Chronic Bedridden Condition Is Reflected by Substantial Changes in Plasma Inflammatory Profile
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Subjects Characteristics
3.2. Differential Plasma Protein Profiles
3.3. Pathway Analysis
4. Discussion
5. Conclusions and Clinical Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walsh, K.; Roberts, J.; Bennett, G. Mobility in old age. Gerodontology 1999, 16, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining chronic inflammation in aging and age-related diseases: Proposal of the senoinflammation concept. Int. Soc. Aging Dis. 2019, 10, 367–382. Available online: https://pubmed.ncbi.nlm.nih.gov/31011483/ (accessed on 23 May 2020). [CrossRef] [PubMed] [Green Version]
- Bruunsgaard, H.; Ladelund, S.; Pedersen, A.N.; Schroll, M.; Jørgensen, T.; Pedersen, B.K. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin. Exp. Immunol. 2003, 132, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Rowsey, P.J.; Bishop, B.L.; Ward, W.O.; MacPhail, R.C. Serum biomarkers of aging in the Brown Norway rat. Exp. Gerontol. 2011, 46, 953–957. Available online: https://pubmed.ncbi.nlm.nih.gov/21835237/ (accessed on 25 May 2020). [CrossRef] [PubMed]
- Swenson, B.L.; Meyer, C.F.; Bussian, T.J.; Baker, D.J. Senescence in aging and disorders of the central nervous system. Transl. Med. Aging 2019, 3, 17–25. [Google Scholar] [CrossRef]
- Carreno, G.; Guiho, R.; Martinez-Barbera, J.P. Cell senescence in neuropathology: A focus on neurodegeneration and tumours. Neuropathol. Appl. Neurobiol. 2021, 47, 359–378. Available online: https://pubmed.ncbi.nlm.nih.gov/33378554/ (accessed on 4 April 2020). [CrossRef] [PubMed]
- Venturelli, M.; Morgan, G.R.; Donato, A.J.; Reese, V.; Bottura, R.; Tarperi, C.; Milanese, C.; Schena, F.; Reggiani, C.; Naro, F.; et al. Cellular aging of skeletal muscle: Telomeric and free radical evidence that physical inactivity is responsible and not age. Clin. Sci. 2014, 127, 415–421. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24708050%5Cnhttp://clinsci.org/lookup/doi/10.1042/CS20140051 (accessed on 15 May 2020). [CrossRef] [PubMed] [Green Version]
- Magliozzi, R.; Howell, O.W.; Nicholas, R.; Cruciani, C.; Castellaro, M.; Romualdi, C.; Rossi, S.; Pitteri, M.; Benedetti, M.D.; Gajofatto, A.; et al. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann. Neurol. 2018, 83, 739–755. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29518260 (accessed on 29 April 2020). [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’Ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. Available online: https://pubmed.ncbi.nlm.nih.gov/23586463/ (accessed on 15 May 2020). [CrossRef] [PubMed] [Green Version]
- Magliozzi, R.; Hametner, S.; Facchiano, F.; Marastoni, D.; Rossi, S.; Castellaro, M.; Poli, A.; Lattanzi, F.; Visconti, A.; Nicholas, R.; et al. Iron homeostasis, complement, and coagulation cascade as CSF signature of cortical lesions in early multiple sclerosis. Ann. Clin. Transl. Neurol. 2019, 6, 2150–2163. Available online: https://pubmed.ncbi.nlm.nih.gov/31675181/ (accessed on 1 June 2020). [CrossRef] [PubMed]
- Moaddel, R.; Ubaida-Mohien, C.; Tanaka, T.; Lyashkov, A.; Basisty, N.; Schilling, B.; Semba, R.D.; Franceschi, C.; Gorospe, M.; Ferrucci, L. Proteomics in aging research: A roadmap to clinical, translational research. Aging Cell 2021, 20, e13325. Available online: https://pubmed.ncbi.nlm.nih.gov/33730416/ (accessed on 1 June 2020). [CrossRef] [PubMed]
- Ruocco, G.; Rossi, S.; Motta, C.; Macchiarulo, G.; Barbieri, F.; De Bardi, M.; Borsellino, G.; Finardi, A.; Grasso, M.G.; Ruggieri, S.; et al. T helper 9 cells induced by plasmacytoid dendritic cells regulate interleukin-17 in multiple sclerosis. Clin. Sci. 2015, 129, 291–303. Available online: https://pubmed.ncbi.nlm.nih.gov/25700150/ (accessed on 4 April 2020). [CrossRef] [PubMed] [Green Version]
- Valentine, M.S.; Link, P.A.; Herbert, J.A.; Kamga Gninzeko, F.J.; Schneck, M.B.; Shankar, K.; Nkwocha, J.; Reynolds, A.M.; Heise, R.L. Inflammation and Monocyte Recruitment Due to Aging and Mechanical Stretch in Alveolar Epithelium are Inhibited by the Molecular Chaperone 4-Phenylbutyrate. Cell. Mol. Bioeng. 2018, 11, 495–508. Available online: https://pubmed.ncbi.nlm.nih.gov/30581495/ (accessed on 15 May 2020). [CrossRef] [PubMed]
- Warwick, C.A.; Keyes, A.L.; Woodruff, T.M.; Usachev, Y.M. The complement cascade in the regulation of neuroinflammation, nociceptive sensitization, and pain. J. Biol. Chem. 2021, 297, 101085. Available online: https://pubmed.ncbi.nlm.nih.gov/34411562/ (accessed on 1 June 2020). [CrossRef] [PubMed]
- Camougrand, N.; Rigoulet, M. Aging and oxidative stress: Studies of some genes involved both in aging and in response to oxidative stress. Respir. Physiol. 2001, 128, 393–401. Available online: https://pubmed.ncbi.nlm.nih.gov/11718766/ (accessed on 1 June 2020). [CrossRef] [PubMed]
- Ouyang, L.; Zhang, K.; Chen, J.; Wang, J.; Huang, H. Roles of platelet-derived growth factor in vascular calcification. J. Cell. Physiol. 2018, 233, 2804–2814. Available online: https://pubmed.ncbi.nlm.nih.gov/28467642/ (accessed on 29 April 2020). [CrossRef] [PubMed]

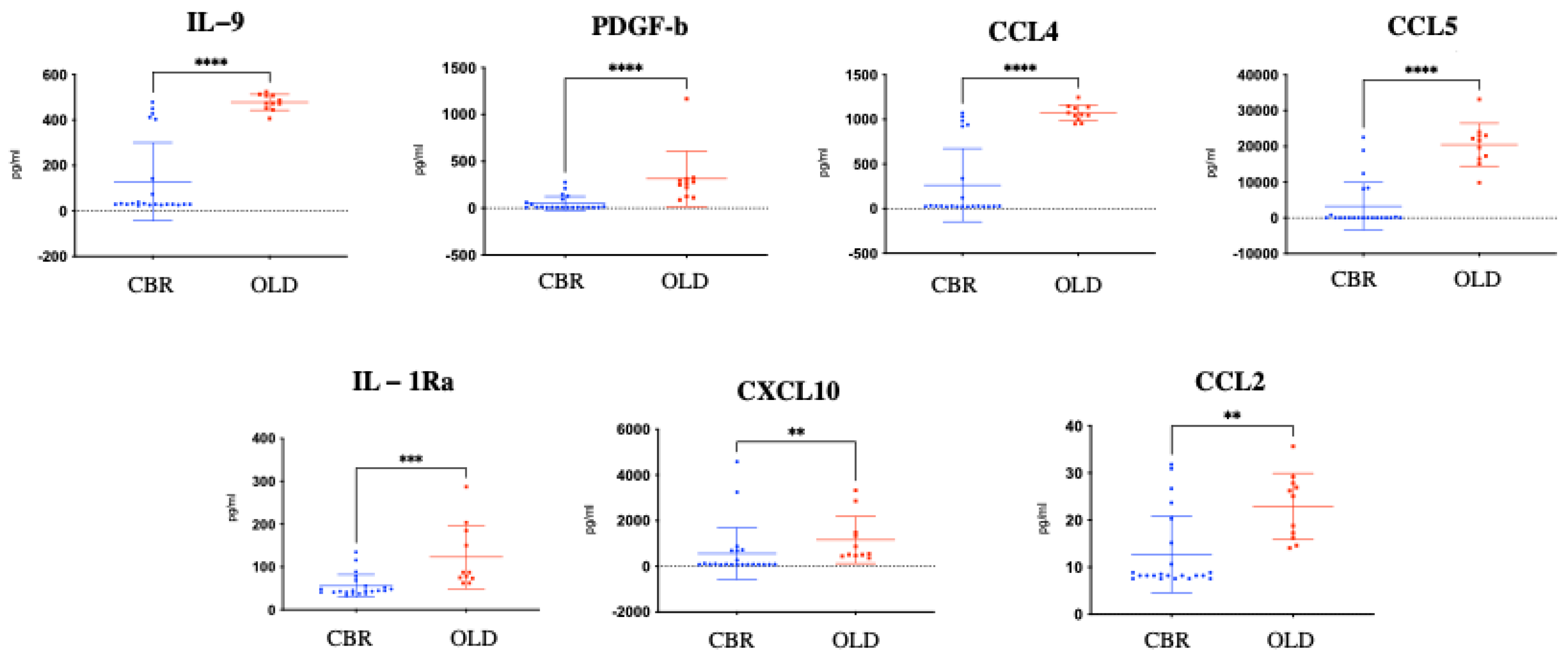

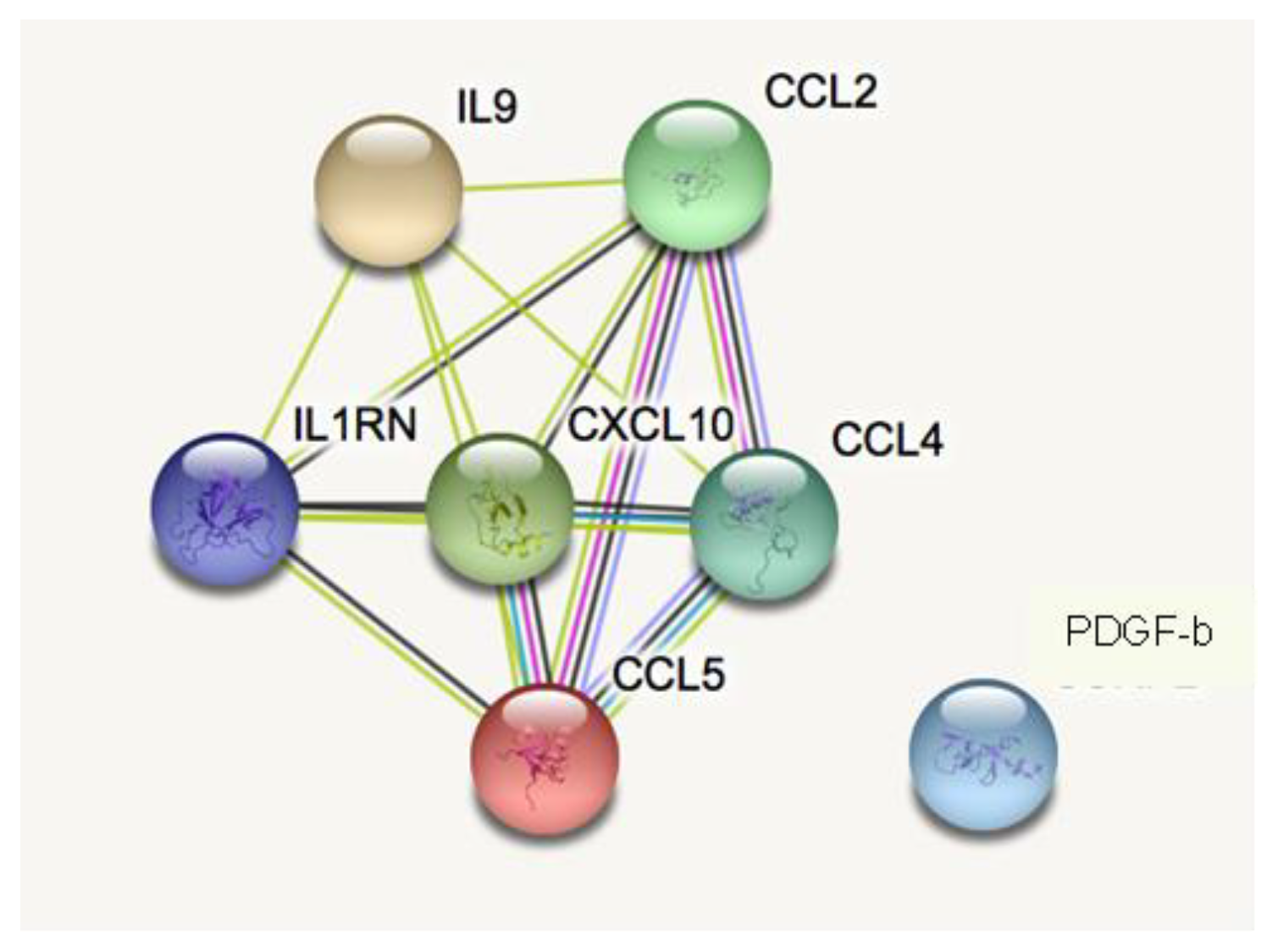
| Index | Name | p-Value | Adjusted p-Value | Odds Ratio | Combined Score |
|---|---|---|---|---|---|
| 1 | positive regulation of interleukin-5 production (GO:0032754) | 0.002448 | 0.01958 | 555.19 | 3338.15 |
| 2 | positive regulation of cytokine biosynthetic process (GO:0042108) | 0.01392 | 0.05567 | 85.27 | 364.51 |
| 3 | regulation of cytokine production (GO:0001817) | 0.03720 | 0.09920 | 30.98 | 101.95 |
| 4 | inflammatory response (GO:0006954) | 0.08495 | 0.1699 | 13.11 | 32.32 |
| 5 | positive regulation of cell proliferation (GO:0008284) | 0.1393 | 0.2229 | 7.71 | 15.20 |
| 6 | positive regulation of cellular process (GO:0048522) | 0.1681 | 0.2242 | 6.27 | 11.17 |
| 7 | cytokine-mediated signaling pathway (GO:0019221) | 0.2016 | 0.2304 | 5.11 | 8.18 |
| 8 | regulation of cell proliferation (GO:0042127) | 0.2320 | 0.2320 | 4.34 | 6.34 |
| Index | Name | p-Value | Adjusted p-Value | Odds Ratio | Combined Score |
|---|---|---|---|---|---|
| 1 | positive regulation of B cell proliferation (GO:0030890) | 0.000006519 | 0.0002255 | 1426.36 | 17,031.88 |
| 2 | regulation of T cell differentiation (GO:0045580) | 0.000007912 | 0.0002255 | 1288.13 | 15,131.87 |
| 3 | negative regulation of B cell apoptotic process (GO:0002903) | 0.001050 | 0.005485 | 1665.92 | 11,427.00 |
| 4 | regulation of B cell proliferation (GO:0030888) | 0.00001353 | 0.0002570 | 973.46 | 10,913.37 |
| 5 | positive regulation of tissue remodeling (GO:0034105) | 0.001200 | 0.005485 | 1427.86 | 9603.49 |
| 6 | bone resorption (GO:0045453) | 0.001200 | 0.005485 | 1427.86 | 9603.49 |
| 7 | negative regulation of lymphocyte apoptotic process (GO:0070229) | 0.001349 | 0.005485 | 1249.31 | 8255.55 |
| 8 | regulation of B cell apoptotic process (GO:0002902) | 0.001499 | 0.005485 | 1110.44 | 7220.95 |
| 9 | interleukin-2-mediated signaling pathway (GO:0038110) | 0.001649 | 0.005485 | 999.35 | 6403.33 |
| 10 | cellular response to interleukin-2 (GO:0071352) | 0.001649 | 0.005485 | 999.35 | 6403.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magliozzi, R.; Pedrinolla, A.; Rossi, S.; Stabile, A.M.; Danese, E.; Lippi, G.; Schena, F.; Calabrese, M.; Venturelli, M.V. Chronic Bedridden Condition Is Reflected by Substantial Changes in Plasma Inflammatory Profile. Biomolecules 2022, 12, 1867. https://doi.org/10.3390/biom12121867
Magliozzi R, Pedrinolla A, Rossi S, Stabile AM, Danese E, Lippi G, Schena F, Calabrese M, Venturelli MV. Chronic Bedridden Condition Is Reflected by Substantial Changes in Plasma Inflammatory Profile. Biomolecules. 2022; 12(12):1867. https://doi.org/10.3390/biom12121867
Chicago/Turabian StyleMagliozzi, Roberta, Anna Pedrinolla, Stefania Rossi, Anna Maria Stabile, Elisa Danese, Giuseppe Lippi, Federico Schena, Massimiliano Calabrese, and Massimo Venturelli Venturelli. 2022. "Chronic Bedridden Condition Is Reflected by Substantial Changes in Plasma Inflammatory Profile" Biomolecules 12, no. 12: 1867. https://doi.org/10.3390/biom12121867
APA StyleMagliozzi, R., Pedrinolla, A., Rossi, S., Stabile, A. M., Danese, E., Lippi, G., Schena, F., Calabrese, M., & Venturelli, M. V. (2022). Chronic Bedridden Condition Is Reflected by Substantial Changes in Plasma Inflammatory Profile. Biomolecules, 12(12), 1867. https://doi.org/10.3390/biom12121867











