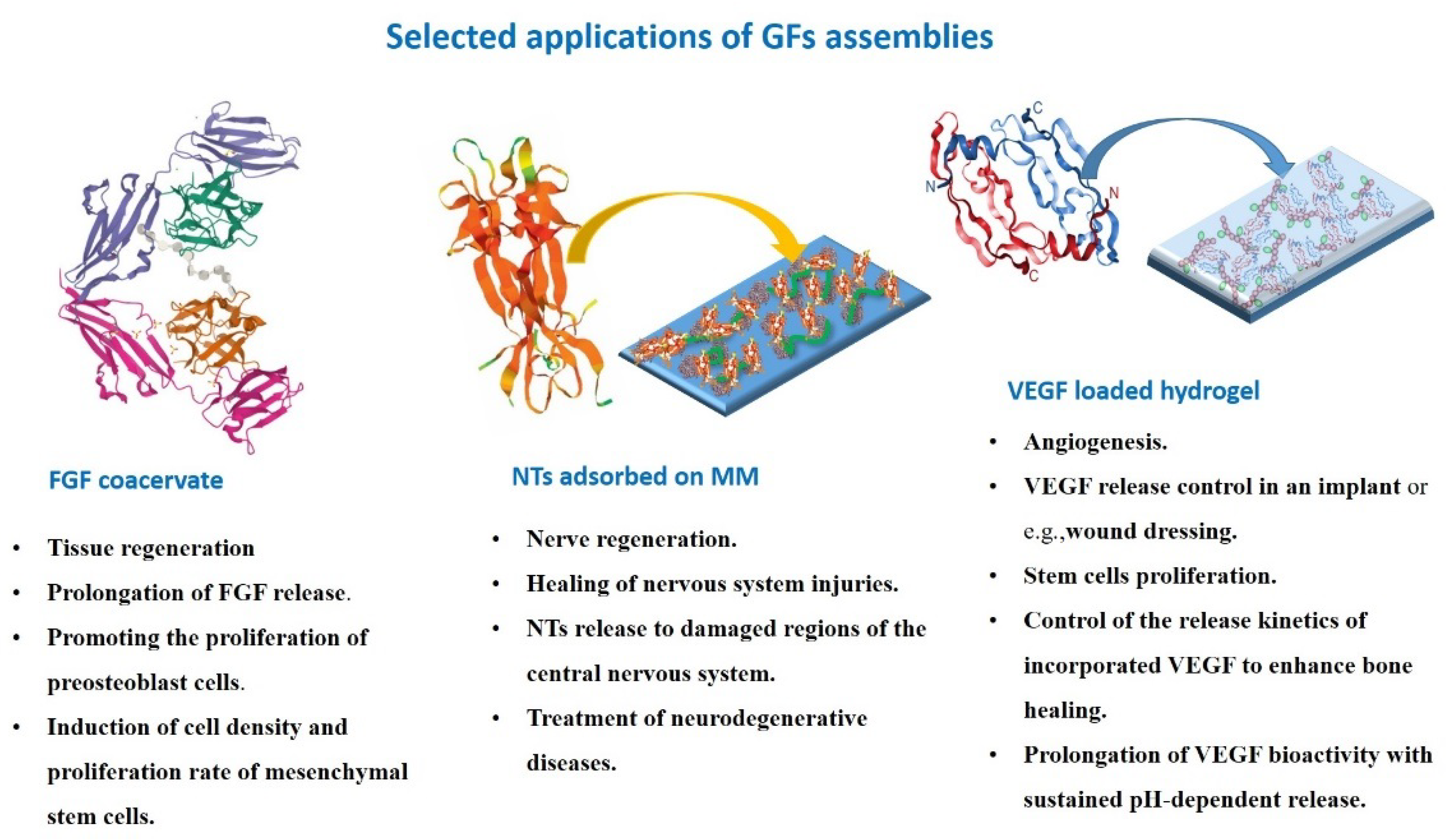
| Synthetic Macroions | Polysaccharides | |
|---|---|---|
| Advantages | Defined size, shape, charge density, structure, mechanical properties [43,44,45], High purity, quality, reproducibility, high mechanical stability [4,45] Biocompatibility [45] Low polydispersity index [43] High solubility in water [46] Well-solubility in solvents of various polarity [47] Broad working range of pH and ionic strength [4,48,49,50] Low production cost [4,45] Non-biodegradable and toxic [4,45] Complicated synthesis, high production cost [45] Limited bioactivity [4] Possible harmful degradation products [4,45] | Natural macroions (biomimetism) [4,9,51] Easy accessibility, easy processing [9,45,51] Excellent biocompatibility, biodegradability, low toxicity, non-immunogenicity [4,9,51,52] Interactions with biomolecules [4,13,21,22] Hydrogel formation [4] Broad or mixed molecular mass [51,52] Low charge density and poor solubility in most organic solvents [4,51,52] High polydispersity index [4], Limited working range of pH and ionic strength [4,53] Various solubilities in water [54] Poor batch-to-batch reproducibility, degradation tendency, potential antigenicity [45] Complicated extraction, high production cost [45] |
| Limitations |
| Focus and Major Findings |
|---|
| Review on physicochemical properties of polysaccharide-based films [4] Effective functionalization and construction of carbohydrate nanocarriers for biomedical applications [9] Enhancement of FGF2 activity by incorporation of heparin and ChS into the macroion film [12] Enhancement of the proliferation and migration of skin-related cells induced by administration of GFs via coacervates [21] Effective chronic wound treatment by using the novel delivery system based on gelatin/sodium alginate/epidermal growth factor (EGF) assemblies [22] Review on molecular-level control of size, shape, surface chemistry, topology, and flexibility of starburst dendrimers [43] Review on physicochemical properties of branched and linear poly(ethylene imine)-based conjugates and their applications in gene delivery [44] A book on the role of macroions in the drug delivery system [45] Review on the role of polysaccharides [51] and synthetic polypeptides [55] in functional drug delivery Review on strategies to modify polysaccharides for the development of polysaccharide-based biomaterials [52] Review on kinetics and mechanism of PDADMAC and its copolymers syntheses, chemical structures, behavior in solution, molecular characterization, and interactions in solution and at interfaces [46] Solvent quality for the hydrophobic macroions in aqueous solutions can be improved by adding to water a miscible organic good solvent [47] Streaming potential measurements are useful for determining adsorption mechanisms and physicochemical characteristics of MM on solid surfaces, especially their stability [48] PDADMAC and poly-L-lysine (PLL) monolayers are more stable than PAH layer [49] PAMAM dendrimer layers can be exploited for studies of their acid-base properties [50] LBL technique can be used to tune the structure of heparin/CS films [53] Polysaccharides are more soluble than purely homogeneous macroions. Ionized linear polysaccharides easily form gels [54] |
| Macroion | Physicochemical Properties | Biological Properties | |
|---|---|---|---|
| Synthetic macroions | PDADMAC | strongly positively charged hydrophilic macrocation [70] | water treatment [46] |
| effective ionization degree: 13–8% [70] | dental material component [69] | ||
| linear shape [70] | DP gelator formation [65] | ||
| “side-on” adsorption (low ionic strength) [70] | ‘‘anchor layer’’ formation [66] | ||
| formation of loops and tails (high ionic strength) [73] | |||
| PAH | weak polybase, high-pH-dependent charge, prolate spheroid (moderate ionic strength), semicircle (high ionic strength) [75] | growth factors carriers [80] | |
| “side-on” adsorption [75] | bioimaging, drug carrier [18,81,82] | ||
| reversible swelling/shirking of layer [76] | enhancement of adhesion of proteins and cells [76] | ||
| PAH derivatives | strongly charged macroions [83] | gene delivery carriers [83] | |
| controlled drug release [84] | |||
| photoreactive growth factor formation [85] | |||
| PAEs | Thermoresponsive [87] | gene/drug carrier [89,90] | |
| Polydisperse [86] | tissue engineering [86] | ||
| bPEI | weak polybase with primary, secondary and tertiary amino groups [98] | plasmid DNA vector [103] | |
| spherical shape in solution, flatten after adsorption [101] | growth factor carrier [104] | ||
| PAMAM dendrimers | monodisperse, nano-sized, radially symmetric, pH-dependent charge [106] | genes/drugs nanocarriers [106,109,110] | |
| easy modification of terminal functional groups [107] | lung diseases treatment [110] | ||
| molecules flatten after adsorption [50,121] | amyloidogenesis disturbing [127] | ||
| pH dependent conformation changes [122] | treatment skin wounds and infection treatment [128,129] | ||
| swelling/shrinking behavior [115,118,119,123] | drug solubility enhancement [137] | ||
| PAA | weak, linear polyacid [144] | growth factor [155] | |
| flexible rod (high ionization degree) and sphere (low ionization degree) formation [145] | drug carriers [152] | ||
| “side-on” adsorption (low molecular mass); formation of loops and tails (higher molecular mass) [147] | |||
| ionic strength and molecular mass layer thickness dependency [150,151] | |||
| Natural macroions (polysaccharides) | CS | positively charged, well soluble in an acidic medium; poorly charged, insoluble at high pH [165] | drugs/growth factors/stem cells/peptides carriers [160] |
| formation of rods, random and stiff coils in bulk [168] | surface-induced thrombosis and blood coagulation enhancement [173] | ||
| “side-on” adsorption, formation rigid and thin layers (low pH) and thick layers and gels (neutral pH) [169,170] | tissue growth [174] | ||
| wound healing supporting [185] | |||
| HA | highly hydrophilic polyanion, loose hydrated network formation [236] | drug/gene carriers [237] | |
| stiff coil in aqueous solution [238] | cytokines/chemokines/growth factors production stimulation [25] | ||
| drug carriers [184] | |||
| anticancer therapy [183] | |||
| Heparin | highly negatively charged, high polydispersity [186] | involved in cell adhesion, migration, proliferation differentiation, effective anticoagulant and anti-inflammatory agent [187] | |
| elongated shape [186,195] | lipid transport, clearance, wound healing [186] | ||
| acceleration or inhibition of protein adsorption depending on protein charge [76,190] | FGFs and VEGF binding [186] | ||
| angiogenesis inhibitor [189] | |||
| FGF carriers [57] | |||
| cell adhesion increasing [76] | |||
| λ-carrageenan | high molar mass [204,205] | drug delivery and release [196] | |
| negatively charged, high solubility in water [219] | inhibitors of viruses and bacterial infections [158,197,198,199] | ||
| extended and flexible conformation in bulk [204,205] | growth factor binding inhibitor [208] | ||
| “side-on” adsorption (low initial bulk concentrations), highly hydrated quasi “polymeric brushes (high bulk concentrations) [219] | HPV acquisition prevention, HIV inhibitor [158,197] | ||
| protection of growth factors against denaturation [59] | |||
| antibacterial properties [199] | |||
| ChS | linear shape, negatively charged [222,223] | bone healing and growth, osteoarthritis treatment [220,225] | |
| growth factor binding [222,226] |
| Neurotrophin Type | Application in Medicine | Ref. |
|---|---|---|
| NGF | attenuation of chronic pain behaviour in osteoarthritis, relieving symptoms and promoting healing in ophthalmological diseases (neurotrophic keratitis and dry eye disease) | [277,278] |
| BDNF | recovery from brain injury, treating deafness, potential drug for Parkinson’s disease treatment | [279,280,281] |
| NT3 | nerve regeneration, spinal cord injury treatment, potential hypoglycemic agent | [254,265,282] |
| NT4/5 | retinopathies treatment | [252] |
| FGF Type | Application in Medicine | Ref. |
|---|---|---|
| FGF1 | treating neuropathic pain | [291] |
| wound repair | [292] | |
| improving cardiac function (new blood vessels in the damaged heart) | [293] | |
| diabetic retinopathy treatment | [294] | |
| FGF2 | wound repair | [292] |
| surface modification and restoration of bone defects | [295] | |
| tissue engineering | [246] | |
| dermal filler | [296] | |
| regenerative endodontic procedures | [175] | |
| muscle regeneration | [297] | |
| FGF3 (expressed in embryonic development) | regulation of the guidance of thalamocortical axons | [298] |
| FGF4 (expressed in embryonic development) | angiogenic gene therapy | [299] |
| FGF5 | potential therapeutic medication for alopecia (based on FGF5 mutant protein) | [300] |
| FGF6 | muscle development and regeneration | [301] |
| preventing and treating metabolic diseases | [302] | |
| FGF7 | signal mediator during liver regeneration | [303] |
| wound healing acceleration | [304] | |
| FGF8 (expressed in embryonic development) | promote healing of the dental pulp | [305] |
| FGF9 | Parkinson’s and Huntington's disease therapy | [306] |
| FGF10 | wound healing and tissue repair, regenerative medicine (FGF10 enables the differentiation of ES cells) | [287,307] |
| FGF11 | not established | - |
| FGF12 | potential drug in pulmonary arterial hypertension | [308] |
| FGF13 | prognostic biomarker for cancer therapy | [309] |
| inflammatory pain treatment | [310] | |
| FGF14 | potential drug for the regulation of motor coordination and balance | [311] |
| FGF15 (expressed in embryonic development) | not identified in humans | [283] |
| FGF16 | protection of the neonatal heart | [312] |
| protection of the heart from cancer drug-induced heart dysfunction | [313] | |
| FGF17 (expressed in embryonic development) | potential drug for neuropsychiatric disorder treatment | [314] |
| FGF18 | biomarker for detection of ovarian cancer | [315] |
| FGF19 (expressed in embryonic development) | potential biomarker for detection of cancer (hepatocellular carcinoma) | [316] |
| treatment of muscle wasting | [317] | |
| FGF20 | FGF20-based adjuvant therapy to cure Parkinson’s disease | [318] |
| FGF21 | potential biomarker for the early detection of cardiometabolic diseases | [319] |
| obesity treatment | [320] | |
| promoting the recovery after spinal cord injury | [321] | |
| potential drug for prophylaxis and treatment of thrombotic disease | [322] | |
| FGF22 | relieving symptoms of depression | [323] |
| FGF23 | early biomarker of chronic kidney disease | [324,325] |
| Biomaterial | Growth Factor | Application of Biomaterial/GF Assemblies | Ref. |
|---|---|---|---|
| PAH/PSS-based films | VEGF | stimulation of endothelial cells proliferation (pro-angiogenic coating) | [336] |
| PAH/PSS-based films | BDNF | stimulation of motoneurons adhering and spreading | [337] |
| bPEI | VEGF | enhancement of VEGF transfection efficiency | [338] |
| bPEI, PAMAM dendrimers | VEGF | myocardial ischemia and infarction treatment | [339] |
| PEI and CS- based complexes | VEGF | generation of new blood vessels | [340] |
| PAMAM dendrimers | FGF1 | wound healing | [126] |
| PAMAM dendrimers | FGF3 | enhancement of gene expression efficiency | [341] |
| PAMAM dendrimers | VEGF | breast cancer treatment | [342] |
| PAMAM dendrimers | VEGF | anti-cancer therapy | [343] |
| PAMAM dendrimers | NT4/5 | treatment of damaged retinal neurons | [270] |
| ARG-grafted PAMAM dendrimers | VEGF | treatment of diabetic skin wounds | [128] |
| PEG-grafted PAMAM dendrimers | BDNF | delivery of neuroprotective proteins | [266] |
| heparin,carrageenan, ChS | FGF1, FGF2 | FGF-based drug delivery | [59] |
| heparin glycodendrimers | FGF2 | tissue repair and regeneration | [58] |
| heparin-based hydrogel | VEGF | diabetic wound treatment | [362] |
| heparin layers | NGF, BDNF | formation of bioactive surfaces stimulated neurite outgrowth | [356] |
| heparin-based films | FGF2 | stimulation of aortic heart valve regeneration | [355] |
| PAE/collagen/heparin/PAA- based films | FGF2 | novel platform for the culture of human pluripotent stem cells | [344] |
| heparin-functionalized CS/alginate gels | FGF2 | tissue regeneration | [347] |
| heparin/HA/ChS-based hydrogels | FGF2 | wound repair | [363] |
| heparin/HA- based hydrogels | FGF2 | damaged tissue reparing | [368] |
| PEI (PSS)/heparin-based films | FGF2 | enhancement of bone formation | [13] |
| bPEI/heparin- based films | FGF1 | formation of bioactive materials | [351] |
| albumin/heparin- based films | FGF2 | formation of biofunctional surface coating | [353] |
| fibrin/heparin-based films | NGF, NT-3, BDNF | enhancement of peripheral nerve regeneration | [357] |
| CS-based hydrogels | FGF1, FGF2, VEGF | wound occlusive dressings for dermal and peptic ulcera | [360] |
| CS-based hydrogel | FGF2 | wound dressing | [361] |
| CS/heparin- based films | FGF2 | formation of biofunctional surface coating | [354] |
| quaternized CS/heparin-based films | FGF2 | formation of biofunctional surface coating | [352] |
| CS/alginate/carrageenan/heparin | FGF2, VEGF, PDGF | tunable incorporation of platelet lysate | [215] |
| CS/HA nanoparticles | VEGF | treatment of ischemia-related diseases | [366] |
| CS/ collagen-based films | rhBMP-2 | enhancement of implant osseointegration | [348] |
| brushite/CS-based films | VEGF | bone regeneration | [349] |
| PAE/ChS/PAA-based films | VEGF | bone tissue engineering | [249] |
| PLL/ChS and PLL/HA- based films | FGF2 | functionalized tissue engineering | [358] |
| ChS/collagen-based films | FGF2 | formation of biofunctional surface coating | [12] |
| HA- based hydrogels | VEGF | drug delivery system | [367] |
| HA- based hydrogels | BDNF | localized gene therapies | [369] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michna, A.; Pomorska, A.; Ozcan, O. Biocompatible Macroion/Growth Factor Assemblies for Medical Applications. Biomolecules 2023, 13, 609. https://doi.org/10.3390/biom13040609
Michna A, Pomorska A, Ozcan O. Biocompatible Macroion/Growth Factor Assemblies for Medical Applications. Biomolecules. 2023; 13(4):609. https://doi.org/10.3390/biom13040609
Chicago/Turabian StyleMichna, Aneta, Agata Pomorska, and Ozlem Ozcan. 2023. "Biocompatible Macroion/Growth Factor Assemblies for Medical Applications" Biomolecules 13, no. 4: 609. https://doi.org/10.3390/biom13040609
APA StyleMichna, A., Pomorska, A., & Ozcan, O. (2023). Biocompatible Macroion/Growth Factor Assemblies for Medical Applications. Biomolecules, 13(4), 609. https://doi.org/10.3390/biom13040609