The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury
Abstract
1. Introduction
2. Molecular Mechanisms of Peripheral Nerve Regeneration
3. Pre-Clinical Review of Electrical Stimulation for Nerve Regeneration
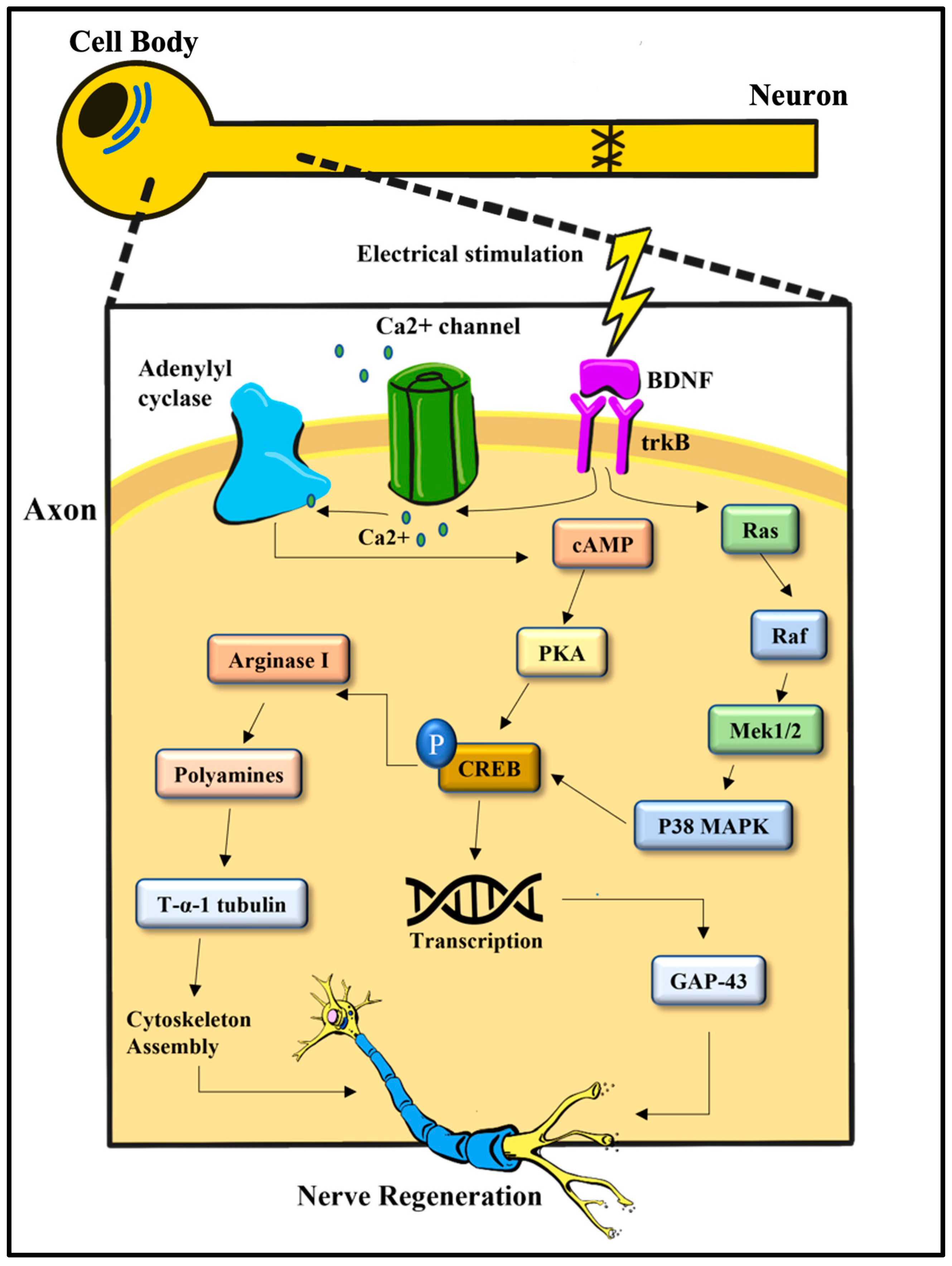
4. Electrical Stimulation on a Molecular Level
5. Delayed Nerve Repair
6. Nerve Defects
7. Duration of ES Delivery
8. Conditioning Lesion Enhances the Effects of Electrical Stimulation
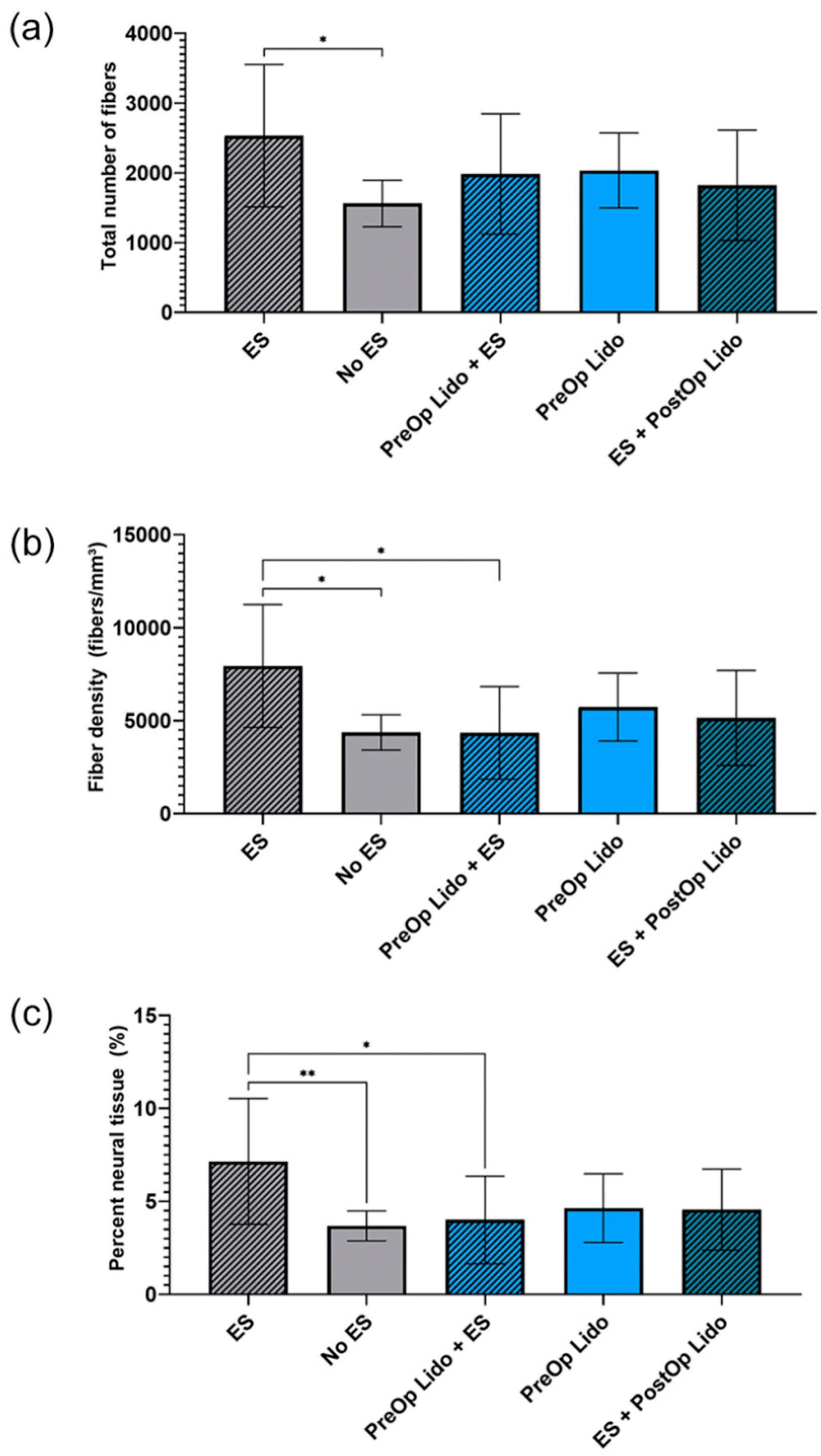
9. Electrical Stimulation and Peripheral Nerve Blocks
10. Perioperative Electrical Stimulation in Clinical Trials
| Trial | Indication | Target Nerve | Surgical Intervention | Trial Size | Duration of ES (20 Hz) | ES Location | Follow Up | Motor Measures | Sensory Measures | Electrophysiology | Surveys |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gordon et al., 2010 [106] | Chronic compression | Median nerve | Decompression (carpal tunnel release) | 21 (11 ES, 10 control) | 1 h | Outside OR (lab) | 12 mo | Purdue pegboard test | SWMT | NCS * MUNE * | Levine’s self-assessment questionnaire |
| Wong et al., 2015 [107] | Transection | Digital nerve | Epineurial repair | 31 (16 ES, 15 control) | 1 h | PACU | 6 mo | - | CDT # WDT # S2 PD # SWMT # | - | DASH |
| Barber et al., 2018 [108] | Traction neurapraxia | Spinal accessory nerve | N/A | 54 (27 ES, 27 control) | 1 h | OR | 12 mo | - | - | NCS | Constant Murley Score (CMS) * Neck Dissection Impairment Index (NDII) |
| Power et al., 2020 [109] | Chronic compression | Ulnar nerve | Decompression (cubital tunnel release) | 31 (20 ES, 11 control) | 1 h | PACU | 36 mo | Grip strength * Pinch strength * | McGowan-Goldberg grade * | NCS * MUNE * | - |
| Chan et al. [112] | Complete Denervation | Brachial plexus | Nerve repair/transfer | 80 (estimated) | 1 h | PACU | 24 mo | Grip Strength Pinch strength Purdue pegboard test Moberg Pick-up Test | SWMT S2PD | NCS MUNE | - |
| Davidge & Zucker et al.—1st Stage [113] | Hemifacial Paralysis/Bell Palsy | Facial nerve | Cross-Facial Nerve Graft | 20 children (estimated) | 1 h | OR | 12 mo | - | - | - | FACEGRAM FaCE |
| Moore et al. [114] | Chronic compression | Ulnar nerve | Decompression (cubital tunnel release) | 100 (estimated) | 10 min | OR | 12 mo | Grip strength Pinch strength MRC grading Finger Spread | SWMT S2PD | NCS | PROMIS (Upper Extremity) PROMIS (Pain) MHQ |
| Chan et al. [102] | Transection | Digital nerve | End-to-end repair | 66 (estimated) | Pre-op: 1 h +/− Post-op: 1 h | Pre-op: Lab Post-op: PACU | 6 mo | - | SWMT S2PD CASE CDT VT | NCS | DASH |
| Chan et al. [103] | Chronic compression | Median Nerve | Decompression (carpal tunnel release) | 60 (estimated) | Pre-op: 1 h Post-op: 1 h | Pre-op: Lab Post-op: PACU | 12 mo | Purdue Pegboard test | SWMT | DASH | |
| Chan et al. [104] | Chronic compression | Ulnar nerve | Decompression (cubital tunnel release) | 30 (estimated) | 1 h | Lab | 36 mo | Pinch strength | MUNE | DASH |
11. Future Directions: Ongoing Trials
11.1. Nerve Defects
11.2. Duration of ES Delivery
11.3. Conditioning Lesions
11.4. Peripheral Nerve Stimulator Devices
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Javeed, S.; Faraji, A.; Dy, C.; Ray, W.; MacEwan, M. Application of electrical stimulation for peripheral nerve regeneration: Stimulation parameters and future horizons. Interdiscip. Neurosurg. 2021, 24, 101117. [Google Scholar] [CrossRef]
- Novak, C.B.; Anastakis, D.J.; Beaton, D.E.; Katz, J. Patient-reported outcome after peripheral nerve injury. J. Hand Surg. Am. 2009, 34, 281–287. [Google Scholar] [CrossRef]
- Padovano, W.M.; Dengler, J.; Patterson, M.M.; Yee, A.; Snyder-Warwick, A.K.; Wood, M.D.; Moore, A.M.; Mackinnon, S.E. Incidence of Nerve Injury After Extremity Trauma in the United States. Hand 2022, 17, 615–623. [Google Scholar] [CrossRef]
- Pestronk, A.; Drachman, D.B.; Griffin, J.W. Effects of aging on nerve sprouting and regeneration. Exp. Neurol. 1980, 70, 65–82. [Google Scholar] [CrossRef]
- Mietto, B.S.; Mostacada, K.; Martinez, A.M. Neurotrauma and inflammation: CNS and PNS responses. Mediat. Inflamm. 2015, 2015, 251204. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Sanes, J.R.; Lichtman, J.W. Pre-existing pathways promote precise projection patterns. Nat. Neurosci. 2002, 5, 861–867. [Google Scholar] [CrossRef]
- Bregeon, F.; Alliez, J.R.; Héry, G.; Marqueste, T.; Ravailhe, S.; Jammes, Y. Motor and sensory re-innervation of the lung and heart after re-anastomosis of the cervical vagus nerve in rats. J. Physiol. 2007, 581, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Ciaramitaro, P.; Mondelli, M.; Logullo, F.; Grimaldi, S.; Battiston, B.; Sard, A.; Scarinzi, C.; Migliaretti, G.; Faccani, G.; Cocito, D.; et al. Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst. 2010, 15, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.Z.; Mackinnon, S.E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged axotomy. J. Neurosci. 1995, 15, 3876–3885. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged denervation. J. Neurosci. 1995, 15, 3886–3895. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, S. Rate of regeneration in human peripheral nerves; analysis of the interval between injury and onset of recovery. Arch. Neurol. Psychiatry 1947, 58, 251–295. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, E.; Guttman, L.; Medawar, P.B.; Young, J.Z. The rate of regeneration of nerve. J. Exp. Biol. 1942, 19, 14–44. [Google Scholar] [CrossRef]
- Seddon, H.J.; Medawar, P.B.; Smith, H. Rate of regeneration of peripheral nerves in man. J. Physiol. 1943, 102, 191–215. [Google Scholar] [CrossRef]
- Huckhagel, T.; Nüchtern, J.; Regelsberger, J.; Lefering, R.; DGU, T. Nerve injury in severe trauma with upper extremity involvement: Evaluation of 49,382 patients from the TraumaRegister DGU® between 2002 and 2015. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 76. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, M.; Zhi, J.; Wu, S.; Wang, Y.; Pei, F. Research Hotspots and Trends of Peripheral Nerve Injuries Based on Web of Science From 2017 to 2021: A Bibliometric Analysis. Front. Neurol. 2022, 13, 872261. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Neumann, C.M.; Brushart, T.M.; Gordon, T. Brief Electrical Stimulation Promotes the Speed and Accuracy of Motor Axonal Regeneration. J. Neurosci. 2000, 20, 2602–2608. [Google Scholar] [CrossRef]
- Geremia, N.M.; Gordon, T.; Brushart, T.M.; Al-Majed, A.A.; Verge, V.M.K. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp. Neurol. 2007, 205, 347–359. [Google Scholar] [CrossRef]
- Elzinga, K.; Tyreman, N.; Ladak, A.; Savaryn, B.; Olson, J.; Gordon, T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp. Neurol. 2015, 269, 142–153. [Google Scholar] [CrossRef]
- Brushart, T.M.; Hoffman, P.N.; Royall, R.M.; Murinson, B.B.; Witzel, C.; Gordon, T. Electrical Stimulation Promotes Motoneuron Regeneration without Increasing Its Speed or Conditioning the Neuron. J. Neurosci. 2002, 22, 6631–6638. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Brushart, T.M.; Gordon, T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 2000, 12, 4381–4390. [Google Scholar] [CrossRef] [PubMed]
- Wenjin, W.; Wenchao, L.; Hao, Z.; Feng, L.; Yan, W.; Wodong, S.; Xianqun, F.; Wenlong, D. Electrical stimulation promotes BDNF expression in spinal cord neurons through Ca(2+)- and Erk-dependent signaling pathways. Cell Mol. Neurobiol. 2011, 31, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Park, E.; Kim, T.; Kang, M.; Lee, K.S.; Park, S.M. Effectiveness of electrical stimulation on nerve regeneration after crush injury: Comparison between invasive and non-invasive stimulation. PLoS ONE 2020, 15, e0233531. [Google Scholar] [CrossRef]
- Witzel, C.; Brushart, T.M.; Koulaxouzidis, G.; Infanger, M. Electrical Nerve Stimulation Enhances Perilesional Branching after Nerve Grafting but Fails to Increase Regeneration Speed in a Murine Model. J. Reconstr. Microsurg. 2016, 32, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Keane, G.C.; Pan, D.; Roh, J.; Larson, E.L.; Schellhardt, L.; Hunter, D.A.; Snyder-Warwick, A.K.; Moore, A.M.; Mackinnon, S.E.; Wood, M.D. The Effects of Intraoperative Electrical Stimulation on Regeneration and Recovery After Nerve Isograft Repair in a Rat Model. Hand 2022, 17, 540–548. [Google Scholar] [CrossRef]
- Knott, E.P.; Assi, M.; Pearse, D.D. Cyclic AMP signaling: A molecular determinant of peripheral nerve regeneration. Biomed. Res. Int. 2014, 2014, 651625. [Google Scholar] [CrossRef] [PubMed]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Gumy, L.F.; Yeo, G.S.; Tung, Y.C.; Zivraj, K.H.; Willis, D.; Coppola, G.; Lam, B.Y.; Twiss, J.L.; Holt, C.E.; Fawcett, J.W. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. RNA 2011, 17, 85–98. [Google Scholar] [CrossRef]
- Bosse, F.; Hasenpusch-Theil, K.; Küry, P.; Müller, H.W. Gene expression profiling reveals that peripheral nerve regeneration is a consequence of both novel injury-dependent and reactivated developmental processes. J. Neurochem. 2006, 96, 1441–1457. [Google Scholar] [CrossRef]
- Bradke, F.; Fawcett, J.W.; Spira, M.E. Assembly of a new growth cone after axotomy: The precursor to axon regeneration. Nat. Rev. Neurosci. 2012, 13, 183–193. [Google Scholar] [CrossRef]
- Waller, A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. Lond. 1850, 140, 423–429. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Brück, W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997, 7, 741–752. [Google Scholar] [CrossRef]
- Gordon, T.; English, A.W. Strategies to promote peripheral nerve regeneration: Electrical stimulation and/or exercise. Eur. J. Neurosci. 2016, 43, 336–350. [Google Scholar] [CrossRef]
- Arthur-Farraj, P.J.; Latouche, M.; Wilton, D.K.; Quintes, S.; Chabrol, E.; Banerjee, A.; Woodhoo, A.; Jenkins, B.; Rahman, M.; Turmaine, M.; et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 2012, 75, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, J.A.; Pilch, K.S.; van der Lans, M.; Fazal, S.V.; Benito, C.; Wagstaff, L.J.; Mirsky, R.; Jessen, K.R. After Nerve Injury, Lineage Tracing Shows That Myelin and Remak Schwann Cells Elongate Extensively and Branch to Form Repair Schwann Cells, Which Shorten Radically on Remyelination. J. Neurosci. 2017, 37, 9086–9099. [Google Scholar] [CrossRef] [PubMed]
- Höke, A.; Redett, R.; Hameed, H.; Jari, R.; Zhou, C.; Li, Z.B.; Griffin, J.W.; Brushart, T.M. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J. Neurosci. 2006, 26, 9646–9655. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Arthur-Farraj, P. Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 2019, 67, 421–437. [Google Scholar] [CrossRef]
- Namgung, U. The role of Schwann cell-axon interaction in peripheral nerve regeneration. Cells Tissues Organs 2014, 200, 6–12. [Google Scholar] [CrossRef]
- Höke, A.; Gordon, T.; Zochodne, D.W.; Sulaiman, O.A. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp. Neurol. 2002, 173, 77–85. [Google Scholar] [CrossRef]
- Witzel, C.; Rohde, C.; Brushart, T.M. Pathway sampling by regenerating peripheral axons. J. Comp. Neurol. 2005, 485, 183–190. [Google Scholar] [CrossRef] [PubMed]
- McGregor, C.E.; English, A.W. The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met. Front. Cell Neurosci. 2018, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; You, S.; Cassar, S.L.; Tetzlaff, W. Reduced expression of regeneration associated genes in chronically axotomized facial motoneurons. Exp. Neurol. 2015, 264, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Eggers, R.; Tannemaat, M.R.; Ehlert, E.M.; Verhaagen, J. A spatio-temporal analysis of motoneuron survival, axonal regeneration and neurotrophic factor expression after lumbar ventral root avulsion and implantation. Exp. Neurol. 2010, 223, 207–220. [Google Scholar] [CrossRef]
- Brushart, T.M.; Aspalter, M.; Griffin, J.W.; Redett, R.; Hameed, H.; Zhou, C.; Wright, M.; Vyas, A.; Höke, A. Schwann cell phenotype is regulated by axon modality and cent.t.tral-peripheral location, and persists in vitro. Exp. Neurol. 2013, 247, 272–281. [Google Scholar] [CrossRef]
- Batt, J.; Bain, J.; Goncalves, J.; Michalski, B.; Plant, P.; Fahnestock, M.; Woodgett, J. Differential gene expression profiling of short and long term denervated muscle. FASEB J. 2006, 20, 115–117. [Google Scholar] [CrossRef]
- Liu, F.; Tang, W.; Chen, D.; Li, M.; Gao, Y.; Zheng, H.; Chen, S. Expression of TGF-β1 and CTGF Is Associated with Fibrosis of Denervated Sternocleidomastoid Muscles in Mice. Tohoku J. Exp. Med. 2016, 238, 49–56. [Google Scholar] [CrossRef]
- Moore, A.M.; Wagner, I.J.; Fox, I.K. Principles of nerve repair in complex wounds of the upper extremity. Semin Plast Surg. 2015, 29, 40–47. [Google Scholar] [CrossRef]
- Lee, S.K.; Wolfe, S.W. Peripheral nerve injury and repair. J. Am. Acad Orthop. Surg. 2000, 8, 243–252. [Google Scholar] [CrossRef]
- de Ruiter, G.C.; Malessy, M.J.; Alaid, A.O.; Spinner, R.J.; Engelstad, J.K.; Sorenson, E.J.; Kaufman, K.R.; Dyck, P.J.; Windebank, A.J. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp. Neurol. 2008, 211, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Ingvar, S. Reaction of cells to the galvanic current in tissue cultures. Proc. Soc. Exp. Biol. Med. 1920, 17, 198–199. [Google Scholar] [CrossRef]
- Zuo, K.J.; Gordon, T.; Chan, K.M.; Borschel, G.H. Electrical stimulation to enhance peripheral nerve regeneration: Update in molecular investigations and clinical translation. Exp. Neurol. 2020, 332, 113397. [Google Scholar] [CrossRef] [PubMed]
- Hyden, H. Protein metabolism in the nerve cell during growth and function. Acta Physiol. Scand. 1943, 6, 88–97. [Google Scholar]
- Barr, M.L.; Bertram, E.G. The behaviour of nuclear structures during depletion and restoration of Nissl material in motor neurons. J. Anat. 1951, 85, 171–181. [Google Scholar]
- Hinkle, L.; McCaig, C.D.; Robinson, K.R. The direction of growth of differentiating neurones and myoblasts from frog embryos in an applied electric field. J. Physiol. 1981, 314, 121–135. [Google Scholar] [CrossRef]
- McCaig, C.D. Nerve branching is induced and oriented by a small applied electric field. J. Cell Sci. 1990, 95, 605–615. [Google Scholar] [CrossRef]
- Jaffe, L.F.; Poo, M.-M. Neurites grow faster towards the cathode than the anode in a steady field. J. Exp. Zool. 1979, 209, 115–127. [Google Scholar] [CrossRef]
- Hoffman, H.; Binet, F.E. Acceleration and retardation of the process of axon-sprouting in partially denervated muscles. Aust. J. Exp. Biol. Med. Sci. 1952, 30, 541–566. [Google Scholar] [CrossRef]
- Wilson, D.H.; Jagadeesh, P. Experimental regeneration in peripheral nerves and the spinal cord in laboratory animals exposed to a pulsed electromagnetic field. Spinal. Cord 1976, 14, 12–20. [Google Scholar] [CrossRef][Green Version]
- Nix, W.A.; Hopf, H.C. Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res. 1983, 272, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Pockett, S.; Gavin, R.M. Acceleration of peripheral nerve regeneration after crush injury in rat. Neurosci. Lett. 1985, 59, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Wujek, J.R.; Lasek, R.J. Correlation of axonal regeneration and slow component B in two branches of a single axon. J. Neurosci. 1983, 3, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Schellhardt, L.; Keane, G.C.; Hunter, D.A.; Moore, A.M.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. Short-Duration, Pulsatile, Electrical Stimulation Therapy Accelerates Axon Regeneration and Recovery following Tibial Nerve Injury and Repair in Rats. Plast. Reconstr. Surg. 2022, 149, 681e–690e. [Google Scholar] [CrossRef]
- Mar, F.M.; Bonni, A.; Sousa, M.M. Cell intrinsic control of axon regeneration. EMBO Rep. 2014, 15, 254–263. [Google Scholar] [CrossRef]
- Ghosh-Roy, A.; Wu, Z.; Goncharov, A.; Jin, Y.; Chisholm, A.D. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J. Neurosci. 2010, 30, 3175–3183. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Tam, S.L.; Gordon, T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol. Neurobiol. 2004, 24, 379–402. [Google Scholar] [CrossRef]
- Soppet, D.; Escandon, E.; Maragos, J.; Middlemas, D.S.; Reid, S.W.; Blair, J.; Burton, L.E.; Stanton, B.R.; Kaplan, D.R.; Hunter, T.; et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 1991, 65, 895–903. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Huang, J.; Huang, M.; He, F.; Ye, Z.; Xiao, W.; Hu, X.; Luo, Z. Electrical stimulation induces calcium-dependent neurite outgrowth and immediate early genes expressions of dorsal root ganglion neurons. Neurochem. Res. 2014, 39, 129–141. [Google Scholar] [CrossRef]
- Richner, M.; Ulrichsen, M.; Elmegaard, S.L.; Dieu, R.; Pallesen, L.T.; Vaegter, C.B. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol. Neurobiol. 2014, 50, 945–970. [Google Scholar] [CrossRef]
- Boyd, J.G.; Gordon, T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol. Neurobiol. 2003, 27, 277–324. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Schwartz, G.; Meador, W.; Sabatier, M.J.; Mulligan, A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev. Neurobiol. 2007, 67, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.; Henley, J.; Tessier-Lavigne, M.; Song, H.; Poo, M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron 2001, 29, 441–452. [Google Scholar] [CrossRef]
- Neumann, S.; Bradke, F.; Tessier-Lavigne, M.; Basbaum, A.I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 2002, 34, 885–893. [Google Scholar] [CrossRef]
- Udina, E.; Furey, M.; Busch, S.; Silver, J.; Gordon, T.; Fouad, K. Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections. Exp. Neurol. 2008, 210, 238–247. [Google Scholar] [CrossRef]
- Han, P.J.; Shukla, S.; Subramanian, P.S.; Hoffman, P.N. Cyclic AMP elevates tubulin expression without increasing intrinsic axon growth capacity. Exp. Neurol. 2004, 189, 293–302. [Google Scholar] [CrossRef]
- Kawamura, K.; Kano, Y. Electrical stimulation induces neurite outgrowth in PC12m3 cells via the p38 mitogen-activated protein kinase pathway. Neurosci. Lett. 2019, 698, 81–84. [Google Scholar] [CrossRef]
- Singh, B.; Krishnan, A.; Micu, I.; Koshy, K.; Singh, V.; Martinez, J.A.; Koshy, D.; Xu, F.; Chandrasekhar, A.; Dalton, C.; et al. Peripheral neuron plasticity is enhanced by brief electrical stimulation and overrides attenuated regrowth in experimental diabetes. Neurobiol. Dis. 2015, 83, 134–151. [Google Scholar] [CrossRef]
- Christie, K.J.; Webber, C.A.; Martinez, J.A.; Singh, B.; Zochodne, D.W. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J. Neurosci. 2010, 30, 9306–9315. [Google Scholar] [CrossRef]
- Huang, J.; Ye, Z.; Hu, X.; Lu, L.; Luo, Z. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia 2010, 58, 622–631. [Google Scholar] [CrossRef]
- Chang, Y.J.; Hsu, C.M.; Lin, C.H.; Lu, M.S.; Chen, L. Electrical stimulation promotes nerve growth factor-induced neurite outgrowth and signaling. Biochim. Biophys. Acta 2013, 1830, 4130–4136. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T. Neurotrophic factor expression in denervated motor and sensory Schwann cells: Relevance to specificity of peripheral nerve regeneration. Exp. Neurol. 2014, 254, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Jari, R.; Verge, V.; Rohde, C.; Gordon, T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp. Neurol. 2005, 194, 221–229. [Google Scholar] [CrossRef]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus 2004, 16, E1. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Lu, L.; Hu, X.; Luo, Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur. J. Neurosci. 2013, 38, 3691–3701. [Google Scholar] [CrossRef] [PubMed]
- Hoben, G.M.; Ee, X.; Schellhardt, L.; Yan, Y.; Hunter, D.A.; Moore, A.M.; Snyder-Warwick, A.K.; Stewart, S.; Mackinnon, S.E.; Wood, M.D. Increasing Nerve Autograft Length Increases Senescence and Reduces Regeneration. Plast. Reconstr. Surg. 2018, 142, 952–961. [Google Scholar] [CrossRef]
- Zuo, K.J.; Shafa, G.; Antonyshyn, K.; Chan, K.; Gordon, T.; Borschel, G.H. A single session of brief electrical stimulation enhances axon regeneration through nerve autografts. Exp. Neurol. 2020, 323, 113074. [Google Scholar] [CrossRef]
- Sayanagi, J.; Acevedo-Cintrón, J.A.; Pan, D.; Schellhardt, L.; Hunter, D.A.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. Brief Electrical Stimulation Accelerates Axon Regeneration and Promotes Recovery Following Nerve Transection and Repair in Mice. J. Bone Jt. Surg. Am. 2021, 103, e80. [Google Scholar] [CrossRef]
- Senger, J.B.; Verge, V.M.K.; Chan, K.M.; Webber, C.A. The nerve conditioning lesion: A strategy to enhance nerve regeneration. Ann. Neurol. 2018, 83, 691–702. [Google Scholar] [CrossRef]
- Senger, J.L.B.; Verge, V.M.K.; Macandili, H.S.J.; Olson, J.L.; Chan, K.M.; Webber, C.A. Electrical stimulation as a conditioning strategy for promoting and accelerating peripheral nerve regeneration. Exp. Neurol. 2018, 302, 75–84. [Google Scholar] [CrossRef]
- McQuarrie, I.G. Effect of conditioning lesion on axonal sprout formation at nodes of Ranvier. J. Comp. Neurol. 1985, 231, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.M.; Verge, V.M. Axonal regeneration in dorsal spinal roots is accelerated by peripheral axonal transection. Brain Res. 1987, 411, 406–408. [Google Scholar] [CrossRef]
- Hoffman, P.N. A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp. Neurol. 2010, 223, 11–18. [Google Scholar] [CrossRef]
- Senger, J.L.; Chan, K.M.; Macandili, H.; Chan, A.W.M.; Verge, V.M.K.; Jones, K.E.; Webber, C.A. Conditioning electrical stimulation promotes functional nerve regeneration. Exp. Neurol. 2019, 315, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Salegio, E.A.; Pollard, A.N.; Smith, M.; Zhou, X.F. Macrophage presence is essential for the regeneration of ascending afferent fibres following a conditioning sciatic nerve lesion in adult rats. BMC Neurosci. 2011, 12, 11. [Google Scholar] [CrossRef]
- Niemi, J.P.; DeFrancesco-Lisowitz, A.; Roldán-Hernández, L.; Lindborg, J.A.; Mandell, D.; Zigmond, R.E. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J. Neurosci. 2013, 33, 16236–16248. [Google Scholar] [CrossRef]
- Kwon, M.J.; Shin, H.Y.; Cui, Y.; Kim, H.; Thi, A.H.; Choi, J.Y.; Kim, E.Y.; Hwang, D.H.; Kim, B.G. CCL2 Mediates Neuron-Macrophage Interactions to Drive Proregenerative Macrophage Activation Following Preconditioning Injury. J. Neurosci. 2015, 35, 15934–15947. [Google Scholar] [CrossRef]
- Senger, J.B.; Rabey, K.N.; Morhart, M.J.; Chan, K.M.; Webber, C.A. Conditioning Electrical Stimulation Accelerates Regeneration in Nerve Transfers. Ann. Neurol. 2020, 88, 363–374. [Google Scholar] [CrossRef]
- Webber, C.; Acton, L.; Chan, K.M.; Senger, J.-L. Determining the mechanism through which conditioning electrical stimulation promotes nerve regeneration. In Proceedings of the American Society of Peripheral Nerve Conference, Virtual Meeting, 15–22 January 2021. [Google Scholar]
- Senger, J.B.; Chan, A.W.M.; Chan, K.M.; Kwan-Wong, T.; Acton, L.; Olson, J.; Webber, C.A. Conditioning Electrical Stimulation Is Superior to Postoperative Electrical Stimulation in Enhanced Regeneration and Functional Recovery Following Nerve Graft Repair. Neurorehabil. Neural Repair 2020, 34, 299–308. [Google Scholar] [CrossRef]
- Senger, J.B.; Rabey, K.N.; Acton, L.; Lin, Y.S.; Lingrell, S.; Chan, K.M.; Webber, C.A. Recovering the regenerative potential in chronically injured nerves by using conditioning electrical stimulation. J. Neurosurg. 2021, 136, 1442–1454. [Google Scholar] [CrossRef]
- Chan, M. The Effect of Pre-Operative Electrical Stimulation on Peripheral Nerve Regeneration. Available online: https://ClinicalTrials.gov/show/NCT03205124 (accessed on 7 September 2022).
- Chan, M. Conditioning Electrical Stimulation to Improve Outcomes in Carpal Tunnel Syndrome. Available online: https://ClinicalTrials.gov/show/NCT04191538 (accessed on 23 November 2022).
- Chan, M. Conditioning Electrical Stimulation to Improve Outcomes in Cubital Tunnel Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT05395715 (accessed on 23 November 2022).
- Keane, G.C.; Marsh, E.B.; Hunter, D.A.; Schellhardt, L.; Walker, E.R.; Wood, M.D. Lidocaine Nerve Block Diminishes the Effects of Therapeutic Electrical Stimulation to Enhance Nerve Regeneration in Rats. Hand 2022. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Amirjani, N.; Edwards, D.C.; Chan, K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2010, 223, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.N.; Olson, J.L.; Morhart, M.J.; Chan, K.M. Electrical stimulation enhances sensory recovery: A randomized controlled trial. Ann. Neurol. 2015, 77, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Barber, B.; Seikaly, H.; Ming Chan, K.; Beaudry, R.; Rychlik, S.; Olson, J.; Curran, M.; Dziegielewski, P.; Biron, V.; Harris, J.; et al. Intraoperative Brief Electrical Stimulation of the Spinal Accessory Nerve (BEST SPIN) for prevention of shoulder dysfunction after oncologic neck dissection: A double-blinded, randomized controlled trial. J. Otolaryngol. Head Neck. Surg. 2018, 47, 7. [Google Scholar] [CrossRef] [PubMed]
- Power, H.A.; Morhart, M.J.; Olson, J.L.; Chan, K.M. Postsurgical Electrical Stimulation Enhances Recovery Following Surgery for Severe Cubital Tunnel Syndrome: A Double-Blind Randomized Controlled Trial. Neurosurgery 2020, 86, 769–777. [Google Scholar] [CrossRef]
- Jain, A.; Dunlop, R.; Hems, T.; Tang, J.B. Outcomes of surgical repair of a single digital nerve in adults. J. Hand Surg. Eur. Vol. 2019, 44, 560–565. [Google Scholar] [CrossRef]
- Khedr, E.M.; Fawi, G.; Allah Abbas, M.A.; El-Fetoh, N.A.; Zaki, A.F.; Gamea, A. Prevalence of Common Types of Compression Neuropathies in Qena Governorate/Egypt: A Population-Based Survey. Neuroepidemiology 2016, 46, 253–260. [Google Scholar] [CrossRef]
- Chan, M. Electrical Stimulation to Enhance Peripheral Nerve Regeneration. Available online: https://ClinicalTrials.gov/show/NCT02403661 (accessed on 7 September 2022).
- Davidge, K.; Zucker, R.; Borschel, G. Electrical Stimulation to Improve Recovery after Peripheral Nerve Injury. Available online: https://ClinicalTrials.gov/show/NCT03996525 (accessed on 7 September 2022).
- Moore, A.M. Promoting Healing of Nerves through Electrical Stimulation (PHONES). Available online: https://ClinicalTrials.gov/show/NCT04662320 (accessed on 7 September 2022).
- Checkpoint Surgical Receives FDA Breakthrough Designation for Nerve Regeneration Device. Available online: https://checkpointsurgical.com/news/checkpoint-surgical-receives-fda-breakthrough-designation-for-nerve-regeneration-device/ (accessed on 10 September 2022).
- Coroneos, C. Feasibility Study of a Temporary Peripheral Nerve Stimulator. Available online: https://ClinicalTrials.gov/show/NCT04732936 (accessed on 7 September 2022).
- Koo, J.; MacEwan, M.R.; Kang, S.K.; Won, S.M.; Stephen, M.; Gamble, P.; Xie, Z.; Yan, Y.; Chen, Y.Y.; Shin, J.; et al. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 2018, 24, 1830–1836. [Google Scholar] [CrossRef]
- Wang, L.; Lu, C.; Yang, S.; Sun, P.; Wang, Y.; Guan, Y.; Liu, S.; Cheng, D.; Meng, H.; Wang, Q.; et al. A fully biodegradable and self-electrified device for neuroregenerative medicine. Sci. Adv. 2020, 6, eabc6686. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juckett, L.; Saffari, T.M.; Ormseth, B.; Senger, J.-L.; Moore, A.M. The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury. Biomolecules 2022, 12, 1856. https://doi.org/10.3390/biom12121856
Juckett L, Saffari TM, Ormseth B, Senger J-L, Moore AM. The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury. Biomolecules. 2022; 12(12):1856. https://doi.org/10.3390/biom12121856
Chicago/Turabian StyleJuckett, Luke, Tiam Mana Saffari, Benjamin Ormseth, Jenna-Lynn Senger, and Amy M. Moore. 2022. "The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury" Biomolecules 12, no. 12: 1856. https://doi.org/10.3390/biom12121856
APA StyleJuckett, L., Saffari, T. M., Ormseth, B., Senger, J.-L., & Moore, A. M. (2022). The Effect of Electrical Stimulation on Nerve Regeneration Following Peripheral Nerve Injury. Biomolecules, 12(12), 1856. https://doi.org/10.3390/biom12121856







