Production of Recombinant Alpha-Synuclein: Still No Standardized Protocol in Sight
Abstract
:1. aSyn Species Are Widely Used in PD Research
2. Putative Diagnostic Assays Based on aSyn Seeding and Aggregation
3. aSyn Is Intrinsically Disordered
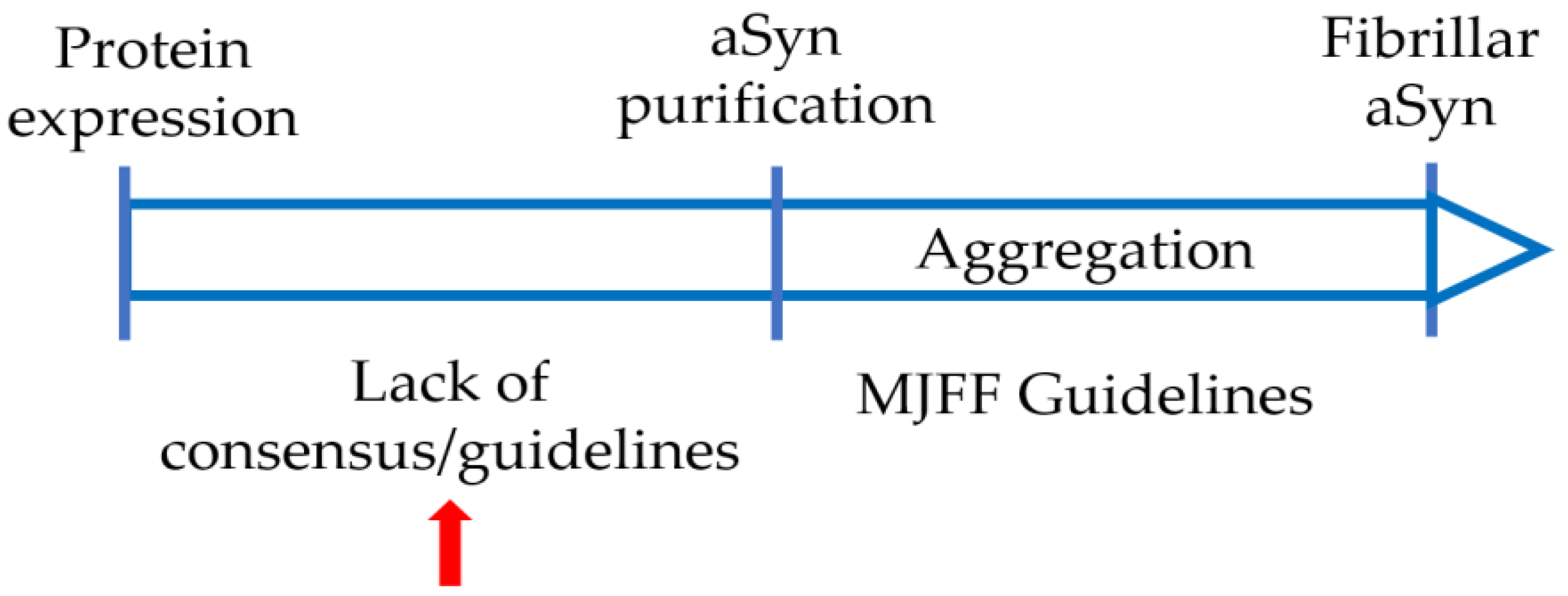
4. Varying Protocols Are in Use to Produce Recombinant aSyn
| aSyn Expression | aSyn Extraction | Purification | Storage | Refs. |
|---|---|---|---|---|
| LB medium, 1 mM IPTG, 4 h induction | 40 mM Tris acetate buffer, sonication, and 10 min boiling | IEX, SEC | Lyophilized | [4] |
| TB medium, no IPTG, ON induction | High salt buffer, sonication, and 15 min boiling | SEC, IEX | Frozen | [19] |
| LB medium, 1 mM IPTG, 5 h induction | Periplasmic lysis by osmotic shock buffer | IEX | Lyophilized | [40] |
| LB medium, 0.5 mM IPTG, ON induction at 25 °C | 10 mM Tris lysis buffer, 30 min boiling, and ammonium sulfate precipitation | IEX, SEC | Frozen | [37] |
| IPTG, other details unstated | Cell lysate precipitated in ammonium sulfate | IEX, SEC | Lyophilized | [41] |
| Details not stated | 20 mM Tris and 100 mm NaCl, acid precipitation | IEX | Not stated | [42] |
| LB medium, 1 mM IPTG, 4 h induction | Periplasmic lysis by osmotic shock buffer | IEX, HIC | Lyophilized | [43] |
| LB medium, 1 mM IPTG, 4 h induction | 20 mM Tris, sonication and 15 min boiling | IEX | Lyophilized | [44] |
| LB medium, IPTG concentration not stated, 4 h induction | 10 mM Tris, freeze–thaw, sonication, 20 min boiling, and ammonium sulfate precipitation | IEX | Not stated | [14] |
| LB medium, 0.5 mM IPTG, ON induction at 25 °C | 500 mM NaCl and 100 mm Tris, 10 min boiling, and acid precipitation | IEX | Not stated | [45] |
| LB medium, 0.44 mM IPTG, 2 h induction at 37 °C | 10 mM Tris, freeze–thaw, sonication, and ammonium sulfate precipitation | IEX, SEC | Not stated | [46] |
| TB medium, 0.5 mM IPTG induction for 16 h at 37 | 100 Mm tris and 500 mm NaCl, freeze–thaw, 10 min boiling, and acid precipitation | RP-HPLC | Lyophilized | [47] |
| LB medium at 37 °C, 4 h IPTG induction | Sonication, 5 min boiling | IEX, SEC, RP-HPLC | Lyophilized | [48] |
| M9 medium at 37 °C, 1 mM IPTG induction for 4–5 h | 10 mM tris, sonication, 20 min boiling, and ammonium sulfate precipitation | IEX, SEC | Frozen | [38] |
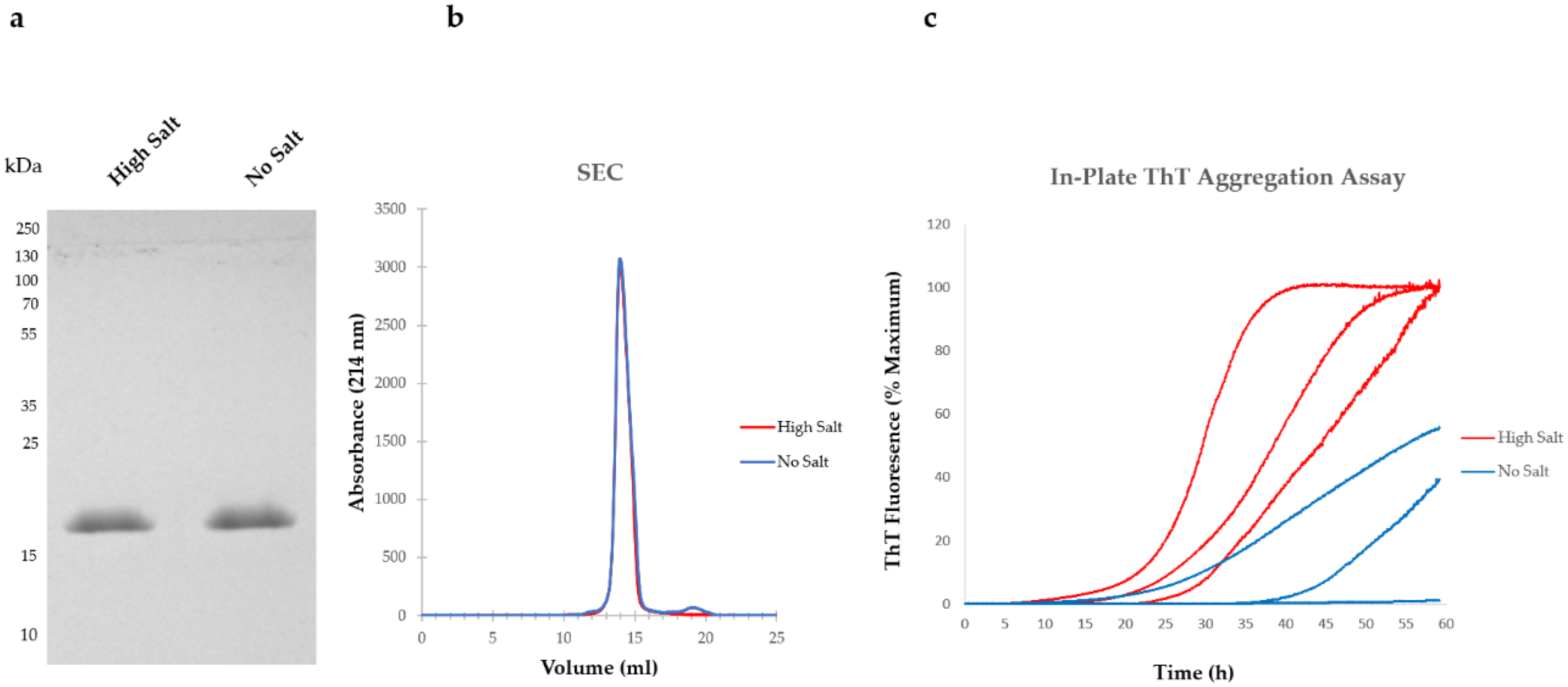
5. Variations in Purification Protocols Can Change the Outcome of Downstream Applications
6. Collaborative Efforts Will Help with Comparing and Standardizing aSyn Production
7. Materials and Methods
7.1. Expression and Purification of aSyn
7.2. Thioflavin-T-Based Aggregation Assays
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinho, R.; Paiva, I.; Jerčić, K.G.; Fonseca-Ornelas, L.; Gerhardt, E.; Fahlbusch, C.; Esparcia, P.G.; Kerimoglu, C.; Pavlou, M.A.S.; Villar-Piqué, A.; et al. Nuclear localization and phosphorylation modulate pathological effects of alpha-Synuclein. Hum. Mol. Genet. 2019, 28, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E.; Schuck, T.M.; Lee, V.M.-Y.; Trojanowski, J.Q. Differential expression and distribution of α-, β-, and γ-synuclein in the developing human substantia nigra. Exp. Neurol. 2001, 168, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.M.; Wassouf, Z.; Zafar, F.; Sastre, D.; Outeiro, T.; Schüle, B. The role of alpha-Synuclein and other parkinson’s genes in neurodevelopmental and neurodegenerative disorders. Int. J. Mol. Sci. 2020, 21, 5724. [Google Scholar] [CrossRef] [PubMed]
- Fauvet, B.; Mbefo, M.K.; Fares, M.-B.; Desobry, C.; Michael, S.; Ardah, M.T.; Tsika, E.; Coune, P.; Prudent, M.; Lion, N.; et al. Alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J. Biol. Chem. 2012, 287, 15345–15364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braak, H.; De Vos, R.A.; Bohl, J.; Del Tredici, K. Gastric alpha-Synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is alpha-Synuclein in the colon a biomarker for premotor Parkinson’s Disease? Evidence from 3 cases. Mov. Disord. 2012, 27, 716–719. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Mutlu, E.; Ms, H.B.D.; Daian, D.; Rn, J.A.J.; Kordower, J.H. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord. 2011, 27, 709–715. [Google Scholar] [CrossRef]
- Donadio, V.; Incensi, A.; Leta, V.; Giannoccaro, M.; Scaglione, C.; Cappellari, S.; Avoni, P.; Baruzzi, A.; Liguori, R. Skin nerve alpha-Synuclein deposits: A biomarker for idiopathic Parkinson’s disease. Clin. Neurophysiol. 2015, 126. [Google Scholar] [CrossRef]
- Miranda, H.V.; Cássio, R.; Guedes, L.C.; Gomes, M.A.; Chegão, A.; Miranda, E.; Soares, T.; Coelho, M.; Rosa, M.M.; Ferreira, J.J.; et al. Posttranslational modifications of blood-derived alpha-Synuclein as biochemical markers for Parkinson’s disease. Sci. Rep. 2017, 7, 13713. [Google Scholar] [CrossRef] [Green Version]
- Weinreb, P.H.; Zhen, W.; Poon, A.W.; Conway, K.A.; Lansbury, P.T. NACP, A protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry 1996, 35, 13709–13715. [Google Scholar] [CrossRef]
- Kim, J. Evidence that the precursor protein of non-Ap component of Alzheimer’s disease amyloid (NACP) has an extended structure primarily composed of random-coil. Mol. Cells 1997, 7, 78–83. [Google Scholar] [PubMed]
- Chandra, S.; Chen, X.; Rizo, J.; Jahn, R.; Südhof, T.C. A broken α-helix in folded alpha-Synuclein. J. Biol. Chem. 2003, 278, 15313–15318. [Google Scholar] [CrossRef] [Green Version]
- Vilar, M.; Chou, H.T.; Lührs, T.; Maji, S.K.; Riek-Loher, D.; Verel, R.; Manning, G.; Stahlberg, H.; Riek, R. The Fold of alpha-Synuclein Fibrils. Proc. Natl. Acad. Sci. USA 2008, 105, 8637–8642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyer, W.; Antony, T.; Cherny, D.; Heim, G.; Jovin, T.M.; Subramaniam, V. Dependence of alpha-Synuclein aggregate morphology on solution conditions. J. Mol. Biol. 2002, 322, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Peelaerts, W.; Bousset, L.; Van der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van den Haute, C.; Melki, R.; Baekelandt, V. Alpha-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Van Der Perren, A.; Gelders, G.; Fenyi, A.; Bousset, L.; Brito, F.; Peelaerts, W.; Haute, C.V.D.; Gentleman, S.; Melki, R.; Baekelandt, V. The structural differences between patient-derived alpha-Synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol. 2020, 139, 977–1000. [Google Scholar] [CrossRef]
- Strohäker, T.; Jung, B.C.; Liou, S.-H.; Fernandez, C.O.; Riedel, D.; Becker, S.; Halliday, G.M.; Bennati, M.; Kim, W.S.; Lee, S.-J.; et al. Structural heterogeneity of alpha-Synuclein fibrils amplified from patient brain extracts. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Luk, K.C.; Song, C.; O’Brien, P.; Stieber, A.; Branch, J.R.; Brunden, K.R.; Trojanowski, J.Q.; Lee, V.M.-Y. Exogenous alpha-Synuclein fibrils seed the formation of lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA 2009, 106, 20051–20056. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli-Daley, L.A.; Luk, K.C.; Lee, V.M.-Y. Addition of exogenous alpha-Synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-Synuclein to Lewy body and Lewy neurite–like aggregates. Nat. Protoc. 2014, 9, 2135–2146. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli-Daley, L.A.; Luk, K.C.; Patel, T.P.; Tanik, S.A.; Riddle, D.M.; Stieber, A.; Meaney, D.F.; Trojanowski, J.Q.; Lee, V.M.Y. Exogenous alpha-Synuclein fibrils induce lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Luk, K.; Kehm, V.M.; Zhang, B.; O’Brien, P.; Trojanowski, J.Q.; Lee, V.M. Intracerebral inoculation of pathological alpha-Synuclein initiates a rapidly progressive neurodegenerative alpha-Synucleinopathy in mice. J. Exp. Med. 2012, 209, 975–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paumier, K.L.; Luk, K.; Manfredsson, F.P.; Kanaan, N.; Lipton, J.W.; Collier, T.J.; Steece-Collier, K.; Kemp, C.J.; Celano, S.; Schulz, E.; et al. Intrastriatal injection of pre-formed mouse alpha-Synuclein fibrils into rats triggers alpha-Synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol. Dis. 2015, 82, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polinski, N.K.; Volpicelli-Daley, L.A.; Sortwell, C.E.; Luk, K.C.; Cremades, N.; Gottler, L.M.; Froula, J.; Duffy, M.F.; Lee, V.M.; Martinez, T.N.; et al. Best practices for generating and using alpha-Synuclein pre-formed fibrils to model Parkinson’s disease in rodents. J. Park. Dis. 2018, 8, 303–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairfoul, G.; McGuire, L.I.; Pal, S.; Ironside, J.W.; Neumann, J.; Christie, S.; Joachim, C.; Esiri, M.; Evetts, S.G.; Rolinski, M.; et al. Alpha-synuclein RT -Qu IC in the CSF of patients with alpha-Synucleinopathies. Ann. Clin. Transl. Neurol. 2016, 3, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz, M.; Tokuda, T.; Waragai, M.; Mendez, N.; Ishii, R.; Trenkwalder, C.; Mollenhauer, B.; Soto, C. Development of a biochemical diagnosis of Parkinson disease by detection of alpha-Synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 2017, 74, 163–172. [Google Scholar] [CrossRef]
- Van Rumund, A.; Green, A.J.E.; Fairfoul, G.; Esselink, R.A.J.; Bloem, B.R.; Verbeek, M.M. Alpha-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann. Neurol. 2019, 85, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Kang, U.J.; Boehme, A.K.; Bs, G.F.; Shahnawaz, M.; Ma, T.; Hutten, S.J.; Green, A.; Soto, C. Comparative study of cerebrospinal fluid alpha-Synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov. Disord. 2019, 34, 536–544. [Google Scholar] [CrossRef]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orrù, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef]
- Paciotti, S.; Bellomo, G.; Gatticchi, L.; Parnetti, L. Are We Ready for Detecting alpha-Synuclein prone to aggregation in patients? The case of “Protein-Misfolding Cyclic Amplification” and “Real-Time Quaking-Induced Conversion” as diagnostic tools. Front. Neurol. 2018, 9, 415. [Google Scholar] [CrossRef] [Green Version]
- Brockmann, K.; Quadalti, C.; Lerche, S.; Rossi, M.; Wurster, I.; Baiardi, S.; Roeben, B.; Mammana, A.; Zimmermann, M.; Hauser, A.-K.; et al. Association between CSF alpha-Synuclein seeding activity and genetic status in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 2021, 9, 1–11. [Google Scholar] [CrossRef]
- Perra, D.; Bongianni, M.; Novi, G.; Janes, F.; Bessi, V.; Capaldi, S.; Sacchetto, L.; Tagliapietra, M.; Schenone, G.; Morbelli, S.; et al. Alpha-synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun. 2021, 3, fcab045. [Google Scholar] [CrossRef]
- Poggiolini, I.; Gupta, V.; Lawton, M.; Lee, S.; El-Turabi, A.; Querejeta-Coma, A.; Trenkwalder, C.; Sixel-Döring, F.; Foubert-Samier, A.; Pavy, A.; et al. Diagnostic value of cerebrospinal fluid alpha-Synuclein seed quantification in synucleinopathies. Brain J. Neurol. 2021, awab431. [Google Scholar] [CrossRef]
- Russo, M.J.; Orru, C.D.; Concha-Marambio, L.; Giaisi, S.; Groveman, B.R.; Farris, C.M.; Holguin, B.; Hughson, A.G.; LaFontant, D.-E.; Caspell-Garcia, C.; et al. High diagnostic performance of independent alpha-Synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol. Commun. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Oliveira, L.M.A.; Gasser, T.; Edwards, R.; Zweckstetter, M.; Melki, R.; Stefanis, L.; Lashuel, H.A.; Sulzer, D.; Vekrellis, K.; Halliday, G.M.; et al. Alpha-synuclein research: Defining strategic moves in the battle against Parkinson’s disease. NPJ Park. Dis. 2021, 7, 1–23. [Google Scholar] [CrossRef]
- Fauvet, B.; Fares, M.-B.; Samuel, F.; Dikiy, I.; Tandon, A.; Eliezer, D.; Lashuel, H.A. Characterization of semisynthetic and naturally Nα-acetylated alpha-Synuclein in vitro and in intact cells. J. Biol. Chem. 2012, 287, 28243–28262. [Google Scholar] [CrossRef] [Green Version]
- Theillet, F.-X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H.M.; Stuiver, M.; Verzini, S.; Lorenz, D.; van Rossum, M.; Goldfarb, D.; et al. Structural disorder of monomeric alpha-Synuclein persists in mammalian cells. Nature 2016, 530, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Bousset, L.; Pieri, L.; Ruiz-Arlandis, G.; Gath, J.; Jensen, P.H.; Habenstein, B.; Madiona, K.; Olieric, V.; Böckmann, A.; Meier, B.H.; et al. Structural and functional characterization of two alpha-Synuclein strains. Nat. Commun. 2013, 4, 2575. [Google Scholar] [CrossRef] [Green Version]
- De Giorgi, F.; Laferrière, F.; Zinghirino, F.; Faggiani, E.; Lends, A.; Bertoni, M.; Yu, X.; Grélard, A.; Morvan, E.; Habenstein, B.; et al. Novel self-replicating alpha-Synuclein polymorphs that escape ThT monitoring can spontaneously emerge and acutely spread in neurons. Sci. Adv. 2020, 6, eabc4364. [Google Scholar] [CrossRef]
- Giehm, L.; Lorenzen, N.; Otzen, D.E. Assays for alpha-Synuclein aggregation. Methods 2011, 53, 295–305. [Google Scholar] [CrossRef]
- Huang, C.; Ren, G.; Zhou, H.; Wang, C.-C. A new method for purification of recombinant human alpha-Synuclein in Escherichia coli. Protein Expr. Purif. 2005, 42, 173–177. [Google Scholar] [CrossRef]
- Conway, K.A.; Harper, J.D.; Lansbury, P.T. Accelerated in vitro fibril formation by a mutant alpha-Synuclein linked to early-onset Parkinson disease. Nat. Med. 1998, 4, 1318–1320. [Google Scholar] [CrossRef]
- Narhi, L.; Wood, S.J.; Steavenson, S.; Jiang, Y.; Wu, G.M.; Anafi, D.; Kaufman, S.A.; Martin, F.; Sitney, K.; Denis, P. Both familial Parkinson’s disease mutations accelerate alpha-Synuclein aggregation. J. Biol. Chem. 1999, 274, 9843–9846. [Google Scholar] [CrossRef] [Green Version]
- Campioni, S.; Carret, G.; Jordens, S.; Nicoud, L.; Mezzenga, R.; Riek, R. The presence of an air–water interface affects formation and elongation of alpha-Synuclein fibrils. J. Am. Chem. Soc. 2014, 136, 2866–2875. [Google Scholar] [CrossRef]
- Kumar, S.T.; Jagannath, S.; Francois, C.; Vanderstichele, H.; Stoops, E.; Lashuel, H.A. How specific are the conformation-specific alpha-Synuclein antibodies? Characterization and validation of 16 alpha-Synuclein conformation-specific antibodies using well-characterized preparations of alpha-Synuclein monomers, fibrils and oligomers with distinct structures and morphology. Neurobiol. Dis. 2020, 146, 105086. [Google Scholar] [CrossRef]
- Der-Sarkissian, A.; Jao, C.C.; Chen, J.; Langen, R. Structural Organization of alpha-Synuclein fibrils studied by site-directed spin labeling. J. Biol. Chem. 2003, 278, 37530–37535. [Google Scholar] [CrossRef] [Green Version]
- Ghee, M.; Melki, R.; Michot, N.; Mallet, J. PA700, the regulatory complex of the 26S proteasome, interferes with alpha-Synuclein assembly. FEBS J. 2005, 272, 4023–4033. [Google Scholar] [CrossRef]
- Galesic, A.; Rakshit, A.; Cutolo, G.; Pacheco, R.P.; Balana, A.T.; Moon, S.P.; Pratt, M.R. Comparison of N-acetyl-glucosamine to other monosaccharides reveals structural differences for the inhibition of alpha-Synuclein aggregation. ACS Chem. Biol. 2021, 16, 14–19. [Google Scholar] [CrossRef]
- Fares, M.B.; Maco, B.; Oueslati, A.; Rockenstein, E.; Ninkina, N.; Buchman, V.L.; Masliah, E.; Lashuel, H.A. Induction of de novo alpha-Synuclein fibrillization in a neuronal model for Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2016, 113, E912–E921. [Google Scholar] [CrossRef] [Green Version]
- Stephens, A.D.; Nespovitaya, N.; Zacharopoulou, M.; Kaminski, C.; Phillips, J.J.; Schierle, G.S.K. Different structural conformers of monomeric alpha-Synuclein identified after lyophilizing and freezing. Anal. Chem. 2018, 90, 6975–6983. [Google Scholar] [CrossRef]
- Stephens, A.D.; Zacharopoulou, M.; Moons, R.; Fusco, G.; Seetaloo, N.; Chiki, A.; Woodhams, P.J.; Mela, I.; Lashuel, H.A.; Phillips, J.J.; et al. Extent of N-terminus exposure of monomeric alpha-Synuclein determines its aggregation propensity. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Stephens, A.D.; Matak-Vinkovic, D.; Fernandez-Villegas, A.; Schierle, G.S.K. Purification of recombinant alpha-Synuclein: A comparison of commonly used protocols. Biochemistry 2020, 59, 4563–4572. [Google Scholar] [CrossRef]
- Giasson, B.I.; Murray, I.V.; Trojanowski, J.Q.; Lee, V.M. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-Synuclein is essential for filament assembly. J. Biol. Chem. 2001, 276, 2380–2386. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Azzani, M.; König, A.; Outeiro, T.F. Production of Recombinant Alpha-Synuclein: Still No Standardized Protocol in Sight. Biomolecules 2022, 12, 324. https://doi.org/10.3390/biom12020324
Al-Azzani M, König A, Outeiro TF. Production of Recombinant Alpha-Synuclein: Still No Standardized Protocol in Sight. Biomolecules. 2022; 12(2):324. https://doi.org/10.3390/biom12020324
Chicago/Turabian StyleAl-Azzani, Mohammed, Annekatrin König, and Tiago Fleming Outeiro. 2022. "Production of Recombinant Alpha-Synuclein: Still No Standardized Protocol in Sight" Biomolecules 12, no. 2: 324. https://doi.org/10.3390/biom12020324
APA StyleAl-Azzani, M., König, A., & Outeiro, T. F. (2022). Production of Recombinant Alpha-Synuclein: Still No Standardized Protocol in Sight. Biomolecules, 12(2), 324. https://doi.org/10.3390/biom12020324







