Hormonal Crosstalk and Root Suberization for Drought Stress Tolerance in Plants
Abstract
:1. Introduction
2. Hormonal Regulation in Drought Stress
2.1. ABA-Biosynthesis and Signaling
2.2. Cytokinin (CK) Signaling
2.3. Auxin Signaling
2.4. Ethylene Signaling
3. Soluble Sugars in Drought and Sugar-Responsive Metabolism
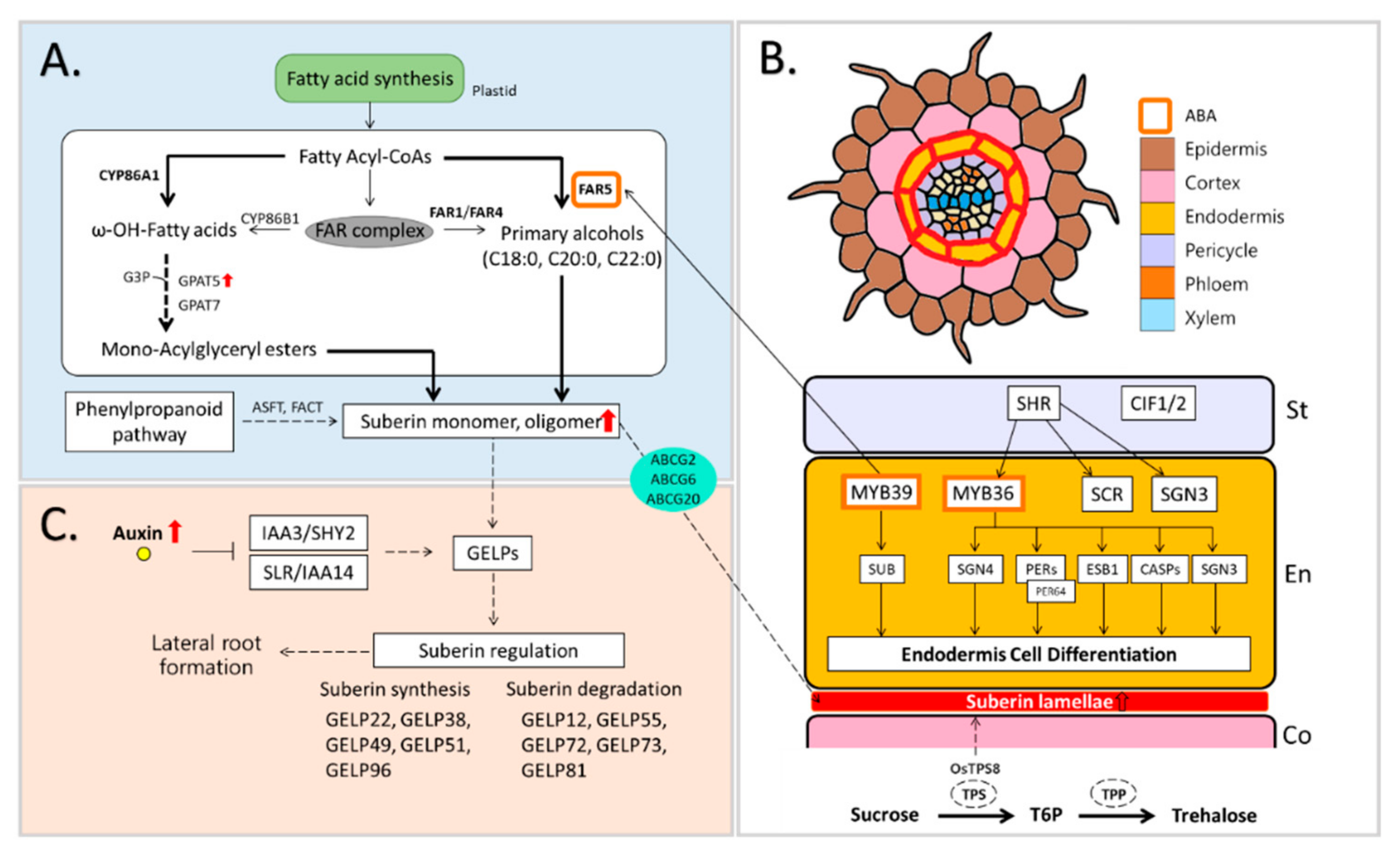
4. Suberin Biosynthesis in Plant Roots and Drought-Derived Modification
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAO3 | ABA-aldehyde oxidase3 |
| ABAs | ABA DEFICIENT |
| ABCG | ATP-binding cassette (ABC) transporters |
| ABI | ABA-INSENSITIVE |
| AHK | Histidine kinase |
| AHP | Histidine phosphotransfer proteins |
| AREB | ABA-responsive element-binding proteins |
| ARF | ABA binding factor |
| ARRs | Arabidopsis type response regulators |
| ASFT | Aliphatic suberin feruloyl transferase |
| CASPs | Casparian strip membrane domain proteins |
| CIF | Casparian strip integrity factor |
| ESB1 | enhanced suberin 1 |
| FACT | Aliphatic suberin feruloyl transferase |
| FARs | fatty acyl reductases |
| Fru | fructose |
| GELPs | GDSL-type esterase/lipase |
| GH3 | Gretchen Hagen 3 |
| Glc | galactose |
| Glu | glucose |
| GPATs | Glycerol-3-phosphate acyltransferase |
| IAAs | Indole-3-acetic acid |
| LBDs | the LATERAL ORGAN BOUNDARIES DOMAIN transcription factors |
| LST8 | LETHAL WITH SEC THIRTEEN 8 |
| NECD3 | 9-cis-epoxycarotenoid dioxygenase |
| PODs | peroxidase |
| PP2C | Protein phosphatase 2C |
| PYL | pyrabactin resistance-like |
| RAPTOR | REGULATORY-ASSOCIATED PROTEIN OF TOR |
| SCR | SCARECROW |
| SGN | SCHENGEN |
| SHR | SHORT-ROOT |
| SHY2 | IAA3 |
| SLR | IAA14 |
| SnRKs | SNF1-RELATED KINASE |
| T6P | Trehalose-6-phosphate |
| TOR | TARGET OF RAPAMYCIN |
| TPP | T6P-phosphatase |
| TPPE | Probable trehalose-phosphate phosphatase E |
| TPS | trehalose-6-phosphate synthase |
References
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Anjum, S.A.; Xie, X.-y.; Wang, L.-c.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Fu, B.-Y.; Xiong, J.-H.; Zhu, L.-H.; Zhao, X.-Q.; Xu, H.-X.; Gao, Y.-M.; Li, Y.-S.; Xu, J.-L.; Li, Z.-K. Identification of functional candidate genes for drought tolerance in rice. Mol. Genet. Genom. 2007, 278, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, P.; Xu, G.C.; Xiao, L.; Ren, Z.P.; Li, Z.B. Growth, morphological, and physiological responses to drought stress in Bothriochloa ischaemum. Front. Plant Sci. 2017, 8, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of Abscisic Acid-Mediated Drought Stress Responses in Plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef] [PubMed]
- Lisar, S.; Motafakkerazad, R.; Hossain, M.M.; Rahman, I. Causes, Effects and Responses. Water Stress 2012, 25. [Google Scholar] [CrossRef] [Green Version]
- Henry, A.; Cal, A.J.; Batoto, T.C.; Torres, R.O.; Serraj, R. Root attributes affecting water uptake of rice (Oryza sativa) under drought. J. Exp. Bot. 2012, 63, 4751–4763. [Google Scholar] [CrossRef]
- Dalal, M.; Sahu, S.; Tiwari, S.; Rao, A.R.; Gaikwad, K. Transcriptome analysis reveals interplay between hormones, ROS metabolism and cell wall biosynthesis for drought-induced root growth in wheat. Plant Physiol. Biochem. 2018, 130, 482–492. [Google Scholar] [CrossRef]
- Cardoso, A.A.; Gori, A.; Da-Silva, C.J.; Brunetti, C. Abscisic acid biosynthesis and signaling in plants: Key targets to improve water use efficiency and drought tolerance. Appl. Sci. 2020, 10, 6322. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.; Zhang, H.; Tian, S.; Chang, X.; Jing, R. TaSnRK2. 4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis. J. Exp. Bot. 2010, 61, 683–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-j.; Kang, J.-y.; Park, H.-J.; Kim, M.D.; Bae, M.S.; Choi, H.-i.; Kim, S.Y. DREB2C interacts with ABF2, a bZIP protein regulating abscisic acid-responsive gene expression, and its overexpression affects abscisic acid sensitivity. Plant Physiol. 2010, 153, 716–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.S.; Kim, Y.S.; Baek, K.H.; Jung, H.; Ha, S.-H.; Do Choi, Y.; Kim, M.; Reuzeau, C.; Kim, J.-K. Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol. 2010, 153, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Kang, N.Y.; Cho, C.; Kim, N.Y.; Kim, J. Cytokinin receptor-dependent and receptor-independent pathways in the dehydration response of Arabidopsis thaliana. J. Plant Physiol. 2012, 169, 1382–1391. [Google Scholar] [CrossRef]
- Nishiyama, R.; Watanabe, Y.; Leyva-Gonzalez, M.A.; Van Ha, C.; Fujita, Y.; Tanaka, M.; Seki, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Herrera-Estrella, L. Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response. Proc. Natl. Acad. Sci. USA 2013, 110, 4840–4845. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.H.; Van Ha, C.; Nishiyama, R.; Watanabe, Y.; Leyva-González, M.A.; Fujita, Y.; Tran, U.T.; Li, W.; Tanaka, M.; Seki, M. Arabidopsis type B cytokinin response regulators ARR1, ARR10, and ARR12 negatively regulate plant responses to drought. Proc. Natl. Acad. Sci. USA 2016, 113, 3090–3095. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Liu, H.; Xiong, L. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, J.; Zhang, W.; Yan, S.; Wang, R.; Zhao, J.; Li, Y.; Qi, Z.; Sun, Z.; Zhu, Z. The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J. 2012, 72, 805–816. [Google Scholar] [CrossRef]
- Kinoshita, N.; Wang, H.; Kasahara, H.; Liu, J.; MacPherson, C.; Machida, Y.; Kamiya, Y.; Hannah, M.A.; Chua, N.-H. IAA-Ala Resistant3, an evolutionarily conserved target of miR167, mediates Arabidopsis root architecture changes during high osmotic stress. Plant Cell 2012, 24, 3590–3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Xiong, L. A plant microRNA regulates the adaptation of roots to drought stress. FEBS Lett. 2012, 586, 1742–1747. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Liao, P.-M.; Kuo, W.-W.; Lin, T.-P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, M.; Skirycz, A.; Claeys, H.; Maleux, K.; Dhondt, S.; De Bodt, S.; Vanden Bossche, R.; De Milde, L.; Yoshizumi, T.; Matsui, M. ETHYLENE RESPONSE FACTOR6 acts as a central regulator of leaf growth under water-limiting conditions in Arabidopsis. Plant Physiol. 2013, 162, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Joong, P.; Hyun, S.; Reveche, M.C.F.; Shic, Y.; Won, J.; Kon, J. Overexpression of OsERF48 causes regulation of OsCML16, a calmodulinâ like protein gene that enhances root growth and drought tolerance. Plant Biotechnol. J. 2017, 15, 1295–1308. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.-K.; Jung, H.; Jang, G.; Jeong, J.S.; Kim, Y.S.; Ha, S.-H.; Do Choi, Y.; Kim, J.-K. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol. 2016, 172, 575–588. [Google Scholar] [CrossRef] [Green Version]
- Quan, R.; Hu, S.; Zhang, Z.; Zhang, H.; Zhang, Z.; Huang, R. Overexpression of an ERF transcription factor TSRF1 improves rice drought tolerance. Plant Biotechnol. J. 2010, 8, 476–488. [Google Scholar] [CrossRef]
- Ikegami, K.; Okamoto, M.; Seo, M.; Koshiba, T. Activation of abscisic acid biosynthesis in the leaves of Arabidopsis thaliana in response to water deficit. J. Plant Res. 2009, 122, 235–243. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic acid synthesis and response. Arab. Book/Am. Soc. Plant Biol. 2013, 11, e0166. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Hou, L.; Meng, J.; You, H.; Li, Z.; Gong, Z.; Yang, S.; Shi, Y. The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.-K.; Yoon, S.; Kim, Y.S.; Kim, J.-K. Rice OsERF71-mediated root modification affects shoot drought tolerance. Plant Signal. Behav. 2017, 12, e1268311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Zhang, J.; Zhang, H.; Zhang, Z.; Quan, R.; Zhou, S.; Huang, R. Transcriptional activation of OsDERF1 in OsERF3 and OsAP2-39 negatively modulates ethylene synthesis and drought tolerance in rice. PLoS ONE 2011, 6, e25216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A. Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol. 2003, 131, 1591–1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Li, Y.; Zhang, J.; Xiao, Y.; Yue, Y.; Duan, L.; Zhang, M.; Li, Z. Overexpression of Arabidopsis molybdenum cofactor sulfurase gene confers drought tolerance in maize (Zea mays L.). PLoS ONE 2013, 8, e52126. [Google Scholar] [CrossRef]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef]
- Singh, D.; Laxmi, A. Transcriptional regulation of drought response: A tortuous network of transcriptional factors. Front. Plant Sci. 2015, 6, 895. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, X.; Zhang, K.; An, H.; Hu, K.; Wen, J.; Shen, J.; Ma, C.; Yi, B.; Tu, J. Comparative analysis of the Brassica napus root and leaf transcript profiling in response to drought stress. Int. J. Mol. Sci. 2015, 16, 18752–18777. [Google Scholar] [CrossRef] [Green Version]
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F.; Yamaguchi-Shinozaki, K.; Ishihama, Y.; Hirayama, T.; Shinozaki, K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588–17593. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef]
- Seo, P.J.; Xiang, F.; Qiao, M.; Park, J.-Y.; Lee, Y.N.; Kim, S.-G.; Lee, Y.-H.; Park, W.J.; Park, C.-M. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol. 2009, 151, 275–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef] [Green Version]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant Cell 2015, 27, 44–63. [Google Scholar] [CrossRef] [Green Version]
- Prerostova, S.; Dobrev, P.I.; Gaudinova, A.; Knirsch, V.; Körber, N.; Pieruschka, R.; Fiorani, F.; Brzobohatý, B.; Černý, M.; Spichal, L. Cytokinins: Their impact on molecular and growth responses to drought stress and recovery in Arabidopsis. Front. Plant Sci. 2018, 9, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soba, D.; Zhou, B.; Arrese-Igor, C.; Munné-Bosch, S.; Aranjuelo, I. Physiological, hormonal and metabolic responses of two alfalfa cultivars with contrasting responses to drought. Int. J. Mol. Sci. 2019, 20, 5099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. Plant Cell 2010, 22, 3905–3920. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-M.; Zheng, H.-X.; Zhang, X.-S.; Sui, N. Cytokinins as central regulators during plant growth and stress response. Plant Cell Rep. 2021, 40, 271–282. [Google Scholar] [CrossRef]
- Leyser, O. Dynamic integration of auxin transport and signalling. Curr. Biol. 2006, 16, R424–R433. [Google Scholar] [CrossRef] [Green Version]
- Sharma, E.; Sharma, R.; Borah, P.; Jain, M.; Khurana, J.P. Emerging roles of auxin in abiotic stress responses. In Elucidation of Abiotic Stress Signaling in Plants; Springer: New York, NY, USA, 2015; pp. 299–328. [Google Scholar]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Reed, J.W. Roles and activities of Aux/IAA proteins in Arabidopsis. Trends Plant Sci. 2001, 6, 420–425. [Google Scholar] [CrossRef]
- Vierstra, R.D. The ubiquitin–26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009, 10, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Růźićka, K.; Ljung, K.; Vanneste, S.; Podhorská, R.; Beeckman, T.; Friml, J.i.; Benková, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant Sci. 2012, 17, 181–195. [Google Scholar] [CrossRef]
- Wang, K.L.-C.; Li, H.; Ecker, J.R. Ethylene biosynthesis and signaling networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef] [Green Version]
- Lacey, R.F.; Binder, B.M. How plants sense ethylene gas—The ethylene receptors. J. Inorg. Biochem. 2014, 133, 58–62. [Google Scholar] [CrossRef]
- Kendrick, M.D.; Chang, C. Ethylene signaling: New levels of complexity and regulation. Curr. Opin. Plant Biol. 2008, 11, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.-C.; Sheen, J. Sugar sensing in higher plants. Trends Plant Sci. 1997, 2, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Pego, J.V.; Kortstee, A.J.; Huijser, C.; Smeekens, S.C. Photosynthesis, sugars and the regulation of gene expression. J. Exp. Bot. 2000, 51 (Suppl. S1), 407–416. [Google Scholar] [CrossRef] [Green Version]
- Loreti, E.; Bellis, L.D.; Alpi, A.; Perata, P. Why and how do plant cells sense sugars? Ann. Bot. 2001, 88, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, P.K.; Sharma, A.D.; Singh, P.; Bhullar, S.S. Effect of various abiotic stresses on the growth, soluble sugars and water relations of sorghum seedlings grown in light and darkness. Bulg. J. Plant Physiol. 2001, 27, 72–84. [Google Scholar]
- Dong, S.; Zhang, J.; Beckles, D.M. A pivotal role for starch in the reconfiguration of 14C-partitioning and allocation in Arabidopsis thaliana under short-term abiotic stress. Sci. Rep. 2018, 8, 9314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadkhani, N.; Heidari, R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J. 2008, 3, 448–453. [Google Scholar]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Deyanira, Q.-M.; Estrada-Luna, A.A.; Altamirano-Hernández, J.; Peña-Cabriales, J.J.; de Oca-Luna, R.M.; Cabrera-Ponce, J.L. Use of trehalose metabolism as a biochemical marker in rice breeding. Mol. Breed. 2012, 30, 469–477. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, K.; Zhang, T.; Li, L.; Wang, J.; Cheng, T.; Zhang, Q. Characteristics and Expression Analyses of Trehalose-6-Phosphate Synthase Family in Prunus mume Reveal Genes Involved in Trehalose Biosynthesis and Drought Response. Biomolecules 2020, 10, 1358. [Google Scholar] [CrossRef]
- Ponnu, J.; Wahl, V.; Schmid, M. Trehalose-6-phosphate: Connecting plant metabolism and development. Front. Plant Sci. 2011, 2, 70. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, C.M.; Lunn, J.E. A tale of two sugars: Trehalose 6-phosphate and sucrose. Plant Physiol. 2016, 172, 7–27. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Chen, W.; Gao, J.; Yang, F.; Zhuang, C. Overexpression of the trehalose-6-phosphate phosphatase OsTPP3 increases drought tolerance in rice. Plant Biotechnol. Rep. 2019, 13, 285–292. [Google Scholar] [CrossRef]
- Vishal, B.; Krishnamurthy, P.; Ramamoorthy, R.; Kumar, P.P. Os TPS 8 controls yield-related traits and confers salt stress tolerance in rice by enhancing suberin deposition. New Phytol. 2019, 221, 1369–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.-D.; Ogé, L.; Hamama, L.; Atanassova, R. The sugar-signaling hub: Overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Hu, T.; Li, X.; Song, C.-P.; Zhu, J.-K.; Chen, L.; Zhao, Y. Phosphorylation of SWEET sucrose transporters regulates plant root: Shoot ratio under drought. Nat. Plants 2022, 8, 68–77. [Google Scholar] [CrossRef]
- Avonce, N.; Mendoza-Vargas, A.; Morett, E.; Iturriaga, G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Cortina, C.; Culiáñez-Macià, F.A. Tomato abiotic stress enhanced tolerance by trehalose biosynthesis. Plant Sci. 2005, 169, 75–82. [Google Scholar] [CrossRef]
- Hlahla, J.M.; Mafa, M.S.; Van der Merwe, R.; Alexander, O.; Duvenhage, M.-M.; Kemp, G.; Moloi, M.J. The photosynthetic efficiency and carbohydrates responses of six edamame (Glycine max. L. Merrill) cultivars under drought stress. Plants 2022, 11, 394. [Google Scholar] [CrossRef]
- Ilhan, S.; Ozdemir, F.; Bor, M. Contribution of trehalose biosynthetic pathway to drought stress tolerance of Capparis ovata Desf. Plant Biol. 2015, 17, 402–407. [Google Scholar] [CrossRef]
- Liu, Y.; He, Z.; Xie, Y.; Su, L.; Zhang, R.; Wang, H.; Li, C.; Long, S. Drought resistance mechanisms of Phedimus aizoon L. Sci. Rep. 2021, 11, 13600. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Xu, J.; Duan, S.; Wang, Q.; Li, G.; Jin, L. Transcriptome profiling reveals effects of drought stress on gene expression in diploid potato genotype P3-198. Int. J. Mol. Sci. 2019, 20, 852. [Google Scholar] [CrossRef] [Green Version]
- Broeckx, T.; Hulsmans, S.; Rolland, F. The plant energy sensor: Evolutionary conservation and divergence of SnRK1 structure, regulation, and function. J. Exp. Bot. 2016, 67, 6215–6252. [Google Scholar] [CrossRef] [PubMed]
- Baena-González, E.; Hanson, J. Shaping plant development through the SnRK1–TOR metabolic regulators. Curr. Opin. Plant Biol. 2017, 35, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Belda-Palazón, B.; Adamo, M.; Valerio, C.; Ferreira, L.J.; Confraria, A.; Reis-Barata, D.; Rodrigues, A.; Meyer, C.; Rodriguez, P.L.; Baena-González, E. A dual function of SnRK2 kinases in the regulation of SnRK1 and plant growth. Nat. Plants 2020, 6, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Goddijn, O.J.; van Dun, K. Trehalose metabolism in plants. Trends Plant Sci. 1999, 4, 315–319. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Li, Z.; Hsu, C.-C.; Liu, X.; Fu, L.; Hou, Y.-J.; Du, Y.; Xie, S.; Zhang, C. Reciprocal regulation of the TOR kinase and ABA receptor balances plant growth and stress response. Mol. Cell 2018, 69, 100–112.e106. [Google Scholar] [CrossRef] [Green Version]
- Janse van Rensburg, H.C.; Van den Ende, W.; Signorelli, S. Autophagy in plants: Both a puppet and a puppet master of sugars. Front. Plant Sci. 2019, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Chen, Q.; Xu, S.; Liu, W.C.; Zhu, X.; Song, C.P. Trehalose-6-phosphate phosphatase E modulates ABA-controlled root growth and stomatal movement in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 1518–1534. [Google Scholar] [CrossRef] [Green Version]
- Graça, J.; Santos, S. Suberin: A biopolyester of plants’ skin. Macromol. Biosci. 2007, 7, 128–135. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; DeBono, A.; Durrett, T.P. Acyl-lipid metabolism. Arab. Book/Am. Soc. Plant Biol. 2013, 11, e0161. [Google Scholar] [CrossRef] [Green Version]
- Domergue, F.; Vishwanath, S.J.; Joubès, J.; Ono, J.; Lee, J.A.; Bourdon, M.; Alhattab, R.; Lowe, C.; Pascal, S.; Lessire, R. Three Arabidopsis fatty acyl-coenzyme A reductases, FAR1, FAR4, and FAR5, generate primary fatty alcohols associated with suberin deposition. Plant Physiol. 2010, 153, 1539–1554. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.; Molina, I.; Ranathunge, K.; Castillo, I.Q.; Rothstein, S.J.; Reed, J.W. ABCG transporters are required for suberin and pollen wall extracellular barriers in Arabidopsis. Plant Cell 2014, 26, 3569–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enstone, D.E.; Peterson, C.A.; Ma, F. Root endodermis and exodermis: Structure, function, and responses to the environment. J. Plant Growth Regul. 2002, 21, 335–351. [Google Scholar] [CrossRef]
- Barberon, M.; Vermeer, J.E.M.; De Bellis, D.; Wang, P.; Naseer, S.; Andersen, T.G.; Humbel, B.M.; Nawrath, C.; Takano, J.; Salt, D.E. Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 2016, 164, 447–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, R.; Schreiber, L. Suberin—a biopolyester forming apoplastic plant interfaces. Curr. Opin. Plant Biol. 2007, 10, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yu, Q.; Gu, X.; Xu, C.; Qi, S.; Wang, H.; Zhong, F.; Baskin, T.I.; Rahman, A.; Wu, S. Construction of a functional casparian strip in non-endodermal lineages is orchestrated by two parallel signaling systems in Arabidopsis thaliana. Curr. Biol. 2018, 28, 2777–2786.e2772. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Calvo-Polanco, M.; Reyt, G.; Barberon, M.; Champeyroux, C.; Santoni, V.; Maurel, C.; Franke, R.B.; Ljung, K.; Novak, O. Surveillance of cell wall diffusion barrier integrity modulates water and solute transport in plants. Sci. Rep. 2019, 9, 4227. [Google Scholar] [CrossRef] [Green Version]
- Drapek, C.; Sparks, E.E.; Marhavy, P.; Taylor, I.; Andersen, T.G.; Hennacy, J.H.; Geldner, N.; Benfey, P.N. Minimum requirements for changing and maintaining endodermis cell identity in the Arabidopsis root. Nat. Plants 2018, 4, 586–595. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Li, P.; Li, H.; Xu, C.; Cohen, H.; Aharoni, A.; Wu, S. Developmental programs interact with abscisic acid to coordinate root suberization in Arabidopsis. Plant J. 2020, 104, 241–251. [Google Scholar] [CrossRef]
- Sozzani, R.; Cui, H.; Moreno-Risueno, M.; Busch, W.; Van Norman, J.; Vernoux, T.; Brady, S.; Dewitte, W.; Murray, J.A.H.; Benfey, P. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature 2010, 466, 128–132. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, P.; Wang, G.; Augstein, F.; de Vries, J.; Carlsbecker, A. Continuous root xylem formation and vascular acclimation to water deficit involves endodermal ABA signalling via miR165. Development 2018, 145, dev159202. [Google Scholar] [CrossRef] [Green Version]
- Cohen, H.; Fedyuk, V.; Wang, C.; Wu, S.; Aharoni, A. SUBERMAN regulates developmental suberization of the Arabidopsis root endodermis. Plant J. 2020, 102, 431–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, V.; Han, J.-P.; Cléard, F.; Lefebvre-Legendre, L.; Gully, K.; Flis, P.; Berhin, A.; Andersen, T.G.; Salt, D.E.; Nawrath, C. Suberin plasticity to developmental and exogenous cues is regulated by a set of MYB transcription factors. Proc. Natl. Acad. Sci. USA 2021, 118, e2101730118. [Google Scholar] [CrossRef] [PubMed]
- Ursache, R.; De Jesus Vieira Teixeira, C.; Dénervaud Tendon, V.; Gully, K.; De Bellis, D.; Schmid-Siegert, E.; Grube Andersen, T.; Shekhar, V.; Calderon, S.; Pradervand, S. GDSL-domain proteins have key roles in suberin polymerization and degradation. Nat. Plants 2021, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.D.; Kimura, S.; Wu, Q.; Franke, R.B.; Kamiya, T.; Kasahara, H. Regulation of suberin biosynthesis and Casparian strip development in the root endodermis by two plant auxins. bioRxiv 2021. [Google Scholar] [CrossRef]
- Shani, E.; Binenbaum, J.; Wulff, N.; Camut, L.; Kiradjiev, K.; Tal, I.; Vasuki, H.; Anfang, M.; Zhang, Y.; Sakvarelidze-Achard, L. Gibberellin and abscisic acid transporters facilitate endodermal suberin formation in Arabidopsis. Res. Sq. 2022, preprint. [Google Scholar]
- Kreszies, T.; Shellakkutti, N.; Osthoff, A.; Yu, P.; Baldauf, J.A.; Zeisler-Diehl, V.V.; Ranathunge, K.; Hochholdinger, F.; Schreiber, L. Osmotic stress enhances suberization of apoplastic barriers in barley seminal roots: Analysis of chemical, transcriptomic and physiological responses. New Phytol. 2019, 221, 180–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Guo, Y.; Zhong, J.; Zhang, T.; Li, D.; Ba, T.; Xu, T.; Chang, L.; Zhang, Q.; Sun, M. Root physiological traits and transcriptome analyses reveal that root zone water retention confers drought tolerance to Opisthopappus taihangensis. Sci. Rep. 2020, 10, 2627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grünhofer, P.; Schreiber, L.; Kreszies, T. Suberin in monocotyledonous crop plants: Structure and function in response to abiotic stresses. In Rhizobiology: Molecular Physiology of Plant Roots; Springer: Cham, Switzerland, 2021; pp. 333–378. [Google Scholar]
- de Silva, N.D.; Murmu, J.; Chabot, D.; Hubbard, K.; Ryser, P.; Molina, I.; Rowland, O. Root Suberin Plays Important Roles in Reducing Water Loss and Sodium Uptake in Arabidopsis thaliana. Metabolites 2021, 11, 735. [Google Scholar] [CrossRef]
- Reynoso, M.A.; Borowsky, A.T.; Pauluzzi, G.C.; Yeung, E.; Zhang, J.; Formentin, E.; Velasco, J.; Cabanlit, S.; Duvenjian, C.; Prior, M.J. Gene regulatory networks shape developmental plasticity of root cell types under water extremes in rice. Dev. Cell 2022, 57, 1177–1192.e6. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Ge, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Mur, L.A.J.; Jiang, D. The different root apex zones contribute to drought priming induced tolerance to a reoccurring drought stress in wheat. Crop J. 2021, 9, 1088–1097. [Google Scholar] [CrossRef]
- Ramachandran, P.; Augstein, F.; Nguyen, V.; Carlsbecker, A. Coping with water limitation: Hormones that modify plant root xylem development. Front. Plant Sci. 2020, 11, 570. [Google Scholar] [CrossRef] [PubMed]
- Bielach, A.; Podlešáková, K.; Marhavý, P.; Duclercq, J.; Cuesta, C.; Müller, B.; Grunewald, W.; Tarkowski, P.; Benková, E. Spatiotemporal regulation of lateral root organogenesis in Arabidopsis by cytokinin. Plant Cell 2012, 24, 3967–3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moubayidin, L.; Perilli, S.; Ioio, R.D.; Di Mambro, R.; Costantino, P.; Sabatini, S. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr. Biol. 2010, 20, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Swarup, K.; Benková, E.; Swarup, R.; Casimiro, I.; Péret, B.; Yang, Y.; Parry, G.; Nielsen, E.; De Smet, I.; Vanneste, S. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 2008, 10, 946–954. [Google Scholar] [CrossRef]
- Goh, T.; Kasahara, H.; Mimura, T.; Kamiya, Y.; Fukaki, H. Multiple AUX/IAA–ARF modules regulate lateral root formation: The role of Arabidopsis SHY2/IAA3-mediated auxin signalling. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1461–1468. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, Y.-C.; Wang, C.-Y.; Luo, Y.-C.; Huang, Q.-J.; Chen, S.-Y.; Zhou, H.; Qu, L.-H.; Chen, Y.-Q. Expression analysis of phytohormone-regulated microRNAs in rice, implying their regulation roles in plant hormone signaling. FEBS Lett. 2009, 583, 723–728. [Google Scholar] [CrossRef] [Green Version]
- Bakhshi, B.; Salekdeh, G.H.; Bihamta, M.R.; Tohidfar, M. Characterization of three key microRNAs in rice root architecture under drought stress using in silico analysis and quantitative real-time PCR. Biosci. Biotechnol. Res. Asia 2014, 11, 555–565. [Google Scholar] [CrossRef]
- Janiak, A.; Kwaśniewski, M.; Szarejko, I. Gene expression regulation in roots under drought. J. Exp. Bot. 2016, 67, 1003–1014. [Google Scholar] [CrossRef] [Green Version]
- Brocard-Gifford, I.; Lynch, T.J.; Garcia, M.E.; Malhotra, B.; Finkelstein, R.R. The Arabidopsis thaliana ABSCISIC ACID-INSENSITIVE8 locus encodes a novel protein mediating abscisic acid and sugar responses essential for growth. Plant Cell 2004, 16, 406–421. [Google Scholar] [CrossRef] [Green Version]
- Brocard, I.M.; Lynch, T.J.; Finkelstein, R.R. Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar, and stress response. Plant Physiol. 2002, 129, 1533–1543. [Google Scholar] [CrossRef] [Green Version]



| Related | Genes | Species | Genetic Manipulation | Effect on Tolerance | Ref. |
|---|---|---|---|---|---|
| ABA | AtNCED3 | Arabidopsis | Over-expression | Transgenic plants were more resistant to drought stress than WT. | [12] |
| ABA | TaSnRK2.4 | Arabidopsis | Over-expression | Under normal conditions, the primary root lengthens. | [13] |
| ABA | AtAREB1, AtAREB2, AtABF3 | Arabidopsis | Knock-out | Transgenic plants were more resistant to ABA compared to primary root growth and displayed reduced drought tolerance. | [14] |
| ABA | AtDREB2A, AtDREB1A, AtDREB2C | Arabidopsis | - | ABA-independent proteins (DREB2A, DREB1A, and DREB2C) interact with each other and play an important role in regulating drought response. | [15] |
| ABA | OsNAC10 | O. sativa | Over-expression | Transgenic plants increase root development and improve the drought tolerance of plants. | [16] |
| CK | AtAHK2 AtAHK3 | Arabidopsis | Knock-out | Transgenic plants more resistant to dehydration than wild-type plants | [17] |
| CK | AtAHP2 AtAHP3 AtAHP5 | Arabidopsis | Knock-out | AHP2, AHP3, and AHP5 responses to drought stress in a negative and redundant manner. | [18] |
| CK | AtARR1 AtARR10 AtARR12 | Arabidopsis | Knock-out | Triple mutant showed a significant increase in drought tolerance versus WT. | [19] |
| Auxin | OsPIN2 | O. sativa | - | Induced by drought. | [20] |
| Auxin | OsPIN5b | O. sativa | - | Induced by drought. | [20] |
| Auxin | OsPIN3t | O. sativa | Over-expression | Transgenic plants improved drought tolerance and led to root development. | [21] |
| O. sativa | Knock-out | Transgenic plants resulted in slightly shorter adventitious roots. | [21] | ||
| Auxin | AtIAR3 | Arabidopsis | Knock-out | Transgenic plants were significantly more sensitive to drought than WT and formed fewer lateral roots. | [22] |
| Auxin | DRO1 | O. sativa | Over-expression | Transgenic plants have higher drought tolerance. | [23] |
| Auxin | miR393 | Arabidopsis | - | miR393-mediated attenuation of auxin signaling is essential for the inhibition of lateral root growth by ABA or osmotic stress. | [24] |
| Ethlyene | AtERF1 | Arabidopsis thaliana. | Over-expression | ERF1 could enhance drought survival. | [25] |
| Ethlyene | AtERF5 AtERF6 | Arabidopsis thaliana. | Knock-out | Double mutants grow better under osmotic stress. | [26] |
| Ethlyene | OsERF48 | O. sativa | Over-expression | Transgenic plants improved drought tolerance and root growth. | [27] |
| Ethlyene | OsERF71 | O. sativa | Over-expression | Transgenic plant enhanced drought tolerance by enabling root morphological adaptation | [28] |
| Ethlyene | TSRF1 | O. sativa | Over-expression | Transgenic plants increase root length and weight and improve drought tolerance. | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Ryu, H.; Sung, J. Hormonal Crosstalk and Root Suberization for Drought Stress Tolerance in Plants. Biomolecules 2022, 12, 811. https://doi.org/10.3390/biom12060811
Kim G, Ryu H, Sung J. Hormonal Crosstalk and Root Suberization for Drought Stress Tolerance in Plants. Biomolecules. 2022; 12(6):811. https://doi.org/10.3390/biom12060811
Chicago/Turabian StyleKim, Gaeun, Hojin Ryu, and Jwakyung Sung. 2022. "Hormonal Crosstalk and Root Suberization for Drought Stress Tolerance in Plants" Biomolecules 12, no. 6: 811. https://doi.org/10.3390/biom12060811
APA StyleKim, G., Ryu, H., & Sung, J. (2022). Hormonal Crosstalk and Root Suberization for Drought Stress Tolerance in Plants. Biomolecules, 12(6), 811. https://doi.org/10.3390/biom12060811