Noninvasive Cardiac Imaging in Formerly Preeclamptic Women for Early Detection of Subclinical Myocardial Abnormalities: A 2022 Update
Abstract
1. Introduction
2. Cardiac Magnetic Resonance Imaging
2.1. Cine MRI
2.2. Myocardial Fibrosis
2.3. Myocardial Strain
2.4. Myocardial Perfusion
2.5. D Flow Measurements
3. Transthoracic Cardiac Ultrasonography
3.1. Volumes and Function
3.2. Myocardial Strain
3.3. Myocardial Perfusion
3.4. Diastolic Function
4. Pros and Cons of CMR and Cardiac Ultrasonography
5. Cardiac Imaging Studies in Formerly Preeclamptic Women
5.1. Search Strategy
- A clear focus on maternal postpartum cardiac imaging;
- Specific results of a post-preeclampsia case group;
- Cardiac imaging studies with the exception of imaging of the coronary arteries;
- Inclusion of a control group or comparing results to reported normal values in literature;
- Publication date <20 years;
- Animal studies were excluded.
5.2. Research Findings
| Reference | Subjects (n) (Controls/PE) | PPI (Months) (Controls/PE) | Age (Years) (Controls/PE) | Study Aim | Imaging Modalities Used | Main Outcomes |
|---|---|---|---|---|---|---|
| Al-Nashi et al., 2016 [69] | 16/15 | 134 ± 7/134 ± 7 | 41.2 ± 3.2/39.4 ± 3.6 | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Ambrožič et al., 2021 [70] | 15/25 | 1 day, 12 months/1 day, 12 months | 36 (31–39)/30 (27–37) | Assessment of ventricular structure and function from immediately post delivery to one year postpartum | US |
|
| Birukov et al., 2020 [16] | 22/22 | 48 ± 63/24 ± 12 | Not specified, no significant difference | Assessment of the possibility of early risk stratification with CMR | CMR |
|
| Bokslag et al., 2018 [61] | 56/131 | 170 ± 28/157 ± 5 | 46.5 ± 2.3/44.0 ± 5.6 | Assessment of PE-induced predisposition to HFpEF | US |
|
| Breetveld et al., 2018 [71] | 37/67 | 100 (79–119)/64 (53–77) | 40 (47–43)/36 (33–39) | Assessment of endothelial function and asymptomatic structural cardiac alterations | US |
|
| Ciftci et al., 2014 [72] | 27/40 | 60/60 | 36.44 ± 10.45/33.75 ± 7.95 | Assessment of impaired myocardial function and arterial stiffness | US |
|
| Clemmensen et al., 2018 [73] | 40/53 | 144 ± 56/150 ± 43 | 30 ± 5/30 ± 5 | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| deMartelly et al., 2021 [62] | 25/21 | Not specified | 39.72 ± 6.02/35.76 ± 5.62 | Assessment of ventricular structure and function in formerly preeclamptic women and the association of activin A to impaired global longitudinal strain | US |
|
| Ersbøll et al., 2018 [28] | 28/28 | 101 (25–146)/95 (26–143) | 39.1 ± 5.3/38.8 ± 5.6 | Assessment of the long-term effect of peripartum cardiomyopathy and PE on cardiac function | CMR |
|
| Ersbøll et al., 2021 [74] | 28/28 | 101 (25–146)/95 (26–143) | 39.1 ± 5.3/38.8 ± 5.7 | Assessment of the relation between biomarkers and cardiac function after PE or peripartum cardiomyopathy | CMR |
|
| Ghi et al., 2014 [64] | 18/16 | 6–12/6–12 | 31.0 (24–38)/36.5 (17–49) | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Ghossein-Doha et al., 2013 [75] | 8/20 | 12, 168/12, 168 | 33 (32–34) at 12 months, 45 (44–47) at 168 months/31 (30–32) at 12 months, 43 (42–45) at 168 months | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Ghossein-Doha et al., 2016 [76] | 51 PE | Not specified | 33 (30–35)/28 (25–33) | Assessment of ventricular structure and function in formerly preeclamptic women according to recurrence of PE | US |
|
| Ghossein-Doha et al., 2017 [77] | 41/107 | 4–10 years/4–10 years | Not specified, parous | Assessment of hemodynamical involvement in hypertensive pregnancy disorders | US |
|
| Guirguis et al., 2015 [78] | 27/39 | <5/<5 | <45/<45 | Assessment of PE as a predictor of diastolic dysfunction | US |
|
| Kalapathorakos et al., 2020 [14] | 8/6 | 1–3 days, 1 week, 6 months/1–3 days, 1 week, 6 months | 29 (20–41)/29 (23–36) | Assessment of ventricular structure and function in formerly preeclamptic women | CMR |
|
| Levine et al., 2019 [84] | 29/29 | 7 (6–9) weeks/6 (5–6) weeks | 27.8 ± 5.53/30.7 ± 7.32 | Assessment of ventricular structure and function in formerly preeclamptic women in the early postpartum period | US |
|
| Melchiorre et al., 2011 [10] | 37/27 | 12/12 months | 78 (29–38)/33 (29–37) | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Orabona et al., 2017 [79] | 60/60 | 26 ± 7/30 ± 12 | 36.87 ± 4.28/38.97 ± 3.71 | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Orabona et al., 2017 [85] | 30/60 | 2.2 ± 0.6 years/2.4 ± 0.7 years | 35 ± 4/35 ± 5 | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Orabona et al., 2020 [80] | 17 controls with fetal growth restriction/134 | 6–48/6–48 | 32 ± 3/30 ± 4 | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Rafik Hamad et al., 2009 [65] | 30/35 | 6–12/6–12 | 31 ± 4/31 ± 5 | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Reddy et al., 2019 [60] | Systematic review | Review | Review | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Shahul et al., 2018 [66] | 25/32 | 12/12 | 31.44 ± 4.96/31.50 ± 6.63 | Assessment of ventricular structure and function in formerly preeclamptic women and the association with activin A | US |
|
| Simmons et al., 2002 [81] | 44/15 | 3 ± 1/3 ± 1 months | 29 ± 5/32 ± 6 | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Soma-Pillay et al., 2018 [86] | 45/96 | 12/12 months | 27.2 ± 7.14/28.9 ± 6.83 | Assessment of ventricular diastolic function in formerly preeclamptic women | US |
|
| Spaan et al., 2009 [82] | 29/22 | 276/276 | Not specified, no significant difference | Assessment of remote hemodynamics and renal function in formerly preeclamptic women | US |
|
| Strobl et al., 2011 [87] | 17/14 | 14.94 ± 1.6 years/15.78 ± 2.2 years | 43.9 ± 3.8/43.6 ± 2.9 | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Valensise et al., 2008 [67] | 1119/107 | 12/12 | 32 ± 5/33 ± 4 | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Valensise et al., 2016 [68] | 147/53 | 12–18/12–18 | 34 ± 4/34 ± 4 | Assessment of ventricular structure and function in formerly preeclamptic women | US |
|
| Yuan et al., 2014 [83] | 7/7 | 16–20/16–20 | Not specified, no significant difference | Assessment of ventricular and carotid structure and function in formerly preeclamptic women | US |
|
6. Future Recommendations
6.1. Recommendations for Researchers
6.2. Recommendations for Clinicians
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, T.A.; Kahn, D.A.; Loewendorf, A.I. Maternal—Fetal rejection reactions are unconstrained in preeclamptic women. PLoS ONE 2017, 12, e0188250. [Google Scholar] [CrossRef] [PubMed]
- Ghossein-Doha, C.; Hooijschuur, M.C.E.; Spaanderman, M.E.A. Pre-Eclampsia: A Twilight Zone Between Health and Cardiovascular Disease? J. Am. Coll. Cardiol. 2018, 72, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.; Maas, A.H.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.C.; Liblik, K.; Johri, A.M.; Smith, G.N. Maternal Cardiovascular Function following a Pregnancy Complicated by Preeclampsia. Am. J. Perinatol. 2020. [Google Scholar] [CrossRef]
- Easterling, T.R.; Benedetti, T.J.; Schmucker, B.C.; Millard, S.P. Maternal hemodynamics in normal and preeclamptic pregnancies: A longitudinal study. Obs. Gynecol. 1990, 76, 1061–1069. [Google Scholar]
- Melchiorre, K.; Sharma, R.; Thilaganathan, B. Cardiovascular implications in preeclampsia: An overview. Circulation 2014, 130, 703–714. [Google Scholar] [CrossRef]
- Mulder, E.G.; de Haas, S.; Mohseni, Z.; Schartmann, N.; Abo Hasson, F.; Alsadah, F.; Van Kuijk, S.M.; Van Drongelen, J.; Spaanderman, M.E.; Ghossein-Doha, C. Cardiac output and peripheral vascular resistance during normotensive and hypertensive pregnancy—A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Benschop, L.; Brouwers, L.; Zoet, G.A.; Meun, C.; Boersma, E.; Budde, R.P.; Fauser, B.C.; de Groot, C.M.; van der Schouw, Y.T.; Maas, A.H.; et al. Early Onset of Coronary Artery Calcification in Women with Previous Preeclampsia. Circ. Cardiovasc. Imaging 2020, 13, e010340. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sutherland, G.R.; Liberati, M.; Thilaganathan, B. Preeclampsia Is Associated with Persistent Postpartum Cardiovascular Impairment. Hypertension 2011, 58, 709–715. [Google Scholar] [CrossRef]
- Glisic, M.; Muka, T.; Franco, O.H. Cardiovascular screening and prevention strategies in women with history of preeclampsia: One size does not fit all. Eur. J. Prev. Cardiol. 2020, 27, 1386–1388. [Google Scholar] [CrossRef]
- Zoet, G.A.; Koster, M.P.; Velthuis, B.K.; De Groot, C.J.; Maas, A.H.; Fauser, B.C.; Franx, A.; Van Rijn, B.B. Determinants of future cardiovascular health in women with a history of preeclampsia. Maturitas 2015, 82, 153–161. [Google Scholar] [CrossRef]
- Boardman, H.; Lamata, P.; Lazdam, M.; Verburg, A.; Siepmann, T.; Upton, R.; Bilderbeck, A.; Dore, R.; Smedley, C.; Kenworthy, Y.; et al. Variations in Cardiovascular Structure, Function, and Geometry in Midlife Associated with a History of Hypertensive Pregnancy. Hypertension 2020, 75, 1542–1550. [Google Scholar] [CrossRef]
- Kalapotharakos, G.; Salehi, D.; Steding-Ehrenborg, K.; Andersson, M.E.; Arheden, H.; Hansson, S.R.; Hedström, E. Cardiovascular effects of severe late-onset preeclampsia are reversed within six months postpartum. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Health 2020, 19, 18–24. [Google Scholar] [CrossRef]
- Davis, E.F.; Lewandowski, A.J.; Leeson, P. Cardiac dysfunction and preeclampsia: Can imaging give clues to mechanism? Circ. Cardiovasc. Imaging 2012, 5, 691–692. [Google Scholar] [CrossRef][Green Version]
- Birukov, A.; Wiesemann, S.; Golic, M.; Balogh, A.; Marko, L.; Rakova, N.; Wilck, N.; Blaszczyk, E.; Lim, C.; Weiss, S.; et al. Myocardial Evaluation of Post-Preeclamptic Women by CMR: Is Early Risk Stratification Possible? JACC Cardiovasc. Imaging 2020, 13, 1291–1293. [Google Scholar] [CrossRef]
- Melchiorre, K.; Sutherland, G.R.; Watt-Coote, I.; Liberati, M.; Thilaganathan, B. Severe Myocardial Impairment and Chamber Dysfunction in Preterm Preeclampsia. Hypertens. Pregnancy 2010, 31, 454–471. [Google Scholar] [CrossRef]
- Quesada, O.; Park, K.; Wei, J.; Handberg, E.; Shufelt, C.; Minissian, M.; Cook-Wiens, G.; Zarrini, P.; Pacheco, C.; Tamarappoo, B.; et al. Left ventricular mass and myocardial scarring in women with hypertensive disorders of pregnancy. Open Heart 2020, 7, e001273. [Google Scholar] [CrossRef]
- Sands, M.J.; Levitin, A. Basics of magnetic resonance imaging. Semin. Vasc. Surg. 2004, 17, 66–82. [Google Scholar] [CrossRef]
- Krug, J.; Rose, G.; Stucht, D.; Clifford, G.; Oster, J. Limitations of VCG based gating methods in ultra high field cardiac MRI. J. Cardiovasc. Magn. Reson. 2013, 15, W19. [Google Scholar] [CrossRef]
- CAAS MR Solutions, Version 5.2.1; Pie Medical Imaging: Maastricht, The Netherlands, 2021.
- Luk, W.H.; Au-Yeung, A.W.S.; Lo, A.X.N.; Loke, T.K.L.; Ng, T.W. Comparing left ventricular ejection fraction measurement using cardiovascular magnetic resonance imaging. Radiol. Technol. 2014, 85, 494–499. [Google Scholar]
- Devereux, R.B.; Reichek, N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977, 55, 613–618. [Google Scholar] [CrossRef]
- Simpson, R.; Bromage, D.; Dancy, L.; McDiarmid, A.; Monaghan, M.; McDonagh, T.; Sado, D. 6 Comparing echocardiography and cardiac magnetic resonance measures of ejection fraction: Implications for HFMRF research. Heart 2018, 104, A3. [Google Scholar] [CrossRef]
- Roy, C.; Slimani, A.; de Meester, C.; Amzulescu, M.; Pasquet, A.; Vancraeynest, D.; Beauloye, C.; Vanoverschelde, J.L.; Gerber, B.L.; Pouleur, A.C. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. J. Cardiovasc. Magn. Reson. 2018, 20, 55. [Google Scholar] [CrossRef]
- Su, M.-Y.M.; Lin, L.-Y.; Tseng, Y.-H.E.; Chang, C.-C.; Wu, C.-K.; Lin, J.-L.; Tseng, W.Y.I. CMR-Verified Diffuse Myocardial Fibrosis Is Associated with Diastolic Dysfunction in HFpEF. JACC Cardiovasc. Imaging 2014, 7, 991–997. [Google Scholar] [CrossRef]
- Holtackers, R.J.; Van De Heyning, C.M.; Chiribiri, A.; Wildberger, J.E.; Botnar, R.M.; Kooi, M.E. Dark-blood late gadolinium enhancement cardiovascular magnetic resonance for improved detection of subendocardial scar: A review of current techniques. J. Cardiovasc. Magn. Reson. 2021, 23, 96. [Google Scholar] [CrossRef]
- Ersbøll, A.S.; Bojer, A.S.; Hauge, M.G.; Johansen, M.; Damm, P.; Gustafsson, F.; Vejlstrup, N.G. Long-Term Cardiac Function After Peripartum Cardiomyopathy and Preeclampsia: A Danish Nationwide, Clinical Follow-Up Study Using Maximal Exercise Testing and Cardiac Magnetic Resonance Imaging. J. Am. Heart Assoc. 2018, 7, e008991. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, E.-A.; Lee, W.; Kim, S.Y.; Chung, J.W. Late gadolinium enhancement magnetic resonance imaging for the assessment of myocardial infarction: Comparison of image quality between single and double doses of contrast agents. Int. J. Cardiovasc. Imaging 2014, 30, 129–135. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Valbuena, S.; Hinojar, R.; Petersen, S.E.; Greenwood, J.P.; Kramer, C.M.; Kwong, R.Y.; McCann, G.P.; Berry, C.; Nagel, E.; et al. Society for Cardiovascular Magnetic Resonance (SCMR) expert consensus for CMR imaging endpoints in clinical research: Part I—Analytical validation and clinical qualification. J. Cardiovasc. Magn. Reson. 2018, 20, 1–23. [Google Scholar] [CrossRef]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native T1 Mapping and Extracellular Volume Mapping for the Assessment of Diffuse Myocardial Fibrosis in Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Arvanitaki, A.; Karvounis, H.; Neubauer, S.; Ferreira, V.M. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc. Imaging 2019, 13, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Child, N.; Suna, G.; Dabir, D.; Yap, M.L.; Rogers, T.; Kathirgamanathan, M.; Arroyo-Ucar, E.; Hinojar, R.; Mahmoud, I.; Young, C.; et al. Comparison of MOLLI.; shMOLLLI.; and SASHA in discrimination between health and disease and relationship with histologically derived collagen volume fraction. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Roujol, S.; Weingärtner, S.; Foppa, M.; Chow, K.; Kawaji, K.; Ngo, L.H.; Kellman, P.; Manning, W.J.; Thompson, R.; Nezafat, R. Accuracy, Precision, and Reproducibility of Four T1 Mapping Sequences: A Head-to-Head Comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology 2014, 272, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 1–12. [Google Scholar] [CrossRef]
- Chong, A.; MacLaren, G.; Chen, R.; Connelly, K.A. Perioperative Applications of Deformation (Myocardial Strain) Imaging with Speckle-Tracking Echocardiography. J. Cardiothorac. Vasc. Anesth. 2014, 28, 128–140. [Google Scholar] [CrossRef]
- Mangion, K.; Burke, N.M.M.; McComb, C.; Carrick, D.; Woodward, R.; Berry, C. Feature-tracking myocardial strain in healthy adults—A magnetic resonance study at 3.0 tesla. Sci. Rep. 2019, 9, 3239. [Google Scholar] [CrossRef]
- Amzulescu, M.S.; De Craene, M.; Langet, H.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.C.; Vanoverschelde, J.L.; Gerber, B.L. Myocardial strain imaging: Review of general principles, validation, and sources of discrepancies. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 605–619. [Google Scholar] [CrossRef]
- Zhang, Y.; Mui, D.; Chirinos, J.A.; Zamani, P.; Ferrari, V.A.; Chen, Y.; Han, Y. Comparing cardiovascular magnetic resonance strain software packages by their abilities to discriminate outcomes in patients with heart failure with preserved ejection fraction. J. Cardiovasc. Magn. Reson. 2021, 23, 55. [Google Scholar] [CrossRef]
- Miskinyte, E.; Bucius, P.; Erley, J.; Zamani, S.M.; Tanacli, R.; Stehning, C.; Schneeweis, C.; Lapinskas, T.; Pieske, B.; Falk, V.; et al. Assessment of Global Longitudinal and Circumferential Strain Using Computed Tomography Feature Tracking: Intra-Individual Comparison with CMR Feature Tracking and Myocardial Tagging in Patients with Severe Aortic Stenosis. J. Clin. Med. 2019, 8, 1423. [Google Scholar] [CrossRef]
- Nazir, S.A.; Shetye, A.M.; Khan, J.N.; Singh, A.; Arnold, J.R.; Squire, I.; McCann, G.P. Inter-study repeatability of circumferential strain and diastolic strain rate by CMR tagging, feature tracking and tissue tracking in ST-segment elevation myocardial infarction. Int. J. Cardiovasc. Imaging 2020, 36, 1133–1146. [Google Scholar] [CrossRef]
- Rahman, Z.U.; Sethi, P.; Murtaza, G.; Virk, H.U.H.; Rai, A.; Mahmod, M.; Schoondyke, J.; Albalbissi, K. Feature tracking cardiac magnetic resonance imaging: A review of a novel non-invasive cardiac imaging technique. World J. Cardiol. 2017, 9, 312–319. [Google Scholar] [CrossRef]
- Singh, A.; Steadman, C.D.; Khan, J.N.; Horsfield, M.A.; Bekele, S.; Nazir, S.A.; Kanagala, P.; Masca, N.G.; Clarysse, P.; McCann, G.P. Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 tesla: A comparison of feature-tracking and tagging in patients with aortic stenosis. J. Magn. Reson. Imaging 2014, 41, 1129–1137. [Google Scholar] [CrossRef]
- Utrera-Lagunas, M.; Orea-Tejeda, A.; Castillo-Martínez, L.; Balderas-Muñoz, K.; Keirns-Davis, C.; Espinoza-Rosas, S.; Sánchez-Ortíz, N.A.; Olvera-Mayorga, G. Abnormal myocardial perfusion and risk of heart failure in patients with type 2 diabetes mellitus. Exp. Clin. Cardiol. 2013, 18, e44. [Google Scholar]
- Löffler, A.I.; Pan, J.A.; Balfour, P.C., Jr.; Shaw, P.W.; Yang, Y.; Nasir, M.; Auger, D.A.; Epstein, F.H.; Kramer, C.M.; Gan, L.M.; et al. Frequency of Coronary Microvascular Dysfunction and Diffuse Myocardial Fibrosis (Measured by Cardiovascular Magnetic Resonance) in Patients with Heart Failure and Preserved Left Ventricular Ejection Fraction. Am. J. Cardiol. 2019, 124, 1584–1589. [Google Scholar] [CrossRef]
- Duca, F.; Zotter-Tufaro, C.; Kammerlander, A.A.; Aschauer, S.; Binder, C.; Mascherbauer, J.; Bonderman, D. Gender-related differences in heart failure with preserved ejection fraction. Sci. Rep. 2018, 8, 1080. [Google Scholar] [CrossRef]
- Dyverfeldt, P.; Bissell, M.; Barker, A.J.; Bolger, A.F.; Carlhäll, C.-J.; Ebbers, T.; Francios, C.J.; Frydrychowicz, A.; Geiger, J.; Giese, D.; et al. 4D flow cardiovascular magnetic resonance consensus statement. J. Cardiovasc. Magn. Reason. 2015, 17, 1–19. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Arifi, A.A.; Omran, A. The basics of echocardiography. J. Saudi Heart Assoc. 2010, 22, 71–76. [Google Scholar] [CrossRef][Green Version]
- Johri, A.M.; Passeri, J.J.; Picard, M. Three dimensional echocardiography: Approaches and clinical utility. Heart 2010, 96, 390–397. [Google Scholar] [CrossRef]
- Yingchoncharoen, T.; Agarwal, S.; Popović, Z.; Marwick, T. Normal Ranges of Left Ventricular Strain: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef]
- Porter, T.R.; Xie, F. Myocardial Perfusion Imaging with Contrast Ultrasound. JACC Cardiovasc. Imaging 2010, 3, 176–187. [Google Scholar] [CrossRef]
- Thijssen, J.M.; de Korte, C.L. Cardiological Ultrasound Imaging. Curr. Pharm. Des. 2014, 20, 6150–6161. [Google Scholar] [CrossRef]
- Nikitin, N.P.; Cleland, J.G.F. Use of myocardial tissue Doppler imaging in cardiology. Kardiologiia 2002, 42, 66–79. [Google Scholar]
- Finocchiaro, G.; Dhutia, H.; D’Silva, A.; Malhotra, A.; Sheikh, N.; Narain, R.; Ensam, B.; Papatheodorou, S.; Tome, M.; Sharma, R.; et al. Role of Doppler Diastolic Parameters in Differentiating Physiological Left Ventricular Hypertrophy from Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2018, 31, 606–613.e1. [Google Scholar] [CrossRef]
- Ryu, D.R. Normal Reference Values for Doppler Echocardiography: Influences of Ageing, Gender and Ethnicity. J. Cardiovasc. Ultrasound 2016, 24, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Atalay, M.K. Cardiac magnetic resonance imaging and computed tomography: State of the art in clinical practice. Rhode Isl. Med J. 2014, 97, 28. [Google Scholar] [PubMed]
- Lin, E.; Alessio, A. What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J. Cardiovasc. Comput. Tomogr. 2009, 3, 403–408. [Google Scholar] [CrossRef]
- Sanghavi, M.; Rutherford, J.D. Cardiovascular Physiology of Pregnancy. Circulation 2014, 130, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- De Haas, S.; Ghossein-Doha, C.; Geerts, L.; Van Kuijk, S.M.J.; Van Drongelen, J.; Spaanderman, M.E.A. Cardiac remodeling in normotensive pregnancy and in pregnancy complicated by hypertension: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2017, 50, 683–696. [Google Scholar] [CrossRef]
- Reddy, M.; Wright, L.; Rolnik, D.L.; Li, W.; Mol, B.W.; La Gerche, A.; da SilvaCosta, F.; Wallace, E.; Palmer, K. Evaluation of Cardiac Function in Women With a History of Preeclampsia: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e013545. [Google Scholar] [CrossRef]
- Bokslag, A.; Franssen, C.; Alma, L.J.; Kovačević, I.; Van Kesteren, F.; Teunissen, P.W.; Kamp, O.; Ganzevoort, W.; Hordijk, P.L.; De Groot, C.J.M.; et al. Early-onset preeclampsia predisposes to preclinical diastolic left ventricular dysfunction in the fifth decade of life: An observational study. PLoS ONE 2018, 13, e0198908. [Google Scholar] [CrossRef]
- Demartelly, V.A.; Dreixler, J.; Tung, A.; Mueller, A.; Heimberger, S.; Fazal, A.A.; Naseem, H.; Lang, R.; Kruse, E.; Yamat, M.; et al. Long-Term Postpartum Cardiac Function and Its Association with Preeclampsia. J. Am. Heart Assoc. 2021, 10, e018526. [Google Scholar] [CrossRef]
- Dennis, A.T.; Castro, J.M. Echocardiographic differences between preeclampsia and peripartum cardiomyopathy. Int. J. Obstet. Anesth. 2014, 23, 260–266. [Google Scholar] [CrossRef]
- Ghi, T.; Degli Esposti, D.; Montaguti, E.; Rosticci, M.; De Musso, F.; Youssef, A.; Salsi, G.; Pilu, G.; Borghi, C.; Rizzo, N. Post-partum evaluation of maternal cardiac function after severe preeclampsia. J. Matern. Neonatal Med. 2013, 27, 696–701. [Google Scholar] [CrossRef]
- Hamad, R.R.; Larsson, A.; Pernow, J.; Bremme, K.; Eriksson, M.J. Assessment of left ventricular structure and function in preeclampsia by echocardiography and cardiovascular biomarkers. J. Hypertens. 2009, 27, 2257–2264. [Google Scholar] [CrossRef]
- Shahul, S.; Ramadan, H.; Nizamuddin, J.; Mueller, A.; Patel, V.; Dreixler, J.; Tung, A.; Lang, R.M.; Weinert, L.; Nasim, R.; et al. Activin A and Late Postpartum Cardiac Dysfunction Among Women with Hypertensive Disorders of Pregnancy. Hypertension 2018, 72, 188–193. [Google Scholar] [CrossRef]
- Valensise, H.; Vasapollo, B.; Gagliardi, G.; Novelli, G.P. Early and Late Preeclampsia. Hypertension 2008, 52, 873–880. [Google Scholar] [CrossRef]
- Valensise, H.; Lo Presti, D.; Gagliardi, G.; Tiralongo, G.M.; Pisani, I.; Novelli, G.P.; Vasapollo, B. Persistent Maternal Cardiac Dysfunction After Preeclampsia Identifies Patients at Risk for Recurrent Preeclampsia. Hypertension 2016, 67, 748–753. [Google Scholar] [CrossRef]
- Al-Nashi, M.; Eriksson, M.J.; Östlund, E.; Bremme, K.; Kahan, T. Cardiac structure and function, and ventricular-arterial interaction 11 years following a pregnancy with preeclampsia. J. Am. Soc. Hypertens. 2016, 10, 297–306. [Google Scholar] [CrossRef]
- Ambrožič, J.; Lučovnik, M.; Cvijić, M. Evolution of cardiac geometry and function in women with severe preeclampsia from immediately post-delivery to 1 year postpartum. Int. J. Cardiovasc. Imaging 2021, 37, 2217–2225. [Google Scholar] [CrossRef]
- Breetveld, N.M.; Ghossein-Doha, C.; Van Neer, J.; Sengers, M.J.J.M.; Geerts, L.; Van Kuijk, S.M.J.; Van Dijk, A.P.; Van Der Vlugt, M.J.; Heidema, W.M.; Rocca, H.P.B.-L.; et al. Decreased endothelial function and increased subclinical heart failure in women several years after pre-eclampsia. Ultrasound Obstet. Gynecol. 2017, 52, 196–204. [Google Scholar] [CrossRef]
- Ciftci, F.C.; Ciftci, O.; Gullu, H.; Caliskan, M.; Uckuyu, A.; Ozcimen, E.E. Does mild preeclampsia cause arterial stiffness and ventricular remodeling through inflammation? Ginekol. Pol. 2014, 85, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Clemmensen, T.S.; Christensen, M.; Kronborg, C.J.S.; Knudsen, U.B.; Løgstrup, B.B. Long-term follow-up of women with early onset pre-eclampsia shows subclinical impairment of the left ventricular function by two-dimensional speckle tracking echocardiography. Pregnancy Hypertens 2018, 14, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ersbøll, A.S.; Goetze, J.P.; Johansen, M.; Hauge, M.G.; Sliwa, K.; Vejlstrup, N.; Gustafsson, F.; Damm, P. Biomarkers and Their Relation to Cardiac Function Late After Peripartum Cardiomyopathy. J. Card. Fail. 2021, 27, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ghossein-Doha, C.; Van Kuijk, S.M.J.; Spaanderman, M.E.A.; Delhaas, T.; Peeters, L.L.H. Age-Related Alterations in Cardiac Geometry in Formerly Preeclamptic Women and Healthy Parous Controls: An Explorative Study. Reprod. Sci. 2012, 20, 39–44. [Google Scholar] [CrossRef]
- Ghossein-Doha, C.; Spaanderman, M.E.; Al Doulah, R.; Van Kuijk, S.M.; Peeters, L.L. Maternal cardiac adaptation to subsequent pregnancy in formerly pre-eclamptic women according to recurrence of pre-eclampsia. Ultrasound Obstet. Gynecol. 2016, 47, 96–103. [Google Scholar] [CrossRef]
- Ghossein-Doha, C.; van Neer, J.; Wissink, B.; Breetveld, N.M.; de Windt, L.J.; van Dijk, A.P.J.; van der Vlugt, M.J.; Janssen, M.C.H.; Heidema, W.M.; Scholten, R.R.; et al. Pre-eclampsia: An important risk factor for asymptomatic heart failure. Ultrasound Obstet. Gynecol. 2016, 49, 143–149. [Google Scholar] [CrossRef]
- Guirguis, G.F.; Aziz, M.M.; Liang, C.B.; Williams, S.F.; Apuzzio, J.J.; Bilinski, R.; Mornan, A.J.; Shah, L.P. Is preeclampsia an independent predictor of diastolic dysfunction? A retrospective cohort study. Pregnancy Hypertens. Int. J. Women’s Cardiovasc. Heal. 2015, 5, 359–361. [Google Scholar] [CrossRef]
- Orabona, R.; Vizzardi, E.; Sciatti, E.; Bonadei, I.; Valcamonico, A.; Metra, M.; Frusca, T. Insights into cardiac alterations after pre-eclampsia: An echocardiographic study. Ultrasound Obstet. Gynecol. 2017, 49, 124–133. [Google Scholar] [CrossRef]
- Orabona, R.; Mohseni, Z.; Sciatti, E.; Mulder, E.G.; Prefumo, F.; Lorusso, R.; Frusca, T.; Ghossein-Doha, C.; Spaanderman, M.E. Maternal myocardial dysfunction after normotensive fetal growth restriction compared with hypertensive pregnancies: A speckle-tracking study. J. Hypertens 2020, 38, 1955–1963. [Google Scholar] [CrossRef]
- Simmons, L.A.; Gillin, A.G.; Jeremy, R.W. Structural and functional changes in left ventricle during normotensive and preeclamptic pregnancy. Am. J. Physiol. Circ. Physiol. 2002, 283, H1627–H1633. [Google Scholar] [CrossRef]
- Spaan, J.J.; Ekhart, T.; Spaanderman, M.E.A.; Peeters, L.L.H. Remote Hemodynamics and Renal Function in Formerly Preeclamptic Women. Obstet. Gynecol. 2009, 113, 853–859. [Google Scholar] [CrossRef]
- Yuan, L.-J.; Duan, Y.-Y.; Xue, D.; Cao, T.-S.; Zhou, N. Ultrasound study of carotid and cardiac remodeling and cardiac-arterial coupling in normal pregnancy and preeclampsia: A case control study. BMC Pregnancy Childbirth 2014, 14, 113. [Google Scholar] [CrossRef]
- Levine, L.D.; Lewey, J.; Koelper, N.; Downes, K.L.; Arany, Z.; Elovitz, M.A.; Sammel, M.D.; Ky, B. Persistent cardiac dysfunction on echocar-diography in African American women with severe preeclampsia. Pregnancy Hypertens. 2019, 17, 127–132. [Google Scholar] [CrossRef]
- Orabona, R.; Vizzardi, E.; Sciatti, E.; Prefumo, F.; Bonadei, I.; Valcamonico, A.; Metra, M.; Frusca, T. Maternal cardiac function after HELLP syndrome: An echocardiography study. Ultrasound Obstet. Gynecol. 2017, 50, 507–513. [Google Scholar] [CrossRef]
- Soma-Pillay, P.; Louw, M.; Adeyemo, A.; Makin, J.; Pattinson, R.; Mc, L.; Ao, A.; Rc, P. Cardiac diastolic function after recovery from pre-eclampsia. Cardiovasc. J. Afr. 2018, 29, 26–31. [Google Scholar] [CrossRef]
- Strobl, I.; Windbichler, G.; Strasak, A.; Weiskopf-Schwendinger, V.; Schweigmann, U.; Ramoni, A.; Scheier, M. Left ventricular function many years after recovery from pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 2010, 118, 76–83. [Google Scholar] [CrossRef][Green Version]
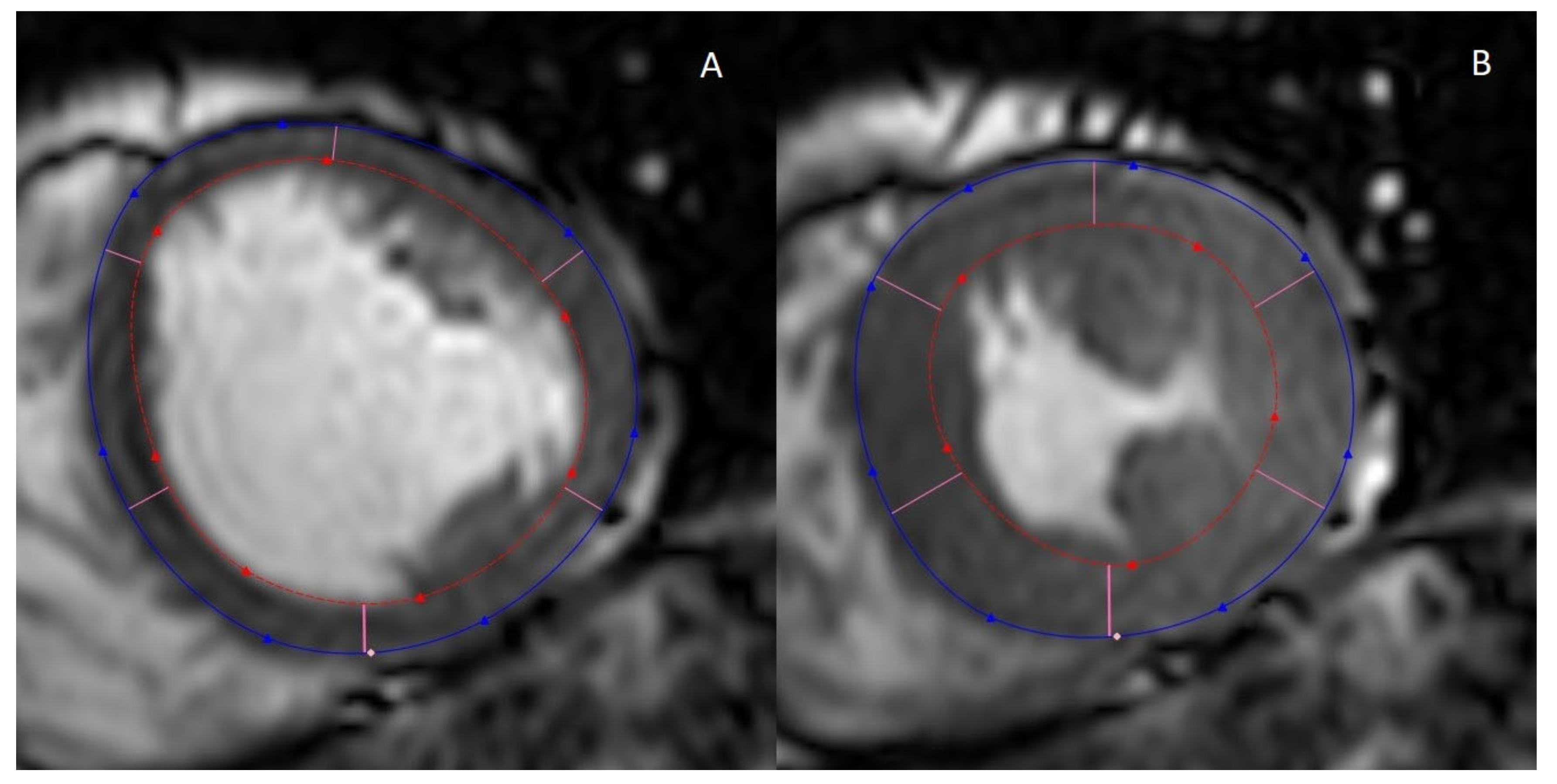
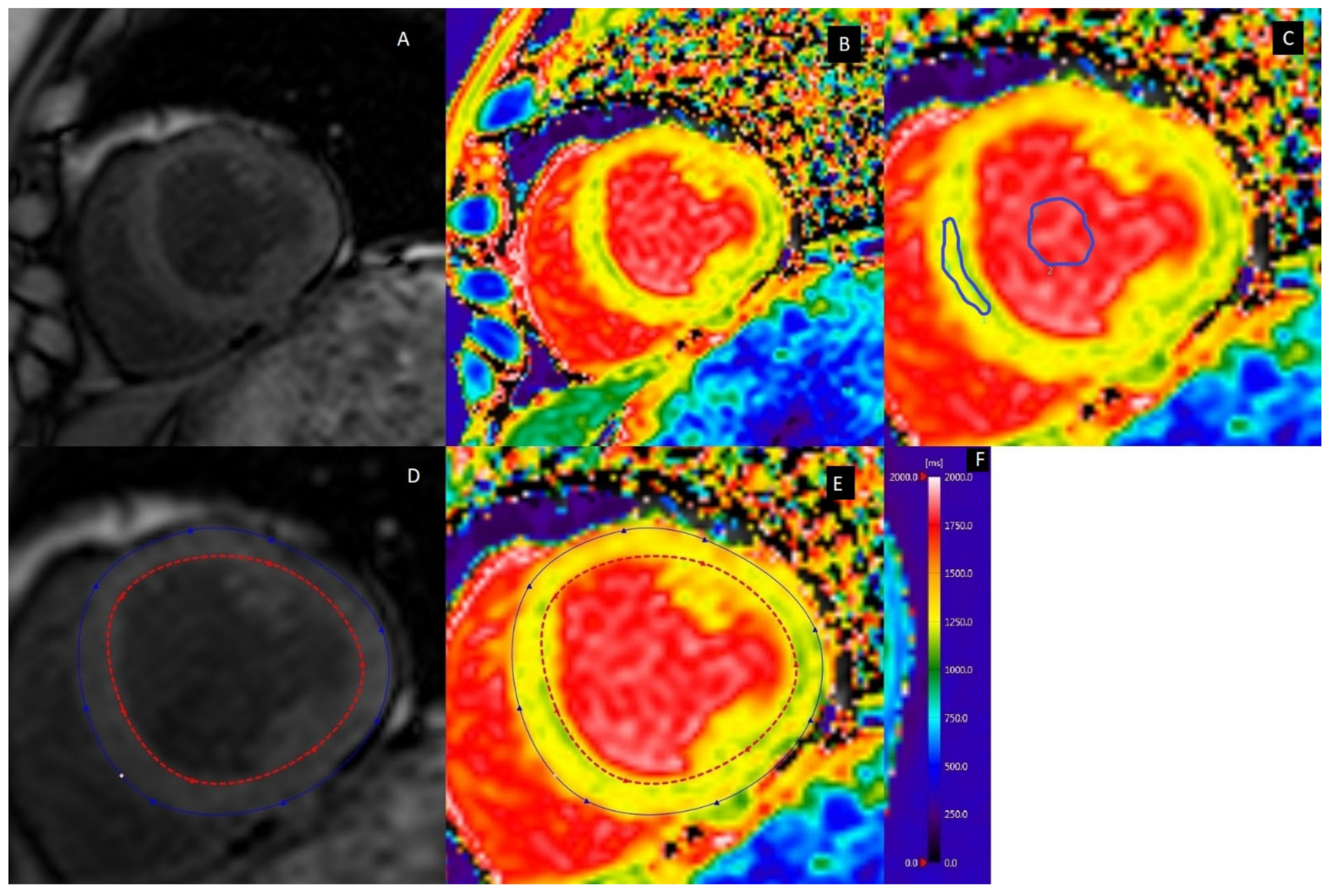
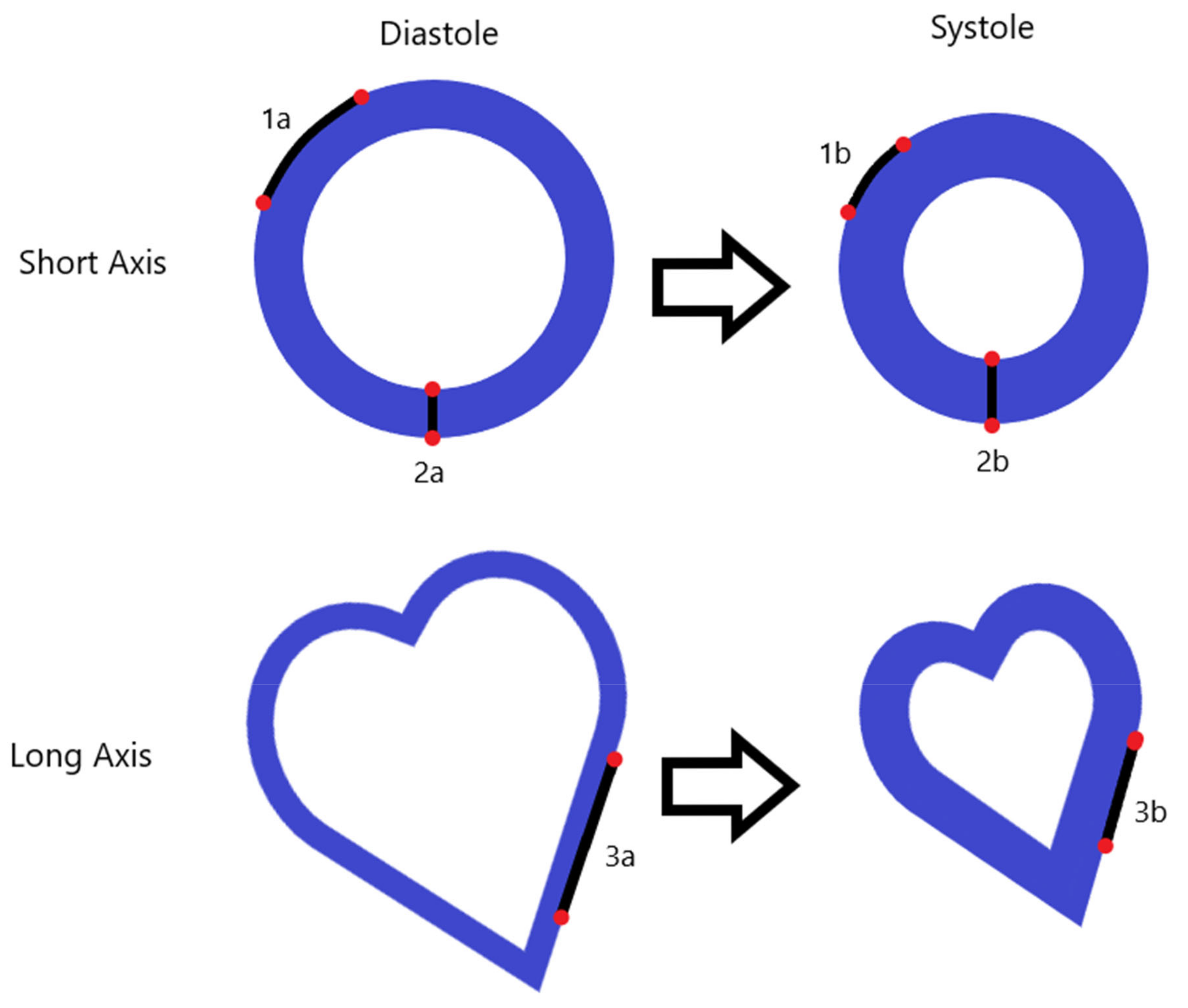
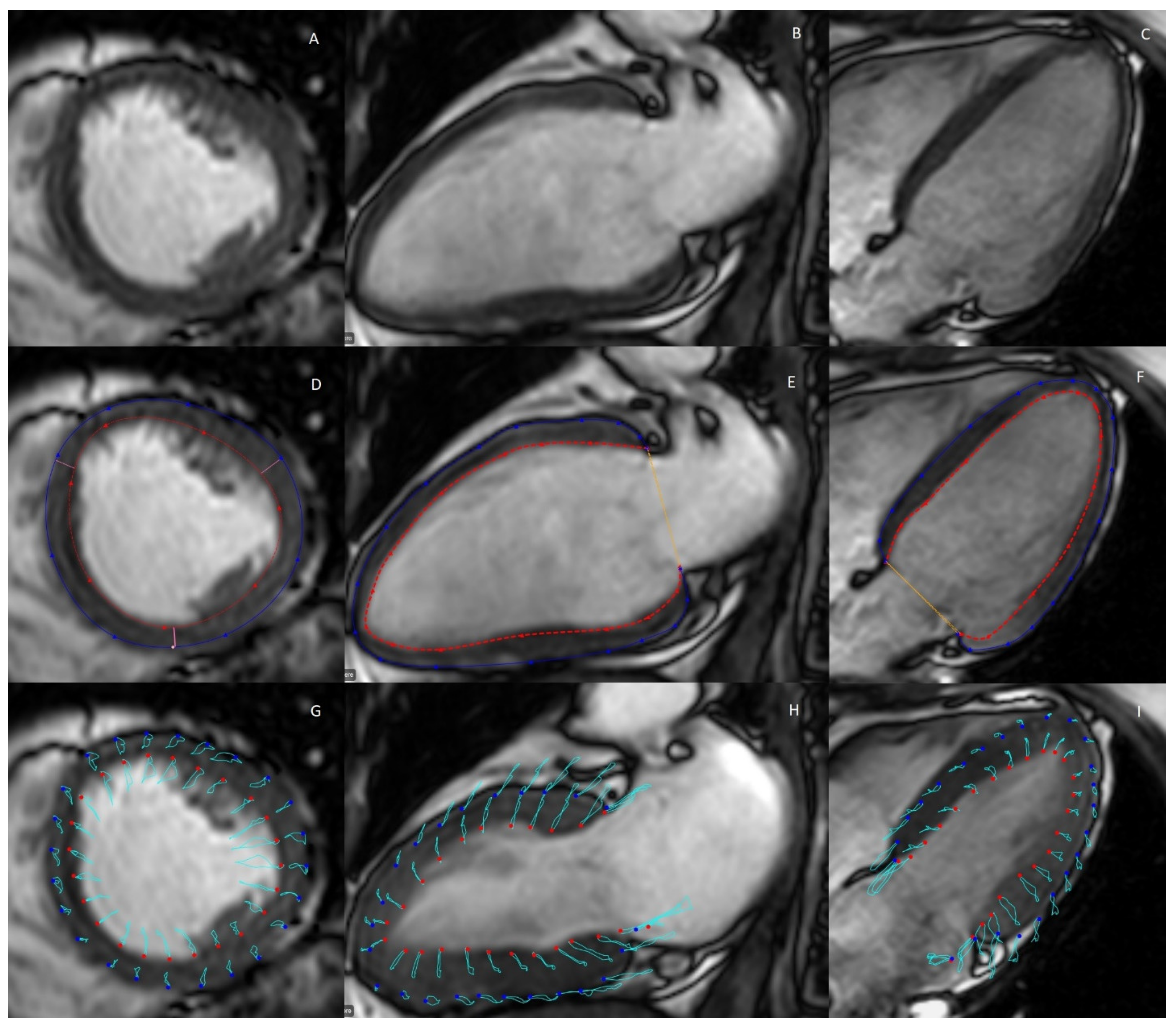

| CMR | Cardiac Ultrasonography |
|---|---|
| Entire left ventricle is depicted | Sectional imaging is usually performed |
| No ionizing radiation | No ionizing radiation |
| Suitable spatial resolution (1–2 mm) [57] | Suitable spatial resolution (0.5–2 mm) [57] |
| Suitable temporal resolution (20–50 ms) [57] | Superior temporal resolution (<5 ms) [57] |
| Superior soft-tissue contrast | Poor soft-tissue contrast |
| Use of navigators or self-gating allows for free breathing, breathholds may also be applied | Free breathing is possible, though sometimes breathholds are required |
| High costs | Lower costs |
| Bedside scan not possible | Bedside scan is possible |
| Long scan times | Short scan times |
| MRI contraindications | No contraindications |
| Accessibility may vary depending on location | Readily accessible |
| Results are operator-independent | Results are highly operator-dependent |
| Enables tissue characterization using T1 and ECV mapping, thus allowing for diffuse and focal fibrosis assessment | Unable to perform tissue characterization |
| Diastolic function is assessable through 4D flow CMR | Diastolic function is readily assessable through Doppler and tissue Doppler ultrasonography |
| Myocardial perfusion is assessable through contrast-based perfusion CMR | Myocardial perfusion is assessable through contrast-based ultrasonography |
| Gold standard for the assessment of ventricular volumes and function (cine MRI) and myocardial strain (tagging) | No gold standard status |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandt, Y.; Ghossein-Doha, C.; Gerretsen, S.C.; Spaanderman, M.E.A.; Kooi, M.E. Noninvasive Cardiac Imaging in Formerly Preeclamptic Women for Early Detection of Subclinical Myocardial Abnormalities: A 2022 Update. Biomolecules 2022, 12, 415. https://doi.org/10.3390/biom12030415
Brandt Y, Ghossein-Doha C, Gerretsen SC, Spaanderman MEA, Kooi ME. Noninvasive Cardiac Imaging in Formerly Preeclamptic Women for Early Detection of Subclinical Myocardial Abnormalities: A 2022 Update. Biomolecules. 2022; 12(3):415. https://doi.org/10.3390/biom12030415
Chicago/Turabian StyleBrandt, Yentl, Chahinda Ghossein-Doha, Suzanne C. Gerretsen, Marc E. A. Spaanderman, and M. Eline Kooi. 2022. "Noninvasive Cardiac Imaging in Formerly Preeclamptic Women for Early Detection of Subclinical Myocardial Abnormalities: A 2022 Update" Biomolecules 12, no. 3: 415. https://doi.org/10.3390/biom12030415
APA StyleBrandt, Y., Ghossein-Doha, C., Gerretsen, S. C., Spaanderman, M. E. A., & Kooi, M. E. (2022). Noninvasive Cardiac Imaging in Formerly Preeclamptic Women for Early Detection of Subclinical Myocardial Abnormalities: A 2022 Update. Biomolecules, 12(3), 415. https://doi.org/10.3390/biom12030415






