Cerebral Ischemic Preconditioning Aggravates Death of Oligodendrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Middle Cerebral Artery Occlusion
2.3. Measurements of Infarct Volume
2.4. Primary Cell Culture
2.5. Lentivirus Transfection, Oxygen-Glucose Deprivation (OGD), and Pharmacological Treatment
2.6. Cell Counts and Viability Assay
2.7. Western Blots
2.8. Immunofluorescent Staining, TUNEL, and Microscopy
2.9. Toluidine Blue Staining and Transmission Electron Microscopy (TEM)
2.10. Statistical Analysis
3. Results
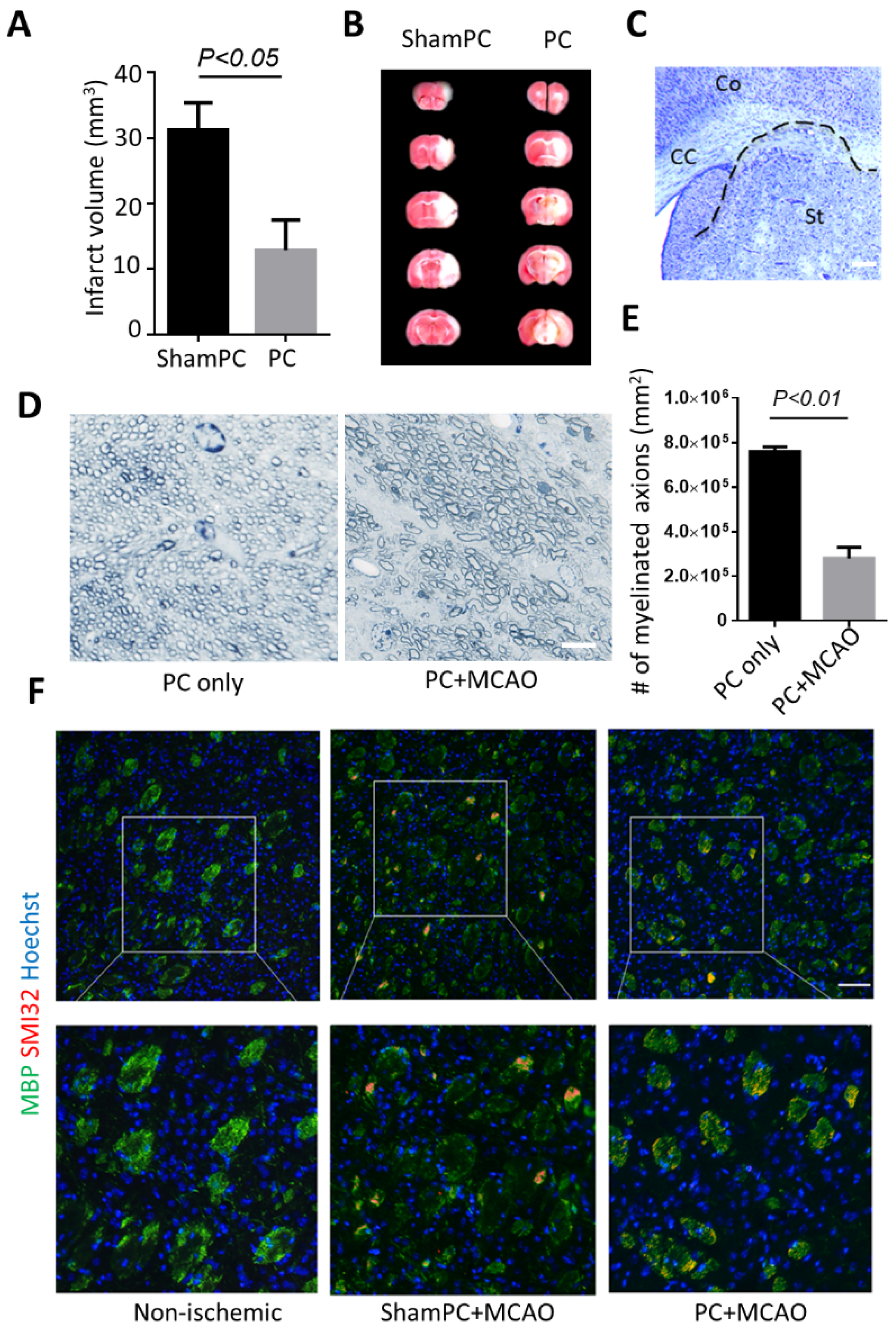
3.1. Preconditioning Does Not Prevent Striatal Infarct after Ischemic Stroke
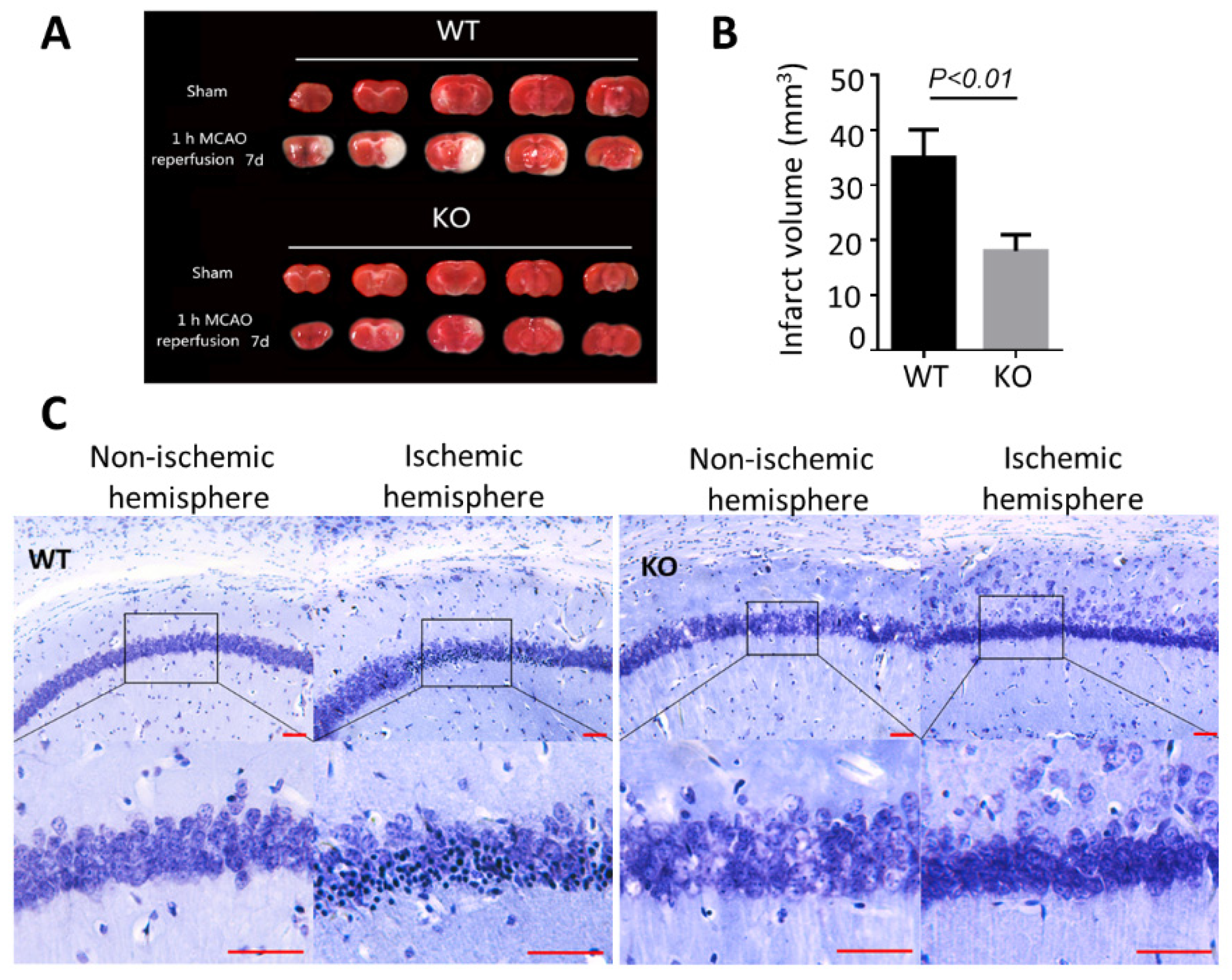
3.2. BNIP3 Knockout Mice Have Less Cerebral Damage Compared with Wild-Type Mice
3.3. BNIP3 Knockout Rescues Myelination Defects after Preconditioning
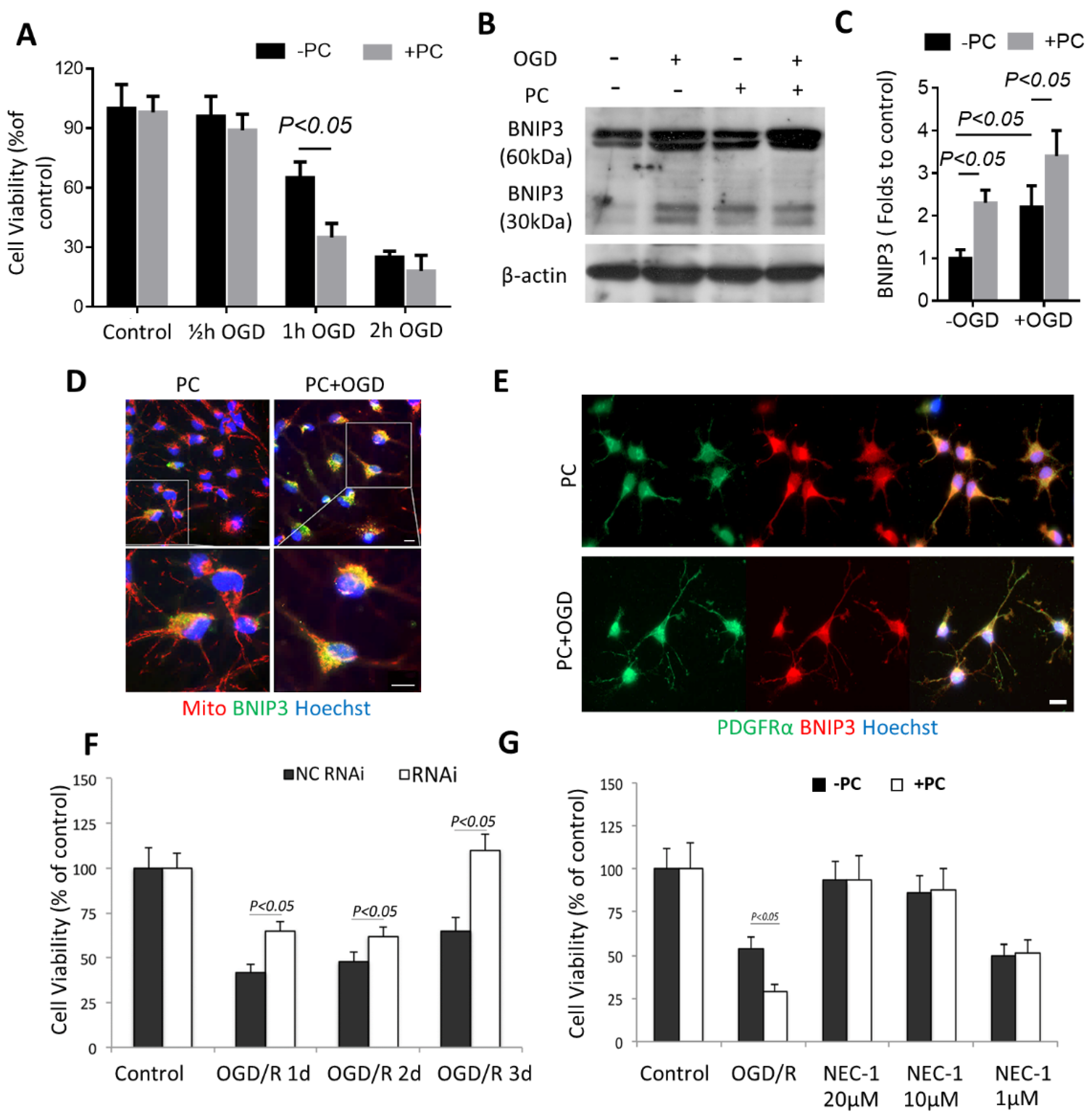
3.4. BNIP3 Is Associated with OPC Vulnerability in Preconditioning
3.5. Preconditioning Triggered Oligodendrocytic MCT1 Loss, and the Toxicity to OPC Is Attenuated by Knocking down BNIP3
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Randhawa, P.K.; Singh, N.; Jaggi, A.S. Role of ATP-Sensitive Potassium Channels in Remote Ischemic Preconditioning Induced Tissue Protection. J. Cardiovasc. Pharmacol. Ther. 2017, 22, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.C.; Cardoso, S.; Santos, R.X.; Carvalho, C.; Santos, M.S.; Perry, G.; Smith, M.A.; Moreira, P.I. New insights into the mechanisms of mitochondrial preconditioning-triggered neuroprotection. Curr. Pharm. Des. 2011, 17, 3381–3389. [Google Scholar] [CrossRef] [PubMed]
- Sisalli, M.J.; Annunziato, L.; Scorziello, A. Novel Cellular Mechanisms for Neuroprotection in Ischemic Preconditioning: A View from Inside Organelles. Front. Neurol. 2015, 6, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bak, L.K.; Walls, A.B.; Schousboe, A.; Waagepetersen, H.S. Astrocytic glycogen metabolism in the healthy and diseased brain. J. Biol. Chem. 2018, 293, 7108–7116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, M.; Miura, M.; Toriyabe, M.; Koyama, M.; Hatakeyama, M.; Ishikawa, M.; Nakajima, T.; Onodera, O.; Takahashi, T.; Nishizawa, M.; et al. Microglia preconditioned by oxygen-glucose deprivation promote functional recovery in ischemic rats. Sci. Rep. 2017, 7, 42582. [Google Scholar] [CrossRef] [Green Version]
- Philips, T.; Rothstein, J.D. Oligodendroglia: Metabolic supporters of neurons. J. Clin. Investig. 2017, 127, 3271–3280. [Google Scholar] [CrossRef] [Green Version]
- Chida, Y.; Kokubo, Y.; Sato, S.; Kuge, A.; Takemura, S.; Kondo, R.; Kayama, T. The alterations of oligodendrocyte, myelin in corpus callosum, and cognitive dysfunction following chronic cerebral ischemia in rats. Brain Res. 2011, 1414, 22–31. [Google Scholar] [CrossRef]
- Lilly, F.R.; Culpepper, J.; Stuart, M.; Steinwachs, D. Stroke survivors with severe mental illness: Are they at-risk for increased non-psychiatric hospitalizations? PLoS ONE 2017, 12, e0182330. [Google Scholar] [CrossRef] [Green Version]
- Guan, T.; Kong, J. Functional regeneration of the brain: White matter matters. Neural Regen. Res. 2015, 10, 355–356. [Google Scholar]
- Li, C.; Guan, T.; Chen, X.; Li, W.; Cai, Q.; Niu, J.; Xiao, L.; Kong, J. BNIP3 mediates pre-myelinating oligodendrocyte cell death in hypoxia and ischemia. J. Neurochem. 2013, 127, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, R.; Wong, A.; Silva, J.; Li, M.; Itoh, A.; Horiuchi, M.; Itoh, T.; Pleasure, D.; Cortopassi, G. Oligodendroglial differentiation induces mitochondrial genes and inhibition of mitochondrial function represses oligodendroglial differentiation. Mitochondrion 2010, 10, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonishvili, S.; Jain, M.R.; Li, H.; Levison, S.W.; Wood, T.L. Identification of Bax-interacting proteins in oligodendrocyte progenitors during glutamate excitotoxicity and perinatal hypoxia-ischemia. ASN Neuro 2013, 5, e00131. [Google Scholar] [CrossRef] [PubMed]
- Moncayo, J.; de Freitas, G.R.; Bogousslavsky, J.; Altieri, M.; van Melle, G. Do transient ischemic attacks have a neuroprotective effect? Neurology 2000, 54, 2089–2094. [Google Scholar] [CrossRef]
- Kiphuth, I.C.; Utz, K.S.; Noble, A.J.; Köhrmann, M.; Schenk, T. Increased prevalence of posttraumatic stress disorder in patients after transient ischemic attack. Stroke 2014, 45, 3360–3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, T.; Qian, Y.; Tang, X.; Huang, M.; Huang, L.; Li, Y.; Sun, H. Maslinic acid, a natural inhibitor of glycogen phosphorylase, reduces cerebral ischemic injury in hyperglycemic rats by GLT-1 up-regulation. J. Neurosci. Res. 2011, 89, 1829–1839. [Google Scholar] [CrossRef]
- Chen, Y.; Balasubramaniyan, V.; Peng, J.; Hurlock, E.C.; Tallquist, M.; Li, J.; Lu, Q.R. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat. Protoc. 2007, 2, 1044–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, K.D.; de Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Ray, R.; Dubik, D.; Shi, L.; Cizeau, J.; Bleackley, R.C.; Saxena, S.; Gietz, R.D.; Greenberg, A.H. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J. Exp. Med. 1997, 186, 1975–1983. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Zhu, S.; Li, V.; Gibson, S.B.; Xu, X.; Kong, J. BNIP3 interacting with LC3 triggers excessive mitophagy in delayed neuronal death in stroke. CNS Neurosci. Ther. 2014, 20, 1045–1055. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Zhang, S.; Ma, X.; Kong, J. BNIP3 upregulation and EndoG translocation in delayed neuronal death in stroke and in hypoxia. Stroke 2007, 38, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Valdez, R.; Martin, L.J.; Flock, D.L.; Northington, F.J. Necrostatin-1 attenuates mitochondrial dysfunction in neurons and astrocytes following neonatal hypoxia-ischemia. Neuroscience 2012, 219, 192–203. [Google Scholar] [CrossRef] [Green Version]
- Dirnagl, U.; Becker, K.; Meisel, A. Preconditioning and tolerance against cerebral ischaemia: From experimental strategies to clinical use. Lancet Neurol. 2009, 8, 398–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahjat, F.R.; Gesuete, R.; Stenzel-Poore, M.P. Steps to translate preconditioning from basic research to the clinic. Transl. Stroke Res. 2013, 4, 89–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirnagl, U.; Simon, R.P.; Hallenbeck, J.M. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003, 26, 248–254. [Google Scholar] [CrossRef]
- Rossi, D.J.; Brady, J.D.; Mohr, C. Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci. 2007, 10, 1377–1386. [Google Scholar] [CrossRef]
- Liu, J.; Bartels, M.; Lu, A.; Sharp, F.R. Microglia/macrophages proliferate in striatum and neocortex but not in hippocampus after brief global ischemia that produces ischemic tolerance in gerbil brain. J. Cereb. Blood Flow Metab. 2001, 21, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Berry, M.; Hubbard, P.; Butt, A.M. Cytology and lineage of NG2-positive glia. J. Neurocytol. 2002, 31, 457–467. [Google Scholar] [CrossRef]
- Deng, W.; Rosenberg, P.A.; Volpe, J.J.; Jensen, F.E. Calcium-permeable AMPA/kainate receptors mediate toxicity and preconditioning by oxygen-glucose deprivation in oligodendrocyte precursors. Proc. Natl. Acad. Sci. USA 2003, 100, 6801–6806. [Google Scholar] [CrossRef] [Green Version]
- Sommer, C. Ischemic preconditioning: Postischemic structural changes in the brain. J. Neuropathol. Exp. Neurol. 2008, 67, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Lehotský, J.; Burda, J.; DanielisovÁ, V.; Gottlieb, M.; Kaplán, P.; Saniová, B. Ischemic tolerance: The mechanisms of neuroprotective strategy. Anat. Rec. (Hoboken) 2009, 292, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Guan, T.; Jiang, Z.; Namaka, M.; Huang, Q.J.; Kong, J.M. Monocarboxylate transporter 1 and the vulnerability of oligodendrocyte lineage cells to metabolic stresses. CNS Neurosci. Ther. 2018, 24, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Guan, T.; Shafiq, K.; Xing, Y.; Sun, B.; Huang, Q.; Kong, J. Compromised Lactate Efflux Renders Vulnerability of Oligodendrocyte Precursor Cells to Metabolic Stresses. ACS Chem. Neurosci. 2020, 11, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kitamura, N.; Shinogi, D.; Yoshida, M.; Goda, O.; Murai, R.; Kamino, H.; Arakawa, H. BNIP3 and NIX mediate Mieap-induced accumulation of lysosomal proteins within mitochondria. PLoS ONE 2012, 7, e30767. [Google Scholar] [CrossRef] [PubMed]
- Webster, K.A.; Graham, R.M.; Bishopric, N.H. BNip3 and signal-specific programmed death in the heart. J. Mol. Cell Cardiol. 2005, 38, 35–45. [Google Scholar] [CrossRef]
- Graham, S.H.; Chen, J. Programmed cell death in cerebral ischemia. J. Cereb. Blood Flow Metab. 2001, 21, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Mu, J.; Weng, J.; Yang, C.; Guan, T.; Deng, L.; Li, M.; Zhang, G.; Kong, J. Necrostatin-1 prevents the proapoptotic protein Bcl-2/adenovirus E1B 19-kDa interacting protein 3 from integration into mitochondria. J. Neurochem. 2021, 156, 929–942. [Google Scholar] [CrossRef]
- Walls, K.C.; Ghosh, A.P.; Ballestas, M.E.; Klocke, B.J.; Roth, K.A. bcl-2/Adenovirus E1B 19-kd interacting protein 3 (BNIP3) regulates hypoxia-induced neural precursor cell death. J. Neuropathol. Exp. Neurol. 2009, 68, 1326–1338. [Google Scholar] [CrossRef] [Green Version]
- Azad, M.B.; Gibson, S.B. Role of BNIP3 in proliferation and hypoxia-induced autophagy: Implications for personalized cancer therapies. Ann. N. Y. Acad. Sci. 2010, 1210, 8–16. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, T.; Guo, Y.; Li, C.; Zhou, T.; Yu, Q.; Yang, C.; Zhang, G.; Kong, J. Cerebral Ischemic Preconditioning Aggravates Death of Oligodendrocytes. Biomolecules 2022, 12, 1872. https://doi.org/10.3390/biom12121872
Guan T, Guo Y, Li C, Zhou T, Yu Q, Yang C, Zhang G, Kong J. Cerebral Ischemic Preconditioning Aggravates Death of Oligodendrocytes. Biomolecules. 2022; 12(12):1872. https://doi.org/10.3390/biom12121872
Chicago/Turabian StyleGuan, Teng, Ying Guo, Chengren Li, Ting Zhou, Qiang Yu, Chaoxian Yang, Guohui Zhang, and Jiming Kong. 2022. "Cerebral Ischemic Preconditioning Aggravates Death of Oligodendrocytes" Biomolecules 12, no. 12: 1872. https://doi.org/10.3390/biom12121872
APA StyleGuan, T., Guo, Y., Li, C., Zhou, T., Yu, Q., Yang, C., Zhang, G., & Kong, J. (2022). Cerebral Ischemic Preconditioning Aggravates Death of Oligodendrocytes. Biomolecules, 12(12), 1872. https://doi.org/10.3390/biom12121872






