Identification, Screening, and Comprehensive Evaluation of Novel DPP-IV Inhibitory Peptides from the Tilapia Skin Gelatin Hydrolysate Produced Using Ginger Protease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ginger Protease
2.3. Comparison of Enzymatic Hydrolysis
2.4. Degree of Hydrolysis (DH)
2.5. In Vitro DPP IV Inhibition Assay
2.6. Identification of Peptide Sequences by Nano LC-ESI-MS/MS
2.7. Screening of Bioactive Peptides Using Bioinformatics Analysis
2.7.1. Peptide Ranker
2.7.2. Molecular Docking
2.8. Surface Plasmon Resonance Experiments
2.9. In Vivo Analysis
2.9.1. In Silico Gastrointestinal Digestion
2.9.2. Oral Administration in Rats
2.10. Statistical Analysis
3. Results and Discussion
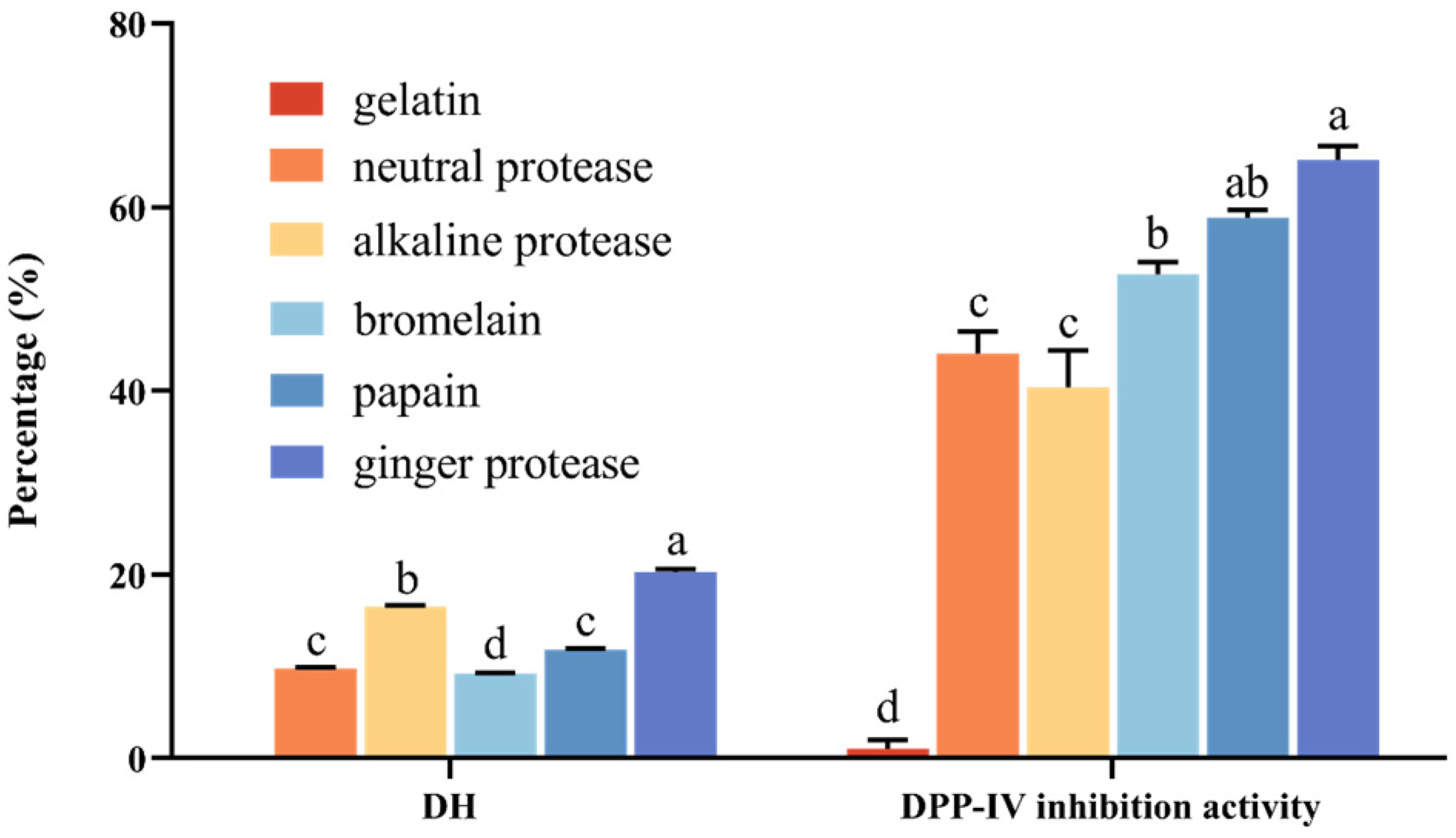
3.1. Comparison of Enzymatic Hydrolysis
3.2. Identification of Peptide Sequences by Nano LC-ESI-MS/MS Coupled with De Novo Sequencing
3.3. Screening of Bioactive Peptides Using Bioinformatics Analysis
3.4. Interactions between GPXGPPGPGP and DPP-IV
3.5. In Vivo Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Wang, L.; Jiang, B.; Li, X.; Guo, C.; Guo, S.; Shi, D. Recent progress of the development of dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Eur. J. Med. Chem. 2018, 151, 145–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.-K.; Yadav, D.; Sharma, N.; Jin, J.-O. Dipeptidyl Peptidase (DPP)-IV Inhibitors with Antioxidant Potential Isolated from Natural Sources: A Novel Approach for the Management of Diabetes. Pharmaceuticals 2021, 14, 586. [Google Scholar] [CrossRef] [PubMed]
- Howse, P.M.; Chibrikova, L.N.; Twells, L.K.; Barrett, B.J.; Gamble, J.-M. Safety and Efficacy of Incretin-Based Therapies in Patients With Type 2 Diabetes Mellitus and CKD: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2016, 68, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Liu, X.; Bai, L.; Yu, Q.; Zhang, Q.; Yu, P.; Yu, D. Efficacy and safety of vildagliptin, Saxagliptin or Sitagliptin as add-on therapy in Chinese patients with type 2 diabetes inadequately controlled with dual combination of traditional oral hypoglycemic agents. Diabetol. Metab. Syndr. 2014, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, L.; He, G.; Wu, J. Molecular targets and mechanisms of bioactive peptides against metabolic syndromes. Food Funct. 2018, 9, 42–52. [Google Scholar] [CrossRef]
- Cheung, I.W.Y.; Li-Chan, E.C.Y. Enzymatic production of protein hydrolysates from steelhead (Oncorhynchus mykiss) skin gelatin as inhibitors of dipeptidyl-peptidase IV and angiotensin-I converting enzyme. J. Funct. Foods 2017, 28, 254–264. [Google Scholar] [CrossRef] [Green Version]
- Rendón-Rosales, M.Á.; Torres-Llanez, M.J.; Mazorra-Manzano, M.A.; González-Córdova, A.F.; Hernández-Mendoza, A.; Vallejo-Cordoba, B. In vitro and in silico evaluation of multifunctional properties of bioactive synthetic peptides identified in milk fermented with Lactococcus lactis NRRL B-50571 and NRRL B-50572. LWT 2022, 154, 112581. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M.; O’ Shea, N.; Gallagher, E.; Lafarga, T. Predicted Release and Analysis of Novel ACE-I, Renin, and DPP-IV Inhibitory Peptides from Common Oat (Avena sativa) Protein Hydrolysates Using in Silico Analysis. Foods 2017, 6, 108. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Investigation of the Potential of Hemp, Pea, Rice and Soy Protein Hydrolysates as a Source of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides. Food Dig. Res. Curr. Opin. 2015, 6, 19–29. [Google Scholar] [CrossRef]
- Ayati, S.; Eun, J.; Atoub, N.; Mirzapour-Kouhdasht, A. Functional yogurt fortified with fish collagen-derived bioactive peptides: Antioxidant capacity, ACE and DPP-IV inhibitory. J. Food Process. Preserv. 2022, 46, e16208. [Google Scholar] [CrossRef]
- Sila, A.; Martinez-Alvarez, O.; Haddar, A.; Gómez-Guillén, M.C.; Nasri, M.; Montero, M.P.; Bougatef, A. Recovery, viscoelastic and functional properties of Barbel skin gelatine: Investigation of anti-DPP-IV and anti-prolyl endopeptidase activities of generated gelatine polypeptides. Food Chem. 2015, 168, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.Y.; Hsieh, C.H.; Hung, C.C.; Jao, C.L.; Chen, M.C.; Hsu, K.C. Fish skin gelatin hydrolysates as dipeptidyl peptidase IV inhibitors and glucagon-like peptide-1 stimulators improve glycaemic control in diabetic rats: A comparison between warm- and cold-water fish. J. Funct. Foods 2015, 19, 330–340. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, L.; Wu, G.; Liu, T.; Qi, X.; Zhang, H. Food-derived dipeptidyl peptidase IV inhibitory peptides: Production, identification, structure-activity relationship, and their potential role in glycemic regulation. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, W.; Li, X.; Qi, D.; Chen, H.; Liu, H.; Yu, S.; Wang, G.; Liu, Y. Evaluating the Properties of Ginger Protease-Degraded Collagen Hydrolysate and Identifying the Cleavage Site of Ginger Protease by Using an Integrated Strategy and LC-MS Technology. Molecules 2022, 27, 5001. [Google Scholar] [CrossRef] [PubMed]
- Taga, Y.; Kusubata, M.; Ogawa-Goto, K.; Hattori, S. Efficient Absorption of X-Hydroxyproline (Hyp)-Gly after Oral Administration of a Novel Gelatin Hydrolysate Prepared Using Ginger Protease. J. Agric. Food Chem. 2016, 64, 2962–2970. [Google Scholar] [CrossRef] [PubMed]
- Swinney, D.C. Biochemical mechanisms of drug action: What does it take for success? Nat. Rev. Drug Discov. 2004, 3, 801–808. [Google Scholar] [CrossRef]
- Copeland, R.A.; Pompliano, D.L.; Meek, T.D. Drug-target residence time and its implications for lead optimization. Nat. Rev. Drug Discov. 2006, 5, 730–739. [Google Scholar] [CrossRef]
- Copeland, R.A. The drug-target residence time model: A 10-year retrospective. Nat. Rev. Drug Discov. 2016, 15, 87–95. [Google Scholar] [CrossRef]
- Strasser, A.; Wittmann, H.-J.; Seifert, R. Binding Kinetics and Pathways of Ligands to GPCRs. Trends Pharm. Sci. 2017, 38, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Yan, W. Binding time--not just affinity--gains stature in drug design. Nat. Med. 2015, 21, 545. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cheng, J.; Wu, H. Discovery of Food-Derived Dipeptidyl Peptidase IV Inhibitory Peptides: A Review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.H.; Laursen, R.A. Amino-acid sequence and glycan structures of cysteine proteases with proline specificity from ginger rhizome Zingiber officinale. Eur. J. Biochem. 2000, 267, 1516–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Hamilton, S.E.; Guddat, L.W.; Overall, C.M. Plant collagenase: Unique collagenolytic activity of cysteine proteases from ginger. Biochim. Et Biophys. Acta 2007, 1770, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides 2013, 39, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Dryáková, A.; Pihlanto, A.; Marnila, P.; Čurda, L.; Korhonen, H.J.T. Antioxidant properties of whey protein hydrolysates as measured by three methods. Eur. Food Res. Technol. 2010, 230, 865–874. [Google Scholar] [CrossRef]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kee, L.T.; Awad, E.A.; Sazili, A.Q. Extraction, characterization and molecular structure of bovine skin gelatin extracted with plant enzymes bromelain and zingibain. J. Food Sci. Technol. 2020, 57, 3772–3781. [Google Scholar] [CrossRef]
- Taga, Y.; Hayashida, O.; Kusubata, M.; Ogawa-Goto, K.; Hattori, S. Production of a novel wheat gluten hydrolysate containing dipeptidyl peptidase-IV inhibitory tripeptides using ginger protease. Biosci. Biotechnol. Biochem. 2017, 81, 1823–1828. [Google Scholar] [CrossRef]
- Jin, Y.; Yan, J.; Yu, Y.; Qi, Y. Screening and identification of DPP-IV inhibitory peptides from deer skin hydrolysates by an integrated approach of LC–MS/MS and in silico analysis. J. Funct. Foods 2015, 18, 344–357. [Google Scholar] [CrossRef]
- Hatanaka, T.; Kawakami, K.; Uraji, M. Inhibitory effect of collagen-derived tripeptides on dipeptidylpeptidase-IV activity. J. Enzym. Inhib. Med. Chem. 2014, 29, 823–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Zheng, L.; Huang, M.; Zhao, M. Exploring structural features of potent dipeptidyl peptidase IV (DPP-IV) inhibitory peptides derived from tilapia (Oreochromis niloticus) skin gelatin by an integrated approach of multivariate analysis and Gly-Pro-based peptide library. Food Chem. 2022, 397, 133821. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, K.; Hendrie, C.; Liang, C.; Li, M.; Doherty-Kirby, A.; Lajoie, G. PEAKS: Powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2337–2342. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, L.; Shan, B.; Chen, W.; Xie, M.; Yuen, D.; Zhang, W.; Zhang, Z.; Lajoie, G.A.; Ma, B. PEAKS DB: De novo sequencing assisted database search for sensitive and accurate peptide identification. Mol. Cell Proteom. 2012, 11, M111.010587. [Google Scholar] [CrossRef] [Green Version]
- Ramshaw, J.A.; Shah, N.K.; Brodsky, B. Gly-X-Y tripeptide frequencies in collagen: A context for host-guest triple-helical peptides. J. Struct. Biol. 1998, 122, 86–91. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing casein-derived peptides. J. Funct. Foods 2013, 5, 1909–1917. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; FitzGerald, R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2019, 43, e12451. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; FitzGerald, R.J. Susceptibility of milk protein-derived peptides to dipeptidyl peptidase IV (DPP-IV) hydrolysis. Food Chem. 2014, 145, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; FitzGerald, R.J. An in silico model to predict the potential of dietary proteins as sources of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Chem. 2014, 165, 489–498. [Google Scholar] [CrossRef]
- Püschel, G.; Mentlein, R.; Heymann, E. Isolation and characterization of dipeptidyl peptidase IV from human placenta. Eur. J. Biochem. 1982, 126, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li-Chan, E.C.Y. Bioactive peptides and protein hydrolysates: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; O’Connor, P.; Hayes, M. Identification of novel dipeptidyl peptidase-IV and angiotensin-I-converting enzyme inhibitory peptides from meat proteins using in silico analysis. Peptides 2014, 59, 53–62. [Google Scholar] [CrossRef]
- Kim, B.-R.; Kim, H.Y.; Choi, I.; Kim, J.-B.; Jin, C.H.; Han, A.-R. DPP-IV Inhibitory Potentials of Flavonol Glycosides Isolated from the Seeds of: In Vitro and Analyses. Molecules 2018, 23, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhou, L.; Zhang, Y.; Sheng, N.-J.; Wang, Z.-K.; Wu, T.-Z.; Wang, X.-Z.; Wu, H. Rapid Identification of Dipeptidyl Peptidase-IV (DPP-IV) Inhibitory Peptides from Ruditapes philippinarum Hydrolysate. Molecules 2017, 22, 1714. [Google Scholar] [CrossRef]
- Patel, B.D.; Ghate, M.D. Recent approaches to medicinal chemistry and therapeutic potential of dipeptidyl peptidase-4 (DPP-4) inhibitors. Eur. J. Med. Chem. 2014, 74, 574–605. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, M.; Pan, F.; Li, J.; Dou, R.; Wang, X.; Wang, Y.; He, Y.; Wang, S.; Cai, S. analysis of novel dipeptidyl peptidase-IV inhibitory peptides released from antimicrobial protein 2 (MiAMP2) and the possible pathways involved in diabetes protection. Curr. Res. Food Sci. 2021, 4, 603–611. [Google Scholar] [CrossRef]
- Araki, M.; Kanegawa, N.; Iwata, H.; Sagae, Y.; Ito, K.; Masuda, K.; Okuno, Y. Hydrophobic interactions at subsite S1’ of human dipeptidyl peptidase IV contribute significantly to the inhibitory effect of tripeptides. Heliyon 2020, 6, e04227. [Google Scholar] [CrossRef]
- Hatanaka, T.; Inoue, Y.; Arima, J.; Kumagai, Y.; Usuki, H.; Kawakami, K.; Kimura, M.; Mukaihara, T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012, 134, 797–802. [Google Scholar] [CrossRef]
- Mann, M.; Jensen, O.N. Proteomic analysis of post-translational modifications. Nat. Biotechnol. 2003, 21, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Taga, Y.; Hayashida, O.; Ashour, A.; Amen, Y.; Kusubata, M.; Ogawa-Goto, K.; Shimizu, K.; Hattori, S. Characterization of Angiotensin-Converting Enzyme Inhibitory Activity of X-Hyp-Gly-Type Tripeptides: Importance of Collagen-Specific Prolyl Hydroxylation. J. Agric. Food Chem. 2018, 66, 8737–8743. [Google Scholar] [CrossRef] [PubMed]
- Alemán, A.; Giménez, B.; Pérez-Santin, E.; Gómez-Guillén, M.C.; Montero, P. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Swinney, D.C. The role of binding kinetics in therapeutically useful drug action. Curr. Opin. Drug Discov. Dev. 2009, 12, 31–39. [Google Scholar]
- Schnapp, G.; Klein, T.; Hoevels, Y.; Bakker, R.A.; Nar, H. Comparative Analysis of Binding Kinetics and Thermodynamics of Dipeptidyl Peptidase-4 Inhibitors and Their Relationship to Structure. J. Med. Chem. 2016, 59, 7466–7477. [Google Scholar] [CrossRef]
- Hussain, H.; Abbas, G.; Green, I.R.; Ali, I. Dipeptidyl peptidase IV inhibitors as a potential target for diabetes: Patent review (2015-2018). Expert Opin. Pat 2019, 29, 535–553. [Google Scholar] [CrossRef]
- Bradley, S.G.; Antalis, T.M.; Bond, J.S. Proteases in the Mammalian Digestive System. In Proteases: Structure and Function; Brix, K., Stöcker, W., Eds.; Springer: Vienna, Austria, 2013; pp. 373–393. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, P.K. Trypsin Detection Strategies: A Review. Crit. Rev. Anal. Chem. 2022, 52, 949–967. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, L.; Song, W.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Separation, identification and molecular binding mechanism of dipeptidyl peptidase IV inhibitory peptides derived from walnut (Juglans regia L.) protein. Food Chem. 2021, 347, 129062. [Google Scholar] [CrossRef]
- Shimizu, K.; Sato, M.; Zhang, Y.; Kouguchi, T.; Takahata, Y.; Morimatsu, F.; Shimizu, M. The bioavailable octapeptide Gly-Ala-Hyp-Gly-Leu-Hyp-Gly-Pro stimulates nitric oxide synthesis in vascular endothelial cells. J. Agric. Food Chem. 2010, 58, 6960–6965. [Google Scholar] [CrossRef]
- Adibi, S.A. The oligopeptide transporter (Pept-1) in human intestine: Biology and function. Gastroenterology 1997, 113, 332–340. [Google Scholar] [CrossRef]
- Abe, M.; Hoshi, T.; Tajima, A. Characteristics of transmural potential changes associated with the proton-peptide co-transport in toad small intestine. J. Physiol. 1987, 394, 481–499. [Google Scholar] [CrossRef] [PubMed]


| Enzymes | pH | Temperature/°C | Time (h) |
|---|---|---|---|
| neutral protease | 7.0 | 55 | 4 |
| alkaline protease | 9.0 | 55 | 4 |
| papain | 6.0 | 55 | 4 |
| bromelain | 6.0 | 55 | 4 |
| ginger protease | 6.0 | 55 | 4 |
| Amino Acid | Number of Residues | Percentage (%) |
|---|---|---|
| Gly | 679 | 30.0 |
| Pro | 537 | 23.7 |
| Hyp | 298 | 13.2 |
| Leu | 164 | 7.2 |
| Ala | 160 | 7.1 |
| Phe | 90 | 4.0 |
| Val | 72 | 3.2 |
| Arg | 69 | 3.0 |
| Lys | 45 | 2.0 |
| Gln | 34 | 1.5 |
| Asp | 31 | 1.4 |
| Ser | 26 | 1.1 |
| Met | 15 | 0.7 |
| Asn | 13 | 0.6 |
| Thr | 11 | 0.5 |
| Tyr | 9 | 0.4 |
| Glu | 5 | 0.2 |
| Trp | 4 | 0.2 |
| His | 4 | 0.2 |
| Num | Peptide Sequence | Formula | Length | Molecular Weight | Peptide Ranker | -Libdock Score | ToxinPred | ALC | tR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | GPXGPPGPGP | C38H56N10O12 | 10 | 844.403 | 0.948 | 221.210 | Non-toxicity | 96 | 16.19 |
| 2 | WFXGPR | C38H50N10O8 | 6 | 774.376 | 0.987 | 212.183 | Non-toxicity | 96 | 13.55 |
| 3 | GPPGPXGPGP | C38H56N10O12 | 10 | 844.403 | 0.948 | 209.382 | Non-toxicity | 97 | 15.15 |
| 4 | GPXGPXGPGX | C38H56N10O14 | 10 | 876.383 | 0.948 | 205.059 | Non-toxicity | 97 | 11.35 |
| 5 | GPXGPXGPGP | C38H56N10O13 | 10 | 860.393 | 0.948 | 204.386 | Non-toxicity | 99 | 13.76 |
| 6 | GFXGFQ | C32H41N7O9 | 6 | 667.292 | 0.951 | 202.225 | Non-toxicity | 97 | 36.35 |
| 7 | GPXGPXGGPR | C39H61N13O13 | 10 | 919.441 | 0.950 | 198.751 | Non-toxicity | 99 | 8.53 |
| 8 | GPXGPGXGMP | C38H58N10O13S | 10 | 894.381 | 0.954 | 194.667 | Non-toxicity | 97 | 18.2 |
| 9 | GPPGPPGPGP | C38H56N10O11 | 10 | 828.413 | 0.948 | 192.787 | Non-toxicity | 97 | 18.68 |
| 10 | FXGFQ | C30H38N6O8 | 5 | 610.270 | 0.971 | 189.562 | Non-toxicity | 95 | 33.49 |
| 11 | FFXGPK | C36H49N7O8 | 6 | 707.359 | 0.957 | 189.253 | Non-toxicity | 99 | 17.38 |
| 12 | FXGMY | C30H39N5O8S | 5 | 629.247 | 0.975 | 187.274 | Non-toxicity | 96 | 34.32 |
| 13 | LXGPLF | C33H50N6O8 | 6 | 658.364 | 0.963 | 185.441 | Non-toxicity | 99 | 44.96 |
| 14 | FXGPR | C27H40N8O7 | 5 | 588.297 | 0.967 | 178.364 | Non-toxicity | 97 | 11.16 |
| 15 | FXGFM | C30H39N5O7S | 5 | 613.252 | 0.994 | 177.207 | Non-toxicity | 95 | 46.23 |
| 16 | GFXGPR | C29H43N9O8 | 6 | 645.319 | 0.961 | 173.383 | Non-toxicity | 97 | 11.97 |
| 17 | FXGPF | C30H37N5O7 | 5 | 579.264 | 0.994 | 165.885 | Non-toxicity | 95 | 41.44 |
| 18 | SGPLF | C25H37N5O7 | 5 | 519.269 | 0.953 | 165.737 | Non-toxicity | 96 | 37.19 |
| 19 | LXGFM | C27H41N5O7S | 5 | 579.268 | 0.974 | 163.263 | Non-toxicity | 98 | 40.22 |
| 20 | FXGGLM | C29H44N6O8S | 6 | 636.289 | 0.963 | 162.622 | Non-toxicity | 96 | 38.19 |
| 21 | LGPF | C22H32N4O5 | 4 | 432.237 | 0.970 | 148.961 | Non-toxicity | 99 | 37.6 |
| 22 | LPGPF | C27H39N5O6 | 5 | 529.290 | 0.973 | 148.511 | Non-toxicity | 99 | 41.31 |
| 23 | LXGPF | C27H39N5O7 | 5 | 545.280 | 0.973 | 148.113 | Non-toxicity | 99 | 34.29 |
| 24 | FXGAP | C24H33N5O7 | 5 | 503.233 | 0.948 | 146.510 | Non-toxicity | 97 | 16.05 |
| 25 | GPLF | C22H32N4O5 | 4 | 432.237 | 0.973 | 136.346 | Non-toxicity | 95 | 34.93 |
| Num. | Peptide | 0.5 h | 1 h |
|---|---|---|---|
| 1 | PX | + | − |
| 2 | GPX | + | − |
| 3 | PXG | + | − |
| 4 | GPXGPPGPGP | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, X.; Yang, W.; Li, X.; Qi, D.; Chen, H.; Liu, H.; Yu, S.; Pan, Y.; Liu, Y.; et al. Identification, Screening, and Comprehensive Evaluation of Novel DPP-IV Inhibitory Peptides from the Tilapia Skin Gelatin Hydrolysate Produced Using Ginger Protease. Biomolecules 2022, 12, 1866. https://doi.org/10.3390/biom12121866
Liu W, Wang X, Yang W, Li X, Qi D, Chen H, Liu H, Yu S, Pan Y, Liu Y, et al. Identification, Screening, and Comprehensive Evaluation of Novel DPP-IV Inhibitory Peptides from the Tilapia Skin Gelatin Hydrolysate Produced Using Ginger Protease. Biomolecules. 2022; 12(12):1866. https://doi.org/10.3390/biom12121866
Chicago/Turabian StyleLiu, Wei, Xinyu Wang, Wenning Yang, Xueyan Li, Dongying Qi, Hongjiao Chen, Huining Liu, Shuang Yu, Yanli Pan, Yang Liu, and et al. 2022. "Identification, Screening, and Comprehensive Evaluation of Novel DPP-IV Inhibitory Peptides from the Tilapia Skin Gelatin Hydrolysate Produced Using Ginger Protease" Biomolecules 12, no. 12: 1866. https://doi.org/10.3390/biom12121866
APA StyleLiu, W., Wang, X., Yang, W., Li, X., Qi, D., Chen, H., Liu, H., Yu, S., Pan, Y., Liu, Y., & Wang, G. (2022). Identification, Screening, and Comprehensive Evaluation of Novel DPP-IV Inhibitory Peptides from the Tilapia Skin Gelatin Hydrolysate Produced Using Ginger Protease. Biomolecules, 12(12), 1866. https://doi.org/10.3390/biom12121866






