Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and Its Metabolic Activity
Abstract
:1. Introduction
2. Gut Microbiota
2.1. Biological Functions of Gut Microbiota
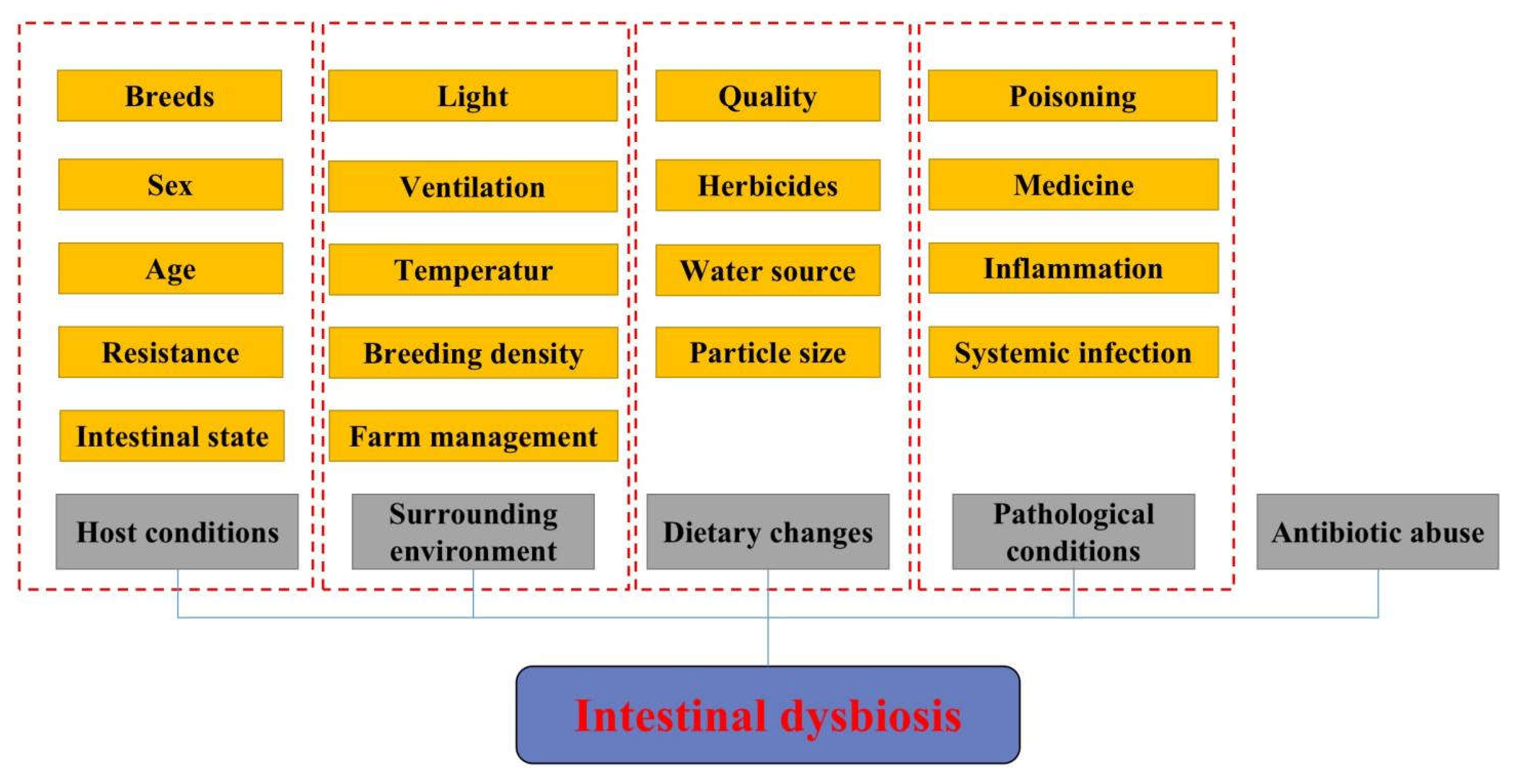
2.2. Main Factors Affecting the Composition of the Animal Gut Microbiota
2.3. Relationship between Gut Microbiota and Numerous Diseases
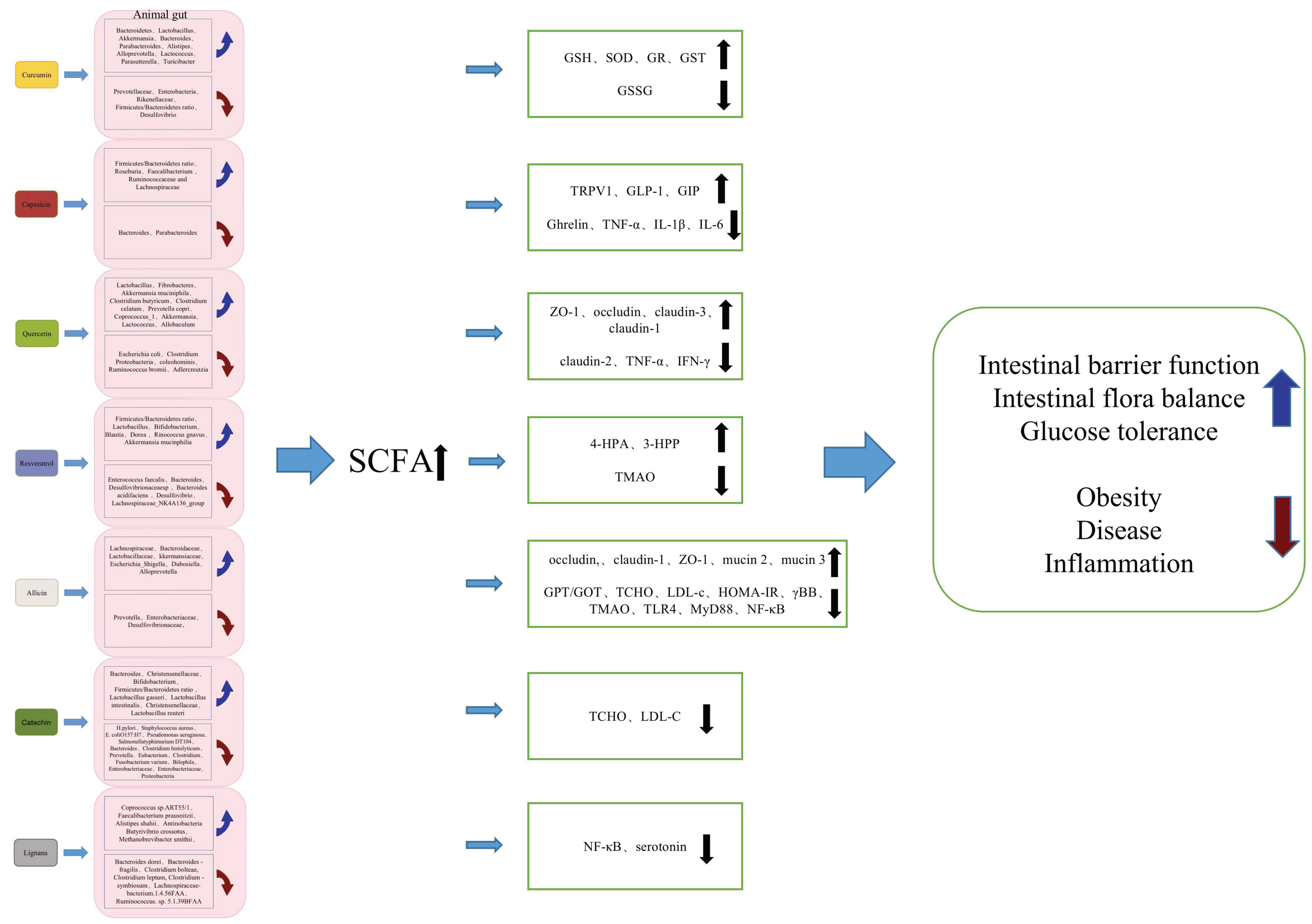
3. The Effect and Application Potential of Plant-Derived Bioactive Components on Gut Microflora
3.1. Effect of Curcumin on Gut Microflora
3.2. Effect of Capsaicin on Gut Microflora
3.3. Effect of Quercetin on Gut Microflora
3.4. Effect of Resveratrol on Gut Microflora
3.5. Effect of Allicin on Gut Microflora
3.6. Effect of Catechin on Gut Microflora
3.7. Effect of Lignans on Gut Microflora
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCFA | short-chain fatty acids |
| ND | normal diet |
| HFD | high fat diet |
| TRPV1 | transient receptor potential vanilloid 1 |
| GLP-1 | glucagon-like peptide-1 |
| GIP | gastric inhibitory polypeptide |
| CB1 | cannabinoid receptor 1 |
| LPS | lipopolysaccharide |
| ZO-1 | zonula occludens 1 |
| TNF-α | tumor necrosis factor-alpha |
| TNF-γ | tumor necrosis factor-gamma |
| TMAO | trimetlylamine oxide |
| Th1 | helper T cell 1 |
| Th17 | helper T cell 17 |
| GPT | glutamic-pyruvic transaminase |
| GOT | glutamic oxaloacetic transa minase |
| TCHO | total cholesterol |
| LDL-c | low-density lipoprotein cholesterol |
| GSH | glutathione |
| SOD | superoxide dismutase |
| GR | glutathione reductase |
| GST | glutathione S-transferase |
| GSSG | oxidized glutathione |
| LCA | lithocholic acid |
| TGR5 | takeda G-protein coupled receptor 5 |
| 4-HPA | 4-hydroxyphenylacetic acid |
| 3-HPP | 3-hydroxyphenylpropionic acid |
| TLR4 | toll-like receptor 4 |
| MyD88 | myeloid differentiation factor 88 |
| LDL | low-density lipoprotein |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| γBB | γ-butyrobetaine |
| TMA | trimethylamine |
| EGCG | epigallocatechin gallate |
| GCG | gallocatechin galleate |
References
- Anomaly, J. Harm to Others: The Social Cost of Antibiotics in Agriculture. J. Agric. Environ. Ethics 2009, 22, 423–435. [Google Scholar] [CrossRef]
- Lange, K.; Buerger, M.; Stallmach, A.; Bruns, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-Bacterial Mutualism in the Human Intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, R. The indigenous gastrointestinal microflora. Trends Microbiol. 1996, 4, 430–435. [Google Scholar] [CrossRef]
- Sittipo, P.; Shim, J.; Lee, Y. Microbial Metabolites Determine Host Health and the Status of Some Diseases. Int. J. Mol. Sci. 2019, 20, 5296. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Mahowald, M.A.; Rey, F.E.; Seedorf, H.; Turnbaugh, P.J.; Fulton, R.S.; Wollam, A.; Shah, N.; Wang, C.; Magrini, V.; Wilson, R.K.; et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc. Natl. Acad. Sci. USA 2009, 106, 5859–5864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimann, E.; Nyman, M.; Degerman, E. Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocyte 2015, 4, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüger, M.; Neuhaus, J.; Herrenthey, A.G.; Gökce, M.M.; Schrödl, W.; Shehata, A.A. Chronic botulism in a Saxony dairy farm: Sources, predisposing factors, development of the disease and treatment possibilities. Anaerobe 2014, 28, 220–225. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [Green Version]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, H.-H.; Chiu, C.-H.; Kong, M.-S.; Chang, C.-J.; Chen, C.-C. Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients 2019, 11, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Yue, Y.; Chen, Y.; Ding, M.; Li, B.; Wang, L.; Wang, Q.; Stanton, C.; Ross, R.P.; Zhao, J.; et al. Lactobacillus plantarum CCFM1143 Alleviates Chronic Diarrhea via Inflammation Regulation and Gut Microbiota Modulation: A Double-Blind, Randomized, Placebo-Controlled Study. Front. Immunol. 2021, 12, 746585. [Google Scholar] [CrossRef] [PubMed]
- Van Tongeren, S.P.; Slaets, J.P.J.; Harmsen, H.J.M.; Welling, G.W. Fecal Microbiota Composition and Frailty. Appl. Environ. Microbiol. 2005, 71, 6438–6442. [Google Scholar] [CrossRef] [Green Version]
- Ohlsson, C.; Sjögren, K. Effects of the gut microbiota on bone mass. Trends Endocrinol. Metab. 2015, 26, 69–74. [Google Scholar] [CrossRef]
- Charles, J.F.; Ermann, J.; Aliprantis, A.O. The intestinal microbiome and skeletal fitness: Connecting bugs and bones. Clin. Immunol. 2015, 159, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Inglis, J.E.; Ilich, J.Z. The Microbiome and Osteosarcopenic Obesity in Older Individuals in Long-Term Care Facilities. Curr. Osteoporos. Rep. 2015, 13, 358–362. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.-F. Bidirectional interactions between dietary curcumin and gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 2896–2902. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.-F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef]
- Sun, Z.-Z.; Li, X.-Y.; Wang, S.; Shen, L.; Ji, H.-F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef]
- Islam, T.; Koboziev, I.; Albracht-Schulte, K.; Mistretta, B.; Scoggin, S.; Yosofvand, M.; Moussa, H.; Zabet-Moghaddam, M.; Ramalingam, L.; Gunaratne, P.H.; et al. Curcumin Reduces Adipose Tissue Inflammation and Alters Gut Microbiota in Diet-Induced Obese Male Mice. Mol. Nutr. Food Res. 2021, 65, e2100274. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. Ott. Ont 2021, 143, 110270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, H.; Zhang, L.; Wang, T. Dietary bisdemethoxycurcumin supplementation attenuates lipopolysaccharide-induced damages on intestinal redox potential and redox status of broilers. Poult. Sci. 2021, 100, 101061. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzler, M.; Pfeiffer, E.; Schulz, S.I.; Dempe, J.S. Curcumin uptake and metabolism. BioFactors 2013, 39, 14–20. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; de la Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant Activity of Fresh and Processed Jalapeño and Serrano Peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef]
- Holzer, P. TRPV1 and the gut: From a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur. J. Pharmacol. 2004, 500, 231–241. [Google Scholar] [CrossRef]
- Song, J.-X.; Ren, H.; Gao, Y.-F.; Lee, C.-Y.; Li, S.-F.; Zhang, F.; Li, L.; Chen, H. Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice. Front. Physiol. 2017, 8, 602. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhu, X.; Liu, K.; Wang, X.; Chen, M.; Wang, J.; Chen, H.; Hui, S.; Huang, L.; et al. Healthy Subjects Differentially Respond to Dietary Capsaicin Correlating with Specific Gut Enterotypes. J. Clin. Endocrinol. Metab. 2016, 101, 4681–4689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, S.; Huang, L.; Wang, X.; Zhu, X.; Zhou, M.; Chen, M.; Yi, L.; Mi, M. Capsaicin improves glucose homeostasis by enhancing glucagon-like peptide-1 secretion through the regulation of bile acid metabolism via the remodeling of the gut microbiota in male mice. FASEB J. 2020, 34, 8558–8573. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. mBio 2017, 8, e00470-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada, T.; Suzuki, T.; Takahashi, M.; Iwai, K. Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats. Toxicol. Appl. Pharmacol. 1984, 72, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release Off. J. Control. Release Soc. 2014, 196, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Abdel-Latif, M.A.; Elbestawy, A.R.; El-Far, A.H.; Noreldin, A.E.; Emam, M.; Baty, R.S.; Albadrani, G.M.; Abdel-Daim, M.M.; Abd El-Hamid, H.S. Quercetin Dietary Supplementation Advances Growth Performance, Gut Microbiota, and Intestinal mRNA Expression Genes in Broiler Chickens. Animals 2021, 11, 2302. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hara, H. Quercetin Enhances Intestinal Barrier Function through the Assembly of Zonnula Occludens-2, Occludin, and Claudin-1 and the Expression of Claudin-4 in Caco-2 Cells. J. Nutr. 2009, 139, 965–974. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Qin, W.; Xu, Y.; Yang, W.; Chen, Y.; Huang, J.; Zhao, J.; Ma, L. Dietary Quercetin Supplementation Attenuates Diarrhea and Intestinal Damage by Regulating Gut Microbiota in Weanling Piglets. Oxid. Med. Cell. Longev. 2021, 2021, 6221012. [Google Scholar] [CrossRef]
- Amasheh, M.; Grotjohann, I.; Amasheh, S.; Fromm, A.; Söderholm, J.D.; Zeitz, M.; Fromm, M.; Schulzke, J.-D. Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: A novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand. J. Gastroenterol. 2009, 44, 1226–1235. [Google Scholar] [CrossRef]
- Su, L.; Zeng, Y.; Li, G.; Chen, J.; Chen, X. Quercetin improves high-fat diet-induced obesity by modulating gut microbiota and metabolites in C57BL/6J mice. Phytother. Res. PTR 2022, 36, 4558–4572. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, O.Q.P.; Zuo, Z.; Chow, M.S.S. Pharmacokinetics and modeling of quercetin and metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, X.H.; Yang, L.; Chen, X.Y.; Jiang, R.S.; Jin, S.H.; Geng, Z.Y. Resveratrol and cardiovascular health--promising therapeutic or hopeless illusion? Pharmacol Res. 2014, 90, 88–115. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Aggarwal, B.B. Resveratrol Suppresses TNF-Induced Activation of Nuclear Transcription Factors NF-κB, Activator Protein-1, and Apoptosis: Potential Role of Reactive Oxygen Intermediates and Lipid Peroxidation. J. Immunol. 2000, 164, 6509–6519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.L.; He, J.H.; Xie, H.B.; Yang, Y.S.; Li, J.C.; Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014, 93, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, X.; Han, X.; Hu, D.; Hu, X.; Li, Y.; Huang, P.; Yao, W. Resveratrol Suppresses Gut-Derived NLRP3 Inflammasome Partly through Stabilizing Mast Cells in a Rat Model. Mediat. Inflamm. 2018, 2018, 6158671. [Google Scholar] [CrossRef]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1201–1211. [Google Scholar] [CrossRef]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241. [Google Scholar] [CrossRef]
- Chen, M.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.; Zhang, Q.; Mi, M. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. MBio 2016, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alrafas, H.R.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P.S. Resveratrol modulates the gut microbiota to prevent murine colitis development through induction of Tregs and suppression of Th17 cells. J. Leukoc. Biol. 2019, 106, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xie, K.; Liu, Z.; Nakasone, Y.; Sakao, K.; Hossain, M.A.; Hou, D.-X. Preventive Effects and Mechanisms of Garlic on Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients 2019, 11, 1225. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Liu, Y.; Dou, Z.; Wu, T.; Liu, R.; Sui, W.; Jin, Y.; Zhang, M. Black garlic melanoidins prevent obesity, reduce serum LPS levels and modulate the gut microbiota composition in high-fat diet-induced obese C57BL/6J mice. Food Funct. 2020, 11, 9585–9598. [Google Scholar] [CrossRef]
- Panyod, S.; Wu, W.-K.; Chen, P.-C.; Chong, K.-V.; Yang, Y.-T.; Chuang, H.-L.; Chen, C.-C.; Chen, R.-A.; Liu, P.-Y.; Chung, C.-H.; et al. Atherosclerosis amelioration by allicin in raw garlic through gut microbiota and trimethylamine-N-oxide modulation. NPJ Biofilms Microbiomes 2022, 8, 4. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, L.; Bo, N.; Chaoyue, Y.; Haiyang, Y. Allicin Ameliorates Intestinal Barrier Damage via Microbiota-Regulated Short-Chain Fatty Acids-TLR4/MyD88/NF-κB Cascade Response in Acrylamide-Induced Rats. J. Agric. Food Chem. 2021, 69, 12837–12852. [Google Scholar] [CrossRef]
- Bancirova, M. Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Res. Int. 2010, 43, 1379–1382. [Google Scholar] [CrossRef]
- Liao, Z.-L.; Zeng, B.-H.; Wang, W.; Li, G.-H.; Wu, F.; Wang, L.; Zhong, Q.-P.; Wei, H.; Fang, X. Impact of the Consumption of Tea Polyphenols on Early Atherosclerotic Lesion Formation and Intestinal Bifidobacteria in High-Fat-Fed ApoE−/− Mice. Front. Nutr. 2016, 3, 42. [Google Scholar] [CrossRef] [Green Version]
- Pastoriza, S.; Mesías, M.; Cabrera, C.; Rufián-Henares, J.A. Healthy properties of green and white teas: An update. Food Funct. 2017, 8, 2650–2662. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Sun, Y.; Hu, B.; Sun, Y.; Jabbar, S.; Zeng, X. Fermentation in vitro of EGCG, GCG and EGCG3”Me isolated from Oolong tea by human intestinal microbiota. Food Res. Int. 2013, 54, 1589–1595. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.-P. Reciprocal Interactions between Epigallocatechin-3-gallate (EGCG) and Human Gut Microbiota In Vitro. J. Agric. Food Chem. 2020, 68, 9804–9815. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Shin, Y.; Kim, S.H.; Jin, M.; Choi, J.J. Dietary Epigallocatechin-3-Gallate Alters the Gut Microbiota of Obese Diabetic db/db Mice: Lactobacillus Is a Putative Target. J. Med. Food 2020, 23, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Wang, M.-N.; Tseng, T.-Y.; Sung, J.-S.; Tsai, T.-H. Pharmacokinetics of (−)-Epigallocatechin-3-gallate in Conscious and Freely Moving Rats and Its Brain Regional Distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Warden, B.A.; Smith, L.S.; Beecher, G.R.; Balentine, D.A.; Clevidence, B.A. Catechins are bioavailable in men and women drinking black tea throughout the day. J. Nutr. 2001, 131, 1731–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tham, D.M.; Gardner, C.D.; Haskell, W.L. Clinical review 97: Potential health benefits of dietary phytoestrogens: A review of the clinical, epidemiological, and mechanistic evidence. J. Clin. Endocrinol. Metab. 1998, 83, 2223–2235. [Google Scholar] [CrossRef] [Green Version]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef]
- Woting, A.; Clavel, T.; Loh, G.; Blaut, M. Bacterial transformation of dietary lignans in gnotobiotic rats. FEMS Microbiol. Ecol. 2010, 72, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Creus-Cuadros, A.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Estruch, R.; Gómez-Gracia, E.; Lapetra, J.; et al. Associations between Both Lignan and Yogurt Consumption and Cardiovascular Risk Parameters in an Elderly Population: Observations from a Cross-Sectional Approach in the PREDIMED Study. J. Acad. Nutr. Diet. 2017, 117, 609–622.e1. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Waśkiewicz, A.; Zujko, M.E.; Szcześniewska, D.; Stepaniak, U.; Pająk, A.; Drygas, W. Are Total and Individual Dietary Lignans Related to Cardiovascular Disease and Its Risk Factors in Postmenopausal Women? A Nationwide Study. Nutrients 2018, 10, 865. [Google Scholar] [CrossRef]
- Soleymani, S.; Habtemariam, S.; Rahimi, R.; Nabavi, S.M. The what and who of dietary lignans in human health: Special focus on prooxidant and antioxidant effects. Trends Food Sci. Technol. 2020, 106, 382–390. [Google Scholar] [CrossRef]
- Li, D.; Luo, F.; Guo, T.; Han, S.; Wang, H.; Lin, Q. Targeting NF-κB pathway by dietary lignans in inflammation: Expanding roles of gut microbiota and metabolites. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Li, J.; Ivey, K.L.; Wilkinson, J.E.; Wang, D.D.; Li, R.; Liu, G.; Eliassen, H.A.; Chan, A.T.; et al. Dietary lignans, plasma enterolactone levels, and metabolic risk in men: Exploring the role of the gut microbiome. BMC Microbiol. 2022, 22, 82. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-H.; Zhu, Y.-X.; Lu, L.; Zhou, L.-P.; Poon, C.C.-W.; Chan, C.-O.; Wang, L.-J.; Cao, S.; Yu, W.-X.; Wong, K.-Y.; et al. The Lignan-Rich Fraction from Sambucus williamsii Hance Exerts Bone Protective Effects via Altering Circulating Serotonin and Gut Microbiota in Rats. Nutrients 2022, 14, 4718. [Google Scholar] [CrossRef]
- Santangelo, R.; Silvestrini, A.; Mancuso, C. Ginsenosides, catechins, quercetin and gut microbiota: Current evidence of challenging interactions. Food Chem. Toxicol. 2019, 123, 42–49. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [Green Version]

| Plant-Derived Bioactive Compounds | Main Active Ingredients | Metabolic Gut Microflora | Reference |
|---|---|---|---|
| Curcumin | Curcumin-O-glucuronide | Lactococcus Parasutterella Turicibacter | [32] |
| Quercetin | 3,4-Dihydroxyphenylacetic acid | B. fragilis, C. perfringens, E. ramulus, Streptococcus S-2, Lactobacillus L-2, Bifidobacterium B-9 Bacteroides JY-6 | [85] |
| Resveratrol | Dihydroresveratrol 3,4′-Dihydroxy-trans-stilbene 3,4′-Dihydroxybibenzyl | Slackia equolifaciens Adlercreutzia equolifacens | [86] |
| Catechin | Valerolactones Hydroxyvaleric acid | Eggertella lenta Flavonifractor plautii | [85] |
| Lignans | Enterolignans enterodiol Enterolactone | Clostridium saccharogumia Eggerthella lenta Blautia producta Lactonifactor longoviformis | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Pan, S.; Li, F.; Xu, X.; Xing, H. Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and Its Metabolic Activity. Biomolecules 2022, 12, 1871. https://doi.org/10.3390/biom12121871
Chen X, Pan S, Li F, Xu X, Xing H. Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and Its Metabolic Activity. Biomolecules. 2022; 12(12):1871. https://doi.org/10.3390/biom12121871
Chicago/Turabian StyleChen, Xinyu, Shifeng Pan, Fei Li, Xinyu Xu, and Hua Xing. 2022. "Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and Its Metabolic Activity" Biomolecules 12, no. 12: 1871. https://doi.org/10.3390/biom12121871
APA StyleChen, X., Pan, S., Li, F., Xu, X., & Xing, H. (2022). Plant-Derived Bioactive Compounds and Potential Health Benefits: Involvement of the Gut Microbiota and Its Metabolic Activity. Biomolecules, 12(12), 1871. https://doi.org/10.3390/biom12121871






