The Regulators of Human Endometrial Stromal Cell Decidualization
Abstract
1. Introduction
2. Functions of Decidualization
3. Regulators for Decidualization
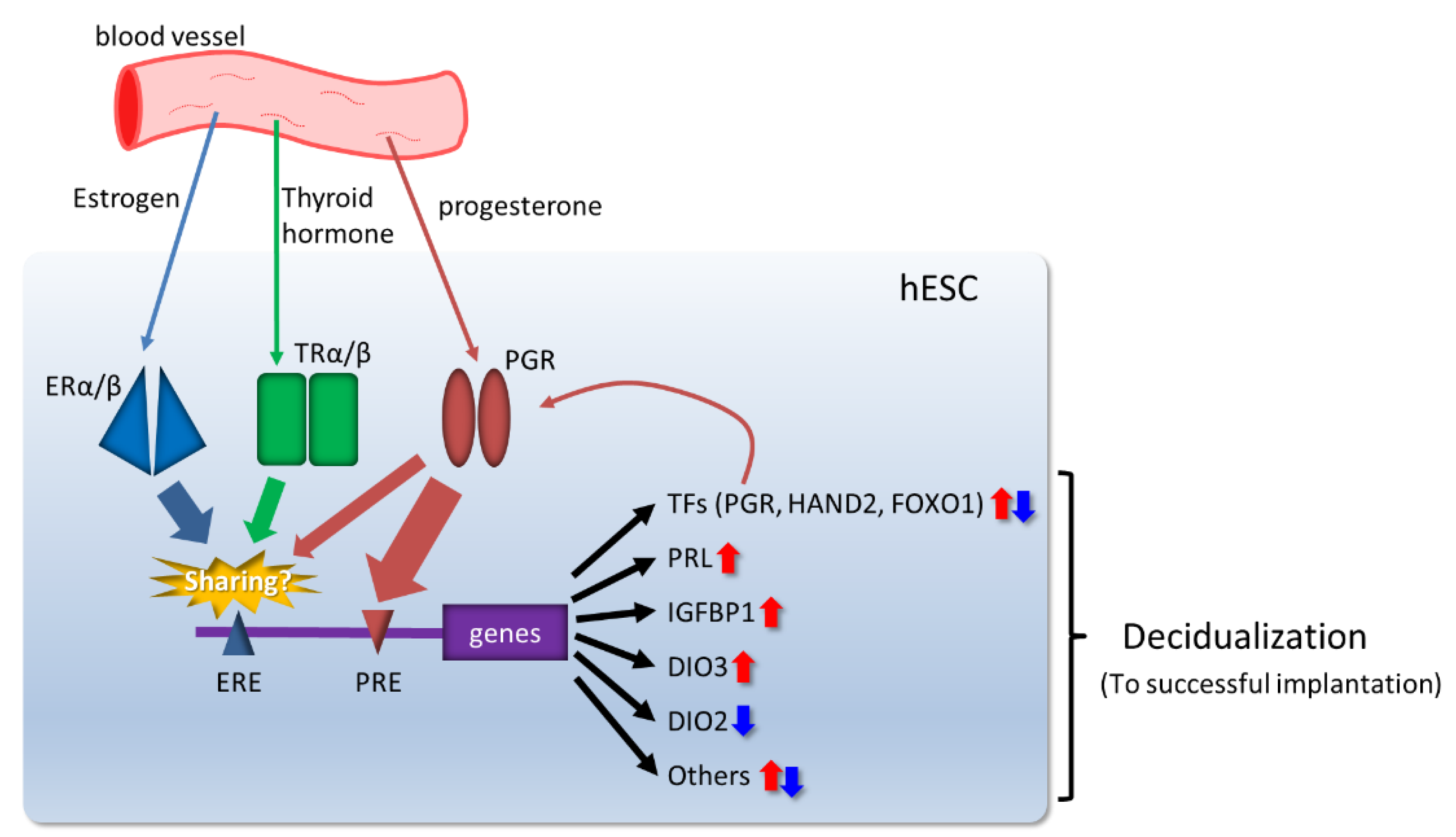
3.1. Ovarian Steroid Hormones
3.2. Transcriptional Regulators Induced by Ovarian Steroid Hormones in Decidualization
3.3. Immune Response in Endometrial Microenvironment
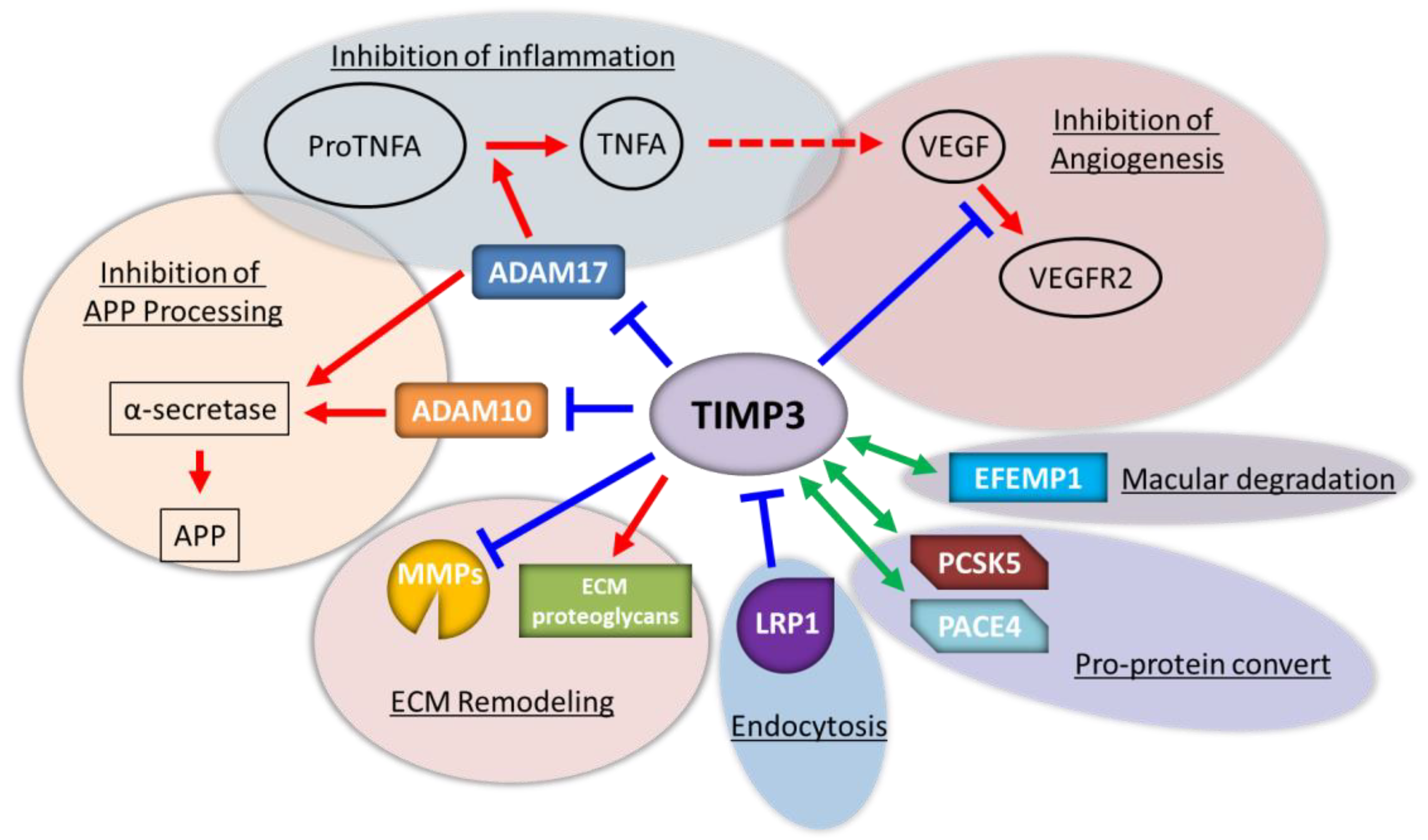
3.4. Matrix Metalloproteinases (MMPs)
3.5. Endometrial Vascular Remodeling and Maturation
3.6. Glucose Metabolism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gellersen, B.; Brosens, J. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Bernal, M.A.; Fazleabas, A.T. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int. J. Mol. Sci. 2020, 21, 1973. [Google Scholar] [CrossRef] [PubMed]
- Vento-Tormo, R.; Efremova, M.A.; Botting, R.; Turco, M.; Vento-Tormo, M.; Meyer, K.B.; Park, J.-E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, E.M.; Houston, M.L.; Harris, J.W. Interactions of the trophoblast and maternal tissues in three closely related primate species. Am. J. Obstet. Gynecol. 1976, 124, 647–652. [Google Scholar] [CrossRef]
- Henriet, P.; Chevronnay, H.P.G.; Marbaix, E. The endocrine and paracrine control of menstruation. Mol. Cell. Endocrinol. 2012, 358, 197–207. [Google Scholar] [CrossRef]
- Evans, J.; Salamonsen, L.A.; Winship, A.; Menkhorst, E.; Nie, G.; Gargett, C.E.; Dimitriadis, E. Fertile ground: Human endometrial programming and lessons in health and disease. Nat. Rev. Endocrinol. 2016, 12, 654–667. [Google Scholar] [CrossRef]
- Petracco, R.G.; Kong, A.; Grechukhina, O.; Krikun, G.; Taylor, H.S. Global Gene Expression Profiling of Proliferative Phase Endometrium Reveals Distinct Functional Subdivisions. Reprod. Sci. 2012, 19, 1138–1145. [Google Scholar] [CrossRef]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef]
- Schatz, F.; Guzeloglu-Kayisli, O.; Arlıer, S.; Kayisli, U.A.; Lockwood, C.J. The role of decidual cells in uterine hemostasis, menstruation, inflammation, adverse pregnancy outcomes and abnormal uterine bleeding. Hum. Reprod. Update 2016, 22, 497–515. [Google Scholar] [CrossRef]
- Su, R.-W.; Fazleabas, A.T. Implantation and Establishment of Pregnancy in Human and Nonhuman Primates. In Regulation of Implantation and Establishment of Pregnancy in Mammals; Advances in Anatomy, Embryology and Cell Biology; Springer: Cham, Switzerland, 2015; Volume 216, pp. 189–213. [Google Scholar] [CrossRef]
- Coulam, C. What about superfertility, decidualization, and natural selection? J. Assist. Reprod. Genet. 2016, 33, 577–580. [Google Scholar] [CrossRef]
- Kajihara, T.; Tanaka, K.; Oguro, T.; Tochigi, H.; Prechapanich, J.; Uchino, S.; Itakura, A.; Šućurović, S.; Murakami, K.; Brosens, J.J.; et al. Androgens Modulate the Morphological Characteristics of Human Endometrial Stromal Cells Decidualized In Vitro. Reprod. Sci. 2013, 21, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Essah, P.; Cheang, K.; Nestler, J. The Pathophysiology of Miscarriage in Women with Polycystic Ovary Syndrome. Review and Proposed Hypothesis of Mechanisms Involved. Hormones 2004, 3, 221–227. [Google Scholar] [CrossRef]
- Gardiner, P.J. Characterization of prostanoid relaxant/inhibitory receptors (psi) using a highly selective agonist, TR4979. Br. J. Pharmacol. 1986, 87, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.C.; Christiansen, O.B.; Kolte, A.M.; Macklon, N. New insights into mechanisms behind miscarriage. BMC Med. 2013, 11, 154. [Google Scholar] [CrossRef]
- Okada, H.; Nakajima, T.; Yoshimura, T.; Yasuda, K.; Kanzaki, H. The inhibitory effect of dienogest, a synthetic steroid, on the growth of human endometrial stromal cells in vitro. Mol. Hum. Reprod. 2001, 7, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Nie, G.; Salamonsen, L.A. Requirement for proprotein convertase 5/6 during decidualization of human endometrial stromal cells in vitro. J. Clin. Endocrinol. Metab. 2005, 90, 1028–1034. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gellersen, B.; Brosens, J.; Salfen, B.; Carroll, J.; Keisler, D. Cyclic AMP and progesterone receptor cross-talk in human endometrium: A decidualizing affair. J. Endocrinol. 2003, 178, 357–372. [Google Scholar] [CrossRef]
- Kajihara, T.; Brosens, J.J.; Ishihara, O. The role of FOXO1 in the decidual transformation of the endometrium and early pregnancy. Med. Mol. Morphol. 2013, 46, 61–68. [Google Scholar] [CrossRef]
- Ujvari, D.; Jakson, I.; Babayeva, S.; Salamon, D.; Rethi, B.; Gidlöf, S.; Hirschberg, A.L. Dysregulation of In Vitro Decidualization of Human Endometrial Stromal Cells by Insulin via Transcriptional Inhibition of Forkhead Box Protein O1. PLoS ONE 2017, 12, e0171004. [Google Scholar] [CrossRef]
- Popovici, R.M.; Kao, L.C.; Giudice, L.C. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology 2000, 141, 3510–3513. [Google Scholar] [CrossRef]
- Shindoh, H.; Okada, H.; Tsuzuki, T.; Nishigaki, A.; Kanzaki, H. Requirement of heart and neural crest derivatives–expressed transcript 2 during decidualization of human endometrial stromal cells in vitro. Fertil. Steril. 2014, 101, 1781–1790.e5. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.; Yin, Y.; Ma, L. Development and utilization of human decidualization reporter cell line uncovers new modulators of female fertility. Proc. Natl. Acad. Sci. USA 2019, 116, 19541–19551. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Tanaka, S.; Okada, H. Immune Tolerance of the Human Decidua. J. Clin. Med. 2021, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef] [PubMed]
- Lucas, E.S.; Dyer, N.P.; Murakami, K.; Lee, Y.H.; Chan, Y.-W.; Grimaldi, G.; Muter, J.; Brighton, P.J.; Moore, J.D.; Patel, G.; et al. Loss of Endometrial Plasticity in Recurrent Pregnancy Loss. Stem Cells 2015, 34, 346–356. [Google Scholar] [CrossRef]
- Lucas, E.S.; Vrljicak, P.; Muter, J.; Diniz-da-Costa, M.M.; Brighton, P.J.; Kong, C.S.; Lipecki, J.; Fishwick, K.J.; Odendaal, J.; Ewington, L.J.; et al. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun. Biol. 2020, 3, 37. [Google Scholar] [CrossRef]
- Garrido-Gomez, T.; Dominguez, F.; Quinonero, A.; Diaz-Gimeno, P.; Kapidzic, M.; Gormley, M.; Ona, K.; Padilla-Iserte, P.; McMaster, M.; Genbacev, O.; et al. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc. Natl. Acad. Sci. USA 2017, 114, E8468–E8477. [Google Scholar] [CrossRef]
- Wu, D.; Kimura, F.; Zheng, L.; Ishida, M.; Niwa, Y.; Hirata, K.; Takebayashi, A.; Takashima, A.; Takahashi, K.; Kushima, R.; et al. Chronic endometritis modifies decidualization in human endometrial stromal cells. Reprod. Biol. Endocrinol. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Edmondson, N.; Bocking, A.; Machin, G.; Rizek, R.; Watson, C.; Keating, S. The Prevalence of Chronic Deciduitis in Cases of Preterm Labor without Clinical Chorioamnionitis. Pediatr. Dev. Pathol. 2009, 12, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 2017, 6, e31274. [Google Scholar] [CrossRef] [PubMed]
- Kusama, K.; Yoshie, M.; Tamura, K.; Nakayama, T.; Nishi, H.; Isaka, K.; Tachikawa, E. The Role of Exchange Protein Directly Activated by Cyclic AMP 2-mediated Calreticulin Expression in the Decidualization of Human Endometrial Stromal Cells. Endocrinology 2014, 155, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Cha, J.; Yoshie, M.; Daikoku, T.; Dey, S.K. Heightened uterine mammalian target of rapamycin complex 1 (mTORC1) signaling provokes preterm birth in mice. Proc. Natl. Acad. Sci. USA 2011, 108, 18073–18078. [Google Scholar] [CrossRef]
- Hirota, Y.; Daikoku, T.; Tranguch, S.; Xie, H.; Bradshaw, H.B.; Dey, S.K. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J. Clin. Investig. 2010, 120, 803–815. [Google Scholar] [CrossRef]
- Campisi, J.; Dimri, G.; Hara, E. Handbook of the Biology of Aging; Academic Press: New York, NY, USA, 1996; pp. 121–149. [Google Scholar]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Borodkina, A.V.; Deryabin, P.I.; Giukova, A.A.; Nikolsky, N.N. “Social Life” of Senescent Cells: What Is SASP and Why Study It? Acta Nat. 2018, 10, 4–14. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Ser. Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Borghesan, M.; Hoogaars, W.M.H.; Varela-Eirin, M.; Talma, N.; Demaria, M. A Senescence-Centric View of Aging: Implications for Longevity and Disease. Trends Cell Biol. 2020, 30, 777–791. [Google Scholar] [CrossRef]
- Karin, O.; Alon, U. Senescent cell accumulation mechanisms inferred from parabiosis. GeroScience 2020, 43, 329–341. [Google Scholar] [CrossRef]
- Peter Durairaj, R.R.; Aberkane, A.; Polanski, L.; Maruyama, Y.; Baumgarten, M.; Lucas, E.S.; Quenby, S.; Chan, J.K.Y.; Raine-Fenning, N.; Brosens, J.J.; et al. Deregulation of the endometrial stromal cell secretome precedes embryo implantation failure. Mol. Hum. Reprod. 2017, 23, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Tomari, H.; Kawamura, T.; Asanoma, K.; Egashira, K.; Kawamura, K.; Honjo, K.; Nagata, Y.; Kato, K. Contribution of senescence in human endometrial stromal cells during proliferative phase to embryo receptivity. Biol. Reprod. 2020, 103, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, P.I.; Borodkina, A.V. Stromal cell senescence contributes to impaired endometrial decidualization and defective interaction with trophoblast cells. Hum. Reprod. 2022, 37, 1505–1524. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Jiang, Y.; He, H.; Ni, H.; Tu, Z.; Zhang, S.; Wang, B.; Lou, J.; Quan, S.; Wang, H. NEDD8-mediated neddylation is required for human endometrial stromal proliferation and decidualization. Hum. Reprod. 2015, 30, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Bartos, A.; Egashira, M.; Haraguchi, H.; Saito-Fujita, T.; Leishman, E.; Bradshaw, H.; Dey, S.K.; Hirota, Y. Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. J. Clin. Investig. 2013, 123, 4063–4075. [Google Scholar] [CrossRef]
- Ochiai, A.; Kuroda, K.; Ozaki, R.; Ikemoto, Y.; Murakami, K.; Muter, J.; Matsumoto, A.; Itakura, A.; Brosens, J.J.; Takeda, S. Resveratrol inhibits decidualization by accelerating downregulation of the CRABP2-RAR pathway in differentiating human endometrial stromal cells. Cell Death Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Ochiai, A.; Kuroda, K.; Ikemoto, Y.; Ozaki, R.; Nakagawa, K.; Nojiri, S.; Takeda, S.; Sugiyama, R. Influence of resveratrol supplementation on IVF–embryo transfer cycle outcomes. Reprod. Biomed. Online 2019, 39, 205–210. [Google Scholar] [CrossRef]
- Kuroda, K.; Ochiai, A.; Brosens, J.J. The actions of resveratrol in decidualizing endometrium: Acceleration or inhibition? Biol. Reprod. 2020, 103, 1152–1156. [Google Scholar] [CrossRef]
- Sharma, S.; Godbole, G.; Modi, D. Decidual Control of Trophoblast Invasion. Am. J. Reprod. Immunol. 2016, 75, 341–350. [Google Scholar] [CrossRef]
- Wetendorf, M.; DeMayo, F.J. The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol. Cell. Endocrinol. 2012, 357, 108–118. [Google Scholar] [CrossRef]
- Maruyama, T.; Yoshimura, Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr. J. 2008, 55, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.J.; Arao, Y.; Korach, K.S. Estrogen hormone physiology: Reproductive findings from estrogen receptor mutant mice. Reprod. Biol. 2013, 14, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Koos, R.D. Minireview: Putting Physiology Back into Estrogens’ Mechanism of Action. Endocrinology 2011, 152, 4481–4488. [Google Scholar] [CrossRef] [PubMed]
- Bhurke, A.S.; Bagchi, I.C.; Bagchi, M.K. Progesterone-Regulated Endometrial Factors Controlling Implantation. Am. J. Reprod. Immunol. 2016, 75, 237–245. [Google Scholar] [CrossRef]
- Conneely, O.M.; Mulac-Jericevic, B.; DeMayo, F.; Lydon, J.P.; O’Malley, B.W. Reproductive functions of progesterone receptors. Recent Prog. Horm. Res. 2002, 57, 339–355. [Google Scholar] [CrossRef]
- Large, M.J.; DeMayo, F.J. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol. Cell. Endocrinol. 2011, 358, 155–165. [Google Scholar] [CrossRef]
- Wang, W.; Taylor, R.N.; Bagchi, I.C.; Bagchi, M.K. Regulation of human endometrial stromal proliferation and differentiation by C/EBPbeta involves cyclin E-cdk2 and STAT3. Mol. Endocrinol. 2012, 26, 2016–2030. [Google Scholar] [CrossRef]
- Mantena, S.R.; Kannan, A.; Cheon, Y.P.; Li, Q.; Johnson, P.F.; Bagchi, I.C.; Bagchi, M.K. C/EBPbeta is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc. Natl. Acad. Sci. USA 2006, 103, 1870–1875. [Google Scholar] [CrossRef]
- Wei, Q.; Clair, J.B.S.; Fu, T.; Stratton, P.; Nieman, L.K. Reduced expression of biomarkers associated with the implantation window in women with endometriosis. Fertil. Steril. 2009, 91, 1686–1691. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb. Perspect. Med. 2015, 6, a023002. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Edelshain, B.; Schatz, F.; Lockwood, C.J.; Taylor, H.S. FKBP4 is regulated by HOXA10 during decidualization and in endometriosis. Reproduction 2012, 143, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, Y.; Mazur, E.C.; Lichun, J.; Kommagani, R.; Jiang, L.; Chen, R.; Lanz, R.B.; Kovanci, E.; Gibbons, W.E.; DeMayo, F.J. FOXO1 is Required for Binding of PR on IRF4, Novel Transcriptional Regulator of Endometrial Stromal Decidualization. Mol. Endocrinol. 2015, 29, 421–433. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, X.; Chen, J.; Jiang, Y.; Zhang, Q.; Kong, C.; Xing, J.; Ding, L.; Diao, Z.; Zhen, X.; et al. Krüppel-like factor 12 is a novel negative regulator of forkhead box O1 expression: A potential role in impaired decidualization. Reprod. Biol. Endocrinol. 2015, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Tanaka, S.; Hisamatsu, Y.; Tsubokura, H.; Hashimoto, Y.; Kitada, M.; Okada, H. Transcriptional regulation of LGALS9 by HAND2 and FOXO1 in human endometrial stromal cells in women with regular cycles. Mol. Hum. Reprod. 2021. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Tanaka, S.; Tsuzuki-Nakao, T.; Kido, T.; Kakita-Kobayashi, M.; Kida, N.; Hisamatsu, Y.; Tsubokura, H.; Hash-imoto, Y.; Kitada, M.; et al. The transcription factor HAND2 up-regulates transcription of the IL15 gene in human endometrial stromal cells. J. Biol. Chem. 2020, 295, 9596–9605. [Google Scholar] [CrossRef]
- Huyen, D.V.; Bany, B.M. Evidence for a conserved function of heart and neural crest derivatives expressed transcript 2 in mouse and human decidualization. Reproduction 2011, 142, 353–368. [Google Scholar] [CrossRef]
- Li, Q.; Kannan, A.; DeMayo, F.J.; Lydon, J.P.; Cooke, P.S.; Yamagishi, H.; Srivastava, D.; Bagchi, M.K.; Bagchi, I.C. The Antiproliferative Action of Progesterone in Uterine Epithelium Is Mediated by Hand2. Science 2011, 331, 912–916. [Google Scholar] [CrossRef]
- Fukuda, T.; Shirane, A.; Wada-Hiraike, O.; Oda, K.; Tanikawa, M.; Sakuabashi, A.; Hirano, M.; Fu, H.; Morita, Y.; Miyamoto, Y.; et al. HAND2-mediated proteolysis negatively regulates the function of estrogen receptor α. Mol. Med. Rep. 2015, 12, 5538–5544. [Google Scholar] [CrossRef][Green Version]
- Mestre-Citrinovitz, A.C.; Kleff, V.; Vallejo, G.; Winterhager, E.; Saragueta, P. A Suppressive Antagonism Evidences Progesterone and Estrogen Receptor Pathway Interaction with Concomitant Regulation of Hand2, Bmp2 and ERK during Early Decidualization. PLoS ONE 2015, 10, e0124756. [Google Scholar]
- Sucurovic, S.; Nikolic, T.; Brosens, J.J.; Mulac-Jericevic, B. Analysis of heart and neural crest derivatives-expressed protein 2 (HAND2)-progesterone interactions in peri-implantation endometriumdagger. Biol. Reprod. 2020, 102, 1111–1121. [Google Scholar] [CrossRef]
- Marinic, M.; Mika, K.; Chigurupati, S.; Lynch, V.J. Evolutionary transcriptomics implicates HAND2 in the origins of implantation and regulation of gestation length. Elife 2021, 10, e61257. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Okada, H.; Tsuzuki, T.; Nishigaki, A.; Yasuda, K.; Kanzaki, H. Progestin-induced heart and neural crest derivatives expressed transcript 2 is associated with fibulin-1 expression in human endometrial stromal cells. Fertil. Steril. 2012, 99, 248–255.e2. [Google Scholar] [CrossRef] [PubMed]
- Firulli, B.A.; Krawchuk, D.; Centonze, V.E.; Vargesson, N.; Virshup, D.M.; Conway, S.J.; Cserjesi, P.; Laufer, E.; Firulli, A.B. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nat. Genet. 2005, 37, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Colicchia, M.; Campagnolo, L.; Baldini, E.; Ulisse, S.; Valensise, H.; Moretti, C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Hum. Reprod. Updat. 2014, 20, 884–904. [Google Scholar] [CrossRef]
- Stagnaro-Green, A.; Pearce, E. Thyroid disorders in pregnancy. Nat. Rev. Endocrinol. 2012, 8, 650–658. [Google Scholar] [CrossRef]
- Velkeniers, B.; Van Meerhaeghe, A.; Poppe, K.; Unuane, D.; Tournaye, H.; Haentjens, P. Levothyroxine treatment and pregnancy outcome in women with subclinical hypothyroidism undergoing assisted reproduction technologies: Systematic review and meta-analysis of RCTs. Hum. Reprod. Updat. 2013, 19, 251–258. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeold, A.; Bianco, A.C. Cellular and mo-lecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef]
- Kakita-Kobayashi, M.; Murata, H.; Nishigaki, A.; Hashimoto, Y.; Komiya, S.; Tsubokura, H.; Kido, T.; Kida, N.; Tsuzuki-Nakao, T.; Matsuo, Y.; et al. Thyroid Hormone Facilitates in vitro Decidualization of Human Endometrial Stromal Cells via Thyroid Hormone Receptors. Endocrinology 2020, 161, bqaa049. [Google Scholar] [CrossRef]
- Ren, B.; Zhu, Y. A New Perspective on Thyroid Hormones: Crosstalk with Reproductive Hormones in Females. Int. J. Mol. Sci. 2022, 23, 2708. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.C.; Salazar, E.P.; Kane, S.R.; Liu, N. Effects of thyroid hormones on human breast cancer cell proliferation. J. Steroid Biochem. Mol. Biol. 2008, 109, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Maruo, T.; Matsuo, H.; Mochizuki, M. Thyroid hormone as a biological amplifier of differentiated trophoblast function in early pregnancy. Eur. J. Endocrinol. 1991, 125, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Galton, V.A.; Martinez, E.; Hernandez, A.; Germain, E.A.S.; Bates, J.M.; Germain, D.L.S. Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J. Clin. Investig. 1999, 103, 979–987. [Google Scholar] [CrossRef]
- Vinketova, K.; Mourdjeva, M.; Oreshkova, T. Human Decidual Stromal Cells as a Component of the Implantation Niche and a Modulator of Maternal Immunity. J. Pregnancy 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, C.J.; Kim, D.-J.; Kang, J.-H. Immune Cells in the Female Reproductive Tract. Immune Netw. 2015, 15, 16–26. [Google Scholar] [CrossRef]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- Sojka, D.K.; Plougastel-Douglas, B.; Yang, L.; Pak-Wittel, M.A.; Artyomov, M.N.; Ivanova, Y.; Zhong, C.; Chase, J.; Rothman, P.B.; Yu, J.; et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 2014, 3, e01659. [Google Scholar] [CrossRef]
- Mahajan, D.; Sharma, N.R.; Kancharla, S.; Kolli, P.; Tripathy, A.; Sharma, A.K.; Singh, S.; Kumar, S.; Mohanty, A.K.; Jena, M.K. Role of Natural Killer Cells during Pregnancy and Related Complications. Biomolecules 2022, 12, 68. [Google Scholar] [CrossRef]
- Sojka, D.K.; Yang, L.; Yokoyama, W.M. Uterine Natural Killer Cells. Front. Immunol. 2019, 10, 960. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, S.; Zhao, Y.; Wang, H.; Pan, Q.; Shao, Q. Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy. Front. Immunol. 2021, 12, 1684. [Google Scholar] [CrossRef] [PubMed]
- Crespo, C.; Mulik, S.; Dotiwala, F.; Ansara, J.A.; Santara, S.S.; Ingersoll, K.; Ovies, C.; Junqueira, C.; Tilburgs, T.; Strominger, J.L.; et al. Decidual NK Cells Transfer Granulysin to Selectively Kill Bacteria in Trophoblasts. Cell 2020, 182, 1125–1139.e18. [Google Scholar] [CrossRef]
- Jabrane-Ferrat, N. Features of Human Decidual NK Cells in Healthy Pregnancy and During Viral Infection. Front. Immunol. 2019, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.E.; Fraser, R.; Gurung, S.; Goulwara, S.S.; Whitley, G.S.; Johnstone, A.P.; Cartwright, J.E. Increased angiogenic factor secretion by decidual natural killer cells from pregnancies with high uterine artery resistance alters trophoblast function. Hum. Reprod. 2014, 29, 652–660. [Google Scholar] [CrossRef]
- Montaldo, E.; Vacca, P.; Chiossone, L.; Croxatto, D.; Loiacono, F.; Martini, S.; Ferrero, S.; Walzer, T.; Moretta, L.; Mingari, M.C. Unique Eomes+ NK Cell Subsets Are Present in Uterus and Decidua During Early Pregnancy. Front. Immunol. 2016, 6, 646. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; Rajakumar, A.; Royle, C.M.; Lo, A.; Husain, Z.; Thadhani, R.I.; Sukhatme, V.P.; Karumanchi, S.A.; Kopcow, H.D. Conversion of Peripheral Blood NK Cells to a Decidual NK-like Phenotype by a Cocktail of Defined Factors. J. Immunol. 2013, 190, 3939–3948. [Google Scholar] [CrossRef]
- Vacca, P.; Moretta, L.; Moretta, A.; Mingari, M.C. Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol. 2011, 32, 517–523. [Google Scholar] [CrossRef]
- Chazara, O.; Xiong, S.; Moffett, A. Maternal KIR and fetal HLA-C: A fine balance. J. Leukoc. Biol. 2011, 90, 703–716. [Google Scholar] [CrossRef]
- Ticconi, C.; Pietropolli, A.; Di Simone, N.; Piccione, E.; Fazleabas, A. Endometrial Immune Dysfunction in Recurrent Pregnancy Loss. Int. J. Mol. Sci. 2019, 20, 5332. [Google Scholar] [CrossRef]
- Vujaklija, D.V.; Gulic, T.; Sucic, S.; Nagata, K.; Ogawa, K.; Laskarin, G.; Saito, S.; Haller, H.; Rukavina, D. First Trimester Pregnancy Decidual Natural Killer Cells Contain and Spontaneously Release High Quantities of Granulysin. Am. J. Reprod. Immunol. 2011, 66, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kopcow, H.; Eriksson, M.; Mselle, T.; Damrauer, S.; Wira, C.; Sentman, C.; Strominger, J. Human Decidual NK Cells from Gravid Uteri and NK Cells from Cycling Endometrium are Distinct NK Cell Subsets. Placenta 2010, 31, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Yamaguchi, T.; Honjo, H. Central Role of Interleukin-15 in Postovulatory Recruitment of Peripheral Blood CD16(−) Natural Killer Cells into Human Endometrium. J. Clin. Endocrinol. Metab. 2005, 90, 2932–2940. [Google Scholar] [CrossRef] [PubMed]
- Barber, E.M.; Pollard, J.W. The Uterine NK Cell Population Requires IL-15 but These Cells Are Not Required for Pregnancy nor the Resolution of a Listeria monocytogenes Infection. J. Immunol. 2003, 171, 37–46. [Google Scholar] [CrossRef]
- Henderson, T.A.; Saunders, P.; Moffett-King, A.; Groome, N.P.; Critchley, H.O.D. Steroid Receptor Expression in Uterine Natural Killer Cells. J. Clin. Endocrinol. Metab. 2003, 88, 440–449. [Google Scholar] [CrossRef]
- Okada, S.; Okada, H.; Sanezumi, M.; Nakajima, T.; Yasuda, K.; Kanzaki, H. Expression of interleukin-15 in human endometrium and decidua. Mol. Hum. Reprod. 2000, 6, 75–80. [Google Scholar] [CrossRef]
- Okada, H.; Nakajima, T.; Yasuda, K.; Kanzaki, H. Interleukin-1 inhibits interleukin-15 production by progesterone during in vitro decidualization in human. J. Reprod. Immunol. 2004, 61, 3–12. [Google Scholar] [CrossRef]
- Burton, G.; Woods, A.; Jauniaux, E.; Kingdom, J. Rheological and Physiological Consequences of Conversion of the Maternal Spiral Arteries for Uteroplacental Blood Flow during Human Pregnancy. Placenta 2009, 30, 473–482. [Google Scholar] [CrossRef]
- Cao, E.; Zang, X.; Ramagopal, U.A.; Mukhopadhaya, A.; Fedorov, A.; Fedorov, E.; Zencheck, W.D.; Lary, J.W.; Cole, J.L.; Deng, H.; et al. T Cell Immunoglobulin Mucin-3 Crystal Structure Reveals a Galectin-9-Independent Ligand-Binding Surface. Immunity 2007, 26, 311–321. [Google Scholar] [CrossRef]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- Seki, M.; Oomizu, S.; Sakata, K.M.; Sakata, A.; Arikawa, T.; Watanabe, K.; Ito, K.; Takeshita, K.; Niki, T.; Saita, N.; et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin. Immunol. 2008, 127, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Alluqmani, N.; Buhari, F.H.M.; Wasim, L.; Smith, L.K.; Quaile, A.T.; Shannon, M.; Hakim, Z.; Furmli, H.; Owen, D.M.; et al. Galectin-9 binds IgM-BCR to regulate B cell signaling. Nat. Commun. 2018, 9, 3288. [Google Scholar] [CrossRef]
- Giovannone, N.; Liang, J.; Antonopoulos, A.; Sweeney, J.G.; King, S.L.; Pochebit, S.M.; Bhattacharyya, N.; Lee, G.S.; Dell, A.; Widlund, H.R.; et al. Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans. Nat. Commun. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Thalhamer, T.; Franca, R.F.; Xiao, S.; Wang, C.; Hotta, C.; Zhu, C.; Hirashima, M.; Anderson, A.C.; Kuchroo, V.K. Galectin-9-CD44 Interaction Enhances Stability and Function of Adaptive Regulatory T Cells. Immunity 2014, 41, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhou, W.H.; Tao, Y.; Wang, S.C.; Jiang, Y.L.; Zhang, D.; Piao, H.L.; Fu, Q.; Li, D.J.; Du, M.R. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal-fetal interface in early pregnancy. Cell Mol. Immunol. 2016, 13, 73–81. [Google Scholar] [CrossRef]
- Genbacev, O.; Schubach, S.A.; Miller, R.K. Villous culture of first trimester human placenta-model to study extravillous trophoblast (EVT) differentiation. Placenta 1992, 13, 439–461. [Google Scholar] [CrossRef]
- Menkhorst, E.M.; Van Sinderen, M.L.; Rainczuk, K.; Cuman, C.; Winship, A.; Dimitriadis, E. Invasive trophoblast promote stromal fibroblast decidualization via Profilin 1 and ALOX5. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Halari, C.D.; Nandi, P.; Jeyarajah, M.J.; Renaud, S.J.; Lala, P.K. Decorin production by the human decidua: Role in decidual cell maturation. Mol. Hum. Reprod. 2020, 26, 784–796. [Google Scholar] [CrossRef]
- Pollheimer, J.; Fock, V.; Knofler, M. Review: The ADAM metalloproteinases-novel regulators of trophoblast invasion? Placenta 2014, S57–S63. [Google Scholar] [CrossRef]
- Hisamatsu, Y.; Murata, H.; Tsubokura, H.; Hashimoto, Y.; Kitada, M.; Tanaka, S.; Okada, H. Matrix Metalloproteinases in Human Decidualized Endometrial Stromal Cells. Curr. Issues Mol. Biol. 2021, 43, 146. [Google Scholar] [CrossRef]
- Mazur, E.C.; Vasquez, Y.; Lichun, J.; Kommagani, R.; Jiang, L.; Chen, R.; Lanz, R.B.; Kovanci, E.; Gibbons, W.E.; DeMayo, F.J. Progesterone Receptor Transcriptome and Cistrome in Decidualized Human Endometrial Stromal Cells. Endocrinology 2015, 156, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Mattos, R.; Staples, C.R.; Thatcher, W.W. Effects of dietary fatty acids on reproduction in ruminants. Rev. Reprod. 2000, 5, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Killeen, A.P.; Morris, D.G.; Kenny, D.A.; Mullen, M.P.; Diskin, M.G.; Waters, S.M. Global gene expression in endometrium of high and low fertility heifers during the mid-luteal phase of the estrous cycle. BMC Genom. 2014, 15, 234. [Google Scholar] [CrossRef]
- Chen, C.; Li, C.; Liu, W.; Guo, F.; Kou, X.; Sun, S.; Ye, T.; Li, S.; Zhao, A. Estrogen-induced FOS-like 1 regulates matrix metalloproteinase expression and the motility of human endometrial and decidual stromal cells. J. Biol. Chem. 2020, 295, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; van Mil, A.; Aguor, E.N.; Siddiqi, S.; Vrijsen, K.; Jaksani, S.; Metz, C.; Zhao, J.; Strijkers, G.J.; Doevendans, P.A.; et al. MiR-155 inhibits cell migration of human cardiomyocyte progenitor cells (hCMPCs) via targeting of MMP-16. J. Cell Mol. Med. 2012, 16, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.H.; Ebrahem, Q.; Moore, N.; Murphy, G.; Claesson-Welsh, L.; Bond, M.; Baker, A.; Anand-Apte, B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): Inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat. Med. 2003, 9, 407–415. [Google Scholar] [CrossRef]
- Menghini, R.; Fiorentino, L.; Casagrande, V.; Lauro, R.; Federici, M. The role of ADAM17 in metabolic inflammation. Atherosclerosis 2013, 228, 12–17. [Google Scholar] [CrossRef]
- Amour, A.; Knight, C.; Webster, A.; Slocombe, P.M.; Stephens, P.E.; Knauper, V.; Docherty, A.J.; Murphy, G. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000, 473, 275–279. [Google Scholar] [CrossRef]
- Hoe, H.-S.; Cooper, M.J.; Burns, M.P.; Lewis, P.; Van Der Brug, M.; Chakraborty, G.; Cartagena, C.M.; Pak, D.T.S.; Cookson, M.R.; Rebeck, G.W. The Metalloprotease Inhibitor TIMP-3 Regulates Amyloid Precursor Protein and Apolipoprotein E Receptor Proteolysis. J. Neurosci. 2007, 27, 10895–10905. [Google Scholar] [CrossRef]
- Walter, L.; Rogers, P.A.W.; Girling, J. The role of progesterone in endometrial angiogenesis in pregnant and ovariectomised mice. Reproduction 2005, 129, 765–777. [Google Scholar] [CrossRef][Green Version]
- Girling, J.E.; Lederman, F.L.; Walter, L.M.; Rogers, P.A.W. Progesterone, But Not Estrogen, Stimulates Vessel Maturation in the Mouse Endometrium. Endocrinology 2007, 148, 5433–5441. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.; Bartlett, M.K. Biopsy studies of human endometrium: Criteria of dating and information about amenorrhea, men-orrhagia, and time of ovulation. J. Am. Med. Assoc. 1937, 108, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Gaengel, K.; Genové, G.; Armulik, A.; Betsholtz, C. Endothelial-mural cell signaling in vascular development and angio-genesis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Girling, J.E.; Rogers, P.A.W. Regulation of endometrial vascular remodelling: Role of the vascular endothelial growth factor family and the angiopoietin–TIE signalling system. Reproduction 2009, 138, 883–893. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef]
- Kida, N.; Nishigaki, A.; Kakita-Kobayashi, M.; Tsubokura, H.; Hashimoto, Y.; Yoshida, A.; Hisamatsu, Y.; Tsuzuki-Nakao, T.; Murata, H.; Okada, H. Exposure to cigarette smoke affects endometrial maturation including angiogenesis and decidualization. Reprod. Med. Biol. 2021, 20, 108–118. [Google Scholar] [CrossRef]
- Kida, N.; Matsuo, Y.; Hashimoto, Y.; Nishi, K.; Tsuzuki-Nakao, T.; Bono, H.; Maruyama, T.; Hirota, K.; Okada, H. Cigarette Smoke Extract Activates Hypoxia-Inducible Factors in a Reactive Oxygen Species-Dependent Manner in Stroma Cells from Human Endometrium. Antioxidants 2021, 10, 48. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Okada, H.; Cho, H.; Shimoi, K.; Miyashiro, H.; Yasuda, K.; Kanzaki, H. Divergent regulation of angiopoietin-1, angiopoietin-2, and vascular endothelial growth factor by hypoxia and female sex steroids in human endometrial stromal cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 95–101. [Google Scholar] [CrossRef]
- Murata, H.; Tsuzuki, T.; Kido, T.; Kakita-Kobayashi, M.; Kida, N.; Hisamatsu, Y.; Okada, H. Progestin-induced heart and neural crest derivatives-expressed transcript 2 inhibits angiopoietin 2 via fibroblast growth factor 9 in human endometrial stromal cells. Reprod. Biol. 2019, 19, 14–21. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Y.; Liang, C.; Liu, J.; Wang, H.; Liu, S.; Yan, Q. poFUT1 promotes uterine angiogenesis and vascular remodeling via enhancing the O-fucosylation on uPA. Cell Death Dis. 2019, 10, 775. [Google Scholar] [CrossRef]
- Madunić, I.V.; Karin-Kujundžić, V.; Madunić, J.; Šola, I.M.; Šerman, L. Endometrial Glucose Transporters in Health and Disease. Front. Cell Dev. Biol. 2021, 9, 2381. [Google Scholar] [CrossRef]
- Frolova, A.; Flessner, L.; Chi, M.; Kim, S.T.; Foyouzi-Yousefi, N.; Moley, K.H. Facilitative glucose transporter type 1 is dif-ferentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology 2009, 150, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Tamura, I.; Ohkawa, Y.; Sato, T.; Suyama, M.; Jozaki, K.; Okada, M.; Lee, L.; Maekawa, R.; Asada, H.; Sato, S.; et al. Genome-wide analysis of histone modifications in human endometrial stromal cells. Mol. Endocrinol. 2014, 28, 1656–1669. [Google Scholar] [CrossRef] [PubMed]
- Frolova, A.I.; Moley, K.H. Quantitative Analysis of Glucose Transporter mRNAs in Endometrial Stromal Cells Reveals Critical Role of GLUT1 in Uterine Receptivity. Endocrinology 2011, 152, 2123–2128. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Sakata, M.; Ogura, K.; Miyake, A. Gestational changes of glucose transporter gene expression in the mouse placenta and decidua. J. Endocrinol. Investig. 1996, 19, 567–569. [Google Scholar] [CrossRef]
- Sakata, M.; Kurachi, H.; Imai, T.; Tadokoro, C.; Yamaguchi, M.; Yoshimoto, Y.; Oka, Y.; Miyake, A. Increase in human placental glucose transporter-1 during pregnancy. Eur. J. Endocrinol. 1995, 132, 206–212. [Google Scholar] [CrossRef]
- Tamura, I.; Maekawa, R.; Jozaki, K.; Ohkawa, Y.; Takagi, H.; Doi-Tanaka, Y.; Shirafuta, Y.; Mihara, Y.; Taketani, T.; Sato, S.; et al. Transcription factor C/EBPbeta induces genome-wide H3K27ac and upregulates gene expression during decidualization of human endometrial stromal cells. Mol. Cell Endocrinol. 2021, 520, 111085–111150. [Google Scholar] [CrossRef]
- Neff, A.M.; Yu, J.; Taylor, R.N.; Bagchi, I.C.; Bagchi, M.K. Insulin Signaling Via Progesterone-Regulated Insulin Receptor Substrate 2 is Critical for Human Uterine Decidualization. Endocrinology 2019, 161, bqz021. [Google Scholar] [CrossRef]
- Matsumoto, L.; Hirota, Y.; Saito-Fujita, T.; Takeda, N.; Tanaka, T.; Hiraoka, T.; Akaeda, S.; Fujita, H.; Shimizu-Hirota, R.; Igaue, S.; et al. HIF2α in the uterine stroma permits embryo invasion and luminal epithelium detachment. J. Clin. Investig. 2018, 128, 3186–3197. [Google Scholar] [CrossRef]
- Daikoku, T.; Matsumoto, H.; Gupta, R.A.; Das, S.K.; Gassmann, M.; DuBois, R.N.; Dey, S.K. Expression of Hypoxia-inducible Factors in the Peri-implantation Mouse Uterus Is Regulated in a Cell-specific and Ovarian Steroid Hormone-dependent Manner. J. Biol. Chem. 2003, 278, 7683–7691. [Google Scholar] [CrossRef]
- Kido, T.; Murata, H.; Nishigaki, A.; Tsubokura, H.; Komiya, S.; Kida, N.; Kakita-Kobayashi, M.; Hisamatsu, Y.; Tsuzuki, T.; Hashimoto, Y.; et al. Glucose transporter 1 is important for the glycolytic metabolism of human endometrial stromal cells in hypoxic environment. Heliyon 2020, 6, e03985. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murata, H.; Tanaka, S.; Okada, H. The Regulators of Human Endometrial Stromal Cell Decidualization. Biomolecules 2022, 12, 1275. https://doi.org/10.3390/biom12091275
Murata H, Tanaka S, Okada H. The Regulators of Human Endometrial Stromal Cell Decidualization. Biomolecules. 2022; 12(9):1275. https://doi.org/10.3390/biom12091275
Chicago/Turabian StyleMurata, Hiromi, Susumu Tanaka, and Hidetaka Okada. 2022. "The Regulators of Human Endometrial Stromal Cell Decidualization" Biomolecules 12, no. 9: 1275. https://doi.org/10.3390/biom12091275
APA StyleMurata, H., Tanaka, S., & Okada, H. (2022). The Regulators of Human Endometrial Stromal Cell Decidualization. Biomolecules, 12(9), 1275. https://doi.org/10.3390/biom12091275




