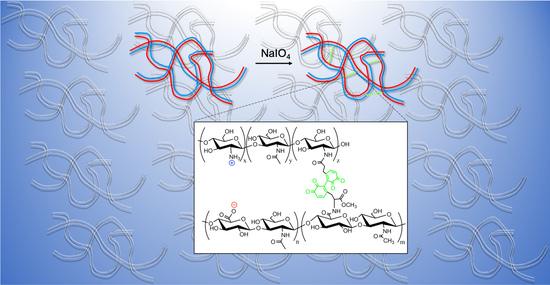
Rheology and Gelation of Hyaluronic Acid/Chitosan Coacervates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coacervates
2.3. Rheology of HA/CHI Coacervates
2.4. Modification of HA and CHI with Catechol Groups
2.5. Estimation of the Degree of Modification for HA–Catechol and CHI–Catechol
3. Results
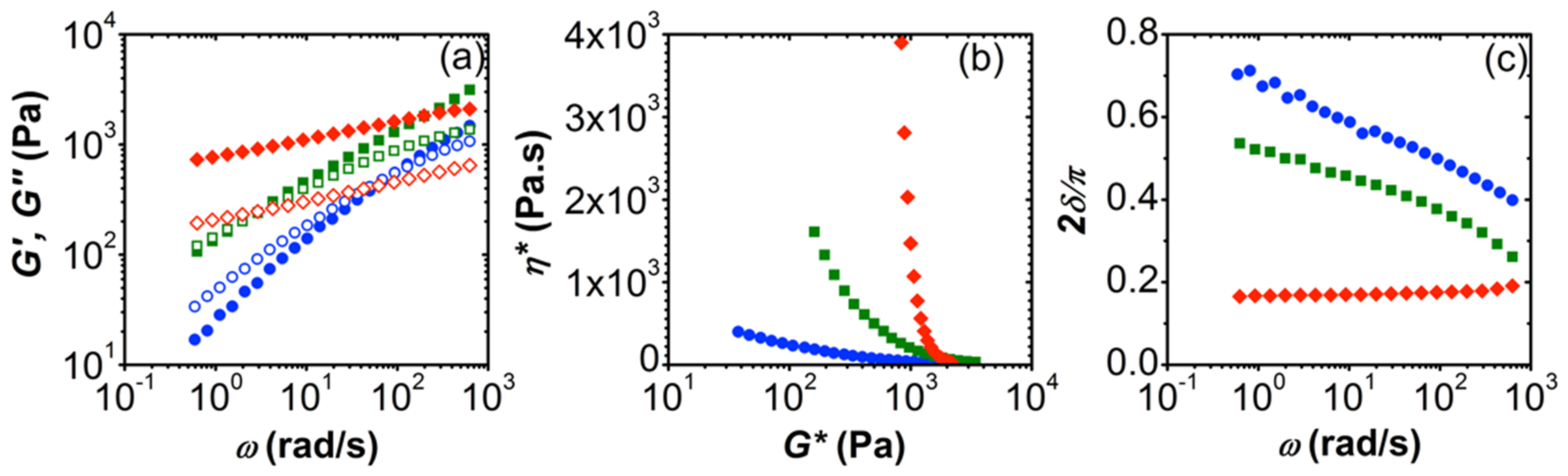
3.1. Effect of Polymer Molecular Weight
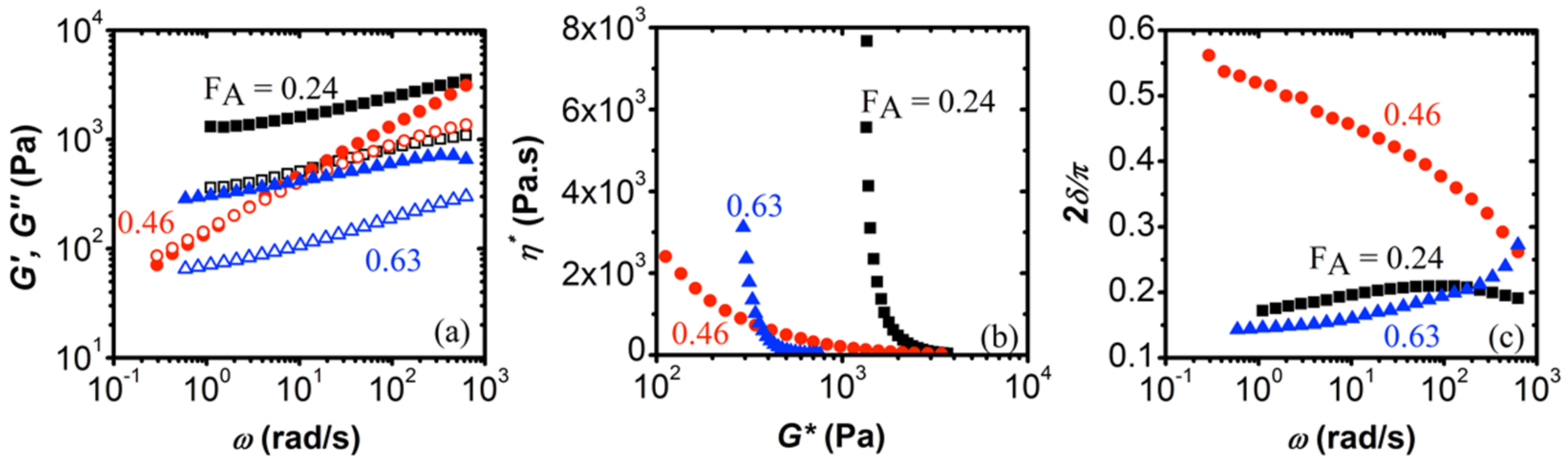
3.2. Effect of Degree of Acetylation
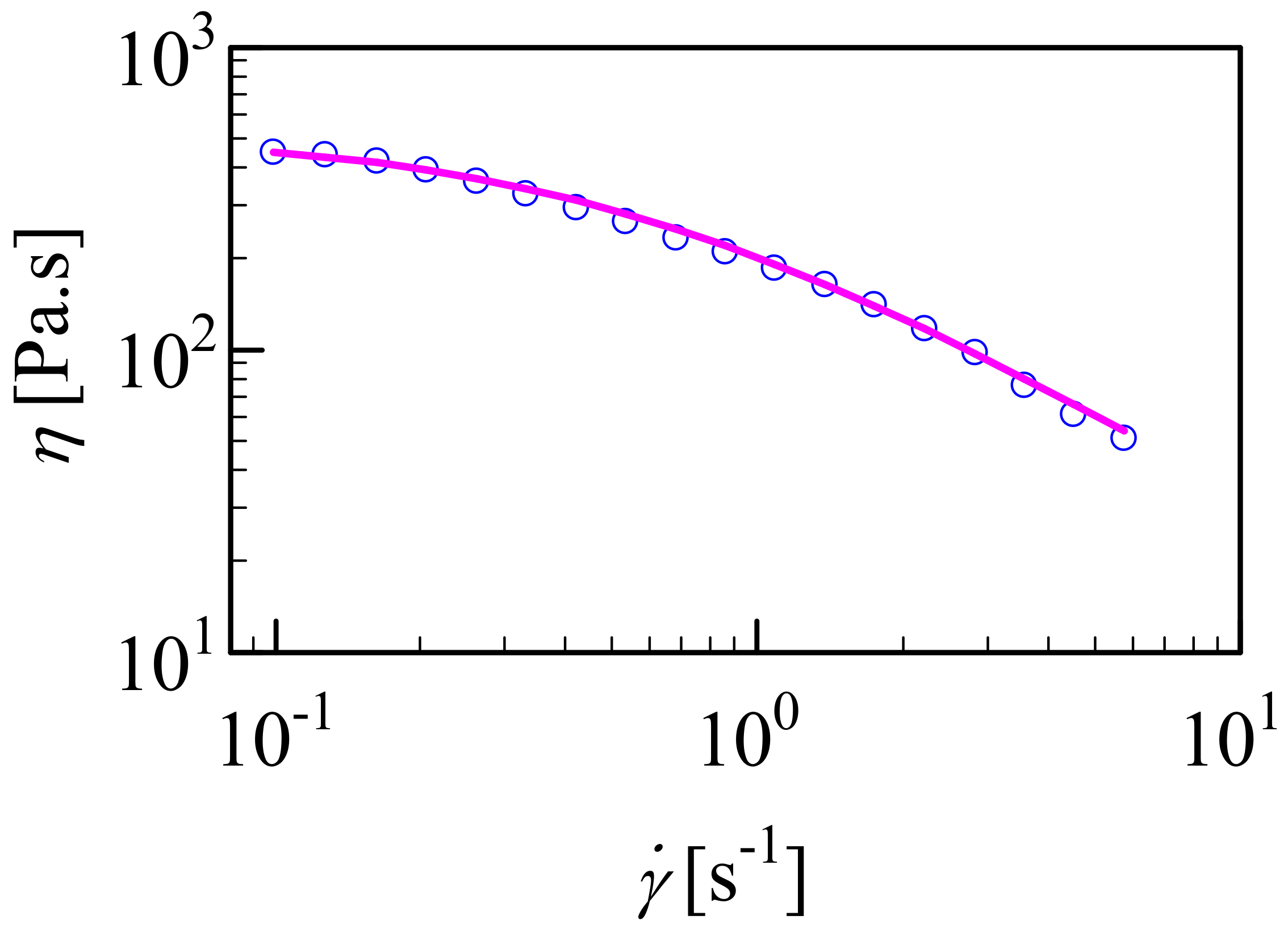
3.3. Shear-Dependent Behavior of HA/CHI Coacervates
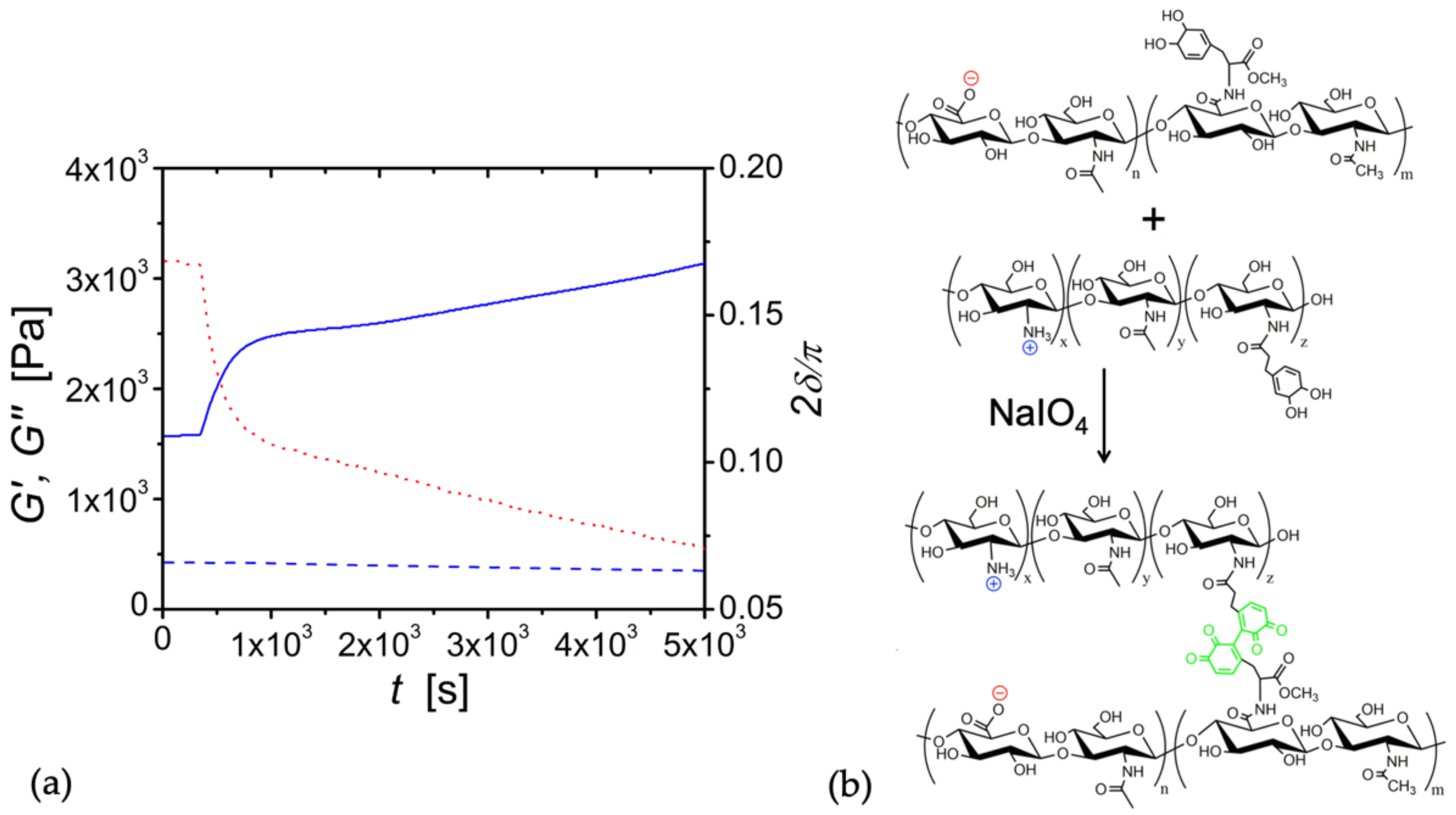
3.4. Conversion of Complex Coacervates into Chemical Gels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmitt, C.; Turgeon, S.L. Protein/Polysaccharide Complexes and Coacervates in Food Systems. Adv. Colloid Interface Sci. 2011, 167, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kizilay, E.; Kayitmazer, A.B.; Dubin, P.L. Complexation and Coacervation of Polyelectrolytes with Oppositely Charged Colloids. Adv. Colloid Interface Sci. 2011, 167, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Comert, F.; Nguyen, D.; Rushanan, M.; Milas, P.; Xu, A.Y.; Dubin, P.L. Precipitate–Coacervate Transformation in Polyelectrolyte–Mixed Micelle Systems. J. Phys. Chem. B 2017, 121, 4466–4473. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, C.; Jiang, H.; Dawadi, M.B.; Vogt, B.D.; Modarelli, D.A.; Zacharia, N.S. Polyelectrolyte–Micelle Coacervates: Intrapolymer-Dominant vs. Interpolymer-Dominant Association, Solute Uptake and Rheological Properties. Soft Matter 2019, 15, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-W.; Lytle, T.K.; Radhakrishna, M.; Madinya, J.J.; Vélez, J.; Sing, C.E.; Perry, S.L. Sequence and Entropy-Based Control of Complex Coacervates. Nat. Commun. 2017, 8, 1273. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Santa Chalarca, C.F.; Carmean, R.N.; Olson, R.A.; Madinya, J.; Sumerlin, B.S.; Sing, C.E.; Emrick, T.; Perry, S.L. Effect of Polymer Chemistry on the Linear Viscoelasticity of Complex Coacervates. Macromolecules 2020, 53, 7851–7864. [Google Scholar] [CrossRef]
- Seal, M.; Weil-Ktorza, O.; Despotović, D.; Tawfik, D.S.; Levy, Y.; Metanis, N.; Longo, L.M.; Goldfarb, D. Peptide-RNA Coacervates as a Cradle for the Evolution of Folded Domains. J. Am. Chem. Soc. 2022, 144, 14150–14160. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Koksal, A.F.; Kilic Iyilik, E. Complex Coacervation of Hyaluronic Acid and Chitosan: Effects of pH, Ionic Strength, Charge Density, Chain Length and the Charge Ratio. Soft Matter 2015, 11, 8605–8612. [Google Scholar] [CrossRef]
- Ferreira, M.; Jing, B.; Lorenzana, A.; Zhu, Y. Effect of Polyampholyte Net Charge on Complex Coacervation between Polyampholytes and Inorganic Polyoxometalate Giant Anions. Soft Matter 2020, 16, 10280–10289. [Google Scholar] [CrossRef]
- Ou, Z.; Muthukumar, M. Entropy and Enthalpy of Polyelectrolyte Complexation: Langevin Dynamics Simulations. J. Chem. Phys. 2006, 124, 154902. [Google Scholar] [CrossRef]
- De Oliveira, V.M.; Caetano, D.L.Z.; da Silva, F.B.; Mouro, P.R.; de Oliveira, A.B.; de Carvalho, S.J.; Leite, V.B.P. pH and Charged Mutations Modulate Cold Shock Protein Folding and Stability: A Constant pH Monte Carlo Study. J. Chem. Theory Comput. 2020, 16, 765–772. [Google Scholar] [CrossRef]
- Barroso da Silva, F.L.; Dias, L.G. Development of Constant-pH Simulation Methods in Implicit Solvent and Applications in Biomolecular Systems. Biophys. Rev. 2017, 9, 699–728. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, D.; Santiso, E.; Gubbins, K.; Barroso da Silva, F.L. Computationally Mapping pKa Shifts due to the Presence of a Polyelectrolyte Chain around Whey Proteins. Langmuir 2017, 33, 11417–11428. [Google Scholar] [CrossRef]
- Caetano, D.L.Z.; de Carvalho, S.J.; Metzler, R.; Cherstvy, A.G. Critical Adsorption of Periodic and Random Polyampholytes onto Charged Surfaces. Phys. Chem. Chem. Phys. 2017, 19, 23397–23413. [Google Scholar] [CrossRef]
- Caetano, D.L.Z.; Metzler, R.; Cherstvy, A.G.; de Carvalho, S.J. Adsorption of Lysozyme into a Charged Confining Pore. Phys. Chem. Chem. Phys. 2021, 23, 27195–27206. [Google Scholar] [CrossRef]
- Lunkad, R.; Barroso da Silva, F.L.; Košovan, P. Both Charge-Regulation and Charge-Patch Distribution Can Drive Adsorption on the Wrong Side of the Isoelectric Point. J. Am. Chem. Soc. 2022, 144, 1813–1825. [Google Scholar] [CrossRef]
- Tian, Q.; Zhou, W.; Cai, Q.; Pan, X.; Ma, G.; Lian, G. In Situ Complex Coacervation Supported by Self-Coated Polydopamine Interlayer on Uniform-Sized Essential Oils Droplet. J. Colloid Interface Sci. 2022, 623, 1027–1038. [Google Scholar] [CrossRef]
- Waltmann, C.; Mills, C.E.; Wang, J.; Qiao, B.; Torkelson, J.M.; Tullman-Ercek, D.; Olvera de la Cruz, M. Functional Enzyme–Polymer Complexes. Proc. Natl. Acad. Sci. USA 2022, 119, e2119509119. [Google Scholar] [CrossRef]
- Mohsen, A.M.; El-Hashemy, H.A.; Salama, A.; Darwish, A.B. Formulation of Tizanidine Hydrochloride–Loaded Provesicular System for Improved Oral Delivery and Therapeutic Activity Employing a 23 Full Factorial Design. Drug Deliv. Transl. Res. 2022. [Google Scholar] [CrossRef]
- Shirzadian, T.; Nourbakhsh, M.S.; Fattahi, A.; Bahrami, G.; Mohammadi, G. Characterization and Optimization of De-esterified Tragacanth-chitosan Nanocomposite as a Potential Carrier for Oral Delivery of Insulin: In Vitro and Ex Vivo Studies. J. Biomed. Mater. Res. A 2021, 109, 2164–2172. [Google Scholar] [CrossRef]
- Park, T.Y.; Jeon, E.Y.; Kim, H.J.; Choi, B.-H.; Cha, H.J. Prolonged Cell Persistence with Enhanced Multipotency and Rapid Angiogenesis of Hypoxia Pre-Conditioned Stem Cells Encapsulated in Marine-Inspired Adhesive and Immiscible Liquid Micro-Droplets. Acta Biomater. 2019, 86, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Spruijt, E.; Sprakel, J.; Lemmers, M.; Stuart, M.A.C.; van der Gucht, J. Relaxation Dynamics at Different Time Scales in Electrostatic Complexes: Time-Salt Superposition. Phys. Rev. Lett. 2010, 105, 208301. [Google Scholar] [CrossRef] [PubMed]
- Bohidar, H.; Dubin, P.L.; Majhi, P.R.; Tribet, C.; Jaeger, W. Effects of Protein–Polyelectrolyte Affinity and Polyelectrolyte Molecular Weight on Dynamic Properties of Bovine Serum Albumin–Poly(Diallyldimethylammonium Chloride) Coacervates. Biomacromolecules 2005, 6, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Callow, J.A. (Eds.) Biological Adhesives; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 978-3-540-31048-8. [Google Scholar]
- Athanasiou, K.A.; Darling, E.M.; Hu, J.C. Articular Cartilage Tissue Engineering. Synth. Lect. Tissue Eng. 2009, 1, 1–182. [Google Scholar] [CrossRef]
- Peter, S.J.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Polymer Concepts in Tissue Engineering. J. Biomed. Mater. Res. 1998, 43, 422–427. [Google Scholar] [CrossRef]
- Tsang, V.L.; Bhatia, S.N. Fabrication of Three-Dimensional Tissues. In Tissue Engineering II; Springer: Berlin/Heidelberg, Germany, 2007; Volume 103, pp. 189–205. [Google Scholar]
- Karabiyik Acar, O.; Kayitmazer, A.B.; Torun Kose, G. Hyaluronic Acid/Chitosan Coacervate-Based Scaffolds. Biomacromolecules 2018, 19, 1198–1211. [Google Scholar] [CrossRef]
- Karabıyık Acar, Ö.; Bedir, S.; Kayitmazer, A.B.; Kose, G.T. Chondro-Inductive Hyaluronic Acid/Chitosan Coacervate-Based Scaffolds for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2021, 188, 300–312. [Google Scholar] [CrossRef]
- Hayashi, K.; Tsutsumi, K.; Nakajima, F.; Norisuye, T.; Teramoto, A. Chain-Stiffness and Excluded-Volume Effects in Solutions of Sodium Hyaluronate at High Ionic Strength. Macromolecules 1995, 28, 3824–3830. [Google Scholar] [CrossRef]
- Berth, G.; Cölfen, H.; Dautzenberg, H. Physicochemical and Chemical Characterisation of Chitosan in Dilute Aqueous Solution. In Analytical Ultracentrifugation VI; Borchard, W., Straatmann, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 50–57. [Google Scholar]
- Liu, X.; Haddou, M.; Grillo, I.; Mana, Z.; Chapel, J.-P.; Schatz, C. Early Stage Kinetics of Polyelectrolyte Complex Coacervation Monitored through Stopped-Flow Light Scattering. Soft Matter 2016, 12, 9030–9038. [Google Scholar] [CrossRef]
- Danielsen, S.P.O.; Panyukov, S.; Rubinstein, M. Ion Pairing and the Structure of Gel Coacervates. Macromolecules 2020, 53, 9420–9442. [Google Scholar] [CrossRef]
- Priftis, D.; Megley, K.; Laugel, N.; Tirrell, M. Complex Coacervation of Poly(Ethylene-Imine)/Polypeptide Aqueous Solutions: Thermodynamic and Rheological Characterization. J. Colloid Interface Sci. 2013, 398, 39–50. [Google Scholar] [CrossRef]
- Friedowitz, S.; Lou, J.; Barker, K.P.; Will, K.; Xia, Y.; Qin, J. Looping-in Complexation and Ion Partitioning in Nonstoichiometric Polyelectrolyte Mixtures. Sci. Adv. 2021, 7, eabg8654. [Google Scholar] [CrossRef]
- Sun, J.; Schiffman, J.D.; Perry, S.L. Linear Viscoelasticity and Time–Alcohol Superposition of Chitosan/Hyaluronic Acid Complex Coacervates. ACS Appl. Polym. Mater. 2022, 4, 1617–1625. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-Molecule Mechanics of Mussel Adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverman, H.G.; Roberto, F.F. Understanding Marine Mussel Adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Lee, J.; Huh, K.M.; Lee, S.H.; Lee, H. Toxicity-Attenuated Glycol Chitosan Adhesive Inspired by Mussel Adhesion Mechanisms. Adv. Health Mater. 2019, 8, 1900275. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.S.; Lee, C.; Park, H.-J.; Yang, K.; Jin, Y.; Ryu, J.H.; Hong, K.S.; Moon, S.-H.; Chung, H.-M.; et al. Tissue Adhesive Catechol-Modified Hyaluronic Acid Hydrogel for Effective, Minimally Invasive Cell Therapy. Adv. Funct. Mater. 2015, 25, 3814–3824. [Google Scholar] [CrossRef]
- Park, H.-J.; Jin, Y.; Shin, J.; Yang, K.; Lee, C.; Yang, H.S.; Cho, S.-W. Catechol-Functionalized Hyaluronic Acid Hydrogels Enhance Angiogenesis and Osteogenesis of Human Adipose-Derived Stem Cells in Critical Tissue Defects. Biomacromolecules 2016, 17, 1939–1948. [Google Scholar] [CrossRef]
- Lim, Z.W.; Varma, V.B.; Ramanujan, R.V.; Miserez, A. Magnetically Responsive Peptide Coacervates for Dual Hyperthermia and Chemotherapy Treatments of Liver Cancer. Acta Biomater. 2020, 110, 221–230. [Google Scholar] [CrossRef]
- Balkenende, D.W.R.; Winkler, S.M.; Messersmith, P.B. Marine-Inspired Polymers in Medical Adhesion. Eur. Polym. J. 2019, 116, 134–143. [Google Scholar] [CrossRef]
- Conejo-Cuevas, G.; Ruiz-Rubio, L.; Sáez-Martínez, V.; Pérez-González, R.; Gartziandia, O.; Huguet-Casquero, A.; Pérez-Álvarez, L. Spontaneous Gelation of Adhesive Catechol Modified Hyaluronic Acid and Chitosan. Polymers 2022, 14, 1209. [Google Scholar] [CrossRef] [PubMed]
- Poh, L.; Narimissa, E.; Wagner, M.H.; Winter, H.H. Interactive Shear and Extensional Rheology—25 Years of IRIS Software. Rheol. Acta 2022, 61, 259–269. [Google Scholar] [CrossRef]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the Degree of Acetylation and the Electrostatic Properties of Chitin and Chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Gatej, I.; Popa, M.; Rinaudo, M. Role of the pH on Hyaluronan Behavior in Aqueous Solution. Biomacromolecules 2005, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Heuzey, M.-C.; Bégin, A.; Carreau, P.J. Viscoelastic Properties of Chitosan Solutions: Effect of Concentration and Ionic Strength. J. Food Eng. 2006, 74, 500–515. [Google Scholar] [CrossRef]
- Winter, H.H.; Chambon, F. Analysis of Linear Viscoelasticity of a Crosslinking Polymer at the Gel Point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Winter, H.H. Three Views of Viscoelasticity for Cox–Merz Materials. Rheol. Acta 2009, 48, 241–243. [Google Scholar] [CrossRef]
- Winter, H.H. Glass Transition as the Rheological Inverse of Gelation. Macromolecules 2013, 46, 2425–2432. [Google Scholar] [CrossRef]
- Cousin, F.; Gummel, J.; Ung, D.; Boué, F. Polyelectrolyte–Protein Complexes: Structure and Conformation of Each Specie Revealed by SANS. Langmuir 2005, 21, 9675–9688. [Google Scholar] [CrossRef]
- Spruijt, E.; Cohen Stuart, M.A.; van der Gucht, J. Linear Viscoelasticity of Polyelectrolyte Complex Coacervates. Macromolecules 2013, 46, 1633–1641. [Google Scholar] [CrossRef]
- Hamad, F.G.; Chen, Q.; Colby, R.H. Linear Viscoelasticity and Swelling of Polyelectrolyte Complex Coacervates. Macromolecules 2018, 51, 5547–5555. [Google Scholar] [CrossRef]
- Ru, Q.; Wang, Y.; Lee, J.; Ding, Y.; Huang, Q. Turbidity and Rheological Properties of Bovine Serum Albumin/Pectin Coacervates: Effect of Salt Concentration and Initial Protein/Polysaccharide Ratio. Carbohydr. Polym. 2012, 88, 838–846. [Google Scholar] [CrossRef]
- Qazvini, N.T.; Bolisetty, S.; Adamcik, J.; Mezzenga, R. Self-Healing Fish Gelatin/Sodium Montmorillonite Biohybrid Coacervates: Structural and Rheological Characterization. Biomacromolecules 2012, 13, 2136–2147. [Google Scholar] [CrossRef]
- Ambrosio, L.; Borzacchiello, A.; Netti, P.A.; Nicolais, L. Rheological Study on Hyaluronic Acid and Its Derivative Solutions. J. Macromol. Sci. Part A 1999, 36, 991–1000. [Google Scholar] [CrossRef]
- Mucha, M. Rheological Characteristics of Semi-Dilute Chitosan Solutions. Macromol. Chem. Phys. 1997, 198, 471–484. [Google Scholar] [CrossRef]
- Yasuda, K.; Armstrong, R.C.; Cohen, R.E. Shear Flow Properties of Concentrated Solutions of Linear and Star Branched Polystyrenes. Rheol. Acta 1981, 20, 163–178. [Google Scholar] [CrossRef]
- Kaur, S.; Weerasekare, G.M.; Stewart, R.J. Multiphase Adhesive Coacervates Inspired by the Sandcastle Worm. ACS Appl. Mater. Interfaces 2011, 3, 941–944. [Google Scholar] [CrossRef] [Green Version]
- Weinbreck, F.; Wientjes, R.H.W.; Nieuwenhuijse, H.; Robijn, G.W.; de Kruif, C.G. Rheological Properties of Whey Protein/Gum Arabic Coacervates. J. Rheol. 2004, 48, 1215–1228. [Google Scholar] [CrossRef]
- Singh, S.S.; Aswal, V.K.; Bohidar, H.B. Structural Studies of Agar–Gelatin Complex Coacervates by Small Angle Neutron Scattering, Rheology and Differential Scanning Calorimetry. Int. J. Biol. Macromol. 2007, 41, 301–307. [Google Scholar] [CrossRef]
- Momeni, A.; Filiaggi, M.J. Rheology of Polyphosphate Coacervates. J. Rheol. 2016, 60, 25–34. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Strand, S.P.; Tribet, C.; Jaeger, W.; Dubin, P.L. Effect of Polyelectrolyte Structure on Protein–Polyelectrolyte Coacervates: Coacervates of Bovine Serum Albumin with Poly(Diallyldimethylammonium Chloride) versus Chitosan. Biomacromolecules 2007, 8, 3568–3577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Hwang, J.; Deming, T.J. Role of L-3,4-Dihydroxyphenylalanine in Mussel Adhesive Proteins. J. Am. Chem. Soc. 1999, 121, 5825–5826. [Google Scholar] [CrossRef]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and Gelation of DOPA-Modified Poly(Ethylene Glycol) Hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Bilston, L.E.; Sinkus, R. In Vivo Brain Viscoelastic Properties Measured by Magnetic Resonance Elastography. NMR Biomed. 2008, 21, 755–764. [Google Scholar] [CrossRef]
- Jiao, T.; Clifton, R.J.; Converse, G.L.; Hopkins, R.A. Measurements of the Effects of Decellularization on Viscoelastic Properties of Tissues in Ovine, Baboon, and Human Heart Valves. Tissue Eng. Part A 2012, 18, 423–431. [Google Scholar] [CrossRef]
- Dong, L.; Li, Z.; Leffler, N.R.; Asch, A.S.; Chi, J.-T.; Yang, L.V. Acidosis Activation of the Proton-Sensing GPR4 Receptor Stimulates Vascular Endothelial Cell Inflammatory Responses Revealed by Transcriptome Analysis. PLoS ONE 2013, 8, e61991. [Google Scholar] [CrossRef] [Green Version]
- Krogstad, D.V.; Lynd, N.A.; Miyajima, D.; Gopez, J.; Hawker, C.J.; Kramer, E.J.; Tirrell, M.V. Structural Evolution of Polyelectrolyte Complex Core Micelles and Ordered-Phase Bulk Materials. Macromolecules 2014, 47, 8026–8032. [Google Scholar] [CrossRef]
- Aubry, S.; Pellet-Rostaing, S.; Lemaire, M. Oxidative Nucleophilic Substitution (SNOX) of the Benzylic Position as a Tunable Synthesis of Tetrahydroisoquinoline Natural Alkaloid Analogues. Eur. J. Org. Chem. 2007, 2007, 5212–5225. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Hu, B.H.; Messersmith, P.B. Convenient Synthesis of Acetonide Protected 3,4-Dihydroxyphenylalanine (DOPA) for Fmoc Solid-Phase Peptide Synthesis. Tetrahedron Lett. 2008, 49, 5519–5521. [Google Scholar] [CrossRef]


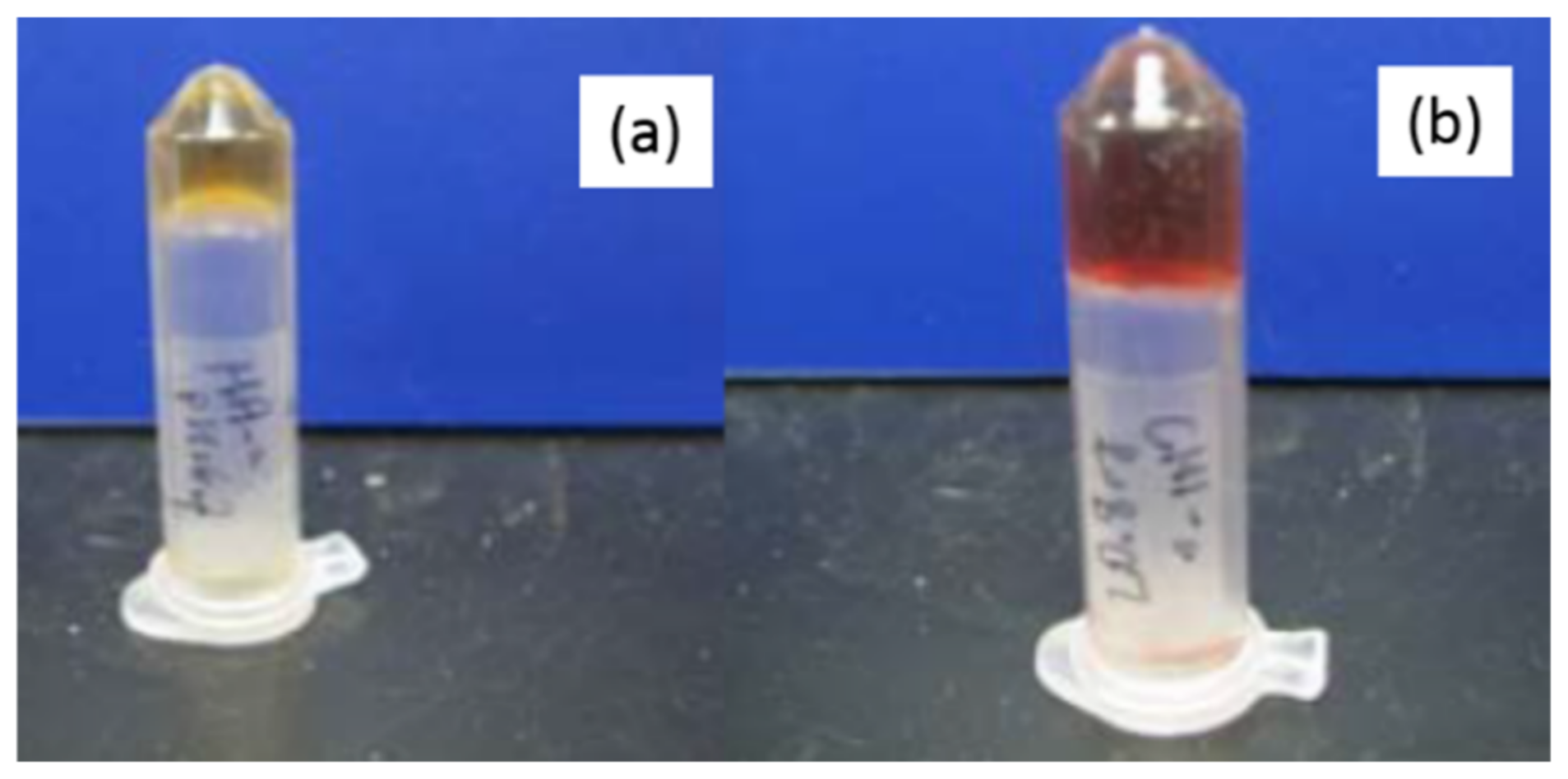
| Polymer | Mw (kDa) | Mn (kDa) |
|---|---|---|
| Chitosan, FA = 0.24 a | 345.6 | 125.5 |
| Chitosan, FA = 0.42 a | 123.5 | 104.5 |
| Chitosan, FA = 0.46 a | 365.1 | 302.8 |
| Chitosan, FA = 0.63 a | 332.4 | 209.5 |
| Hyaluronic acid b | 750 | N/A |
| Hyaluronic acid b | 132.3 | N/A |
| FA | MW of Chitosan Repeat Unit (g/mole) | Degree of Ionization * (β) at pH = 6.7 | [−]/[+] at pH = 6.7 |
|---|---|---|---|
| 0.24 0.42 0.46 0.63 | 196.1 194.9 194.7 193.6 | 0.34 0.44 0.45 0.58 | 1.89 1.90 2.00 2.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayitmazer, A.B.; Comert, F.; Winter, H.H.; Messersmith, P.B. Rheology and Gelation of Hyaluronic Acid/Chitosan Coacervates. Biomolecules 2022, 12, 1817. https://doi.org/10.3390/biom12121817
Kayitmazer AB, Comert F, Winter HH, Messersmith PB. Rheology and Gelation of Hyaluronic Acid/Chitosan Coacervates. Biomolecules. 2022; 12(12):1817. https://doi.org/10.3390/biom12121817
Chicago/Turabian StyleKayitmazer, A. Basak, Fatih Comert, Henning H. Winter, and Phillip B. Messersmith. 2022. "Rheology and Gelation of Hyaluronic Acid/Chitosan Coacervates" Biomolecules 12, no. 12: 1817. https://doi.org/10.3390/biom12121817
APA StyleKayitmazer, A. B., Comert, F., Winter, H. H., & Messersmith, P. B. (2022). Rheology and Gelation of Hyaluronic Acid/Chitosan Coacervates. Biomolecules, 12(12), 1817. https://doi.org/10.3390/biom12121817