Phenolipids, Amphipilic Phenolic Antioxidants with Modified Properties and Their Spectrum of Applications in Development: A Review
Abstract
1. Introduction
2. Synthesis of Phenolipids
3. Phenolipids and Antioxidant Capacity
4. Potential Applications of Phenolipids
4.1. Preservation and Stability in Food
4.2. Antimicrobial Capacity against Foodborne Pathogens
4.3. Health Benefits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride reagent |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid reagent |
| DNA | Deoxyribonucleic acid |
| FRAP | Ferric Reducing Antioxidant Power assay |
| MTT | 3(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide reagent |
References
- Losada, B.S.; Bravo-Díaz, C.; Paiva-Martins, F.; Romsted, L. Maxima in Antioxidant Distributions and Efficiencies with Increasing Hydrophobicity of Gallic Acid and Its Alkyl Esters. The Pseudophase Model Interpretation of the “Cutoff Effect”. J. Agric. Food Chem. 2013, 61, 6533–6543. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.-D.M.; Petersen, L.K.; de Diego, S.; Nielsen, N.S.; Lue, B.-M.; Yang, Z.; Xu, X.; Jacobsen, C. The antioxidative effect of lipophilized rutin and dihydrocaffeic acid in fish oil enriched milk. Eur. J. Lipid Sci. Technol. 2012, 114, 434–445. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety-Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Carregosa, D.; Mota, S.; Ferreira, S.; Alves-Dias, B.; Loncarevic-Vasiljkovic, N.; Crespo, C.L.; Menezes, R.; Teodoro, R.; Santos, C. Overview of Beneficial Effects of (Poly)phenol Metabolites in the Context of Neurodegenerative Diseases on Model Organisms. Nutrients 2021, 13, 2940. [Google Scholar] [CrossRef] [PubMed]
- Dirimanov, S.; Högger, P. Screening of Inhibitory Effects of Polyphenols on Akt-Phosphorylation in Endothelial Cells and Determination of Structure-Activity Features. Biomolecules 2019, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavonones, flavanones and human health: Epidemological evidence. J. Med. Food 2005, 8, 81–90. [Google Scholar] [CrossRef]
- Kang, G.; Francis, N.; Hill, R.; Waters, D.; Blanchard, C.; Shanthakumar, A. Dietary Polyphenols and Gene Expression in Molecular Pathways Associated with Type 2 Diabetes Mellitus: A Review. Int. J. Mol. Sci 2020, 21, 140. [Google Scholar] [CrossRef]
- Lue, B.-M.; Sørensen, A.-D.M.; Jacobsen, C.; Guo, Z.; Xu, X. Antioxidant efficacies of rutin and rutin esters in bulk oil and oil-in-water emulsion. Eur. J. Lipid Sci. Technol. 2017, 119, 1600049. [Google Scholar] [CrossRef]
- Mosele, J.I.; Motilva, M.-J. Phenol Biological Metabolites as Food Intake Biomarkers, a Pending Signature for a Complete Understanding of the Beneficial Effects of the Mediterranean Diet. Nutrients 2021, 13, 3051. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Rabiej, D. Effect of new antioxidants: Phenolipids on quality of fried French fries and rapeseed oil. J. Food Sci. Technol. 2021, 58, 2589–2598. [Google Scholar] [CrossRef]
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Andreea, S.; Ayvaz, H.; Andreea, S.; Mihai, S.; Diaconeasa, Z.; et al. Phytochemical Characterization of Five Edible Purple-Reddish Vegetables: Anthocyanins, Flavonoids, and Phenolic Acid Derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef] [PubMed]
- Panya, A.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; McClements, D.J.; Decker, E.A. An investigation of the versatile antioxidant mechanisms of action of rosmarinate alkyl esters in oil-in-water emulsions. J. Agric. Food Chem. 2012, 60, 2692–2700. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Durand, E.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; Jacobsen, C. Antioxidant Properties and Efficacies of Synthesized Alkyl Caffeates, Ferulates, and Coumarates. J. Agric. Food Chem. 2014, 62, 12553–12562. [Google Scholar] [CrossRef]
- Benincasa, C.; La Torre, C.; Fazio, A.; Perri, E.; Caroleo, M.C.; Plastina, P.; Cione, E. Identification of Tyrosyl Oleate as a Novel Olive Oil Lipophenol with Proliferative and Antioxidant Properties in Human Keratinocytes. Antioxidants 2021, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xu, M. Natural Antioxidants in Foods. In Encyclopedia of Food Chemistry; Academic Press: Cambridge, MA, USA; Elsevier: Oxford, UK, 2019; pp. 180–188. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Laguerre, M.; Villeneuve, P.; Lecomte, J. From phenolics to phenolipids: Optimizing antioxidants in lipid dispersions. Lipid Technol. 2013, 25, 131–134. [Google Scholar] [CrossRef]
- Kahveci, D.; Laguerre, M.; Villeneuve, P. 7-Phenolipids as New Antioxidants: Production, Activity, and Potential Applications. In Polar Lipids; Ahmad, M.U., Xu, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 185–214. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Bourlieu, C.; Durand, E.; Lecomte, J.; Villeneuve, P. Lipophilized Antioxidants. In Encyclopedia of Food Chemistry; Academic Press: Cambridge, MA, USA, 2019; pp. 193–201. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Panya, A.; Figueroa-Espinoza, M.C. Antioxidant activity of protocatechuates evaluated by DPPH, ORAC, and CAT methods. Food Chem. 2016, 194, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Grajeda-Iglesias, C.; Salas, E.; Barouh, N.; Baréa, B.; Figueroa-Espinoza, M.C. Lipophilization and MS characterization of the main anthocyanins purified from hibiscus flowers. Food Chem. 2017, 230, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, A.; Skouridou, V.; Sereti, V.; Stamatis, H.; Kolisis, F.N. Lipase-catalyzed esterification of rutin and naringin with fatty acids of medium carbon chain. J. Mol. Catal. 2003, 21, 59–62. [Google Scholar] [CrossRef]
- Guyot, B.; Bosquette, B.; Pina, M. Esterification of phenolic acids from green coffee with an immobilized lipase from Candida antarctica in solvent-free medium. Biotechnol. Lett. 1997, 19, 529–532. [Google Scholar] [CrossRef]
- Kikugawa, M.; Tsuchiyama, M.; Kai, K.; Sakamoto, T. Synthesis of highly water-soluble feruloyl diglycerols by esterification of an Aspergillus niger feruloyl esterase. Appl. Microbiol. Biotechnol. 2012, 95, 615–622. [Google Scholar] [CrossRef]
- Laguerre, M.; Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Chain length affects antioxidant properties of chlorogenate esters in emulsion: The cutoff theory behind the polar paradox. J. Agric. Food Chem. 2009, 57, 11335–11342. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Lecomte, J.; Baréa, B.; Dubreucq, E.; Lortie, R.; Villeneuve, P. Evaluation of deep eutectic solvent-water binary mixtures for lipase-catalyzed lipophilization of phenolic acids. Green Chem. 2013, 15, 2275–2282. [Google Scholar] [CrossRef]
- Buisman, G.; van Helteren, C.; Kramer, G.; Veldsink, J.W.; Derksen, J.T.P.; Cuperus, F.P. Enzymatic esterifications of functionalized phenols for the synthesis of lipophilic antioxidants. Biotechnol. Lett. 1998, 20, 131–136. [Google Scholar] [CrossRef]
- Chalas, J.; Claise, C.; Edeas, M.; Messaoudi, C.; Vergnes, L.; Abella, A.; Lindenbaum, A. Effect of ethyl esterification of phenolic acids on low-density lipoprotein oxidation. Biomed. Pharmacother. 2001, 55, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Katsoura, M.H.; Polydera, A.C.; Tsironis, L.D.; Petraki, M.P.; Rajacić, S.K.; Tselepis, A.D.; Stamatis, H. Efficient enzymatic preparation of hydroxycinnamates in ionic liquids enhances their antioxidant effect on lipoproteins oxidative modification. New Biotechnol. 2009, 26, 83–91. [Google Scholar] [CrossRef]
- Reis, B.; Martins, M.; Barreto, B.; Milhazes, N.; Garrido, E.M.; Silva, P.; Garrido, J.; Borges, F. Structure-property-activity relationship of phenolic acids and derivatives. Protocatechuic acid alkyl esters. J. Agric. Food Chem. 2010, 58, 6986–6993. [Google Scholar] [CrossRef]
- Zieniuk, B.; Białecka-Florjańczyk, E.; Fabiszewska, A. Anti-Listerial Effect of 4-Hydroxyphenylpropanoic Acid Esters Synthesized by Lipase-Catalyzed Esterification. Proceedings 2021, 70, 17. [Google Scholar] [CrossRef]
- Compton, D.L.; Laszlo, J.A.; Berhow, M.A. Lipase-catalyzed synthesis of ferulate esters. J. Am. Oil Chem. Soc. 2000, 77, 513–519. [Google Scholar] [CrossRef]
- Sabally, K.; Karboune, S.; Yeboah, F.K.; Kermasha, S. Lipase-catalyzed esterification of selected phenolic acids with linolenyl alcohols in organic solvent media. Appl. Biochem. Biotechnol. 2005, 127, 17–27. [Google Scholar] [CrossRef]
- Lee, G.S.; Widjaja, A.; Ju, Y.H. Enzymatic Synthesis of Cinnamic Acid Derivatives. Biotechnol. Lett. 2006, 28, 581–585. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Baréa, B.; Villeneuve, P. Towards a better understanding of how to improve lipase-catalyzed reactions using deep eutectic solvents based on choline chloride. Eur. J. Lipid Sci. Technol. 2014, 116, 16–23. [Google Scholar] [CrossRef]
- Chen, B.; Liu, H.; Guo, Z.; Huang, J.; Wang, M.; Xu, X.; Zheng, L. Lipase-catalyzed esterification of ferulic Acid with oleyl alcohol in ionic liquid/iso-octane binary systems. J. Agric. Food Chem. 2011, 59, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. A direct correlation between the antioxidant efficiencies of caffeic acid and its alkyl esters and their concentrations in the interfacial region of olive oil emulsions. The pseudophase model interpretation of the “cut-off” effect. Food Chem. 2015, 175, 233–242. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Magalhães, J.; Monteiro, L.; Bravo-Díaz, C.; Paiva-Martins, F. Effects of the Reactive Moiety of Phenolipids on Their Antioxidant Efficiency in Model Emulsified Systems. Foods 2021, 10, 1228. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Girão da Cruz, M.T.; Cordeiro, M.N.; Milhazes, N.; Borges, F.; Marques, M.P. Phenolic acid derivatives with potential anticancer properties—A structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg. Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, Z.; Xu, X. Ionic Liquid-Assisted Solubilization for Improved Enzymatic Esterification of Phenolic Acids. J. Am. Oil Chem. Soc. 2021, 89, 1049–1055. [Google Scholar] [CrossRef]
- Lecomte, J.; Giraldo, L.J.L.; Laguerre, M.; Baréa, B.; Villeneuve, P. Synthesis, Characterization and Free Radical Scavenging Properties of Rosmarinic Acid Fatty Esters. J. Am. Oil Chem. Soc. 2010, 87, 615–620. [Google Scholar] [CrossRef]
- López-Giraldo, L.; Laguerre, M.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.; Villeneuve, P. Kinetic and Stoichiometry of the Reaction of Chlorogenic Acid and Its Alkyl Esters against the DPPH Radical. J. Agric. Food Chem. 2009, 57, 863–870. [Google Scholar] [CrossRef]
- Cruz, L.; Fernandes, V.C.; Araújo, P.; Mateus, N.; de Freitas, V. Synthesis, characterisation and antioxidant features of procyanidin B4 and malvidin-3-glucoside stearic acid derivatives. Food Chem. 2015, 174, 480–486. [Google Scholar] [CrossRef]
- Marquez-Rodriguez, A.S.; Guimarães, M.; Mateus, N.; de Freitas, V.; Ballinas-Casarrubias, M.L.; Fuentes-Montero, M.E.; Salas, E.; Cruz, L. Disaccharide anthocyanin delphinidin 3-O-sambubioside from Hibiscus sabdariffa L.: Candida antarctica lipase B-catalyzed fatty acid acylation and study of its color properties. Food Chem. 2021, 344, 128603. [Google Scholar] [CrossRef]
- Chen, M.; Yu, S. Characterization of Lipophilized Monomeric and Oligomeric Grape Seed Flavan-3-ol Derivatives. J. Agric. Food Chem. 2017, 65, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Ma, C.M.; Shahidi, F. Antioxidant and antiviral activities of lipophilic epigallocatechin gallate (EGCG) derivatives. JFF 2012, 4, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ambigaipalan, P.; Oh, W.Y.; Shahidi, F. Epigallocatechin (EGC) esters as potential sources of antioxidants. Food Chem. 2020, 309, 125609. [Google Scholar] [CrossRef] [PubMed]
- Aissa, I.; Sghair, R.M.; Bouaziz, M.; Laouini, D.; Sayadi, S.; Gargouri, Y.T. Synthesis of lipophilic tyrosyl esters derivatives and assessment of their antimicrobial and antileishmania activities. Lipids Health Dis. 2012, 11, 13. [Google Scholar] [CrossRef]
- Oh, W.Y.; Shahidi, F. Lipophilization of Resveratrol and Effects on Antioxidant Activities. J. Agric. Food Chem. 2017, 65, 8617–8625. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Figueroa-Espinoza, M.C.; Barouh, N.; Muñoz-Castellanos, L.N.; Salas, E. Polyphenol lipophilisation: A suitable tool for the valorisation of natural by-products. Int. J. Food Sci. Technol. 2022, 57, 6935–6947. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Almeida, J.; Losada-Barreiro, S.; Costa, M.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. Interfacial Concentrations of Hydroxytyrosol and Its Lipophilic Esters in Intact Olive Oil-in-Water Emulsions: Effects of Antioxidant Hydrophobicity, Surfactant Concentration, and the Oil-to-Water Ratio on the Oxidative Stability of the Emulsions. J. Agric. Food Chem. 2016, 64, 5274–5283. [Google Scholar] [CrossRef] [PubMed]
- Alemán, M.; Bou, R.; Guardiola, F.; Durand, E.; Villeneuve, P.; Jacobse, C.; Sørensen, A.-D.M. Antioxidative effect of lipophilized caffeic acid in fish oil enriched mayonnaise and milk. Food Chem. 2015, 167, 236–244. [Google Scholar] [CrossRef]
- Shi, Y.G.; Zhu, Y.J.; Shao, S.Y.; Zhang, R.R.; Wu, Y.; Zhu, C.M.; Liang, X.R.; Cai, W.Q. Alkyl Ferulate Esters as Multifunctional Food Additives: Antibacterial Activity and Mode of Action against Escherichia coli in Vitro. J. Agric. Food Chem. 2018, 66, 12088–12101. [Google Scholar] [CrossRef]
- Li, N.-G.; Shi, Z.-H.; Tang, Y.-P.; Li, B.-Q.; Duan, J.-A. Highly Efficient Esterification of Ferulic Acid Under Microwave Irradiation. Molecules 2009, 14, 2118–2126. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.-D.M.; Nielsen, N.S.; Yang, Z.; Xu, X.; Jacobsen, C. Lipophilization of dihydrocaffeic acid affects its antioxidative properties in fish-oil-enriched emulsions. Eur. J. Lipid Sci. Technol. 2012, 114, 134–145. [Google Scholar] [CrossRef]
- Qiu, X.; Charlotte Jacobsen, C.; Villeneuve, P.; Durand, E.; Sørensen, A.-D.M. Effects of Different Lipophilized Ferulate Esters in Fish Oil-Enriched Milk: Partitioning, Interaction, Protein, and Lipid Oxidation. J. Agric. Food Chem. 2017, 65, 9496–9505. [Google Scholar] [CrossRef] [PubMed]
- Sharif, Z.M.; Mustapha, F.A.; Jai, J.; Mohd, Y.N.; Zaki, N.M.; Shah, A. Review on methods for preservation and natural preservatives for extending the food longevity. Chem. Eng. Res. Bull. 2017, 19, 145–153. [Google Scholar] [CrossRef]
- Shi, Y.; Bian, L.-Q.; Zhu, Y.-J.; Zhang, R.-R.; Shao, S.-Y.; Wu, Y.; Chen, Y.-W.; Dang, Y.; Ding, Y.; Sun, H. Multifunctional alkyl ferulate esters as potential food additives: Antibacterial activity and mode of action against Listeria monocytogenes and its application on American sturgeon caviar preservation. Food Control 2018, 96, 390–402. [Google Scholar] [CrossRef]
- Durand, E.; Lecomte, J.; Upasani, R.; Chabi, B.; Bayrasy, C.; Baréa, B.; Jublanc, E.; Clarke, M.J.; Moore, D.J.; Crowther, J.; et al. Evaluation of the ROS Inhibiting Activity and Mitochondrial Targeting of Phenolic Compounds in Fibroblast Cells Model System and Enhancement of Efficiency by Natural Deep Eutectic Solvent (NADES) Formulation. Pharm. Res. 2017, 34, 1134–1146. [Google Scholar] [CrossRef]
- Bayrasy, C.; Chabi, B.; Laguerre, M.; Lecomte, J.; Jublanc, E.; Villeneuve, P.; Wrutniak-Cabello, C.; Cabello, G. Boosting antioxidants by lipophilization: A strategy to increase cell uptake and target mitochondria. Pharm. Res. 2013, 30, 1979–1989. [Google Scholar] [CrossRef]
- Li, W.; Li, N.; Tang, Y.; Li, B.; Liu, L.; Zhang, X.; Fu, H.; Duan, J. Biological activity evaluation and structure–activity relationships analysis of ferulic acid and caffeic acid derivatives for anticancer. Bioorganic Med. Chem. Lett. 2012, 22, 6085–6088. [Google Scholar] [CrossRef]
- Kaihatsu, K.; Mori, S.; Matsumura, H.; Daidoji, T.; Kawakami, C.; Kurata, H.; Nakaya, T.; Kato, N. Broad and potent anti-influenza virus spectrum of epigallocatechin-3-O-gallate-monopalmitate. J. Mol. Genet. Genom. Med. 2009, 3, 195–197. [Google Scholar] [CrossRef]
- Jantas, D.; Chwastek, J.; Malarz, J.; Stojakowska, A.; Lasoń, W. Neuroprotective Effects of Methyl Caffeate against Hydrogen Peroxide-Induced Cell Damage: Involvement of Caspase 3 and Cathepsin D Inhibition. Biomolecules 2020, 10, 1530. [Google Scholar] [CrossRef]
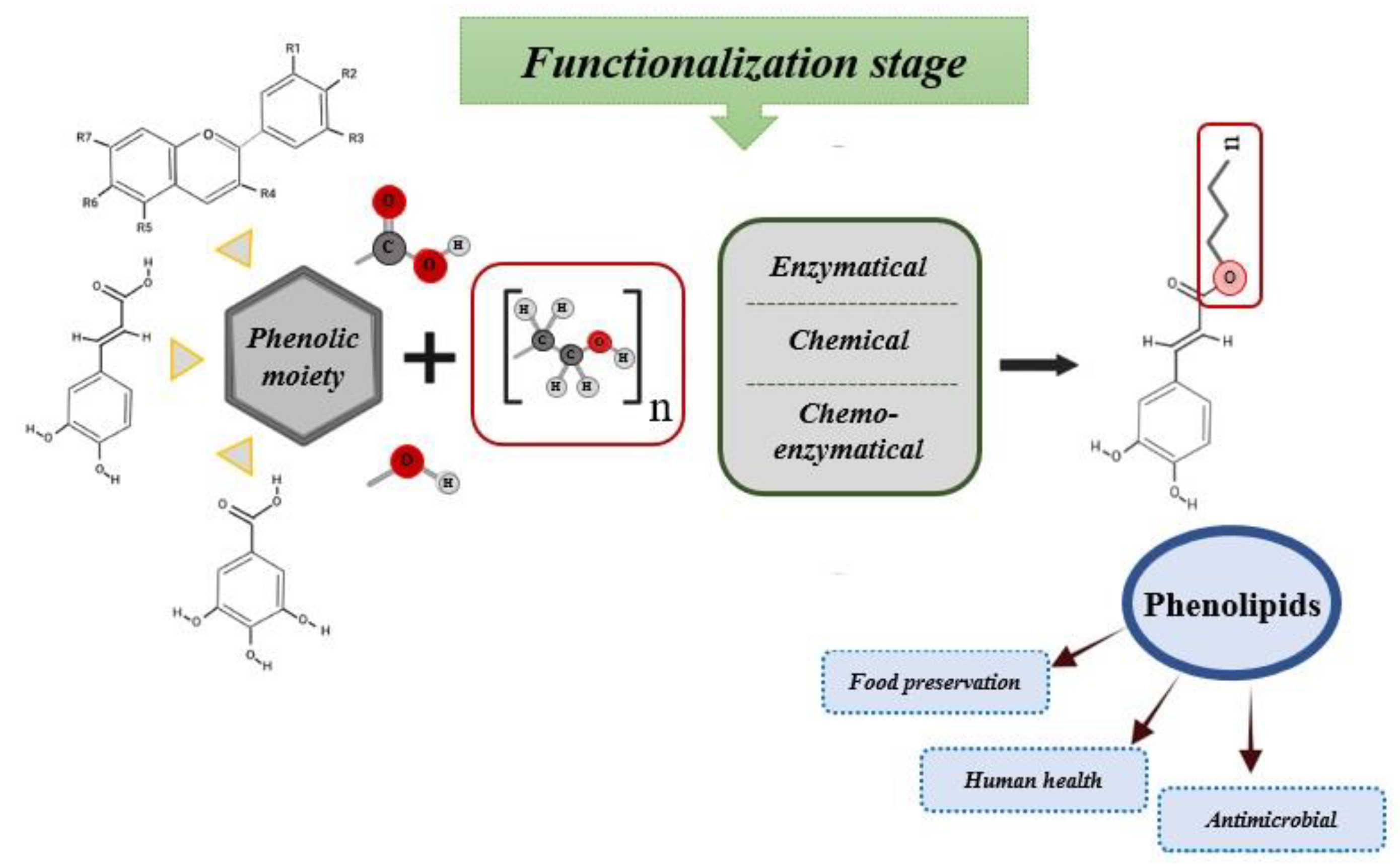
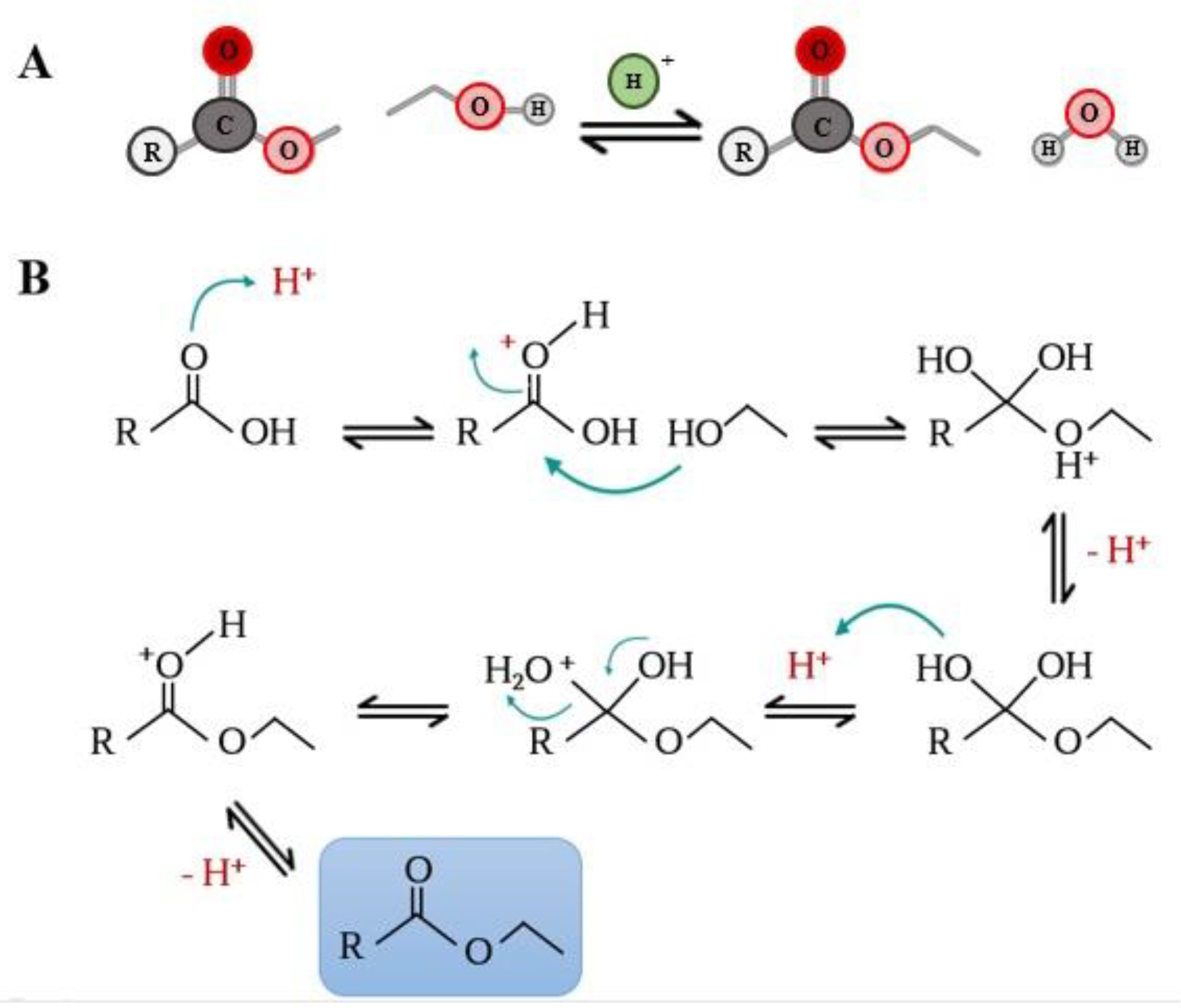
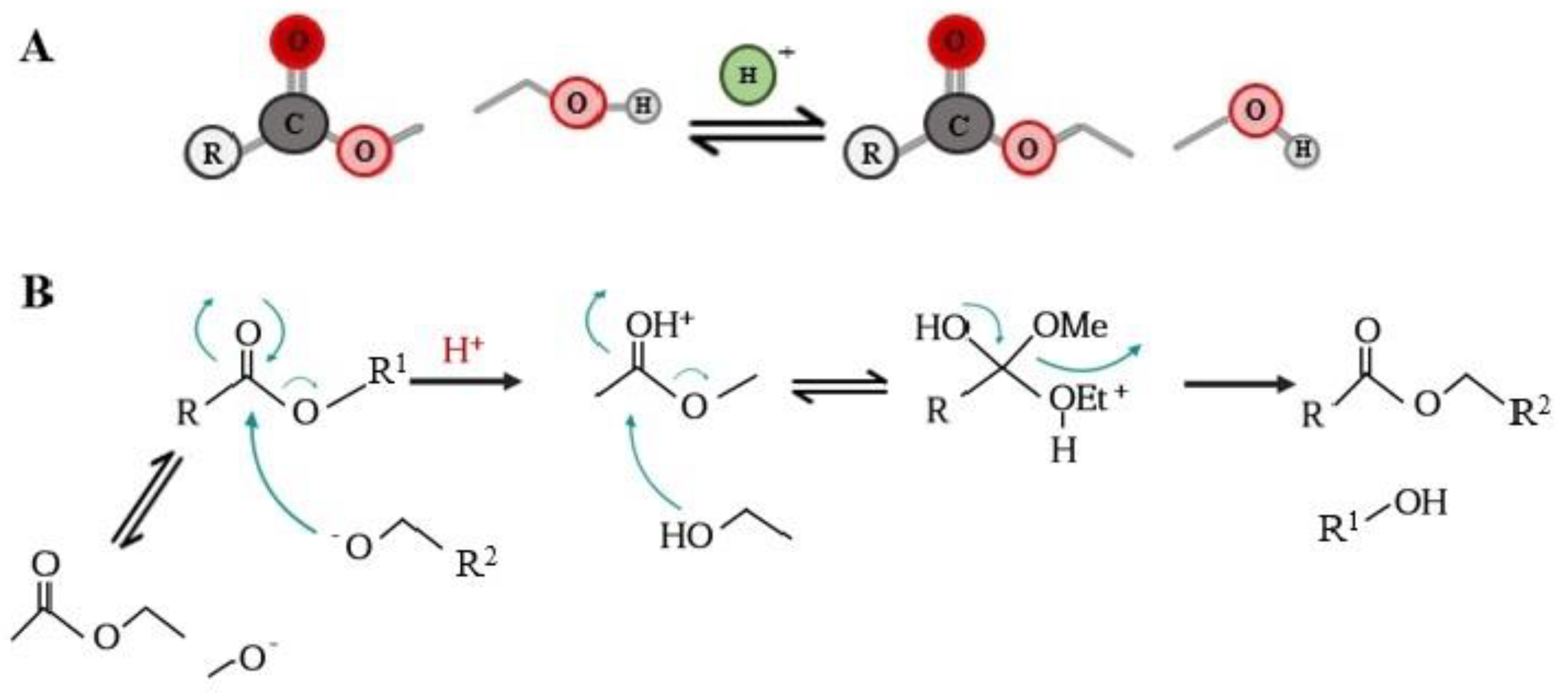
| Polyphenolic Family | Phenolic Core | Lipophilic Domain | Synthesis Procedure | Functionalization Reaction Media | Yield | References |
|---|---|---|---|---|---|---|
| HBZ-PA | Gallic acid | C6 | Enzymatic | Diethyl ether | <2% | [26] |
| C2 | Chemical | Data not available | Data not available | [27] | ||
| HBZ-PA | p-hydroxybenzoic acid | C6 | Enzymatic | Diethyl ether | <2% | [26] |
| HBZ-PA | Syringic acid | C6 | Enzymatic | Diethyl ether | <2% | [26] |
| HBZ-PA | Vanillic acid | C6 | Enzymatic | Diethyl ether | <2% | [26] |
| HBZ-PA | 3,5-di-t-butyl- 4-hydroxybenzylalcohol | C8 | Enzymatic | Cyclohexane | 98% | [26] |
| HBZ-PA | p-Hydroxyphenyl acetic | C1 to C10 | Enzymatic | 1-ethyl-3-methylimidazolium hexafluorophosphate | 13.4–62.6% (C8) | [28] |
| HBZ-PA | Protocatechuic acid | C1, C2, C3 | Chemical | Fatty alcohols | 71–81% | [29] |
| C1, C3,C6,C8, C10, C12, C14, C16, C18 | Chemical | Fatty alcohols | 59–95% | [19] | ||
| HBZ-PA | Gentisic acid | C6 | Enzymatic | Diethyl ether | <2% | [26] |
| HBZ-PA | 4-hydroxyphenylpropanoic | C2, C4, C6, C8, C10 | Enzymatic | Methyl-tert-butyl ether, fatty alcohols | Data not available | [30] |
| HCM-PA | Ferulic acid | C2, C8 | Enzymatic | 2-methyl-2-propanol toluene/hexane | 14–20% 50% (C8) | [31] |
| C18 (linoleyl alcohol) | Enzymatic | Hexane, 2-butanone | 16% | [32] | ||
| C2 | Enzymatic | Isooctane | 87% | [33] | ||
| C4, C8, C12, C16 | Chemo- enzymatic | Methanol, ChCl-urea-water system, 1-alkanol | 98% | [34] | ||
| C1 to C10 | Enzymatic | 1-ethyl-3-methylimidazolium tetrafluoroborate/hexafluorophosphate,1-butyl-3-methylimidazolium tetrafluoroborate/ hexafluorophosphate, 1-octyl-3-methylimidazo methylimidazolium tetrafluoroborate/ hexafluorophosphate | 6.7–62.6% (C8, [emim]PF6) 2.3–4.9% (C4, [emim]BF4) 3.4–14.9 (C4, [bmim]BF4) 7.6–18.2 (C4, [omim]BF4) 23.4–34.1 (C4, [emim]PF6) 32.9–52.6 (C4, [bmim]PF6) 55.6–59.2 (C4, [omim]PF6) 3.9–7.3 (C4, [bmim]BF4/[bmim]PF6 90/10) 10.2–12 (C4, [bmim]BF4/[bmim]PF6 50/50) 31.3–31.5 (C4, [bmim]BF4/[bmim]PF6 10/90) | [28] | ||
| C2, C4, C8, C12, oleyl alcohol | Enzymatic | Fatty alcohols | Traces (C4-C8) 2% (C12-oleyl alcohol) | [22] | ||
| Oleyl alcohol | Enzymatic | tert-butanol isooctane toluene 2-butanone hexane cyclohexane 1-butyl-3-methylimidazoliumbis (trifluoromethylsulfonyl) mide/ isooctane 1-hexyl-3-methylimidazolium hexafluorophosphate/ isooctane 1-methyl-3-octylimidazolium hexafluorophosphate/ isooctane 1-butyl-3-methylimidazolium hexafluorophosphate/ isooctane 1-butyl-3-methylimidazol umtetrafluoroborate/ isooctane | 3.81% 97.68% 25.83% 0% 99.17% 74.02% 16.51 mg/mL 14.67 mg/mL 12.53 mg/mL 4.03 mg/mL 2.37 mg/mL | [35] | ||
| C2 | Chemical | Data not available | Data not available | [27] | ||
| C1, C4, C8, C12, C16, C18, C20 | Chemical | THF, sulfuric acid, ethyl acetate | >90% | [13] | ||
| HCM-PA | p-coumaric acid | C4, C8, C12, C16 | Chemo-enzymatic | Methanol, ChCl-urea-water system | 98% | [34] |
| C2 | Chemical | Data not available | Data not available | [27] | ||
| HCM-PA | Cinnamic acid | C4, C6 and C12 | Enzymatic | n-pentane, cyclohexane Diethyl ether, t-butylmethyl ether, 1-butanol | >85% (C4) 68–70% (C6-C12) <6% | [26] |
| C1 to C10 | Enzymatic | 1-ethyl-3-methylimidazolium hexafluorophosphate | 54.2–56.2% (C8) | [28] | ||
| C2, C4, C8, C12, oleyl alcohol | Enzymatic | Fatty alcohols | 30% (C4-C8-oleyl alcohol) 26% (C12) | [22] | ||
| HCM-PA | Caffeic acid | C1 to C10 | Enzymatic | 1-ethyl-3-methylimidazolium hexafluorophosphate | 8.4–11.6% (C8) | [28] |
| C1, C2, C3, C4, C6, C8, C10,C12, C14, C16 | Chemical | Methyl, ethyl, propyl, butyl, hexyl, octyl, decyl, dodecyl tetradecyl and hexadecyl malonates, 3,4-dihydroxybenzaldehyde | Data not available | [36] | ||
| C8, C16 | Chemical | Monooctyl/ monohexadecyl malonates, 3,4-dihydroxybenzaldehyde | Data not available | [37] | ||
| C2, C4, C8, C12, oleyl alcohol | Enzymatic | Fatty alcohols | 0% | [22] | ||
| C2 | Chemical | Data not available | Data not available | [27] | ||
| C2, C3, C8 | Chemical | Fatty alcohols, sulfuric acid, dimethylformamide, ethyl ether, oxalyl chloride | Data not available | [38] | ||
| C1, C4, C8, C12, C16, C18, C20 | Chemical | THF, sulfuric acid, ethyl acetate | >90% | [13] | ||
| HCM-PA | Sinaptic acid | C1 to C10 | Enzymatic | 1-ethyl-3-methylimidazolium hexafluorophosphate | 0.4–31.2% (C8) | [28] |
| C2 | Chemical | Data not available | Data not available | [27] | ||
| C18 (linoleyl alcohol) | Enzymatic | Hexane, 2-butanone | 99% | [32] | ||
| HCM-PA | Dihydrocaffeic acid | C8, C16 | Chemical | Monooctyl/ monohexadecyl malonates, 3,4-dihydroxybenzaldehyde | Data not available | [37] |
| C2, C4, C8, C12, oleyl alcohol | Enzymatic | Fatty alcohols | 50% (C4), 38% (C8), 30–31% (C12-oleyl alcohol) | [22] | ||
| C8 | Enzymatic | Trioctylmethylammonium trifluoroacetate | 62% | [39] | ||
| HCM-PA | Rosmarinic acid | C1, C4, C8, C12, C18 and C20 | Chemical | Fatty alcohols | 98.5% (C1) 99.3% (C4) 99.5% (C8) 4.4% (C12) 81.6% (C16) 99.0% (C18–20) | [40] |
| C4, C8, C12, C18 and C20 | Chemical | Fatty alcohols | Data not available | [12] | ||
| HCM-PA | Phloretic acid | C1 to C10 | Enzymatic | 1-ethyl-3-methylimidazolium hexafluorophosphate | 38.5–60.6% (C8) | [28] |
| HCM-PA | 4-hydroxicinnamic acid | C8, C16 | Chemical | Monooctyl/ monohexadecyl malonates, 3,4-dihydroxybenzaldehyde | Data not available | [37] |
| HCM-PA | 2,4 dihydroxihydrocinnamic acid | C1 to C10 | Enzymatic | 1-ethyl-3-methylimidazolium hexafluorophosphate | 23.3–27.7% (C8) | [28] |
| HCM-PA | 3,4 dihydroxihydrocinnamic acid | C1 to C10 | Enzymatic | 1-ethyl-3-methylimidazolium hexafluorophosphate | 30.0–35.5% (C8) | [28] |
| HCM-PA | 3,4 dimethoxy cinnamic acid | C2, C4, C8, C12, oleyl alcohol | Enzymatic | Fatty alcohols | 60% (C4) 12% (C8) 10% (C12-oleyl alcohol) | [22] |
| HCM-PA | Clorogenic acid or isomers | C1, C4, C8, C12, C16, C18 and C20 | Chemo-enzymatic | Fatty alcohols | Data not available | [24] |
| C1, C4, C8, C12, C16, C18 and C20 | Chemo-enzymatic | Fatty alcohols | Data not available | [41] | ||
| HCM-PA | p-methoxycinnamic acid | 2-ethyl-hexanol | Enzymatic | Isooctane | 90% | [33] |
| HCM-PA | Coumaric acid | C1, C4, C8, C12, C16, C18, C20 | Chemical | THF, sulfuric acid, ethyl acetate | >90% | [13] |
| C1 to C10 | Enzymatic | 1-ethyl-3-methylimidazolium hexafluorophosphate | 27.4–32.9% (C8) | [28] | ||
| FLV | Naringin | C8, C10, C12 | Enzymatic | Acetone tert-butanol THF Fatty alcohols | 30% (C8), 23% (C10), 24% (C12) 15% (C8), 25% (C10), 16% (C12) 2% (C8/C10), 7% (C12) 8% (C8), 14% (C10), 5% (C12) | [21] |
| FLV | Rutin | C8, C10, C12 | Enzymatic | Acetone tert-butanol THF Fatty alcohols | 18% (C8), 20% (C10), 21% (C12) 19% (C8), 23% (C10), 20% (C12) 2% (C8/C10), <2% (C12) 10% (C8), 8% (C10), 5% (C12) | [21] |
| FLV | Malvidin-3- O-glucoside | Stearoyl chloride | Chemical | Acetonitrile | Data not available | [42] |
| FLV | Procyanidin B4 | Stearoyl chloride | Chemical | Dimethylformamide, benzyl bromide, potassium carbonate, acetonitrile, triethylamine, dichloromethane, dimethylaminopyridine | 70% | [42] |
| FLV | Delphinidin-3- O-sambubioside | Octanoyl chloride | Chemical | Dimethylfomamide | 59–95% | [19] |
| Octanoic acid | Enzymatic | Fatty acid, 2-methyl-2-butanol | 15% | [43] | ||
| FLV | Delphinidin-3- O-glucoside | Octanoic acid | Enzymatic | Fatty acid, 2-methyl-2-butanol | 28% | [43] |
| FLV | Cyanidin-3- O-sambubioside | Octanoyl chloride | Chemical | N,N-dimethylformamide, triethylamine, octanoyl chloride | Data not available | [20] |
| FLV | (+) Catechin | C12 | Enzymatic | Lauric acid. Methanol, ethanol, acetone, isopropanol, tetrahydrofuran, ethyl acetate, termamyl alcohol, pentane, petroleum ether | >70%, >80%, >80%, >60%, >50%, >50%, >40%, <10%, <10% | [44] |
| FLV | (-) Epicatechin | C12 | Enzymatic | Lauric acid. Methanol, ethanol, acetone, isopropanol, tetrahydrofuran, ethyl acetate, termamyl alcohol, pentane, petroleum ether | >70%, >80%, >80%, >60%, >50%, >50%, >40%, <10%, <10% | [44] |
| FLV | (-) epicatechin-3-O-gallate | C12 | Enzymatic | Methanol, ethanol, acetone, isopropanol, tetrahydrofuran, ethyl acetate, termamyl alcohol, pentane, petroleum ether | >70%, >80%, >80%, >60%, >50%, >50%, >40%, <10%, <10% | [44] |
| FLV | (-) epigallocatechin | C12 | Enzymatic | Methanol, ethanol, acetone, isopropanol, tetrahydrofuran, ethyl acetate, termamyl alcohol, pentane, petroleum ether | >70%, >80%, >80%, >60%, >50%, >50%, >40%, <10%, <10% | [44] |
| C18, EPA and DHA | Chemical | stearoyl, eicosapentaenoyl and docosahexaenoyl chloride, ethyl acetate | Data not available | [45] | ||
| C3,C8, C12, C18 chlorides, DHA | Chemical | C3,C8, C12, C18 and DHA chlorides, ethyl acetate, pyridine | Data not available | [46] | ||
| Ph-OH | Tyrosol | C1, C3, C8, C10, C12, C16, C18 and C18:1 | Enzymatic | Fatty acids, 2-methyl-2-propanol/n-hexane | 99.74% (C2), 95.93% (C3), 85.55% (C8), 75.42% (C10), 73.33% (C12), 69.95% (C16), 66.95% (C18), 57% (C18:1) | [47] |
| C18:1 | Enzymatic | Methyl oleate, t-butanol | Data not available | [14] | ||
| Ph-OH | Hydroxytyrosol | C8, C16 | Enzymatic | Fatty acids | Data not available | [37] |
| C8 | Enzymatic | Diethyl ether n-pentane, n-hexane Chloroform, dichloromethane, tetrahydrofuran | 85% 70–80% 20% | [26] | ||
| STB | Resveratrol | C3,C4,C6, C8,C10,C12, C14, C16, C18,C18:1, EPA and DHA chlorides | Chemical | Fatty acids, ethyl acetate, pyridine | Data not available | [48] |
| ELT | Punicalagin | Octanoyl/ dodecyl chloride | Chemical | DMF, acetonitrile, triethylamine, chlorides | Data not available | [49] |
| Phenolipid | Polyphenolic Parent Compound | Lipophilic Domain | Results | References |
|---|---|---|---|---|
| Ferulates | Ferulic acid | C1 to C12 | Ferulates ≈ Ferulic acid | [23] |
| Gallates | Gallic acid | C1, C3, C12 and C18 | Gallates > Gallic acid | [23] |
| Dihydrocaffeate | Dihydrocaffeic acid (DHCA) | C18 | DHCA > linolenyc Dihydrocaffeate | [32] |
| Clorogenates | 5- caffeoylquinic acid (5-CQA) | C1, C4, C8, C12, C16, C18 and C20 | C4/C8 clorogenates > 5-CQA | [41] |
| Rosmarinates | Rosmarinic acid (RA) | C4, C8, C12 and C20 | C8/C12 rosmarinates > RA | [12] |
| Caffeates | Caffeic acid (CA) | C1, C2, C3, C4, C6, C8, C10,C12, C14, C16 | C1-C8 caffeates > CA> C10-C16 caffeates | [31] |
| Hydroxytyrosol esters | Hydroxytyrosol (HTy) | C1 to C16 | HTy esters ≈ Hydroxytyrosol | [51] |
| Sinaptic acid ester | Sinaptic acid | C8 | Octyl sinaptic acid ester > sinaptic acid | [52] |
| Ferulate | Ferulic acid | C8 | Octyl ferulate > ferulic acid | [52] |
| Caffeate | Caffeic acid | C8 | Octyl caffeate > caffeic acid | [52] |
| p-coumarilate | p-coumaric acid (pCA) | C8 and C16 | pCA > C8 pCA > C16 kCA | [37] |
| Dihydrocaffeates | Dihydrocaffeic acid (DHCA) | C8 and C16 | C16 DHCA < C8 DHCA < DHCA | [37] |
| Caffeates | Caffeic acid (CA) | C8 and C16 | C16 CA > C8 CA > CA | [37] |
| Hydroxytyrosol esters | Hydroxytyrosol (HTy) | C8 and C16 | C8 HTy > HTy > C16 HTy | [37] |
| Tyrosol esters | Tyrosol (Ty) | C8 and C16 | Tyrosol > C8/C16 Tyr Esters | [37] |
| (+) Catechin monoester | (+) Catechin ((+)Cat) | C18 | (+) Catechin mono ester > (+)Cat | [42] |
| Malvidin-3-glucoside mono- /di- ester | Malvidin-3-glucoside (Mv3glu) | C18 | Mv3glu di-ester > Mv3glu mono-ester > Mv3glu | [42] |
| Procyanidin B4 di-ester | Procyanidin B4 | C18 | Procyanidin B4 > Procyanidin B4 di-ester | [42] |
| Caffeates | Caffeic acid | C1, C4, C8, C12, C16, C18 and C20 | Caffeates ≈ Cafeic acid | [13] |
| Ferulates | Ferulic acid | C1, C4, C8, C12, C16, C18 and C20 | Ferulates < Ferulic acid | [13] |
| Coumarilates | Coumaric acid (CmA) | C1, C4, C8, C12, C16, C18 and C20 | C8 Coumarilate > CmA > (C1,4,12,16,18) Coumarilates | [13] |
| (+) Catechin mono-ester | (+) Catechin ((+)Cat) | C12 | (+) Catechin mono ester > (+)Cat | [44] |
| Epicatechin mono-ester | (-) Epicatechin ((-) EC) | C12 | Epicatechin mono-ester > (-)EC | [44] |
| Epicatechin-3- O-gallate mono- ester | (-) Epicatechin-3- O-gallate ((-)EC3OG) | C12 | Epicatechin-3- O-gallate mono- ester > (-)EC3OG | [44] |
| Epigallocatechin di-ester | (-) Epigallocatechin ((-)EGC) | C12 | Epigallocatechin di-ester > (-)EGC | [44] |
| Protocatechuates mono-esters | Protocatechuic acid (PA) | C1, C2, C3 | Propyl PA > Ethyl PA > Methyl PA > PA | [29] |
| Epicallocatechin mono-esters | Epigallocatechin (EGC) | C3,C8, C12, C18 chlorides, docosahexanoic acid (DHA) | EGC > C3 EGC > C8 EGC > C12 EGC > DHA EGC | [46] |
| Punicalaginate mono-esters | Punicalagin (PG) | C8, C12 chlorides | C8 PG > PGC > C12 PG | [49] |
| Resveratryl mono-esters | Resveratrol (Rvt) | C3:0, C4:0, C6:0, C8:0, C10:0, C12:0, C14:0, C16:0, C18:0, C18:1, C20:5 and C22:6 Chlorides | Rvt> C18:1 Rvt > C18 Rvt > DHA Rvt > C14 Rvt > C16 Rvt > EPA Rvt > C6 Rvt > C12 Rvt - C8 Rvt-C3 Rvt > C4 Rvt > C10 Rvt | [48] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arzola-Rodríguez, S.I.; Muñoz-Castellanos, L.-N.; López-Camarillo, C.; Salas, E. Phenolipids, Amphipilic Phenolic Antioxidants with Modified Properties and Their Spectrum of Applications in Development: A Review. Biomolecules 2022, 12, 1897. https://doi.org/10.3390/biom12121897
Arzola-Rodríguez SI, Muñoz-Castellanos L-N, López-Camarillo C, Salas E. Phenolipids, Amphipilic Phenolic Antioxidants with Modified Properties and Their Spectrum of Applications in Development: A Review. Biomolecules. 2022; 12(12):1897. https://doi.org/10.3390/biom12121897
Chicago/Turabian StyleArzola-Rodríguez, Silvia Ivonne, Laila-Nayzzel Muñoz-Castellanos, César López-Camarillo, and Erika Salas. 2022. "Phenolipids, Amphipilic Phenolic Antioxidants with Modified Properties and Their Spectrum of Applications in Development: A Review" Biomolecules 12, no. 12: 1897. https://doi.org/10.3390/biom12121897
APA StyleArzola-Rodríguez, S. I., Muñoz-Castellanos, L.-N., López-Camarillo, C., & Salas, E. (2022). Phenolipids, Amphipilic Phenolic Antioxidants with Modified Properties and Their Spectrum of Applications in Development: A Review. Biomolecules, 12(12), 1897. https://doi.org/10.3390/biom12121897










