Identification and Characterization of Hdh-FMRF2 Gene in Pacific Abalone and Its Possible Role in Reproduction and Larva Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal and Sample Collection
2.2. Tissues Collection for Gene Cloning
2.3. Collection of CG and Preparation of Frozen Sections for In Situ Hybridization
2.4. Tissue Collection for mRNA Expression Analysis in Different Experimental Conditions
2.4.1. Collection of Various Organ Tissues of Pacific Abalone
2.4.2. Collection of Ganglion Tissues of Pacific Abalone during Gonadal Development
2.4.3. Collection of Ganglion Tissues of Pacific Abalone Conditioned at Effective Accumulative Temperature (EAT)
2.4.4. Collection of Ganglion of Pacific Abalone during Induced Spawning Events
2.4.5. Collection of Different Embryonic and Larval Developmental Stages Samples of Pacific Abalone
2.5. RNA Extraction and cDNA Synthesis
2.6. Cloning and Sequencing of the Full-length FMRF2 Gene (Hdh-FMRF2) in Pacific Abalone
2.6.1. Cloning of Partial Sequence
2.6.2. Cloning of 5′- and 3′-RACE Sequence
2.7. In-Silico Analysis of Cloned H. discus hannai FMRF2 (Hdh-FMRF2) Sequence
2.8. Analysis of Amino Acid Sequence Alignment and Identity-Similarity Index
2.9. Prediction of Three-Dimensional (3D) Structure and Pairwise 3D Structure Alignment
2.10. Phylogenetic Analysis
2.11. Peptide Identification Using Nano-LC-ESI-MS/MS
2.11.1. Sample Preparation and Peptide Extraction
2.11.2. Nano-LC-ESI-MS/MS and Peptide Identification
2.12. Localization of Hdh-FMRF2 via Fluorescent in Situ Hybridization (FISH)
2.12.1. Synthesis of Hdh-FMRF2 Riboprobe
2.12.2. Fluorescence in Situ Hybridization (FISH)
2.13. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.14. Statistical Analysis
3. Results
3.1. Haliotis discus hannai FMRFamide 2 (Hdh-FMRF2) Sequence
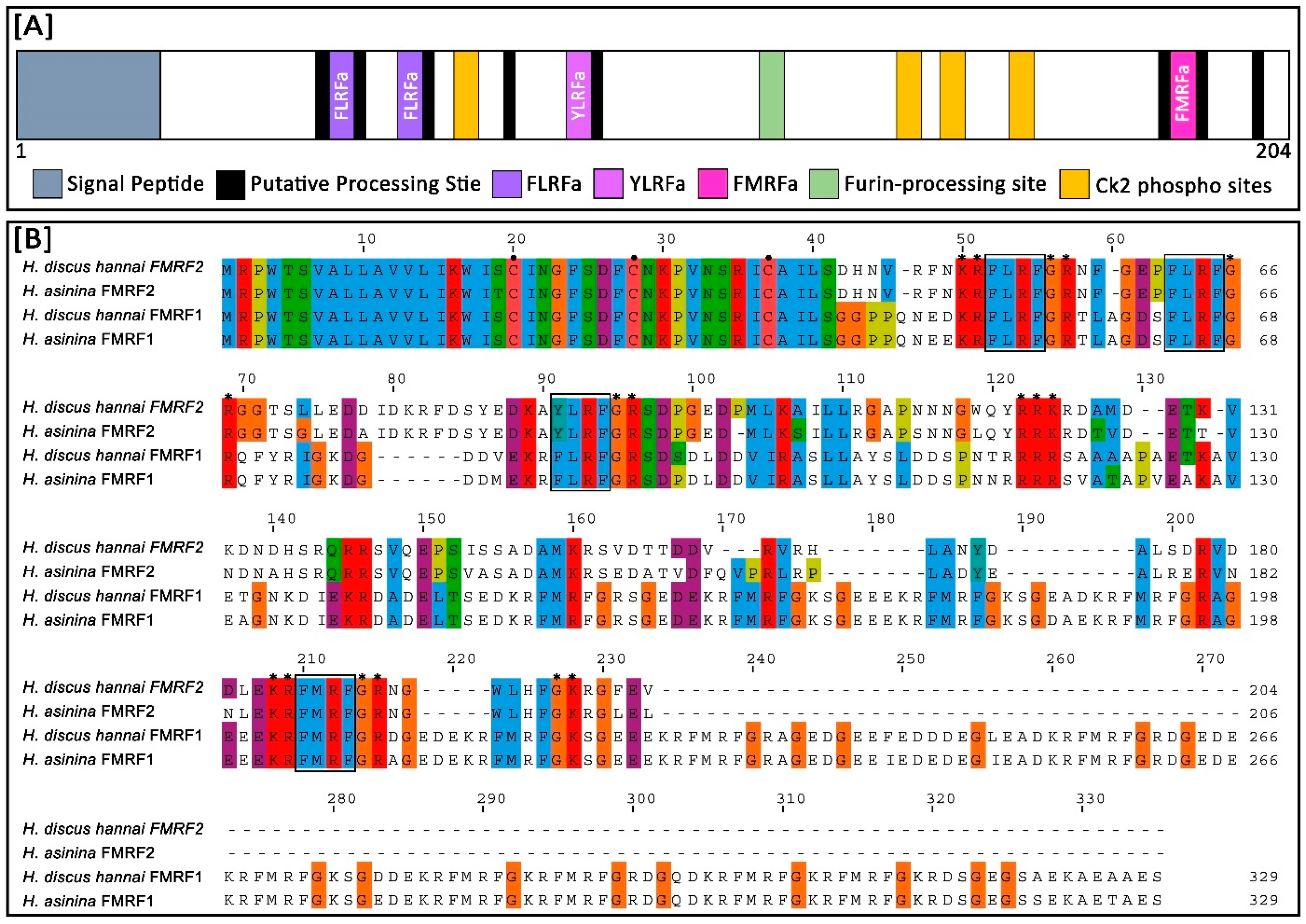
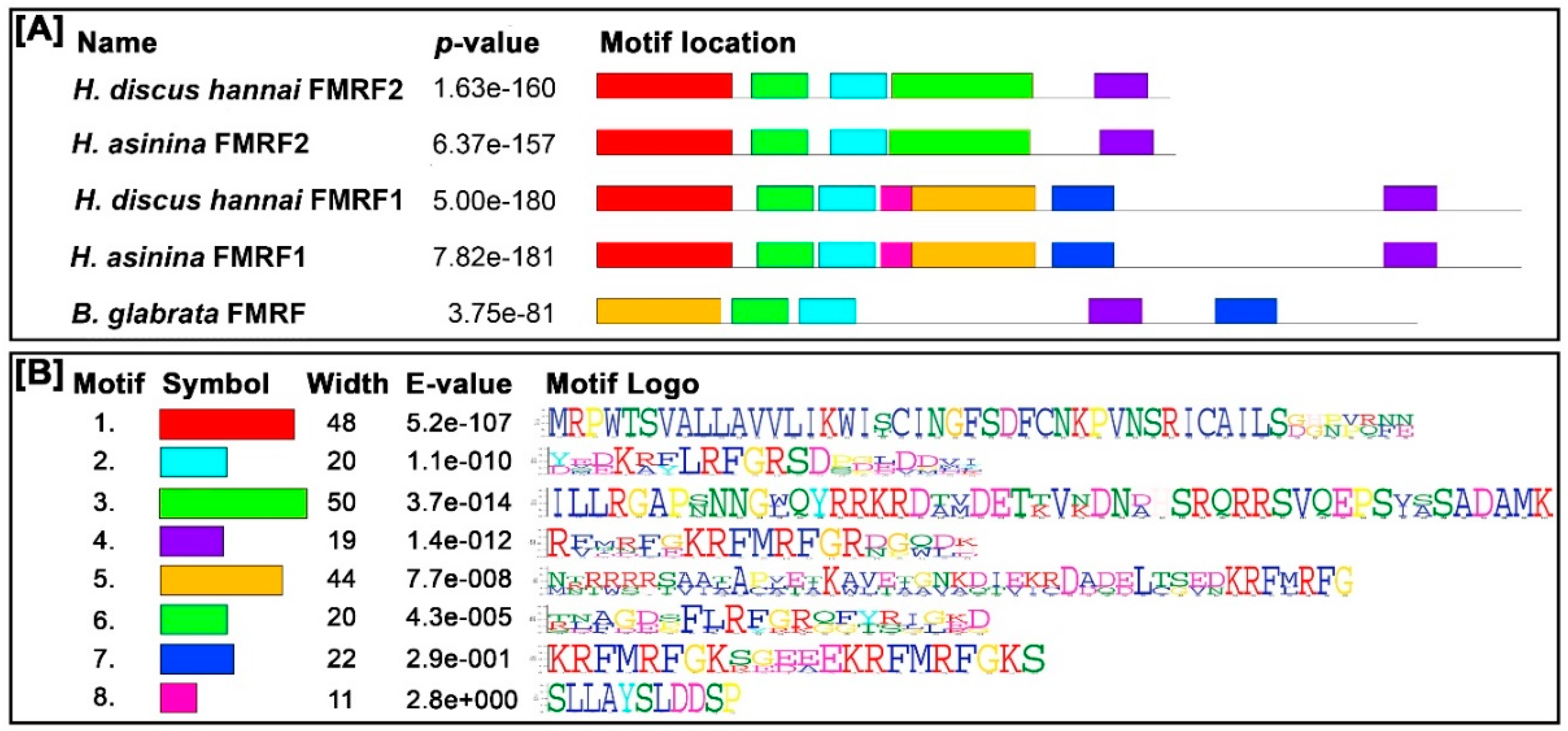
3.2. Features of Hdh-FMRF2 Amino Acid Sequence and Bioinformatic Analysis
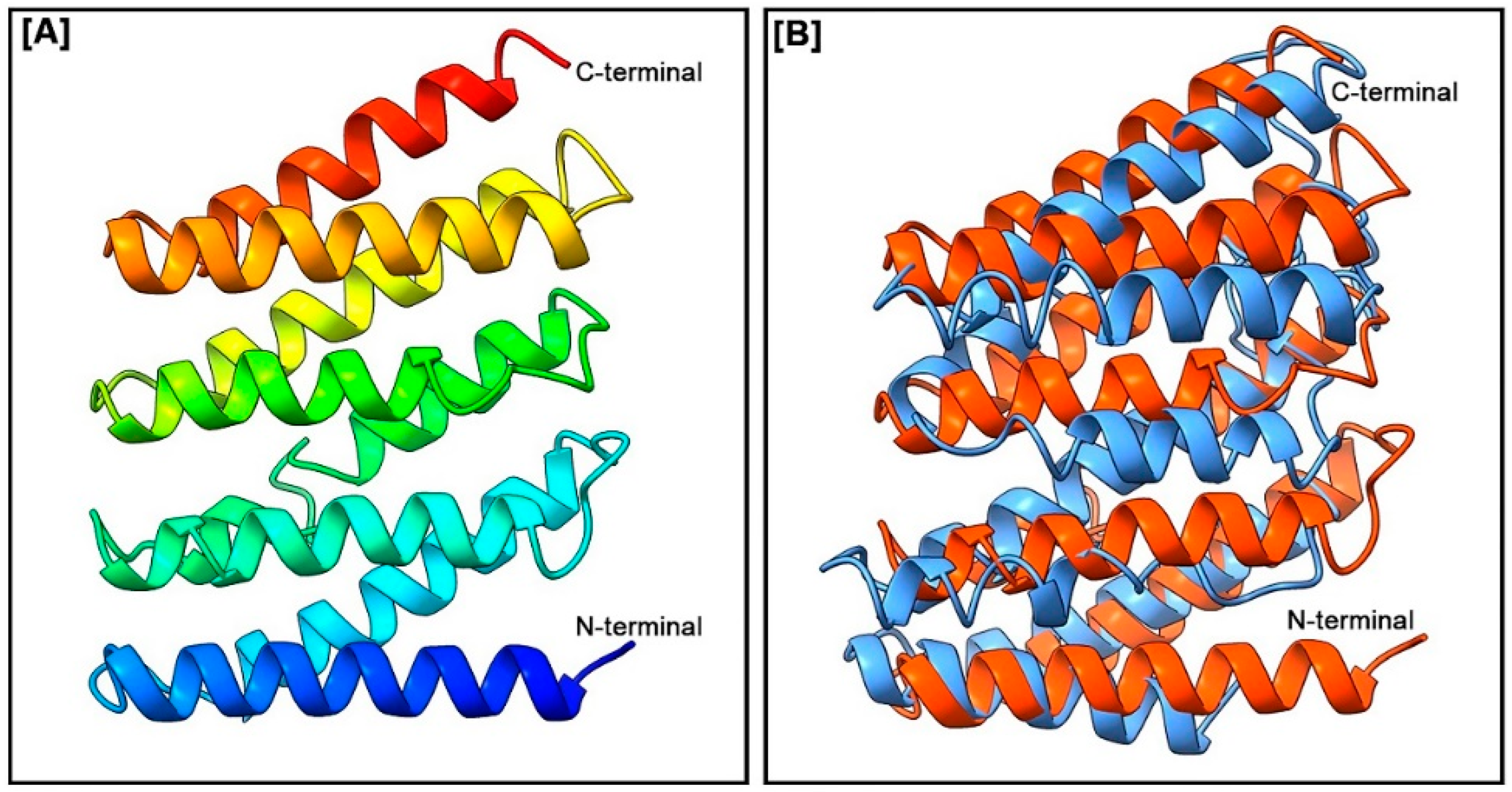
3.3. Three-Dimensional Structure and Pairwise 3D Structure Alignment
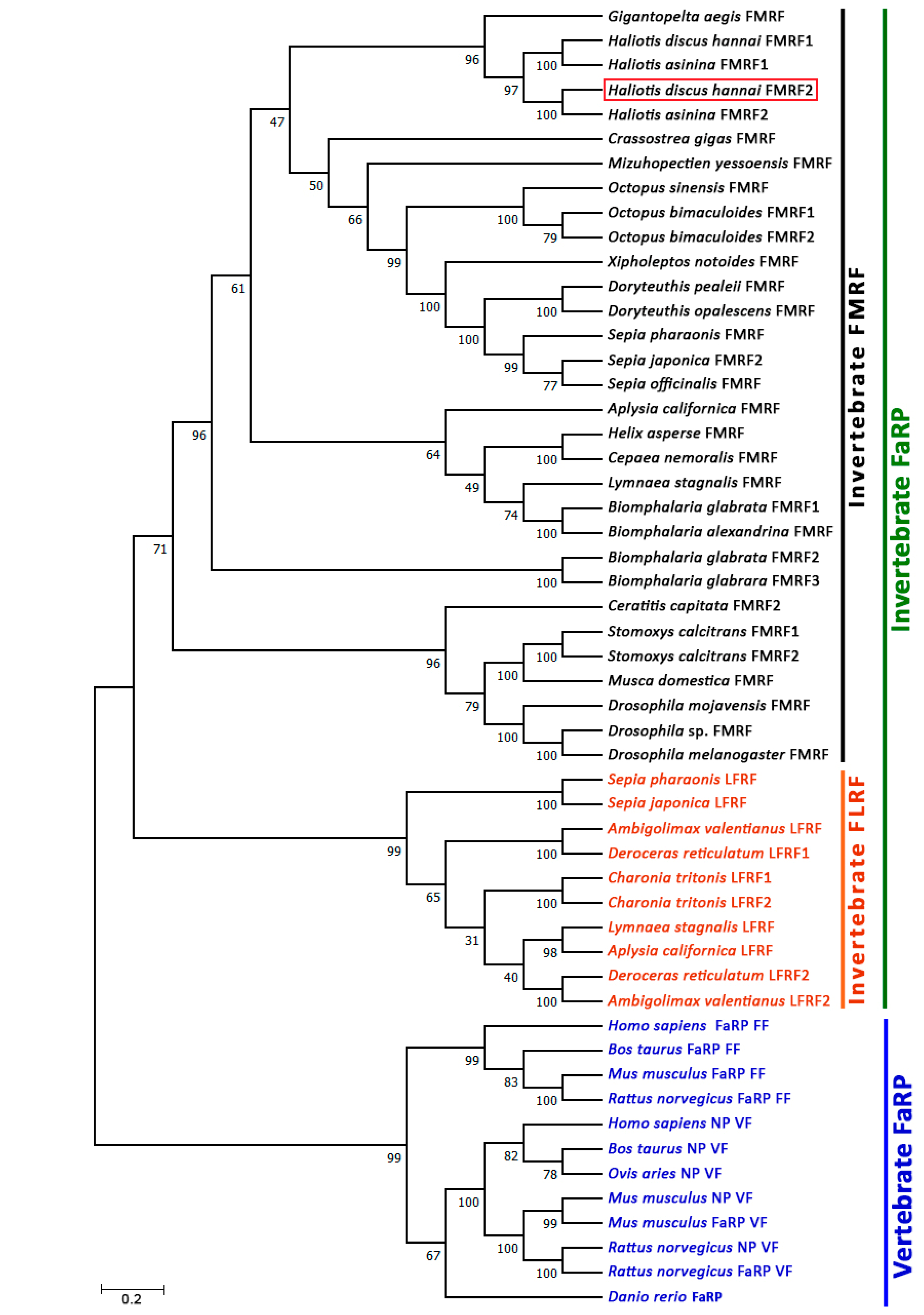
3.4. Phylogenetic Analysis
3.5. Identification of FaRP Peptides
3.6. Fluorescence in Situ Hybridization (FISH) Localization of Hdh-FMRF2
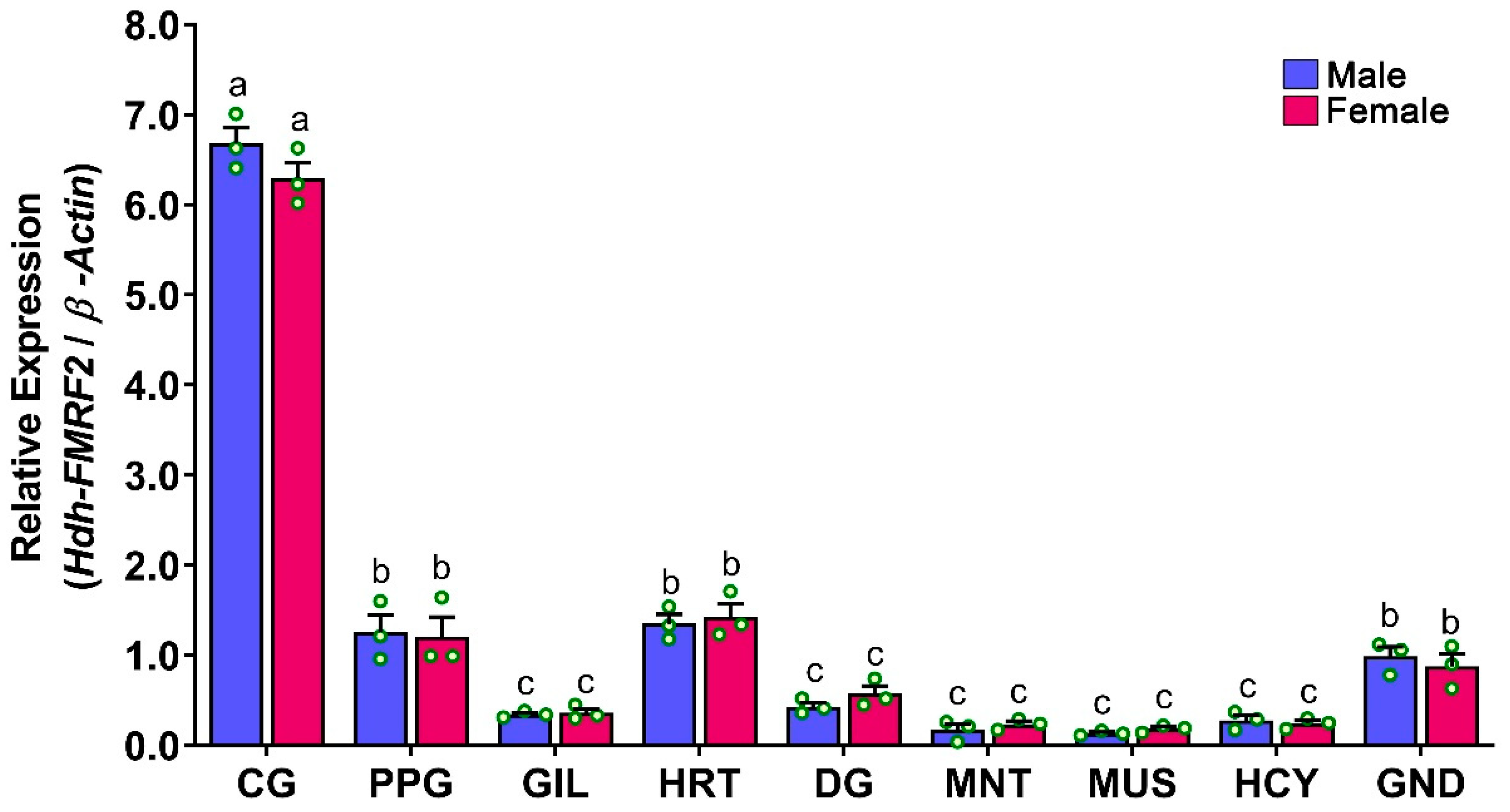
3.7. Expression Levels of Hdh-FMRF2 mRNA in Different Tissues of Pacific Abalone
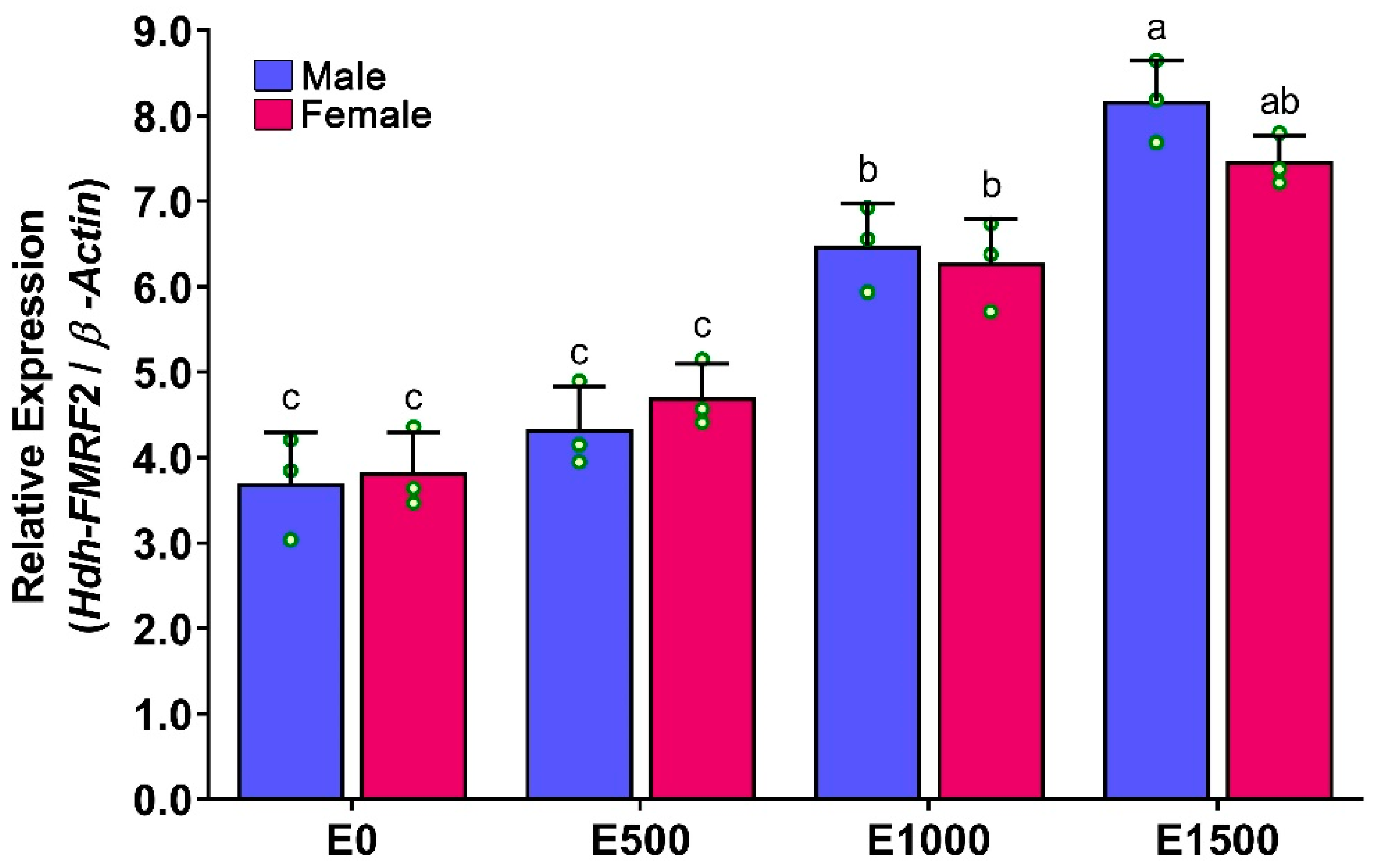
3.8. Expression Levels of Hdh-FMRF2 in CG during Gonadal Development of Pacific Abalone
3.9. Expression Levels of Hdh-FMRF2 in CG during Gonadal Development of Pacific Abalone
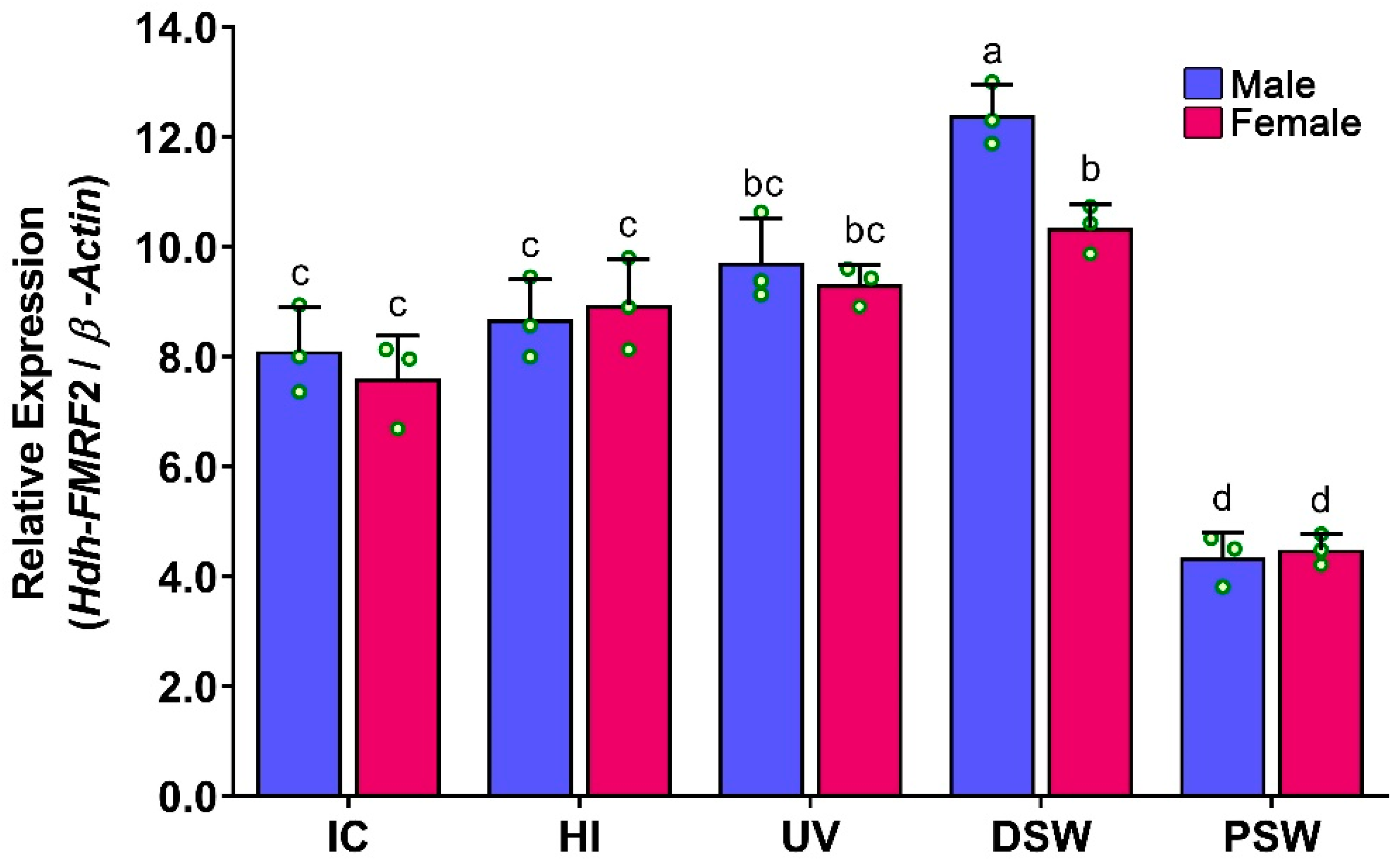
3.10. Expression Levels of Hdh-FMRF2 mRNA in CG during Induced Spawning Events
3.11. Expression Levels of Hdh-FMRF2 mRNA in Embryonic and Larval Developmental Stages of Pacific Abalone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Espinoza, E.; Carrigan, M.; Thomas, S.G.; Shaw, G.; Edison, A.S. A statistical view of FMRFamide neuropeptide diversity. Mol. Neurobiol. 2000, 21, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Cummins, S.F.; Tollenaere, A.; Degnan, B.M.; Croll, R.P. Molecular analysis of two FMRFamide-encoding transcripts expressed during the development of the tropical abalone Haliotis asinina. J. Comp. Neurol. 2011, 519, 2043–2059. [Google Scholar] [CrossRef] [PubMed]
- Wollesen, T.; Cummins, S.F.; Degnan, B.M.; Wanninger, A. FMRFamide gene and peptide expression during central nervous system development of the cephalopod mollusk, Idiosepius notoides. Evol. Dev. 2010, 12, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Dockray, G.J. The expanding family of RFamide peptides and their effects on feeding behaviour. Exp. Physiol. 2004, 89, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Price, D.A.; Greenberg, M.J. Purification and characterization of a cardioexcitatory neuropeptide from the central ganglia of a bivalve mollusk. Prep. Biochem. 1977, 7, 261–281. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Pan, F.; Wang, S.; Wang, Y.; Hu, Y. FMRFamide-like peptide 22 influences the head movement, host finding, and infection of Heterodera glycines. Front. Plant Sci. 2021, 12, 673354. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, L.L.; Wu, J.H.; Liu, H.H.; Zheng, L.B.; Lü, Z.M.; Chi, C.F. An FMRFamide neuropeptide in cuttlefish Sepia pharaonis: Identification, characterization, and potential function. Molecules 2020, 25, 1636. [Google Scholar] [CrossRef]
- Cristo, C.D.; Delli, B.P.; Cosmo, A.D. Role of FMRFamide in the reproduction of Octopus vulgaris: Molecular analysis and effect on visual input. Peptides 2003, 24, 1525–1532. [Google Scholar] [CrossRef]
- Salzet, M.; Bulet, P.; Wattez, C.; Malecha, J. FMRFamide-related peptides in the sex segmental ganglia of the Pharyngobdellid leech Erpobdella octoculata. Identification and involvement in the control of hydric balance. Eur. J. Biochem. 1994, 221, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Peymen, K.; Watteyne, J.; Frooninckx, L.; Schoofs, L.; Beets, I. The FMRFamide-like peptide family in nematodes. Front. Endocrinol. 2014, 5, 90. [Google Scholar] [CrossRef]
- Krajniak, K.G. Invertebrate FMRFamide related peptides. Protein Pept Lett. 2013, 20, 647–670. [Google Scholar] [CrossRef] [PubMed]
- Orchard, I.; Lange, A.B.; Bendena, W.G. FMRFamide-related peptides: A multifunctional family of structurally related neuropeptides in insects. Adv. Insect Physiol. 2001, 28, 267–329. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Z.; Lim, H.; Liu, H.; Lü, Z.; Chi, C. Identification, Characterization, and expression analysis of a FMRFamide-like peptide gene in the common Chinese cuttlefish (Sepiella japonica). Molecules 2018, 23, 742. [Google Scholar] [CrossRef] [PubMed]
- Zatylny-Gaudin, C.; Favrel, P. Diversity of the RFamide peptide family in mollusks. Front. Endocrinol. 2014, 5, 178. [Google Scholar] [CrossRef]
- Burbach, J.P.; Grant, P.; Hellemons, A.J.; Degiorgis, J.A.; Li, K.W.; Pant, H.C. Differential expression of the FMRF gene in adult and hatchling stellate ganglia of the squid Loligo pealei. Biol. Open 2014, 3, 50–58. [Google Scholar] [CrossRef][Green Version]
- López-Vera, E.; Aguilar, M.B.; Heimer de la Cotera, E.P. FMRFamide and related peptides in the phylum mollusca. Peptides 2008, 29, 310–317. [Google Scholar] [CrossRef]
- Santama, N.; Benjamin, P.R. Gene expression and function of FMRFamide-related neuropeptides in the snail Lymnaea. Microsc. Res. Tech. 2000, 49, 547–556. [Google Scholar] [CrossRef]
- Walker, R.J.; Papaioannou, S.; Holden-Dye, L. A review of FMRFamide- and RFamide-like peptides in metazoa. Invert. Neurosci. 2009, 9, 111–153. [Google Scholar] [CrossRef]
- Sukhan, Z.P.; Sharker, M.R.; Cho, Y.; Hossen, S.; Choi, K.S.; Kho, K.H. Thermal stress affects gonadal maturation by regulating GnRH, GnRH receptor, APGWamide, and serotonin receptor gene expression in male pacific abalone, Haliotis discus hannai during breeding season. Front. Mar. Sci. 2021, 8, 664426. [Google Scholar] [CrossRef]
- Hossen, S.; Sukhan, Z.P.; Cho, Y.; Kho, K.H. Effects of cryopreservation on gene expression and post thaw sperm quality of Pacific abalone, Haliotis discus hannai. Front. Mar. Sci. 2021, 8, 652390. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Masci, P.P.; Gobe, G.C.; Osborne, S.A. Therapeutic potential of abalone and status of bioactive molecules: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1742–1748. [Google Scholar] [CrossRef]
- Cook, P.A. Recent Trends in Worldwide Abalone Production. J. Shellfish Res. 2016, 35, 581–583. [Google Scholar] [CrossRef]
- Sukhan, Z.P.; Cho, Y.; Sharker, M.R.; Hossen, S.; Rha, S.-J.; Kho, K.H. Effective accumulative temperature affects gonadal maturation by controlling expression of GnRH, GnRH receptor, serotonin receptor and APGWamide gene in Pacific abalone, Haliotis discus hannai during broodstock conditioning in hatcheries. J. Therm. Biol. 2021, 100, 103037. [Google Scholar] [CrossRef] [PubMed]
- Meusel, E.; Menanteau-Ledouble, S.; Naylor, M.; Kaiser, H.; El-Matbouli, M. Gonad development in farmed male and female South African abalone, Haliotis midae, fed artificial and natural diets under a range of husbandry conditions. Aquacult. Int. 2022, 30, 1279–1293. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Fang, J.; Liu, X.; Mao, Y.; Liu, G.; Bian, D. Reproductive performance of one-year-old Pacific abalone (Haliotis discus hannai) and its crossbreeding effect on offspring growth and survival. Aquaculture 2017, 473, 110–114. [Google Scholar] [CrossRef]
- Takami, H.; Fukazawa, H.; Kawamura, T. Delayed metamorphosis by larval abalone in the field. Bull. Fish. Res. Agency 2006, 5, 97–117. [Google Scholar]
- Kim, M.A.; Markkandan, K.; Han, N.Y.; Park, J.M.; Lee, J.S.; Lee, H.; Sohn, Y.C. Neural ganglia transcriptome and peptidome associated with sexual maturation in female Pacific abalone (Haliotis discus hannai). Genes 2019, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Sukhan, Z.P.; Sharker, M.R.; Kho, K.H. Molecular cloning, in silico characterization and expression analysis of axonemal protein 66.0 in Pacific abalone, Haliotis discus hannai. Eur. Zoolog. J. 2020, 87, 648–658. [Google Scholar] [CrossRef]
- Sukhan, Z.P.; Kitano, H.; Selvaraj, S.; Yoneda, M.; Yamaguchi, A.; Matsuyama, M. Identification and distribution of three gonadotropin-releasing hormone (GnRH) isoforms in the brain of a clupeiform fish, Engraulis japonicus. Zoolog. Sci. 2013, 30, 1081–1091. [Google Scholar] [CrossRef]
- Sukhan, Z.P.; Hossen, S.; Cho, Y.; Lee, W.K.; Kho, K.H. Hdh-tektin-4 regulates motility of fresh and cryopreserved sperm in Pacific abalone, Haliotis discus hannai. Front. Cell Dev. Biol. 2022, 10, 870743. [Google Scholar] [CrossRef]
- Kho, K.H.; Sukhan, Z.P.; Hossen, S.; Cho, Y.; Kim, S.C.; Sharker, M.R.; Jung, H.-J.; Nou, I.-S. Construction of a genetic linkage map based on SNP markers, QTL mapping and detection of candidate genes of growth-related traits in Pacific abalone using genotyping-by-sequencing. Front. Mar. Sci. 2021, 8, 713783. [Google Scholar] [CrossRef]
- Veenstra, J.A. Neurohormones and neuropeptides encoded by the genome of Lottia gigantea, with reference to other mollusks and insects. Gen. Comp. Endocrinol. 2010, 167, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Rolón-Martínez, S.; Habib, M.R.; Mansour, T.A.; Díaz-Ríos, M.; Rosenthal, J.J.C.; Zhou, X.N.; Croll, R.P.; Miller, M.W. FMRF-NH2 -related neuropeptides in Biomphalaria spp., intermediate hosts for schistosomiasis: Precursor organization and immunohistochemical localization. J. Comp./ Neurol. 2021, 529, 3336–3358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Goodwin, E.; Loi, P.K.; Tublitz, N.J. Molecular analysis of a novel FMRFamide-related peptide gene (SOFaRP(2)) and its expression pattern in the brain of the European cuttlefish Sepia officinalis. Peptides 2012, 34, 114–119. [Google Scholar] [CrossRef]
- Kellett, E.; Saunders, S.E.; Li, K.W.; Staddon, J.W.; Benjamin, P.R.; Burke, J.F. Genomic organization of the FMRFamide gene in Lymnaea: Multiple exons encoding novel neuropeptides. J. Neurosci. 1994, 14, 6564–6570. [Google Scholar] [CrossRef]
- Wollesen, T.; Loesel, R.; Wanninger, A. FMRFamide-like immunoreactivity in the central nervous system of the cephalopod mollusc, Idiosepius notoides. Acta Biol. Hung. 2008, 59, 111–116. [Google Scholar] [CrossRef]
- Suzuki, H.; Yamamoto, T.; Nakagawa, M.; Uemura, H. Neuropeptide Y-immunoreactive neuronal system and colocalization with FMRFamide in the optic lobe and peduncle complex of the octopus (Octopus vulgaris). Cell Tissue Res. 2002, 307, 255–264. [Google Scholar] [CrossRef]
- Harris, L.L.; Lesser, W.; Ono, J.K. FMRFamide is endogenous to the Aplysia heart. Cell Tissue Res. 1995, 282, 331–341. [Google Scholar] [CrossRef]
- Lehman, H.K.; Price, D.A. Localization of FMRFamide-like peptides in the snail Helix aspersa. J. Exp. Biol. 1987, 131, 37–53. [Google Scholar] [CrossRef]
- Di Cosmo, A.; Di Cristo, C. Neuropeptidergic control of the optic gland of Octopus vulgaris: FMRF-amide and GnRH immunoreactivity. J. Comp. Neurol. 1998, 398, 1–12. [Google Scholar] [CrossRef]
- Sedra, L.; Lange, A.B. The female reproductive system of the kissing bug, Rhodnius prolixus: Arrangements of muscles, distribution and myoactivity of two endogenous FMRFamide-like peptides. Peptides 2014, 53, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Burton, T.; Ha, L.; Huang, Z.; Olajubelo, A.; Li, C. Modulation of locomotion and reproduction by FLP neuropeptides in the nematode Caenorhabditis elegans. PLoS ONE 2015, 10, e0135164. [Google Scholar] [CrossRef] [PubMed]
- York, P.S.; Cummins, S.F.; Degnan, S.M.; Woodcroft, B.J.; Degnan, B.M. Marked changes in neuropeptide expression accompany broadcast spawnings in the gastropod Haliotis asinina. Front Zool. 2012, 9, 9. [Google Scholar] [CrossRef]
- Brussaard, A.B.; Kits, K.S.; Ter Maat, A.; Van Minnen, J.; Moed, P.J. Dual inhibitory action of FMRFamide on neurosecretory cells controlling egg laying behavior in the pond snail. Brain Res. 1988, 447, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Fisher, T.; Lin, C.H.; Kaczmarek, L.K. The peptide FMRFa terminates a discharge in Aplysia bag cell neurons by modulating calcium, potassium, and chloride conductances. J. Neurophysiol. 1993, 69, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Crofts, D.R. V-The development of Haliotis tuberculata, with special reference to organogenesis during torsion. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1937, 228, 219–268. [Google Scholar] [CrossRef]
- Yurchenko, O.V.; Skiteva, O.I.; Voronezhskaya, E.E.; Dyachuk, V.A. Nervous system development in the Pacific oyster, Crassostrea gigas (Mollusca: Bivalvia). Front. Zool. 2018, 15, 10. [Google Scholar] [CrossRef]
- Croll, R.P. Development of embryonic and larval cells containing serotonin, catecholamines, and FMRFamide-related peptides in the gastropod mollusc Phestilla sibogae. Biol. Bull. 2006, 211, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A. The distribution of cells containing FMRFamide- and 5-HT-related molecules in the embryonic development of Viviparus ater (Mollusca, Gastropoda). Eur. J. Histochem. 2005, 49, 301–308. [Google Scholar] [CrossRef]
- Dickinson, A.J.; Croll, R.P.; Voronezhskaya, E.E. Development of embryonic cells containing serotonin, catecholamines, and FMRFamide-related peptides in Aplysia californica. Biol. Bull. 2000, 199, 305–315. [Google Scholar] [CrossRef]




| Gene Name | % Identity | % Similarity | ||||||
|---|---|---|---|---|---|---|---|---|
| Hdh-FMRF2 | Hdh-FMRF1 | Has-FMRF1 | Has-FMRF2 | Hdh-FMRF2 | Hdh-FMRF1 | Has-FMRF1 | Has-FMRF2 | |
| Hdh-FMRF2 | 100 | 100 | 37.01 | 37.01 | 89.86 | |||
| Hdh-FMRF1 | 28.96 | 100 | 100 | 96.96 | 36.12 | |||
| Has-FMRF1 | 28.96 | 94.53 | 100 | 100 | 35.82 | |||
| Has-FMRF2 | 85.02 | 27.46 | 27.46 | 100 | 100 | |||
| Transcript and Peptide Sequences | No. of Amino Acid or Peptides Encoded | Predicted Monoisotopic Mass (kDa) | Isoelectric Point | Attribute | |||
|---|---|---|---|---|---|---|---|
| FMRF1 | FMRF2 | FMRF1 | FMRF2 | FMRF1 | FMRF2 | ||
| Full sequence | 329 | 204 | 38.38 | 22.77 | 9.54 | 9.70 | - |
| Pre-tetrabasic cleavage site: | |||||||
| FLRFa | 2 | 1 | 0.58 | 0.58 | – | 10.55 | Basic |
| TLAGDSFLRFa | 1 | × | 1.12 | – | 7.81 | – | Neutral |
| p-QFYRIa | 1 | × | 0.71 | – | 9.37 | – | Basic |
| Ac-SDSDLDDVIRASLLAYSLDDSPNT | 1 | × | 2.58 | – | 3.57 | – | Acidic |
| NFGEPFLRFa | × | 1 | – | 1.13 | – | 6.00 | Neutral |
| FDSYEDKAYLRFa | × | 1 | – | 1.55 | – | 4.56 | Acidic |
| Ac-SDPGEDPMLKAILLRGAPNNNGWQY | × | 1 | – | 2.76 | – | 4.56 | Acidic |
| Post-tetrabasic cleavage site: | |||||||
| Ac-SAAAAPAETKAVETGNKDIE | 1 | × | – | 1.97 | – | 4.41 | Acidic |
| DAMDETKVKDNDHSRQ | × | 1 | – | 1.89 | – | 4.75 | Acidic |
| FMRFa | 13 | 1 | 0.60 | 0.60 | 10.55 | 10.55 | Basic |
| NGWLHFa | × | 1 | – | 0.77 | – | 6.74 | Basic |
| Peptide Sequence | # Proteins | # PSMs | # Missed Cleavages | Theorical MH+ [Da] | XCross |
|---|---|---|---|---|---|
| FMRFGR | 1 | 1 | 1 | 829.41 | 0.28 |
| YLRFGR | 1 | 6 | 1 | 882.49 | 0.31 |
| NFGEPFLRFGR | 1 | 2 | 1 | 1339.69 | 0.10 |
| FGRNFGEPFLRFGR | 1 | 4 | 2 | 1699.88 | 0.43 |
| FDSYEDKAYLRFGR | 1 | 3 | 2 | 1766.85 | 0.19 |
| GGTSLLEDDIDKR | 1 | 5 | 1 | 14.1871 | 0.97 |
| SDPGEDPMLKAILLRGAPNNNGWQYR | 1 | 3 | 2 | 2912.44 | 1.02 |
| NGWLHFGKR | 1 | 2 | 1 | 1114.59 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhan, Z.P.; Cho, Y.; Hossen, S.; Lee, W.K.; Kho, K.H. Identification and Characterization of Hdh-FMRF2 Gene in Pacific Abalone and Its Possible Role in Reproduction and Larva Development. Biomolecules 2023, 13, 109. https://doi.org/10.3390/biom13010109
Sukhan ZP, Cho Y, Hossen S, Lee WK, Kho KH. Identification and Characterization of Hdh-FMRF2 Gene in Pacific Abalone and Its Possible Role in Reproduction and Larva Development. Biomolecules. 2023; 13(1):109. https://doi.org/10.3390/biom13010109
Chicago/Turabian StyleSukhan, Zahid Parvez, Yusin Cho, Shaharior Hossen, Won Kyo Lee, and Kang Hee Kho. 2023. "Identification and Characterization of Hdh-FMRF2 Gene in Pacific Abalone and Its Possible Role in Reproduction and Larva Development" Biomolecules 13, no. 1: 109. https://doi.org/10.3390/biom13010109
APA StyleSukhan, Z. P., Cho, Y., Hossen, S., Lee, W. K., & Kho, K. H. (2023). Identification and Characterization of Hdh-FMRF2 Gene in Pacific Abalone and Its Possible Role in Reproduction and Larva Development. Biomolecules, 13(1), 109. https://doi.org/10.3390/biom13010109







