Calnexin Is Involved in Forskolin-Induced Syncytialization in Cytotrophoblast Model BeWo Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Collection of Human Tissue Samples and Preparation of These Samples
2.3. Immunohistochemical Analysis
2.4. Culture of Cells
2.5. Creation of Stable CNX-Knockdown Cells
2.6. Analysis of Immunoblots
2.7. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Real-Time Quantitative RT-PCR (RT-qPCR)
2.8. Analysis of Cell Fusion
2.9. Analysis of Cell Proliferation
2.10. Analysis via Immunofluorescence Microscopy
2.11. Analysis of Cell-Surface LHCGR by Biotinylation
2.12. Preparation of β-hCG-Containing Conditioned Medium
2.13. Statistical Analysis
3. Results
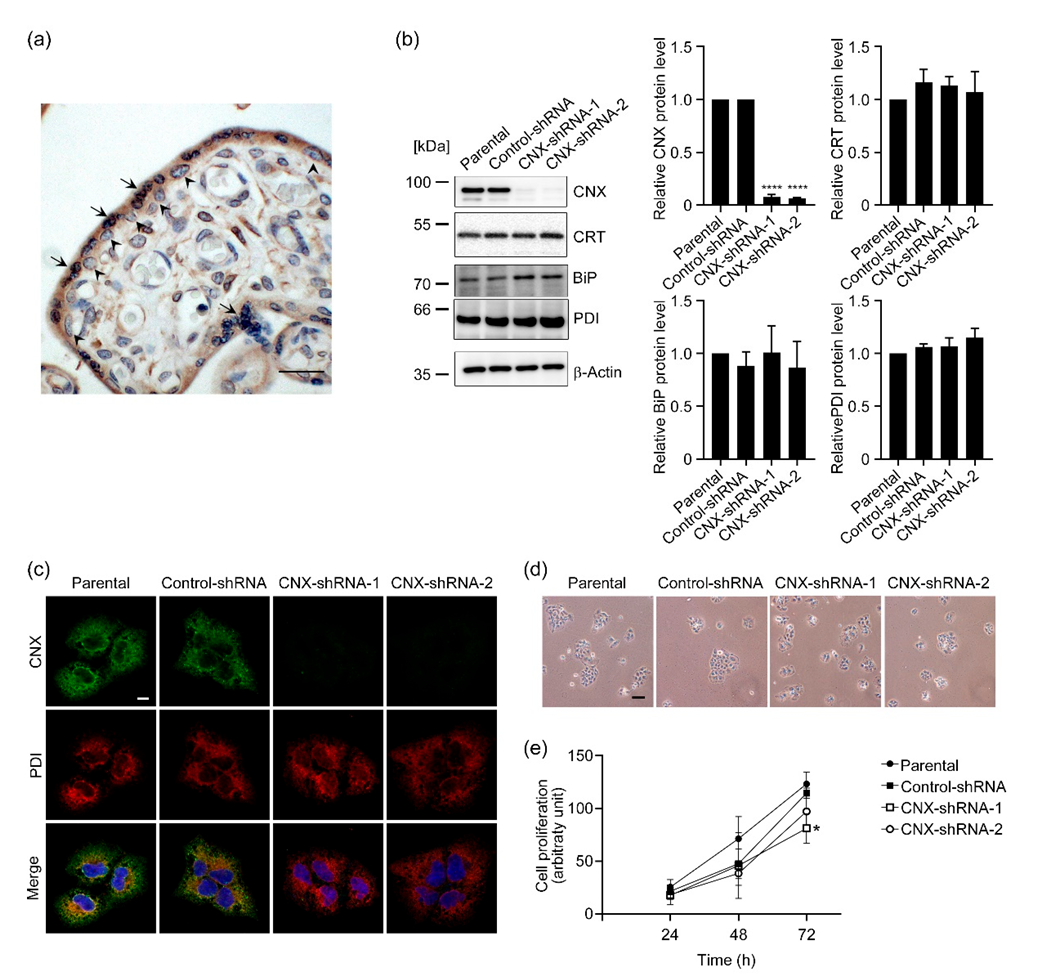
3.1. Establishment of Stable CNX-Knockdown BeWo Cells
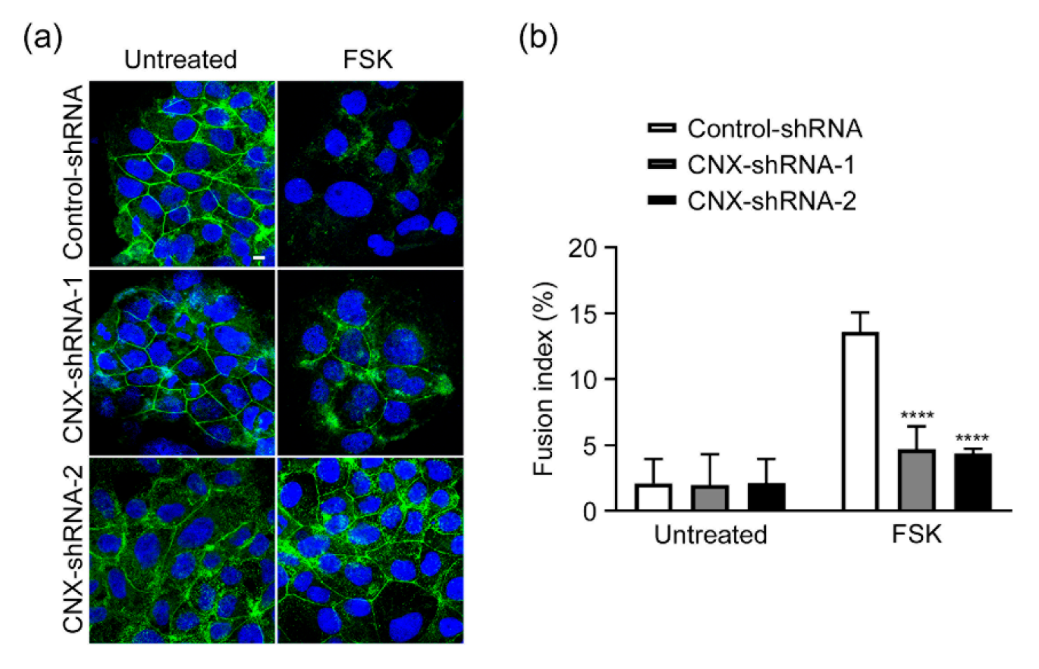
3.2. Effect of CNX Knockdown on FSK-Induced Cell Fusion in BeWo Cells
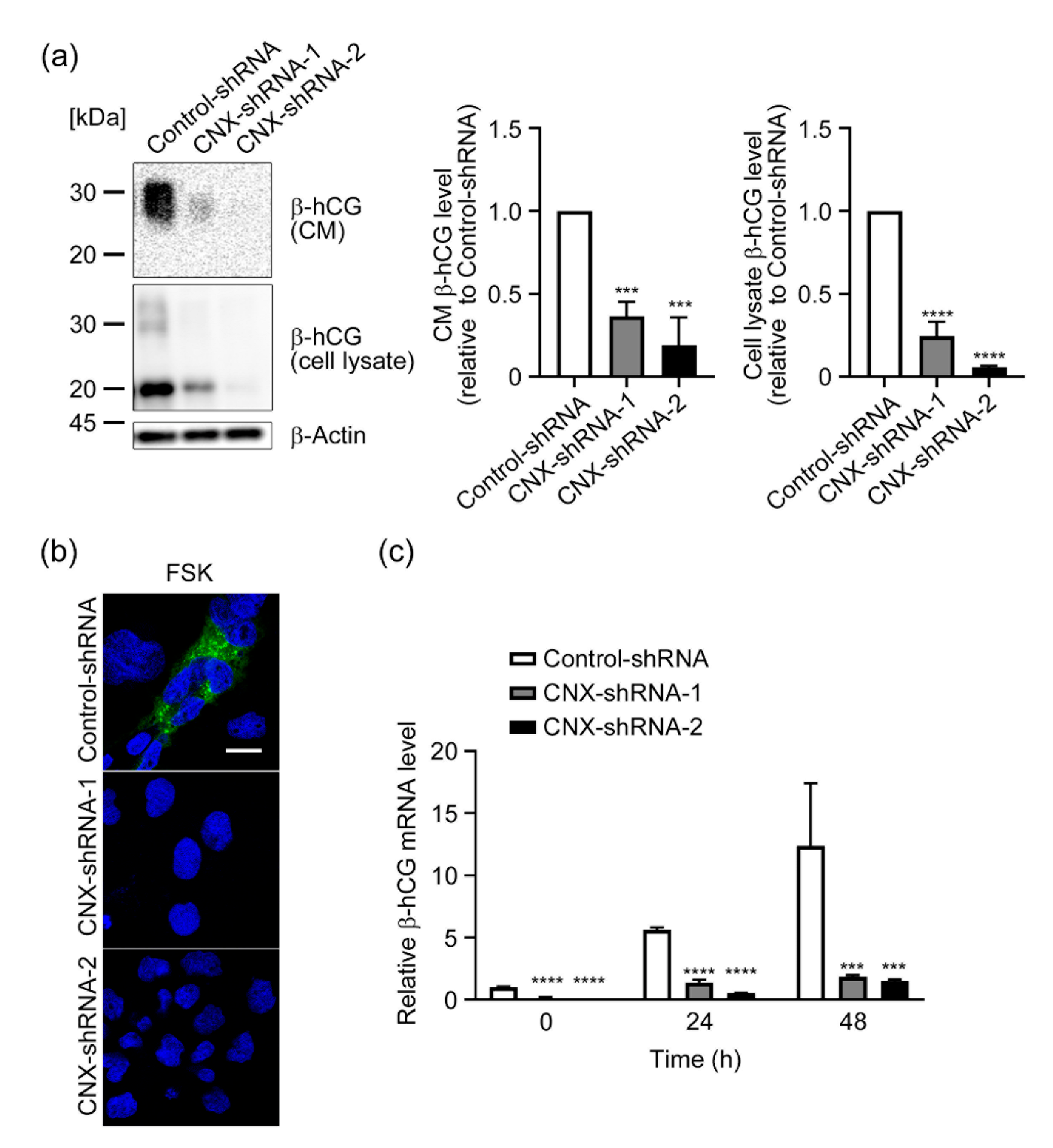
3.3. Effect of CNX Knockdown on Induction of β-hCG by FSK in BeWo Cells
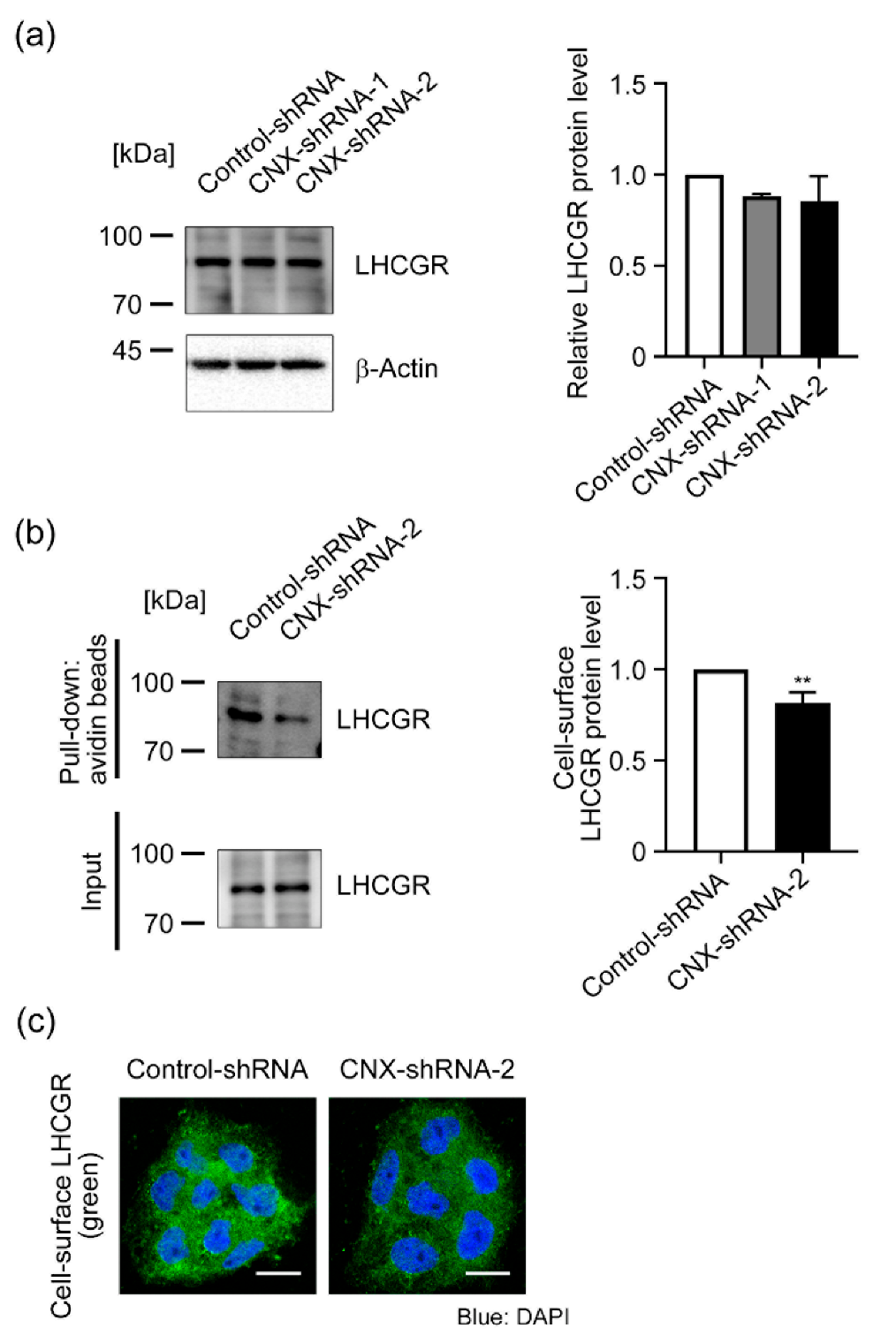
3.4. Effect of CNX Knockdown on LHCGR Expression in BeWo Cells
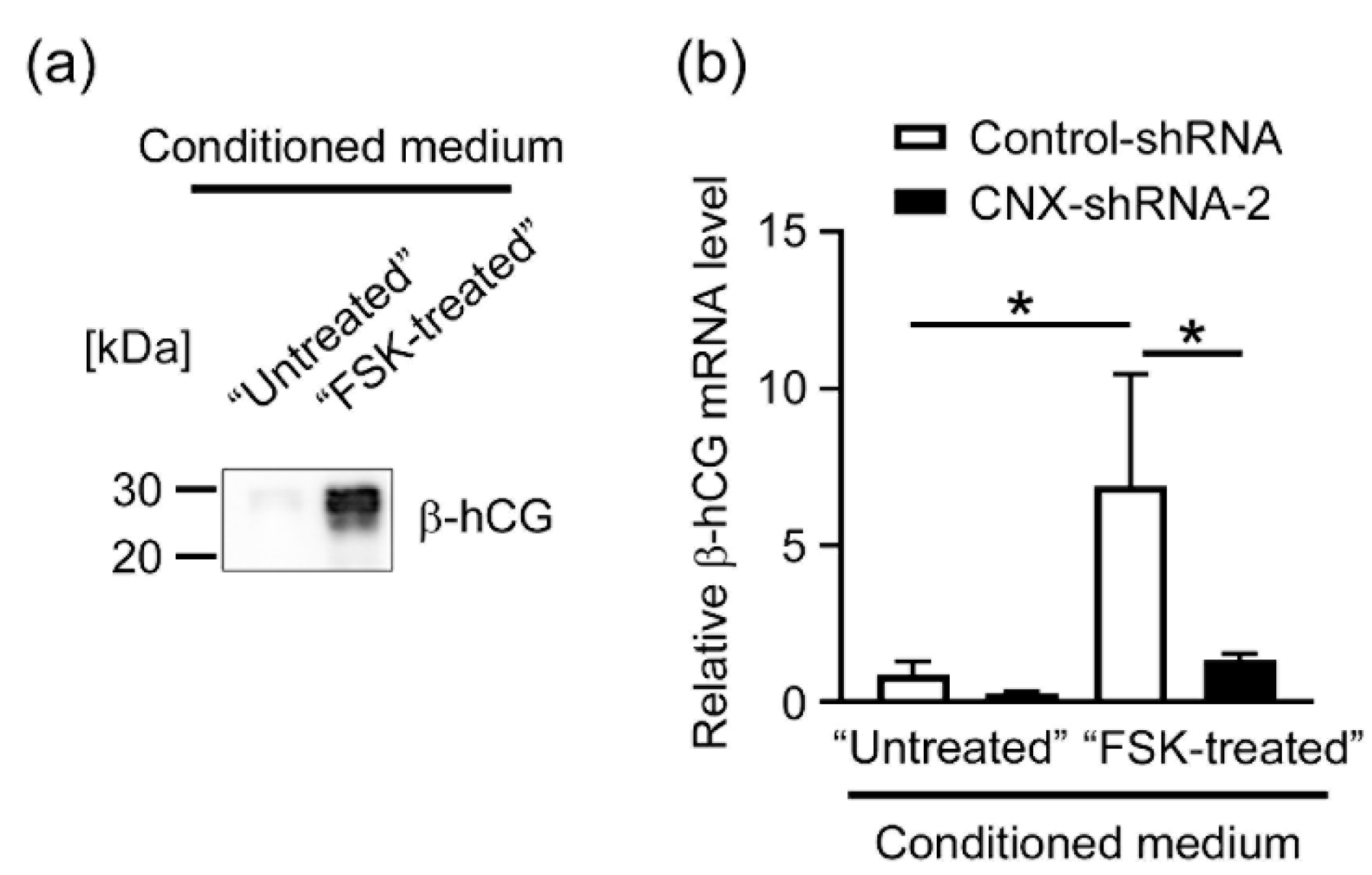
3.5. Effects of CNX Knockdown on the Expression of β-hCG in BeWo Cells Induced by the Conditioned Medium Containing hCG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knofler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A. The endocrine function of human placenta: An overview. Reprod. Biomed. Online 2016, 32, 14–43. [Google Scholar] [CrossRef] [PubMed]
- Licht, P.; Cao, H.; Lei, Z.M.; Rao, C.V.; Merz, W.E. Novel self-regulation of human chorionic gonadotropin biosynthesis in term pregnancy human placenta. Endocrinology 1993, 133, 3014–3025. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.J.; Lei, Z.M.; Rao, C.V.; Lin, J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 1993, 132, 1387–1395. [Google Scholar] [CrossRef]
- Pidoux, G.; Gerbaud, P.; Marpeau, O.; Guibourdenche, J.; Ferreira, F.; Badet, J.; Evain-Brion, D.; Frendo, J.L. Human placental development is impaired by abnormal human chorionic gonadotropin signaling in trisomy 21 pregnancies. Endocrinology 2007, 148, 5403–5413. [Google Scholar] [CrossRef]
- Costa, M.A. Scrutinising the regulators of syncytialization and their expression in pregnancy-related conditions. Mol. Cell Endocrinol. 2016, 420, 180–193. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- Adams, B.M.; Oster, M.E.; Hebert, D.N. Protein quality control in the endoplasmic reticulum. Protein. J. 2019, 38, 317–329. [Google Scholar] [CrossRef]
- Sun, Z.; Brodsky, J.L. Protein quality control in the secretory pathway. J. Cell Biol. 2019, 218, 3171–3187. [Google Scholar] [CrossRef]
- Lian, I.A.; Loset, M.; Mundal, S.B.; Fenstad, M.H.; Johnson, M.P.; Eide, I.P.; Bjorge, L.; Freed, K.A.; Moses, E.K.; Austgulen, R. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta 2011, 32, 823–829. [Google Scholar] [CrossRef]
- Yung, H.W.; Calabrese, S.; Hynx, D.; Hemmings, B.A.; Cetin, I.; Charnock-Jones, D.S.; Burton, G.J. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 2008, 173, 451–462. [Google Scholar] [CrossRef]
- Michalak, M.; Groenendyk, J.; Szabo, E.; Gold, L.I.; Opas, M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 2009, 417, 651–666. [Google Scholar] [CrossRef]
- Hojrup, P.; Roepstorff, P.; Houen, G. Human placental calreticulin characterization of domain structure and post-translational modifications. Eur. J. Biochem. 2001, 268, 2558–2565. [Google Scholar] [CrossRef]
- Gu, V.Y.; Wong, M.H.; Stevenson, J.L.; Crawford, K.E.; Brennecke, S.P.; Gude, N.M. Calreticulin in human pregnancy and pre-eclampsia. Mol. Hum. Reprod. 2008, 14, 309–315. [Google Scholar] [CrossRef][Green Version]
- Shi, Z.; Hou, W.; Hua, X.; Zhang, X.; Liu, X.; Wang, X.; Wang, X. Overexpression of calreticulin in pre-eclamptic placentas: Effect on apoptosis, cell invasion and severity of pre-eclampsia. Cell Biochem. Biophys. 2012, 63, 183–189. [Google Scholar] [CrossRef]
- Crawford, K.E.; Stevenson, J.L.; Wlodek, M.E.; Gude, N.M. No change in calreticulin with fetal growth restriction in human and rat pregnancies. Placenta 2013, 34, 1066–1071. [Google Scholar] [CrossRef]
- Crawford, K.E.; Kalionis, B.; Stevenson, J.L.; Brennecke, S.P.; Gude, N.M. Calreticulin has opposing effects on the migration of human trophoblast and myometrial endothelial cells. Placenta 2012, 33, 416–423. [Google Scholar] [CrossRef]
- Yamamoto, M.; Ikezaki, M.; Toujima, S.; Iwahashi, N.; Mizoguchi, M.; Nanjo, S.; Minami, S.; Ihara, Y.; Ino, K. Calreticulin is involved in invasion of human extravillous trophoblasts through functional regulation of integrin beta1. Endocrinology 2017, 158, 3874–3889. [Google Scholar] [CrossRef]
- Iwahashi, N.; Ikezaki, M.; Matsuzaki, I.; Yamamoto, M.; Toujima, S.; Murata, S.I.; Ihara, Y.; Ino, K. Calreticulin regulates syncytialization through control of the synthesis and transportation of e-cadherin in bewo cells. Endocrinology 2019, 160, 359–374. [Google Scholar] [CrossRef]
- Kozlov, G.; Gehring, K. Calnexin cycle—Structural features of the ER chaperone system. FEBS J. 2020, 287, 4322–4340. [Google Scholar] [CrossRef]
- Hammond, C.; Braakman, I.; Helenius, A. Role of n-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc. Natl. Acad. Sci. USA 1994, 91, 913–917. [Google Scholar] [CrossRef]
- Ware, F.E.; Vassilakos, A.; Peterson, P.A.; Jackson, M.R.; Lehrman, M.A.; Williams, D.B. The molecular chaperone calnexin binds glc1man9glcnac2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J. Biol. Chem. 1995, 270, 4697–4704. [Google Scholar] [CrossRef]
- Spiro, R.G.; Zhu, Q.; Bhoyroo, V.; Soling, H.D. Definition of the lectin-like properties of the molecular chaperone, calreticulin, and demonstration of its copurification with endomannosidase from rat liver golgi. J. Biol. Chem. 1996, 271, 11588–11594. [Google Scholar] [CrossRef]
- Williams, D.B. Beyond lectins: The calnexin/calreticulin chaperone system of the endoplasmic reticulum. J. Cell Sci. 2006, 119, 615–623. [Google Scholar] [CrossRef]
- Lamriben, L.; Graham, J.B.; Adams, B.M.; Hebert, D.N. N-glycan-based er molecular chaperone and protein quality control system: The calnexin binding cycle. Traffic 2016, 17, 308–326. [Google Scholar] [CrossRef]
- Denzel, A.; Molinari, M.; Trigueros, C.; Martin, J.E.; Velmurgan, S.; Brown, S.; Stamp, G.; Owen, M.J. Early postnatal death and motor disorders in mice congenitally deficient in calnexin expression. Mol. Cell Biol. 2002, 22, 7398–7404. [Google Scholar] [CrossRef]
- Kraus, A.; Groenendyk, J.; Bedard, K.; Baldwin, T.A.; Krause, K.H.; Dubois-Dauphin, M.; Dyck, J.; Rosenbaum, E.E.; Korngut, L.; Colley, N.J.; et al. Calnexin deficiency leads to dysmyelination. J. Biol. Chem. 2010, 285, 18928–18938. [Google Scholar] [CrossRef]
- Jung, J.; Eggleton, P.; Robinson, A.; Wang, J.; Gutowski, N.; Holley, J.; Newcombe, J.; Dudek, E.; Paul, A.M.; Zochodne, D.; et al. Calnexin is necessary for t cell transmigration into the central nervous system. JCI Insight 2018, 3, 98410. [Google Scholar] [CrossRef]
- Orendi, K.; Gauster, M.; Moser, G.; Meiri, H.; Huppertz, B. The choriocarcinoma cell line bewo: Syncytial fusion and expression of syncytium-specific proteins. Reproduction 2010, 140, 759–766. [Google Scholar] [CrossRef]
- Sivasubramaniyam, T.; Garcia, J.; Tagliaferro, A.; Melland-Smith, M.; Chauvin, S.; Post, M.; Todros, T.; Caniggia, I. Where polarity meets fusion: Role of par6 in trophoblast differentiation during placental development and preeclampsia. Endocrinology 2013, 154, 1296–1309. [Google Scholar] [CrossRef] [PubMed]
- Cronier, L.; Bastide, B.; Herve, J.C.; Deleze, J.; Malassine, A. Gap junctional communication during human trophoblast differentiation: Influence of human chorionic gonadotropin. Endocrinology 1994, 135, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.X.; Conn, P.M. Chaperoning g protein-coupled receptors: From cell biology to therapeutics. Endocr. Rev. 2014, 35, 602–647. [Google Scholar] [CrossRef] [PubMed]
- Mizrachi, D.; Segaloff, D.L. Intracellularly located misfolded glycoprotein hormone receptors associate with different chaperone proteins than their cognate wild-type receptors. Mol. Endocrinol. 2004, 18, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cai, H.; Fatima, N.; Buczko, E.; Dufau, M.L. Functional glycosylation sites of the rat luteinizing hormone receptor required for ligand binding. J. Biol. Chem. 1995, 270, 21722–21728. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chaudhry, P.; Asselin, E. Bridging endometrial receptivity and implantation: Network of hormones, cytokines, and growth factors. J. Endocrinol. 2011, 210, 5–14. [Google Scholar] [CrossRef]
- Fournier, T. Human chorionic gonadotropin: Different glycoforms and biological activity depending on its source of production. Ann. Endocrinol. 2016, 77, 75–81. [Google Scholar] [CrossRef]
- Olsen, D.T.; Peng, L.; Traeholt, S.D.; Duus, K.; Hojrup, P.; Houen, G. Purification and characterization of a soluble calnexin from human placenta. Protein. Expr. Purif. 2013, 92, 105–111. [Google Scholar] [CrossRef][Green Version]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two hormones for one receptor: Evolution, biochemistry, actions, and pathophysiology of lh and hcg. Endocr. Rev. 2018, 39, 549–592. [Google Scholar] [CrossRef]
- Rozell, T.G.; Davis, D.P.; Chai, Y.; Segaloff, D.L. Association of gonadotropin receptor precursors with the protein folding chaperone calnexin. Endocrinology 1998, 139, 1588–1593. [Google Scholar] [CrossRef][Green Version]
- Davis, D.P.; Rozell, T.G.; Liu, X.; Segaloff, D.L. The six n-linked carbohydrates of the lutropin/choriogonadotropin receptor are not absolutely required for correct folding, cell surface expression, hormone binding, or signal transduction. Mol. Endocrinol. 1997, 11, 550–562. [Google Scholar] [CrossRef]
- Gerbaud, P.; Pidoux, G. Review: An overview of molecular events occurring in human trophoblast fusion. Placenta 2015, 36 (Suppl. 1), S35–S42. [Google Scholar] [CrossRef]
- Robin, M.J.D.; Appelman, M.D.; Vos, H.R.; van Es, R.M.; Paton, J.C.; Paton, A.W.; Burgering, B.; Fickert, P.; Heijmans, J.; van de Graaf, S.F.J. Calnexin depletion by endoplasmic reticulum stress during cholestasis inhibits the na(+)-taurocholate cotransporting polypeptide. Hepatol. Commun. 2018, 2, 1550–1566. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Xu, C.F.; Yu, C.H.; Chen, W.X.; Li, Y.M. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1768–1776. [Google Scholar] [CrossRef]
- Clarke, J.D.; Novak, P.; Lake, A.D.; Hardwick, R.N.; Cherrington, N.J. Impaired n-linked glycosylation of uptake and efflux transporters in human non-alcoholic fatty liver disease. Liver Int. 2017, 37, 1074–1081. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of antigen processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Leone, P.; Shin, E.C.; Perosa, F.; Vacca, A.; Dammacco, F.; Racanelli, V. Mhc class i antigen processing and presenting machinery: Organization, function, and defects in tumor cells. J. Natl. Cancer Inst. 2013, 105, 1172–1187. [Google Scholar] [CrossRef]
- Dissemond, J.; Busch, M.; Kothen, T.; Mors, J.; Weimann, T.K.; Lindeke, A.; Goos, M.; Wagner, S.N. Differential downregulation of endoplasmic reticulum-residing chaperones calnexin and calreticulin in human metastatic melanoma. Cancer Lett. 2004, 203, 225–231. [Google Scholar] [CrossRef]
- Li, H.; Han, L.; Yang, Z.; Huang, W.; Zhang, X.; Gu, Y.; Li, Y.; Liu, X.; Zhou, L.; Hu, J.; et al. Differential proteomic analysis of syncytiotrophoblast extracellular vesicles from early-onset severe preeclampsia, using 8-plex itraq labeling coupled with 2d nano lc-ms/ms. Cell Physiol. Biochem. 2015, 36, 1116–1130. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsukawa, H.; Ikezaki, M.; Nishioka, K.; Iwahashi, N.; Fujimoto, M.; Nishitsuji, K.; Ihara, Y.; Ino, K. Calnexin Is Involved in Forskolin-Induced Syncytialization in Cytotrophoblast Model BeWo Cells. Biomolecules 2022, 12, 1050. https://doi.org/10.3390/biom12081050
Matsukawa H, Ikezaki M, Nishioka K, Iwahashi N, Fujimoto M, Nishitsuji K, Ihara Y, Ino K. Calnexin Is Involved in Forskolin-Induced Syncytialization in Cytotrophoblast Model BeWo Cells. Biomolecules. 2022; 12(8):1050. https://doi.org/10.3390/biom12081050
Chicago/Turabian StyleMatsukawa, Hitomi, Midori Ikezaki, Kaho Nishioka, Naoyuki Iwahashi, Masakazu Fujimoto, Kazuchika Nishitsuji, Yoshito Ihara, and Kazuhiko Ino. 2022. "Calnexin Is Involved in Forskolin-Induced Syncytialization in Cytotrophoblast Model BeWo Cells" Biomolecules 12, no. 8: 1050. https://doi.org/10.3390/biom12081050
APA StyleMatsukawa, H., Ikezaki, M., Nishioka, K., Iwahashi, N., Fujimoto, M., Nishitsuji, K., Ihara, Y., & Ino, K. (2022). Calnexin Is Involved in Forskolin-Induced Syncytialization in Cytotrophoblast Model BeWo Cells. Biomolecules, 12(8), 1050. https://doi.org/10.3390/biom12081050





