An In Vivo Screening Model for Investigation of Pathophysiology of Human Implantation Failure
Abstract
1. Introduction
2. HVJ-E Vector System as a Gene Delivery System
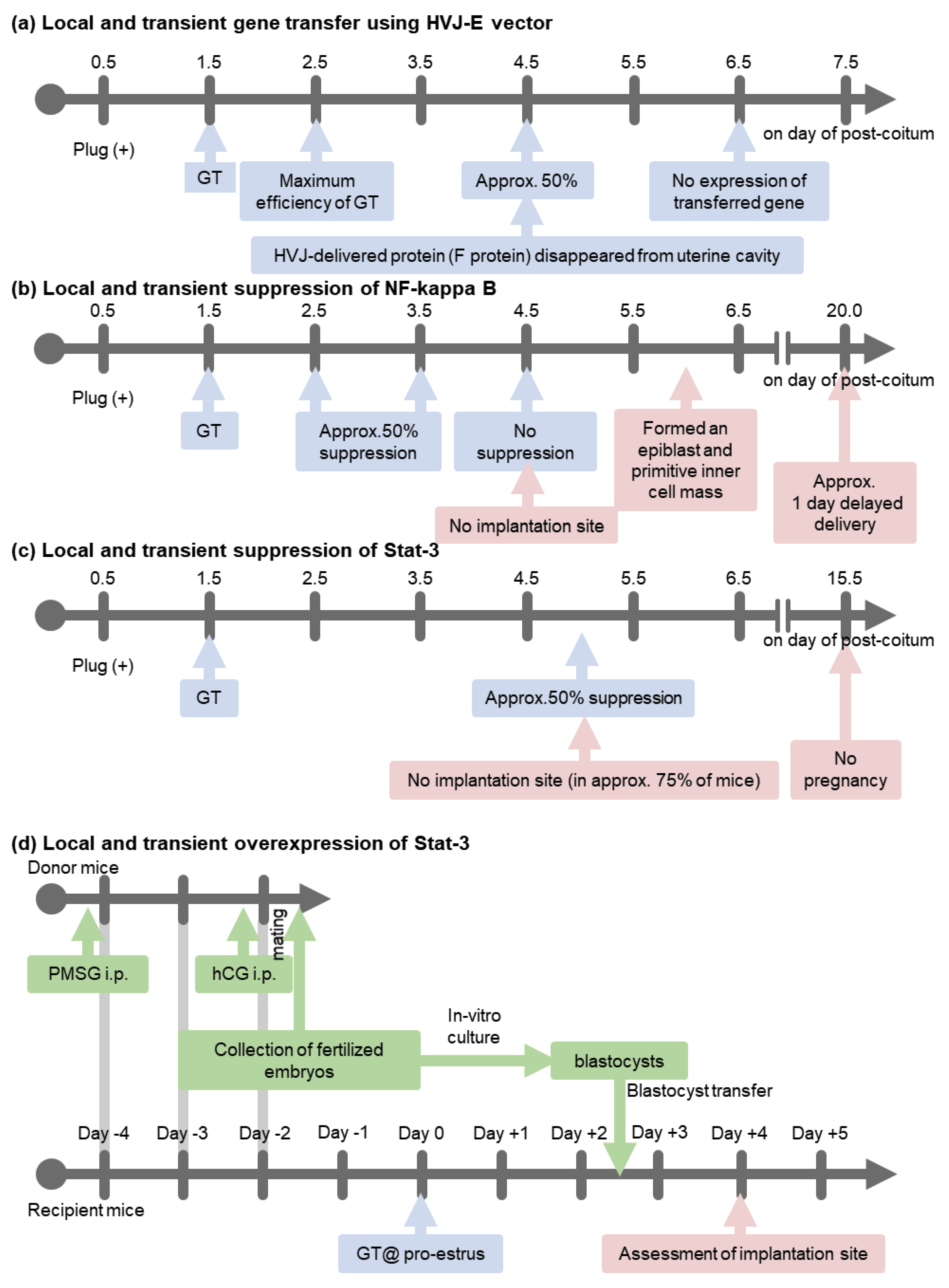
3. A Transient and Local In Vivo Gene Transfer System to Murine Uterine Endometrium Using HVJ-E Vector
4. An In Vivo Screening Model
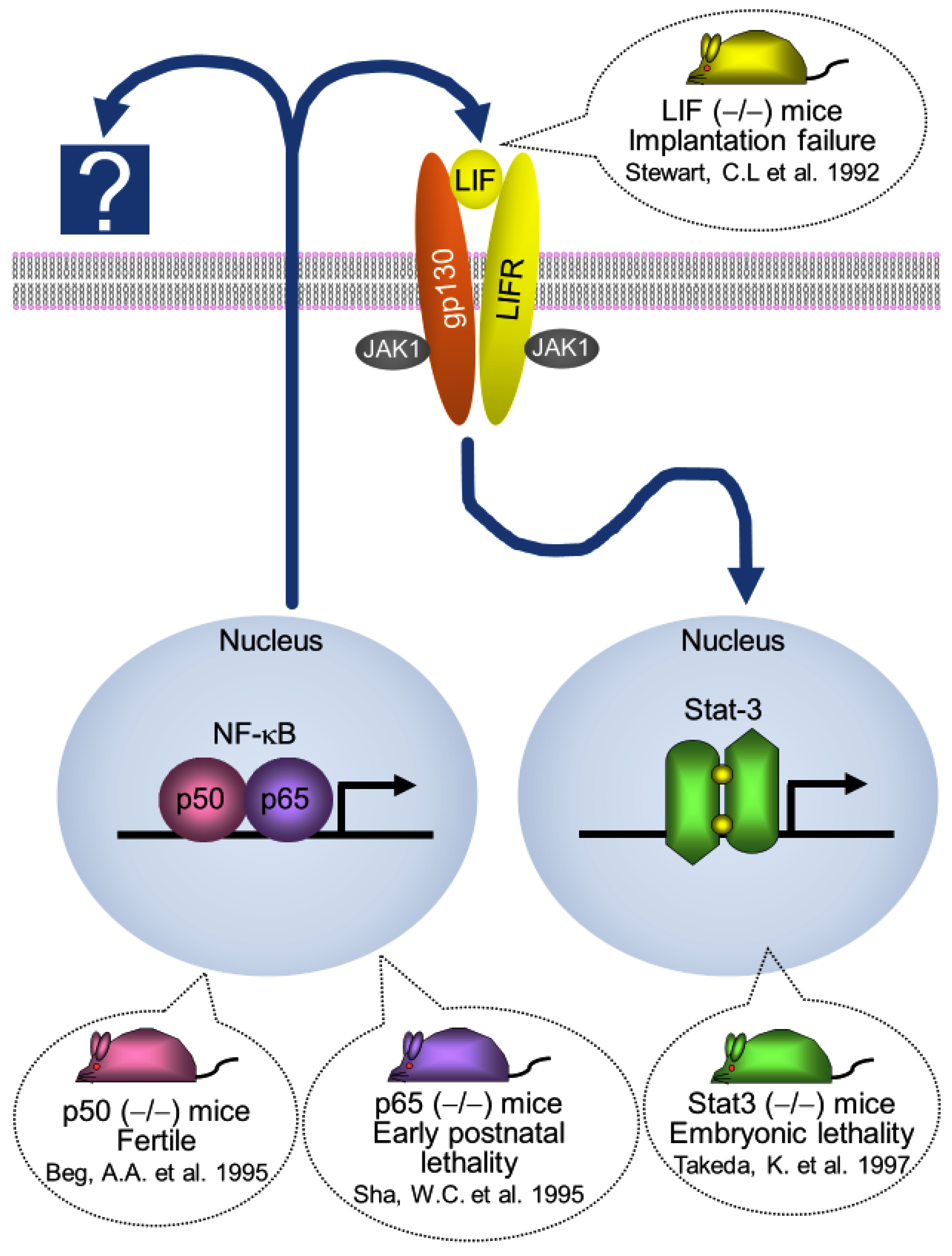
5. NF-κB Activation Determines the Timing of Implantation
6. Interaction between Ovarian Hormones (E2 and P4) and NF-κB Activity
7. Stat-3 Regulates Blastocyst Attachment and Decidualization
8. Interaction between Ovarian Hormones (E2 and P4) and Stat-3 Activity
9. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franasiak, J.M.; Alecsandru, D.; Forman, E.J.; Gemmell, L.C.; Goldberg, J.M.; Llarena, N.; Margolis, C.; Laven, J.; Schoenmakers, S.; Seli, E. A review of the pathophysiology of recurrent implantation failure. Fertil. Steril. 2021, 116, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Garneau, A.S.; Young, S.L. Defining recurrent implantation failure: A profusion of confusion or simply an illusion? Fertil. Steril. 2021, 116, 1432–1435. [Google Scholar] [CrossRef]
- Bui, A.H.; Timmons, D.B.; Young, S.L. Evaluation of endometrial receptivity and implantation failure. Curr. Opin. Obs. Gynecol. 2022, 34, 107–113. [Google Scholar] [CrossRef]
- Li, T.C.; Klentzeris, L.; Barratt, C.; Warren, M.A.; Cooke SCooke, I.D. A study of endometrial morphology in women who failed to conceive in a donor insemination programme. Br. J. Obs. Gynaecol. 1993, 100, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Turocy, J.; Williams, Z. Novel therapeutic options for treatment of recurrent implantation failure. Fertil. Steril. 2021, 116, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Kliman, H.J.; Frankfurter, D. Clinical approach to recurrent implantation failure: Evidence-Based evaluation of the endometrium. Fertil. Steril. 2019, 111, 618–628. [Google Scholar] [CrossRef]
- Bellver, J.; Simon, C. Implantation failure of endometrial origin: What is new? Curr. Opin. Obstet. Gynecol. 2018, 30, 229–236. [Google Scholar] [CrossRef]
- Paria, B.C.; Huet-Hudson, Y.M.; Dey, S.K. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc. Natl. Acad. Sci. USA 1993, 90, 10159–10162. [Google Scholar] [CrossRef]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Fukui, Y.; Hirota, Y.; Matsuo, M.; Gebril, M.; Akaeda, S.; Hiraoka, T.; Osuga, Y. Uterine receptivity, embryo attachment, and embryo invasion: Multistep processes in embryo implantation. Reprod. Med. Biol. 2019, 18, 234–240. [Google Scholar] [CrossRef]
- Ma, W.G.; Song, H.; Das, S.K.; Paria, B.C.; Dey, S.K. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc. Natl. Acad. Sci. USA 2003, 100, 2963–2968. [Google Scholar] [CrossRef] [PubMed]
- DeMayo, F.J.; Lydon, J.P. 90 YEARS OF PROGESTERONE: New insights into progesterone receptor signaling in the endometrium required for embryo implantation. J. Mol. Endocrinol. 2020, 65, T1–T14. [Google Scholar] [CrossRef] [PubMed]
- Ghobara, T.; Gelbaya, T.A.; Ayeleke, R.O. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst. Rev. 2017, 7, CD003414. [Google Scholar] [CrossRef]
- Namiki, T.; Ito, J.; Kashiwazaki, N. Molecular mechanisms of embryonic implantation in mammals: Lessons from the gene manipulation of mice. Reprod. Med. Biol. 2018, 17, 331–342. [Google Scholar] [CrossRef]
- Wattiaux, R.; Laurent, N.; Wattiaux-De Coninck, S.; Jadot, M. Endosomes, lysosomes: Their implication in gene transfer. Adv. Drug. Deliv. Rev. 2000, 41, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y. Paramyxovirus replication and pathogenesis. Reverse genetics transforms understanding. Rev. Med. Virol. 1999, 9, 83–99. [Google Scholar] [CrossRef]
- Samal, S.K. Paramyxoviruses of Animals. In Encyclopedia of Virology, 3rd ed.; Brian, W.J.M., Van Regenmortel, M.H.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 40–47. [Google Scholar]
- Okada, Y. Sendai virus-induced cell fusion. Methods Enzymol. 1993, 221, 18–41. [Google Scholar] [CrossRef]
- Kaneda, Y. Development of liposomes and pseudovirions with fusion activity for efficient gene delivery. Curr. Gene. Ther. 2011, 11, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, Y.; Nakajima, T.; Nishikawa, T.; Yamamoto, S.; Ikegami, H.; Suzuki, N.; Nakamura, H.; Morishita, R.; Kotani, H. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol. Ther. 2002, 6, 219–226. [Google Scholar] [CrossRef]
- Kiyohara, E.; Tanemura, A.; Nishioka, M.; Yamada, M.; Tanaka, A.; Yokomi, A.; Saito, A.; Sakura, K.; Nakajima, T.; Myoui, A.; et al. Intratumoral injection of hemagglutinating virus of Japan-envelope vector yielded an antitumor effect for advanced melanoma: A phase I/IIa clinical study. Cancer Immunol. Immunother. 2020, 69, 1131–1140. [Google Scholar] [CrossRef]
- Nakamura, H.; Kimura, T.; Ikegami, H.; Ogita, K.; Koyama, S.; Shimoya, K.; Tsujie, T.; Koyama, M.; Kaneda, Y.; Murata, Y. Highly efficient and minimally invasive in-vivo gene transfer to the mouse uterus using haemagglutinating virus of Japan (HVJ) envelope vector. Mol. Hum. Reprod. 2003, 9, 603–609. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanemura, A.; Kiyohara, E.; Katayama, I.; Kaneda, Y. Recent advances and developments in the antitumor effect of the HVJ envelope vector on malignant melanoma: From the bench to clinical application. Cancer Gene. Ther. 2013, 20, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Nakamura, H.; Koyama, S.; Ogita, K.; Tabata, C.; Tsutsui, T.; Shimoya, K.; Koyama, M.; Kaneda, Y.; Murata, Y. In Vivo gene transfer into the mouse uterus: A powerful tool for investigating implantation physiology. J. Reprod. Immunol. 2005, 67, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Kimura, T.; Ogita, K.; Nakamura, H.; Tabata, C.; Ali, K.M.A.H.N.; Temma-Asano, K.; Shimoya, K.; Tsutsui, T.; Koyama, M.; et al. Simple and highly efficient method for transient in vivo gene transfer to mid-late pregnant mouse uterus. J. Reprod. Immunol. 2006, 70, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Charnock-Jones, D.S.; Sharkey, A.M.; Jaggers, D.C.; Yoo, H.J.; Heap, R.B.; Smith, S.K. In-Vivo gene transfer to the uterine endometrium. Hum. Reprod. 1997, 12, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Bagot, C.N.; Troy, P.J.; Taylor, H.S. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene. Ther. 2000, 7, 1378–1384. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Lin, C.S.; Sun, Y.L.; Chang, C.C.; Tsai, H.D.; Wu, J.C. In Vivo gene transfer of leukemia inhibitory factor (LIF) into mouse endometrium. J. Assist. Reprod. Genet. 2002, 19, 79–83. [Google Scholar] [CrossRef]
- Xu, B.; Geerts, D.; Qian, K.; Zhang, H.; Zhu, G. Myeloid ecotropic viral integration site 1 (MEIS) 1 involvement in embryonic implantation. Hum. Reprod. 2008, 23, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Laurema, A.; Lumme, S.; Heinonen, S.E.; Heinonen, S.; Yla-Herttuala, S. Transduction patterns and efficiencies in rabbit uterine tissues after intraluminal uterine adenovirus administration vary with the reproductive cycle. Acta. Obstet. Gynecol. Scand. 2007, 86, 1035–1040. [Google Scholar] [CrossRef]
- Tang, M.; Taylor, H.S.; Tabibzadeh, S. In Vivo gene transfer of lefty leads to implantation failure in mice. Hum. Reprod. 2005, 20, 1772–1778. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, R.; Endo, K.; Ohmori, Y.; Hondo, E. A novel method of gene transduction to the murine endometrium using in vivo electroporation. J. Vet. Med. Sci. 2017, 79, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K.; Nakamura, H.; Iijima, M.; Matsuzaki, T.; Somiya, M.; Kumasawa, K.; Kimura, T.; Kuroda, S. In Vivo uterine local gene delivery system using TAT-displaying bionanocapsules. J. Gene Med. 2019, 21, e3140. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kimura, T.; Koyama, S.; Ogita, K.; Tsutsui, T.; Shimoya, K.; Taniguchi, T.; Koyama, M.; Kaneda, Y.; Murata, Y. Mouse model of human infertility: Transient and local inhibition of endometrial STAT-3 activation results in implantation failure. FEBS Lett. 2006, 580, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kimura, T.; Ogita, K.; Koyama, S.; Tsujie, T.; Tsutsui, T.; Shimoya, K.; Koyama, M.; Kaneda, Y.; Murata, Y. Alteration of the timing of implantation by in vivo gene transfer: Delay of implantation by suppression of nuclear factor kappaB activity and partial rescue by leukemia inhibitory factor. Biochem. Biophys. Res. Commun. 2004, 321, 886–892. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. Role of the NF-kappaB pathway in the pathogenesis of human disease states. Curr. Mol. Med. 2001, 1, 287–296. [Google Scholar] [CrossRef]
- Nakamura, H.; Kimura, T.; Ogita, K.; Nakamura, T.; Takemura, M.; Shimoya, K.; Koyama, S.; Tsujie, T.; Koyama, M.; Murata, Y. NF-kappaB activation at implantation window of the mouse uterus. Am. J. Reprod. Immunol. 2004, 51, 16–21. [Google Scholar] [CrossRef]
- Beg, A.A.; Sha, W.C.; Bronson, R.T.; Ghosh, S.; Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 1995, 376, 167–170. [Google Scholar] [CrossRef]
- Sha, W.C.; Liou, H.C.; Tuomanen, E.I.; Baltimore, D. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 1995, 80, 321–330. [Google Scholar] [CrossRef]
- Huet, Y.M.; Dey, S.K. Role of early and late oestrogenic effects on implantation in the mouse. J. Reprod. Fertil. 1987, 81, 453–458. [Google Scholar] [CrossRef]
- Theiler, K. The House Mouse: Atlas of Embryonic Development, 1st ed.; Springer: Berlin, Germany, 1989. [Google Scholar]
- Stewart, C.L.; Kaspar, P.; Brunet, L.J.; Bhatt, H.; Gadi, I.; Köntgen, F.; Abbondanzo, S.J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992, 359, 76–79. [Google Scholar] [CrossRef]
- Takeda, K.; Noguchi, K.; Shi, W.; Tanaka, T.; Matsumoto, M.; Yoshida, N.; Kishimoto, T.; Akira, S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA 1997, 94, 3801–3804. [Google Scholar] [CrossRef]
- Lappas, M.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol. Reprod. 2002, 67, 668–673. [Google Scholar] [CrossRef]
- Lappas, M. Nuclear factor-kappaB mediates placental growth factor induced pro-labour mediators in human placenta. Mol. Hum. Reprod. 2012, 18, 354–361. [Google Scholar] [CrossRef]
- Lim, S.; MacIntyre, D.A.; Lee, Y.S.; Khanjani, S.; Terzidou, V.; Teoh, T.G.; Bennett, P.R. Nuclear factor kappa B activation occurs in the amnion prior to labour onset and modulates the expression of numerous labour associated genes. PLoS ONE 2012, 7, e34707. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz, A. The role of NFkappaB in the three stages of pregnancy—Implantation, maintenance, and labour: A review article. BJOG 2018, 125, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Hammes, S.R.; Levin, E.R. Extranuclear steroid receptors: Nature and actions. Endocr. Rev. 2007, 28, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Stice, J.P.; Knowlton, A.A. Estrogen, NFkappaB, and the heat shock response. Mol. Med. 2008, 14, 517–527. [Google Scholar] [CrossRef]
- Kalaitzidis, D.; Gilmore, T.D. Transcription factor cross-talk: The estrogen receptor and NF-kappaB. Trends Endocrinol. Metab. 2005, 16, 46–52. [Google Scholar] [CrossRef]
- Ray, A.; Prefontaine, K.E.; Ray, P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J. Biol. Chem. 1994, 269, 12940–12946. [Google Scholar] [CrossRef]
- Cerillo, G.; Rees, A.; Manchanda, N.; Reilly, C.; Brogan, I.; White, A.; Needham, M. The oestrogen receptor regulates NFkappaB and AP-1 activity in a cell-specific manner. J. Steroid. Biochem. Mol. Biol. 1998, 67, 79–88. [Google Scholar] [CrossRef]
- Valentine, J.E.; Kalkhoven, E.; White, R.; Hoare, S.; Parker, M.G. Mutations in the estrogen receptor ligand binding domain discriminate between hormone-dependent transactivation and transrepression. J. Biol. Chem. 2000, 275, 25322–25329. [Google Scholar] [CrossRef]
- Kalaitzidis, D.; Ok, J.; Sulak, L.; Starczynowski, D.T.; Gilmore, T.D. Characterization of a human REL-estrogen receptor fusion protein with a reverse conditional transforming activity in chicken spleen cells. Oncogene 2004, 23, 7580–7587. [Google Scholar] [CrossRef]
- Tzagarakis-Foster, C.; Geleziunas, R.; Lomri, A.; An, J.; Leitman, D.C. Estradiol represses human T-cell leukemia virus type 1 Tax activation of tumor necrosis factor-alpha gene transcription. J. Biol. Chem. 2002, 277, 44772–44777. [Google Scholar] [CrossRef] [PubMed]
- Harnish, D.C.; Scicchitano, M.S.; Adelman, S.J.; Lyttle, C.R.; Karathanasis, S.K. The role of CBP in estrogen receptor cross-talk with nuclear factor-kappaB in HepG2 cells. Endocrinology 2000, 141, 3403–3411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsu, S.M.; Chen, Y.C.; Jiang, M.C. 17 beta-estradiol inhibits tumor necrosis factor-alpha-induced nuclear factor-kappa B activation by increasing nuclear factor-kappa B p105 level in MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2000, 279, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Speir, E.; Yu, Z.X.; Takeda, K.; Ferrans, V.J.; Cannon, R.O., 3rd. Antioxidant effect of estrogen on cytomegalovirus-induced gene expression in coronary artery smooth muscle cells. Circulation 2000, 102, 2990–2996. [Google Scholar] [CrossRef]
- Chu, S.; Nishi, Y.; Yanase, T.; Nawata, H.; Fuller, P.J. Transrepression of estrogen receptor beta signaling by nuclear factor-kappab in ovarian granulosa cells. Mol. Endocrinol. 2004, 18, 1919–1928. [Google Scholar] [CrossRef][Green Version]
- Shyamala, G.; Guiot, M.C. Activation of kappa B-specific proteins by estradiol. Proc. Natl. Acad. Sci. USA 1992, 89, 10628–10632. [Google Scholar] [CrossRef]
- Xue, X.-T.; Kou, X.-X.; Li, C.; Bi, R.-Y.; Meng, Z.; Wang, X.-D.; Zhou, Y.-H.; Gan, Y.-H. Progesterone attenuates temporomandibular joint inflammation through inhibition of NF-kappaB pathway in ovariectomized rats. Sci. Rep. 2017, 7, 15334. [Google Scholar] [CrossRef]
- Davies, S.; Dai, D.; Feldman, I.; Pickett, G.; Leslie, K.K. Identification of a novel mechanism of NF-kappaB inactivation by progesterone through progesterone receptors in Hec50co poorly differentiated endometrial cancer cells: Induction of A20 and ABIN-2. Gynecol. Oncol. 2004, 94, 463–470. [Google Scholar] [CrossRef]
- Kalkhoven, E.; Wissink, S.; van der Saag, P.T.; van der Burg, B. Negative interaction between the RelA (p65) subunit of NF-kappaB and the progesterone receptor. J. Biol. Chem. 1996, 271, 6217–6224. [Google Scholar] [CrossRef]
- Pritchett, K.R.; Taft, R.A. Chapter 3—Reproductive Biology of the Laboratory Mouse in The Mouse in Biomedical Research. In Normative Biology, Husbandry, and Models, 2nd ed.; Fox, J.G., Davisson, M.T., Quimby, F.W., Barthold, S.W., Newcomer, C.E., Smith, A.L., Eds.; Academic Press: Cambridge, MA, USA, 2007; Volume III. [Google Scholar]
- Piekorz, R.P.; Gingras, S.; Hoffmeyer, A.; Ihle, J.N.; Weinstein, Y. Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20 alpha-hydroxysteroid dehydrogenase. Mol. Endocrinol. 2005, 19, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Paria, B.C.; Dey, S.K.; Das, S.K. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 1999, 140, 5310–5321. [Google Scholar] [CrossRef] [PubMed]
- Wetendorf, M.; Wu, S.-P.; Wang, X.; Creighton, C.J.; Wang, T.; Lanz, R.B.; Blok, L.; Tsai, S.Y.; Tsai, M.-J.; Lydon, J.P.; et al. Decreased epithelial progesterone receptor A at the window of receptivity is required for preparation of the endometrium for embryo attachment. Biol. Reprod. 2017, 96, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.P.; Li, R.; DeMayo, F.J. Progesterone Receptor Regulation of Uterine Adaptation for Pregnancy. Trends Endocrinol. Metab. 2018, 29, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.E.; Darnell, J.E., Jr. Stats: Transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002, 3, 651–662. [Google Scholar] [CrossRef]
- Stewart, C.L. Leukemia Inhibitory Factor. In Early Pregnancy and Implantation, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 405–411. [Google Scholar] [CrossRef]
- Robb, L.; Li, R.; Hartley, L.; Nandurkar, H.H.; Koentgen, F.; Begley, C.G. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat. Med. 1998, 4, 303–308. [Google Scholar] [CrossRef]
- Bilinski, P.; Roopenian, D.; Gossler, A. Maternal IL-11Ralpha function is required for normal decidua and fetoplacental development in mice. Genes. Dev. 1998, 12, 2234–2243. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.H.; Oh, S.J.; Yoo, J.; Akira, S.; Ku, B.J.; Lydon, J.P.; Jeong, J. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J. 2013, 27, 2553–2563. [Google Scholar] [CrossRef]
- Sun, X.; Bartos, A.; Whitsett, J.A.; Dey, S.K. Uterine deletion of Gp130 or Stat3 shows implantation failure with increased estrogenic responses. Mol. Endocrinol. 2013, 27, 1492–1501. [Google Scholar] [CrossRef]
- Pawar, S.; Starosvetsky, E.; Orvis, G.D.; Behringer, R.R.; Bagchi, I.C.; Bagchi, M.K. STAT3 regulates uterine epithelial remodeling and epithelial-stromal crosstalk during implantation. Mol. Endocrinol. 2013, 27, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Inglese, M.; Waring, P.; Campbell, I.K.; Bao, S.; Clay, F.J.; Alexander, W.S.; Wicks, I.P.; Tarlinton, D.; Novak, U.; et al. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J. Exp. Med. 2001, 194, 189–203. [Google Scholar] [CrossRef]
- Bhurke, A.S.; Bagchi, I.C.; Bagchi, M.K. Progesterone-Regulated Endometrial Factors Controlling Implantation. Am. J. Reprod. Immunol. 2016, 75, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-X.; Liu, L.; Jin, Z.-Y.; Liang, X.-H.; Fu, Y.-S.; Gu, X.-W.; Yang, Z.-M. The high concentration of progesterone is harmful for endometrial receptivity and decidualization. Sci. Rep. 2018, 8, 712. [Google Scholar] [CrossRef]
- Matsuo, M.; Hirota, Y.; Fukui, Y.; Fujita, H.; Saito-Fujita, T.; Kaku, T.; Gebril, M.; Hirata, T.; Akaeda, S.; Hiraoka, T.; et al. Levonorgestrel Inhibits Embryo Attachment by Eliminating Uterine Induction of Leukemia Inhibitory Factor. Endocrinology 2019, 161, bqz005. [Google Scholar] [CrossRef]
- Troude, P.; Bailly, E.; Guibert, J.; Bouyer, J.; de la Rochebrochard, E.; Group, D. Spontaneous pregnancies among couples previously treated by in vitro fertilization. Fertil. Steril. 2012, 98, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Soave, I.; Lo Monte, G.; Marci, R. Spontaneous pregnancy and unexplained infertility: A gift with many whys. N. Am. J. Med. Sci. 2012, 4, 512–513. [Google Scholar] [CrossRef] [PubMed]
- Lande, Y.; Seidman, D.S.; Maman, E.; Baum, M.; Dor, J.; Hourvitz, A. Spontaneous conceptions following successful ART are not associated with premature referral. Hum. Reprod. 2012, 27, 2380–2383. [Google Scholar] [CrossRef][Green Version]
- Volgsten, H.; Schmidt, L. Live birth outcome, spontaneous pregnancy and adoption up to five years after undergoing assisted reproductive technology treatment. Acta. Obstet. Gynecol. Scand. 2017, 96, 954–959. [Google Scholar] [CrossRef]
- Stanford, J.B.; Sanders, J.N.; Simonsen, S.E.; Hammoud, A.; Gibson, M.; Smith, K.R. Methods for a Retrospective Population-based and Clinic-based Subfertility Cohort Study: The Fertility Experiences Study. Paediatr. Perinat. Epidemiol. 2016, 30, 397–407. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Sharkey, A.M.; Tan, Y.L.; Salamonsen, L.A.; Sherwin, J.R. Immunolocalisation of phosphorylated STAT3, interleukin 11 and leukaemia inhibitory factor in endometrium of women with unexplained infertility during the implantation window. Reprod. Biol. Endocrinol. 2007, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, H.-R.; Lim, E.J.; Park, M.; Yoon, J.A.; Kim, Y.S.; Kim, E.-K.; Shin, J.-E.; Kim, J.H.; Kwon, H.; et al. Integrative Analyses of Uterine Transcriptome and MicroRNAome Reveal Compromised LIF-STAT3 Signaling and Progesterone Response in the Endometrium of Patients with Recurrent/Repeated Implantation Failure (RIF). PLoS ONE 2016, 11, e0157696. [Google Scholar] [CrossRef] [PubMed]
- Subramani, E.; Madogwe, E.; Ray, C.D.; Dutta, S.K.; Chakravarty, B.; Bordignon, V.; Duggavathi, R.; Chaudhury, K. Dysregulated leukemia inhibitory factor and its receptor regulated signal transducers and activators of transcription 3 pathway: A possible cause for repeated implantation failure in women with dormant genital tuberculosis? Fertil. Steril. 2016, 105, 1076–1084e5. [Google Scholar] [CrossRef] [PubMed][Green Version]

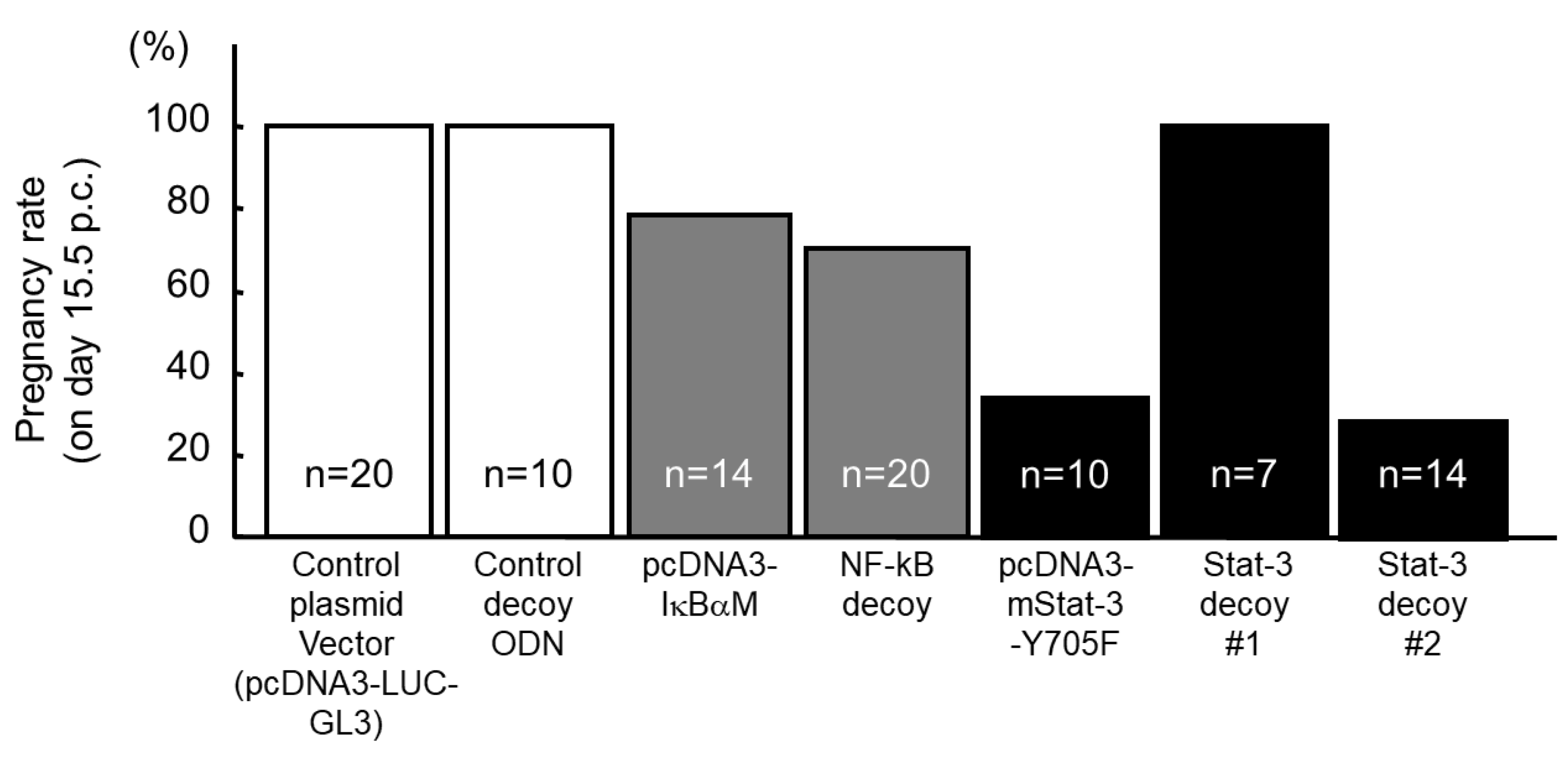
| NF-κB decoy | 5’-CCTTGAAGGGATTTCCCTCC-3’ 3’-GGAGGGAAATCCCTTCAAGG-5’ |
| Stat-3 decoy #1 | 5’-GATCCTTCTGGGAATTCCTAGATC-3’ 3’-CTAGGAAGACCCTTAAGGATCTAG-5’ |
| Stat-3 decoy #2 | 5’-CCTTCCGGGAATTCCTTCCGGGAATTC-3’ 3’-GGAAGGCCCTTAAGGAAGGCCCTTAAG-5’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, H.; Kimura, T. An In Vivo Screening Model for Investigation of Pathophysiology of Human Implantation Failure. Biomolecules 2023, 13, 79. https://doi.org/10.3390/biom13010079
Nakamura H, Kimura T. An In Vivo Screening Model for Investigation of Pathophysiology of Human Implantation Failure. Biomolecules. 2023; 13(1):79. https://doi.org/10.3390/biom13010079
Chicago/Turabian StyleNakamura, Hitomi, and Tadashi Kimura. 2023. "An In Vivo Screening Model for Investigation of Pathophysiology of Human Implantation Failure" Biomolecules 13, no. 1: 79. https://doi.org/10.3390/biom13010079
APA StyleNakamura, H., & Kimura, T. (2023). An In Vivo Screening Model for Investigation of Pathophysiology of Human Implantation Failure. Biomolecules, 13(1), 79. https://doi.org/10.3390/biom13010079





