Systems Biology Analysis of Temporal Dynamics That Govern Endothelial Response to Cyclic Stretch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Endothelial Cell Culture
2.2. Cyclic Stretch
2.3. RNA Sequencing
2.4. Pathway Analysis
2.5. Immunofluorescence and Western Blotting
2.6. Quantitative PCR
2.7. Atomic Force Microscopy
3. Results
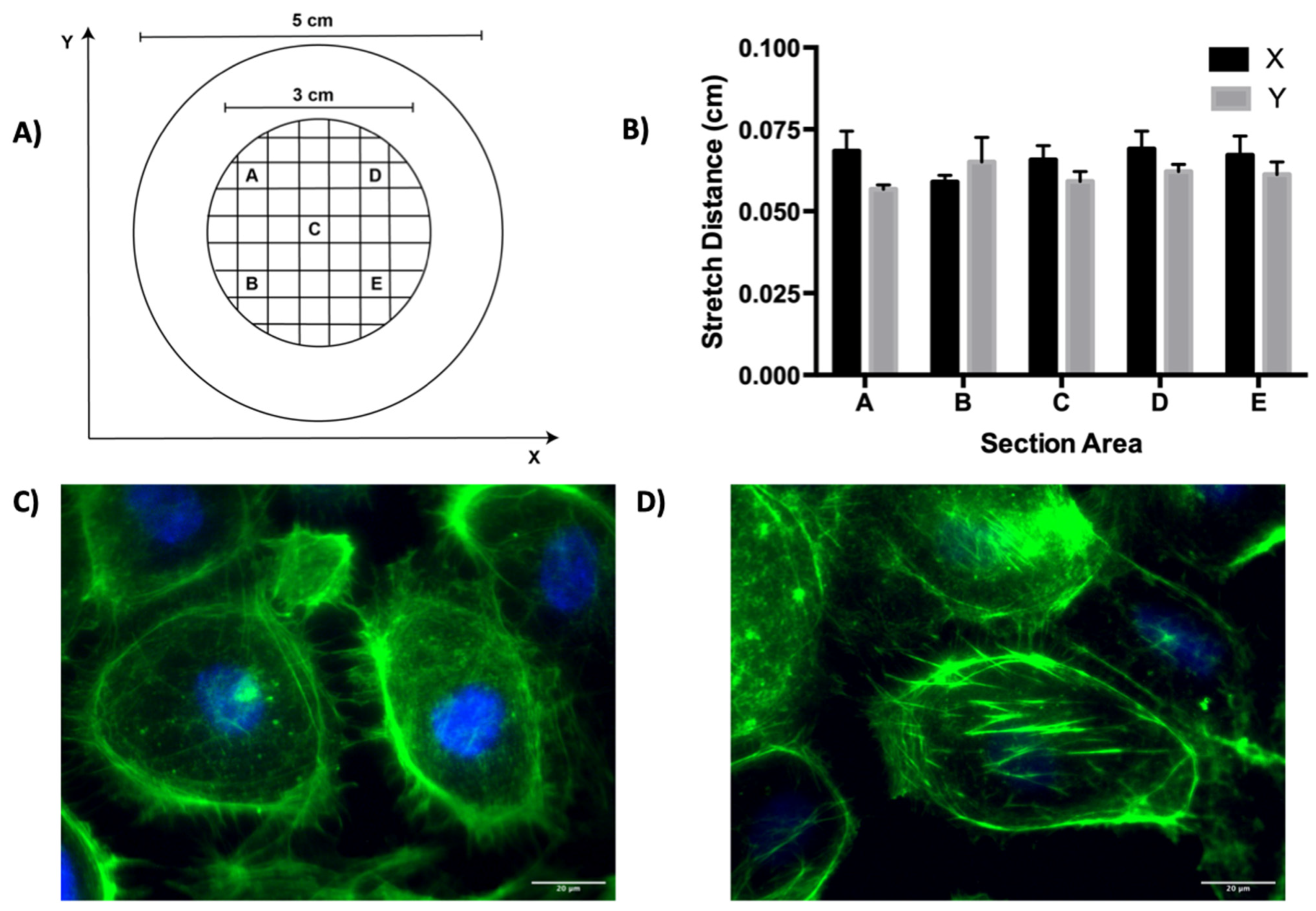
3.1. Cyclic Stretch Validation
3.2. Cell Cycle Regulation
3.3. Inflammatory Response
3.4. Fatty Acid Metabolism
3.5. mTOR Signaling
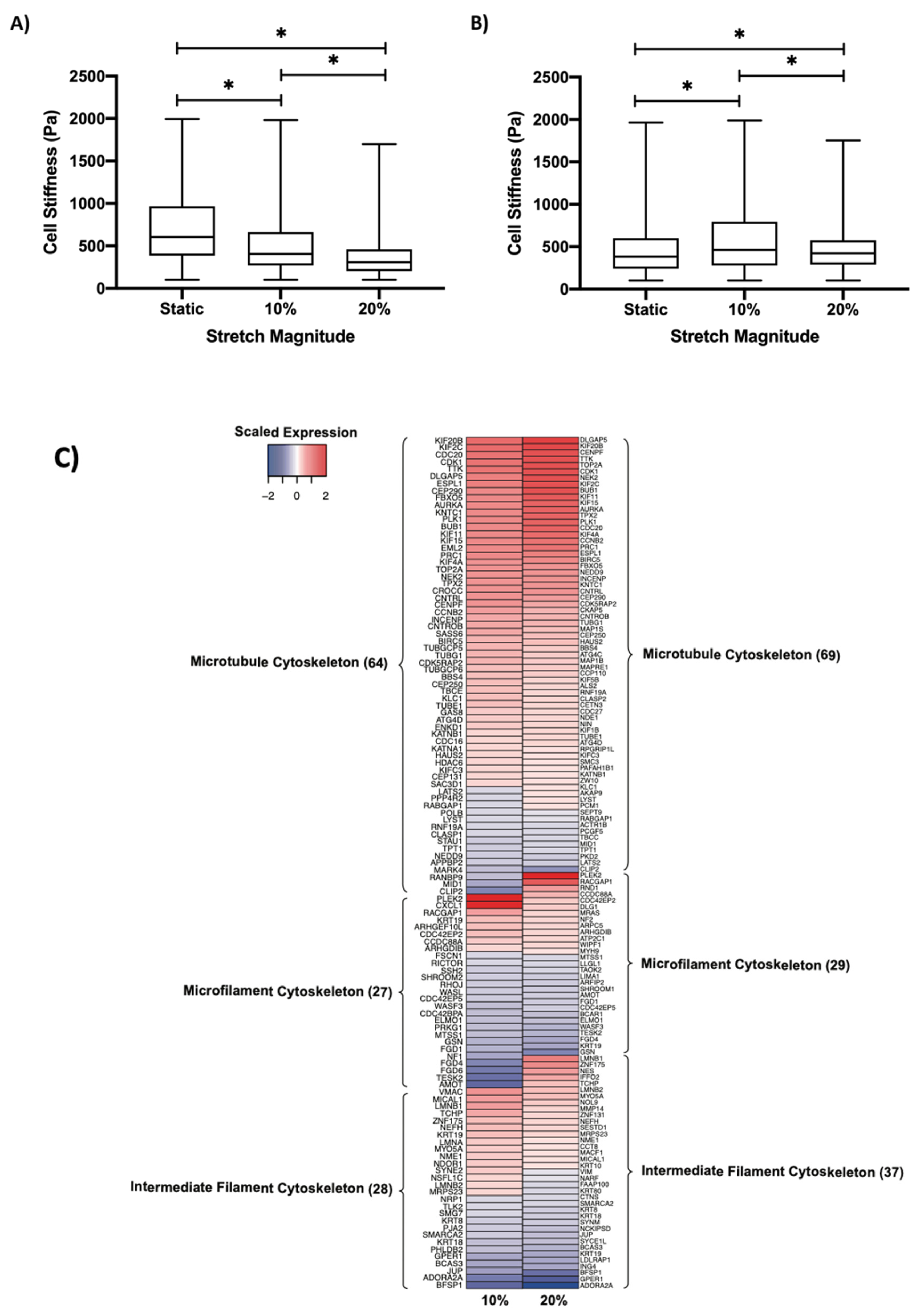
3.6. Regulation of Cell Stiffness by Cyclic Stretch
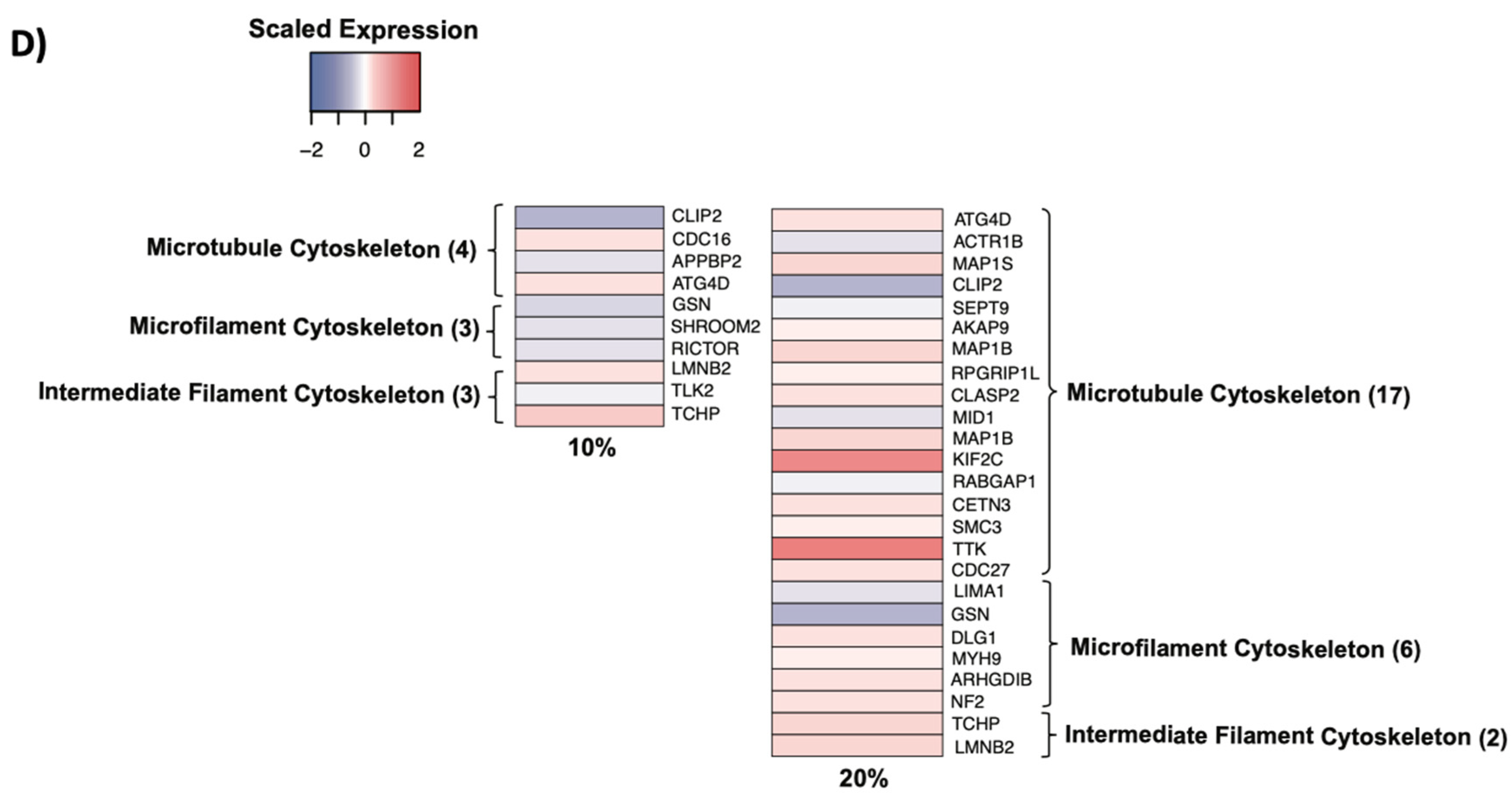
3.7. Cytoskeletal Remodeling
3.8. Shear Stress Comparison
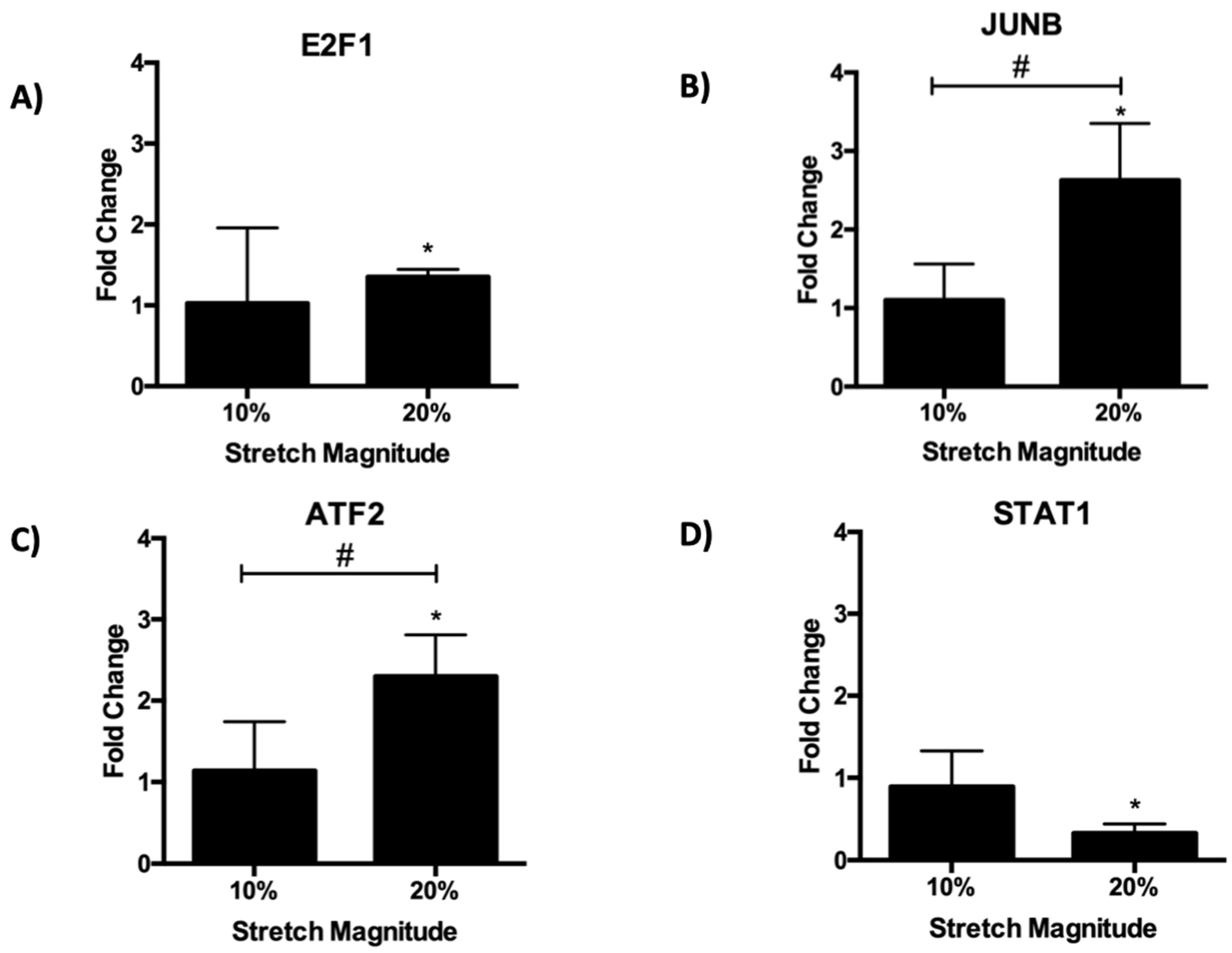
3.9. qPCR Expression Validation
4. Discussion
4.1. Endothelial Response to Cyclic Stretch
4.2. Effect of Cyclic Stretch on the Endothelial Cytoskeleton
4.3. Comparison of Endothelial Response to Cyclic Stretch and Shear Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charbonier, F.; Zamani, M.; Huang, N. Endothelial Cell Mechanotransduction in the Dynamic Vascular Environment. Adv. Biosyst. 2018, 3, 1800252. [Google Scholar] [CrossRef] [PubMed]
- Alberio, L.; Dale, G.L. Platelet-collagen interactions: Membrane receptors and intracellular signalling pathways. Eur. J. Clin. Investig. 1999, 29, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ward, E.; Siegel, R.; Jemal, A. Temporal Trends in Mortality in the United States, 1969–2013. JAMA 2015, 314, 1731. [Google Scholar] [CrossRef] [Green Version]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.; Yun, S.; Schwartz, M. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 2016, 126, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.; Orekhov, A.; Bobryshev, Y. Effects of shear stress on endothelial cells: Go with the flow. Acta Physiol. 2016, 219, 382–408. [Google Scholar] [CrossRef] [PubMed]
- Chiu, J.; Chien, S. Effects of Disturbed Flow on Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimbrone, M.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Ajami, N.; Gupta, S.; Maurya, M.; Nguyen, P.; Li, J.; Shyy, J.; Chen, Z.; Chien, S.; Subramaniam, S. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proc. Natl. Acad. Sci. USA 2017, 114, 10990–10995. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Friedman, M. Adaptive response of vascular endothelial cells to an acute increase in shear stress magnitude. Am. J. Physiol.-Heart Circ. Physiol. 2012, 302, H983–H991. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Chien, S. Shear Stress-Initiated Signaling and Its Regulation of Endothelial Function. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef]
- Figueroa, D.; Kemeny, S.; Clyne, A. Glycated Collagen Decreased Endothelial Cell Fibronectin Alignment in Response to Cyclic Stretch Via Interruption of Actin Alignment. J. Biomech. Eng. 2014, 136, 101010. [Google Scholar] [CrossRef] [PubMed]
- Adapala, R.; Talasila, P.; Bratz, I.; Zhang, D.; Suzuki, M.; Meszaros, J.; Thodeti, C. PKCα mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H757–H765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsumi, A.; Naoe, T.; Matsushita, T.; Kaibuchi, K.; Schwartz, M. Integrin Activation and Matrix Binding Mediate Cellular Responses to Mechanical Stretch. J. Biol. Chem. 2005, 280, 16546–16549. [Google Scholar] [CrossRef] [Green Version]
- Osawa, M.; Masuda, M.; Kusano, K.; Fujiwara, K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction. J. Cell Biol. 2002, 158, 773–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thodeti, C.; Matthews, B.; Ravi, A.; Mammoto, A.; Ghosh, K.; Bracha, A.; Ingber, D. TRPV4 Channels Mediate Cyclic Strain–Induced Endothelial Cell Reorientation Through Integrin-to-Integrin Signaling. Circ. Res. 2009, 104, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaunas, R.; Nguyen, P.; Usami, S.; Chien, S. From the Cover: Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Natl. Acad. Sci. USA 2005, 102, 15895–15900. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, J.; Pike, D.; Gibson, C.; Kubota, A.; Shiu, Y. Differential effects of cyclic stretch on bFGF- and VEGF-induced sprouting angiogenesis. Biotechnol. Prog. 2014, 30, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Pearlstein, D.; Mathieu, C.; Schumacker, P. Mitochondrial requirement for endothelial responses to cyclic strain: Implications for mechanotransduction. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L486–L496. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Hu, Z.; Han, X.; Jiang, B.; Zhang, R.; Zhang, X.; Lu, Y.; Geng, C.; Li, W.; He, Y.; et al. Hypertensive stretch regulates endothelial exocytosis of Weibel-Palade bodies through VEGF receptor 2 signaling pathways. Cell Res. 2013, 23, 820–834. [Google Scholar] [CrossRef] [Green Version]
- Contreras, A.; Dormond, O.; Edelbauer, M.; Calzadilla, K.; Hoerning, A.; Pal, S.; Briscoe, D. mTOR—Understanding the Clinical Effects. Transplant. Proc. 2008, 40, S9–S12. [Google Scholar] [CrossRef]
- Wang, J.; Goldschmidt-Clermont, P.; Wille, J.; Yin, F. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 2001, 34, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Balestrini, J.; Udelsman, B.; Zhou, K.; Zhao, L.; Ferruzzi, J.; Starcher, B.C.; Levene, M.J.; Humphrey, J.D.; Niklason, L.E. Biaxial Stretch Improves Elastic Fiber Maturation, Collagen Arrangement and Mechanical Properties in Engineered Arteries. Tissue Eng. Part C Methods 2016, 22, 524–533. [Google Scholar] [CrossRef]
- Ursekar, C.; Teo, S.; Hirata, H.; Harada, I.; Chiam, K.; Sawada, Y. Design and Construction of an Equibiaxial Cell Stretching System That Is Improved for Biochemical Analysis. PLoS ONE 2014, 9, e90665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwar, M.; Shalhoub, J.; Lim, C.; Gohel, M.; Davies, A. The Effect of Pressure- Induced Mechanical Stretch on Vascular Wall Differential Gene Expression. J. Vasc. Res. 2012, 49, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, Physiological, and Pathological Perspectives, Problems and Promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Seandel, M.; Butler, J.; Kobayashi, H.; Hooper, A.; White, I.; Zhang, F.; Vertes, E.L.; Kobayashi, M.; Zhang, Y.; Shmelkov, S.V.; et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc. Natl. Acad. Sci. USA 2008, 105, 19288–19293. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.; McCarthy, D.; Smyth, G. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Tamayo, P.; Mootha, V.; Mukherjee, S.; Ebert, B.; Gillette, M.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montojo, J.; Zuberi, K.; Rodriguez, H.; Kazi, F.; Wright, G.; Donaldson, S.; Morris, Q.; Bader, G.D. GeneMANIA Cytoscape plugin: Fast gene function predictions on the desktop. Bioinformatics 2010, 26, 2927–2928. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Poulos, M.; Palikuqi, B.; Badwe, C.; Lis, R.; Kunar, B.; Ding, B.-S.; Rabbany, S.Y.; Shido, K.; Butler, J.M.; et al. Endothelial jagged-2 sustains hematopoietic stem and progenitor reconstitution after myelosuppression. J. Clin. Investig. 2017, 127, 4242–4256. [Google Scholar] [CrossRef]
- Tondon, A.; Kaunas, R. The Direction of Stretch-Induced Cell and Stress Fiber Orientation Depends on Collagen Matrix Stress. PLoS ONE 2014, 9, e89592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Bao, H.; Wang, K.; Zhang, P.; Yao, Q.; Chen, X.; Huang, K.; Qi, Y.; Jiang, Z. Secreted miR- 27a Induced by Cyclic Stretch Modulates the Proliferation of Endothelial Cells in Hypertension via GRK6. Sci. Rep. 2017, 7, srep41058. [Google Scholar] [CrossRef]
- Polak-Iwaniuk, A.; Harasim-Symbor, E.; Gołaszewska, K.; Chabowski, A. How Hypertension Affects Heart Metabolism. Front. Physiol. 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamada, M.; Nishio, I.; Baba, A.; Fukuda, K.; Takeda, J.; Ura, M.; Hano, T.; Kuchii, M.; Masuyama, Y. Enhanced DNA synthesis of cultured vascular smooth muscle cells from spontaneously hypertensive rats Difference of response to growth factor, intracellular free calcium concentration and DNA synthesizing cell cycle. Atherosclerosis 1990, 81, 191–198. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nagino, M.; Komatsu, S.; Naruse, K.; Nimura, Y.; Nakanishi, M.; Sokabe, M. Stretch-induced IL-6 secretion from endothelial cells requires NF-κB activation. Biochem. Biophys. Res. Commun. 2003, 308, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Kopitar-Jerala, N. The Role of Interferons in Inflammation and Inflammasome Activation. Front. Immunol. 2017, 8, 873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazon, A.; Goldrath, A.; Nizet, V.; Johnson, R. HIF Transcription Factors, Inflammation and Immunity. Immunity 2014, 41, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Rauch, I.; Müller, M.; Decker, T. The regulation of inflammation by interferons and their STATs. JAK-STAT 2013, 2, e23820. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Lu, M.; Lin, T.; Chen, Z.; Chen, G.; Wang, W.; Marin, T.; Shentu, T.-P.; Wen, L.; Gongol, B.; et al. Sterol Regulatory Element Binding Protein 2 Activation of NLRP3 Inflammasome in Endothelium Mediates Hemodynamic-Induced Atherosclerosis Susceptibility. Circulation 2013, 128, 632–642. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Reilly, M. Anti-Inflammatory Effects of High-Density Lipoprotein Through Activating Transcription Factor 3. Arterioscler. Thromb. Vasc. Biol. 2014, 34, e11–e12. [Google Scholar] [CrossRef]
- Talati, M.; Hemnes, A. Fatty Acid Metabolism in Pulmonary Arterial Hypertension: Role in Right Ventricular Dysfunction and Hypertrophy. Pulm. Circ. 2015, 5, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. New Insights into the Role of mTOR Signaling in the Cardiovascular System. Circ. Res. 2018, 122, 489–505. [Google Scholar] [CrossRef]
- Liu, L.; Luo, Y.; Chen, L.; Shen, T.; Xu, B.; Chen, W.; Zhou, H.; Han, X.; Huang, S. Rapamycin Inhibits Cytoskeleton Reorganization and Cell Motility by Suppressing RhoA Expression and Activity. J. Biol. Chem. 2010, 285, 38362–38373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.; Tempel, D.; van Haperen, R.; van der Baan, A.; Grosveld, F.; Daemen, M.; Krams, R.; De Crom, R. Atherosclerotic Lesion Size and Vulnerability Are Determined by Patterns of Fluid Shear Stress. Circulation 2006, 113, 2744–2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, H.; Sasai, K.; Kloc, M.; Brinkley, B.; Sen, S. Aurora kinase-A regulates kinetochore/chromatin associated microtubule assembly in human cells. Cell Cycle 2008, 7, 2691–2704. [Google Scholar] [CrossRef] [Green Version]
- Thubrikar, M.; Robicsek, F. Pressure-induced arterial wall stress and atherosclerosis. Ann. Thorac. Surg. 1995, 59, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Tanner, F.; Greutert, H.; Barandier, C.; Frischknecht, K.; Lüscher, T.F. Different Cell Cycle Regulation of Vascular Smooth Muscle in Genetic Hypertension. Hypertension 2003, 42, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, Q.; Zhang, Y.; Liu, W.; Gu, B.; Narumi, T.; Siu, K.L.; Youn, J.Y.; Liu, P.; Yang, X.; et al. Novel Treatment of Hypertension by Specifically Targeting E2F for Restoration of Endothelial Dihydrofolate Reductase and eNOS Function under Oxidative Stress. Hypertension 2019, 73, 179–189. [Google Scholar] [CrossRef]
- Bakker, W.; Weijts, B.; Westendorp, B.; de Bruin, A. HIF proteins connect the RB- E2F factors to angiogenesis. Transcription 2013, 4, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Chaussepied, M.; Ginsberg, D. Transcriptional Regulation of AKT Activation by E2F. Mol. Cell 2004, 16, 831–837. [Google Scholar] [CrossRef]
- De Miguel, C.; Rudemiller, N.; Abais, J.; Mattson, D. Inflammation and Hypertension: New Understandings and Potential Therapeutic Targets. Curr. Hypertens. Rep. 2014, 17, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miserez, A.; Muller, P.; Barella, L.; Barella, S.; Staehelin, H.; Leitersdorf, E.; Kark, J.D.; Friedlander, Y. Sterol-regulatory element-binding protein (SREBP)-2 contributes to polygenic hypercholesterolaemia. Atherosclerosis 2002, 164, 15–26. [Google Scholar] [CrossRef]
- De Nardo, D.; Labzin, L.; Kono, H.; Seki, R.; Schmidt, S.; Beyer, M.; Xu, D.; Zimmer, S.; Lahrmann, C.; Schildberg, F.A.; et al. High- density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2013, 15, 152–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronova, A.; Fahey, T., III; Zarnegar, R. Management of hypertension in primary aldosteronism. World J. Cardiol. 2014, 6, 227. [Google Scholar] [CrossRef]
- Su, H.; Gu, Y.; Li, F.; Wang, Q.; Huang, B.; Jin, X.; Ning, G.; Sun, F. The PI3K/AKT/mTOR Signaling Pathway Is Overactivated in Primary Aldosteronism. PLoS ONE 2013, 8, e62399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinh, B.; Hepprich, M.; Betz, M.; Burkard, T.; Cavelti-Weder, C.; Seelig, E.; Meienberg, F.; Kratschmar, D.V.; Beuschlein, F.; Reincke, M.; et al. Treatment of Primary Aldosteronism with mTORC1 Inhibitors. J. Clin. Endocrinol. Metab. 2019, 104, 4703–4714. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Rodier, G.; Kirsh, O.; Houles, T.; Delpech, H.; Seyran, B.; Gayte, L.; Casas, F.; Pessemesse, L.; Heuillet, M.; et al. E4F1 controls a transcriptional program essential for pyruvate dehydrogenase activity. Proc. Natl. Acad. Sci. USA 2016, 113, 10998–11003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westin, M.; Hunt, M.; Alexson, S. The Identification of a Succinyl-CoA Thioesterase Suggests a Novel Pathway for Succinate Production in Peroxisomes. J. Biol. Chem. 2005, 280, 38125–38132. [Google Scholar] [CrossRef] [Green Version]
- Atwal, G.; Sun, Y.; Chen, J.; Komarova, Y. Role of Microtubule Cytoskeleton as a Novel Target Against Hypoxia Induced Pulmonary Hypertension. J. Am. Coll. Cardiol. 2017, 69, 1882. [Google Scholar] [CrossRef]
- Barilli, A.; Visigalli, R.; Sala, R.; Gazzola, G.; Parolari, A.; Tremoli, E.; Bonomini, S.; Simon, A.; Closs, E.; Dall’Asta, V.; et al. In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovasc. Res. 2008, 78, 563–571. [Google Scholar] [CrossRef]
- Gavara, N.; Chadwick, R. Relationship between cell stiffness and stress fiber amount, assessed by simultaneous atomic force microscopy and live-cell fluorescence imaging. Biomech. Model. Mechanobiol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Gawlak, G.; O’Donnell, J.; Birukova, A.; Birukov, K. Activation of Vascular Endothelial Growth Factor (VEGF) Receptor 2 Mediates Endothelial Permeability Caused by Cyclic Stretch. J. Biol. Chem. 2016, 291, 10032–10045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, S.; He, M.; Tong, T.; Wu, H.; Liu, X.; Lau, C.; Wang, J.-H.; Warden, C.; Wu, X.; Signoretti, S.; et al. RNA-seq Reveals Aurora Kinase–Driven mTOR Pathway Activation in Patients with Sarcomatoid Metastatic Renal Cell Carcinoma. Mol. Cancer Res. 2014, 13, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Valle, F.; Badura, M.; Braunstein, S.; Narasimhan, M.; Schneider, R. Mitotic Raptor Promotes mTORC1 Activity, G2/M Cell Cycle Progression, and Internal Ribosome Entry Site-Mediated mRNA Translation. Mol. Cell. Biol. 2010, 30, 3151–3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourest-Lieuvin, A.; Peris, L.; Gache, V.; Garcia-Saez, I.; Juillan-Binard, C.; Lantez, V.; Job, D. Microtubule Regulation in Mitosis: Tubulin Phosphorylation by the Cyclin-dependent Kinase Cdk1. Mol. Biol. Cell 2006, 17, 1041–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacic, J.; Dimmeler, S.; Harvey, R.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A. Endothelial to Mesenchymal Transition in Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef]
- Meza, D.; Musmacker, B.; Steadman, E.; Stransky, T.; Rubenstein, D.; Yin, W. Endothelial Cell Biomechanical Responses are Dependent on Both Fluid Shear Stress and Tensile Strain. Cell. Mol. Bioeng. 2019, 12, 311–325. [Google Scholar] [CrossRef]
- Huan, T.; Esko, T.; Peters, M.; Pilling, L.; Schramm, K.; Schurmann, C.; Chen, B.H.; Liu, C.; Joehanes, R.; Johnson, A.D.; et al. A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension. PLoS Genet. 2015, 11, e1005035. [Google Scholar] [CrossRef]

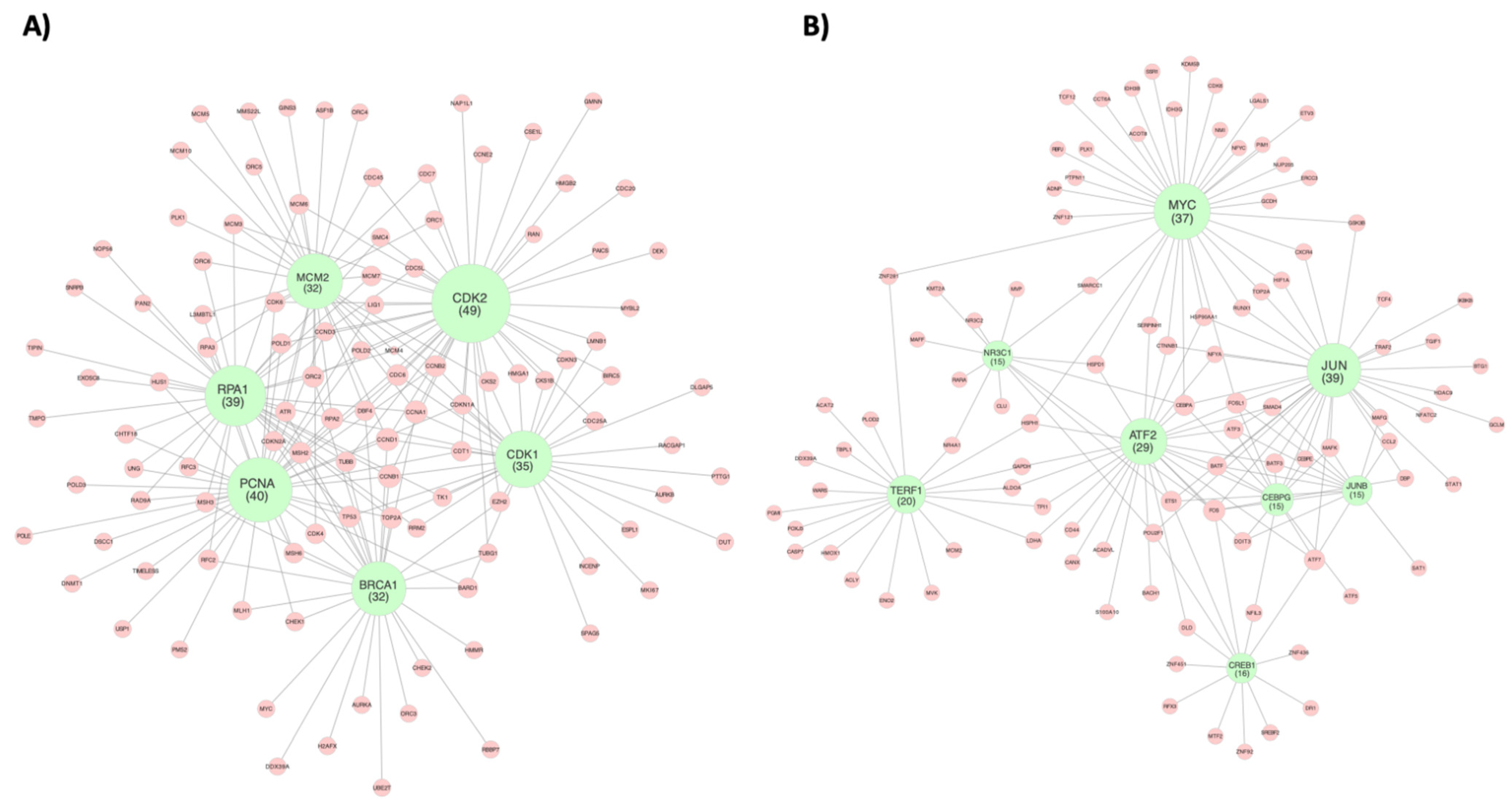



| CDK2 (49) | PCNA (40) | RPA1 (39) | CDK1 (35) | BRCA1 (32) | MCM2 (32) |
|---|---|---|---|---|---|
| PA2G4 | USP1 | TIPIN | TIPIN | ORC2 | RPA3 |
| PAICS | TIMELESS | EXOSC8 | EXOSC8 | MSH6 | ORC2 |
| MYBL2 | POLD3 | SMC4 | SMC4 | CCNB1 | PLK1 |
| HMGA1 | POLD2 | TMPO | TMPO | H2AFX | CDK6 |
| CCNE2 | POLD1 | CHTF18 | CHTF18 | HMMR | ORC5 |
| HMGB2 | RPA1 | UNG | UNG | CDK4 | L3MBTL1 |
| CDKN3 | RPA2 | NOP56 | NOP56 | TUBB | MCM7 |
| CKS2 | RPA3 | RFC2 | RFC2 | EZH2 | ORC4 |
| CCNA1 | RFC2 | RFC3 | RFC3 | MYC | MCM5 |
| CDC25A | RFC3 | SNRPB | SNRPB | UBE2T | GINS3 |
| GMNN | CHTF18 | RPA3 | RPA3 | MLH1 | CDC6 |
| CKS1B | HUS1 | PAN2 | PAN2 | DBF4 | CDK4 |
| CDC20 | MLH1 | RPA2 | RPA2 | MSH3 | ORC6 |
| CDC7 | DSCC1 | POLD1 | POLD1 | CHEK2 | MCM3 |
| DBF4 | POLE | PCNA | PCNA | RBBP7 | DBF4 |
| MCM4 | UNG | TOP2A | TOP2A | DDX39A | CCNB2 |
| MCM6 | PMS2 | MSH3 | MSH3 | ORC3 | CDC45 |
| CDC45 | TOP2A | HUS1 | HUS1 | BARD1 | MCM4 |
| ORC2 | MSH3 | RAD9A | RAD9A | CDKN2A | CKS2 |
| MCM7 | MSH6 | MSH6 | MSH6 | TOP2A | CDKN2A |
| ORC1 | RAD9A | MSH2 | MSH2 | RPA1 | RPA1 |
| CDC6 | DNMT1 | ATR | ATR | TP53 | ASF1B |
| CDT1 | MSH2 | BRCA1 | BRCA1 | TUBG1 | MCM10 |
| CDKN1A | CHEK1 | TK1 | TK1 | MSH2 | RPA2 |
| CCNB1 | BARD1 | CDK1 | CDK1 | ATR | ATR |
| CDK1 | CDK6 | CCNB1 | CCNB1 | CHEK1 | ORC1 |
| TUBG1 | LIG1 | CCNA1 | CCNA1 | CDK1 | CCNA1 |
| LMNB1 | CDKN2A | ORC6 | ORC6 | CCNA1 | CDC7 |
| CCNB2 | TP53 | MCM4 | MCM4 | CCND1 | MMS22L |
| CCND1 | CDK4 | MCM6 | MCM6 | AURKA | CCND1 |
| MCM2 | CCND3 | MCM7 | MCM7 | CDK2 | MCM6 |
| MCM3 | CCND1 | MCM2 | MCM2 | RFC2 | CDK2 |
| POLD1 POLD2 | CDK2 CDC6 | MCM3 ORC2 | MCM3 ORC2 | ||
| RPA1 | CDKN1A | CDK2 | CDK2 | ||
| PCNA | CCNB2 | CDC5L | CDC5L | ||
| BRCA1 | CDK1 | TP53 | TP53 | ||
| TUBB CDC5L | CCNB1 CDT1 | TUBB RRM2 | TUBB RRM2 | ||
| BIRC5 | L3MBTL1 | ||||
| CCND3 | |||||
| CDK6 | |||||
| MSH2 SMC4 |
| JUN (39) | MYC (37) | TERF1 (20) | ATF2 (29) | NR3C1 (15) | JUNB (15) | CREB1 (16) | CEBPG (15) |
|---|---|---|---|---|---|---|---|
| JUN | WARS | HSP90AA1 | NR4A1 | SAT1 | SREBF2 | CEBPE | |
| DDIT3 | HIF1A | PGMI | FOSL1 | MVP | DDIT3 | ZNF451 | DDIT3 |
| TRAF2 CCL2 | HSP90AA1 | FOXJ3 | CEBPA | CLU | ATF3 | JUN | ATF2 |
| FOSL1 | MCM2 | BATF3 | SMARCC1 | CCL2 | MYC | ATF7 | |
| ATF3 TGIF1 JUNB | CEBPA | HMOX1 | JUN | CREB1 | SMAD4 | NR3C1 | BATF |
| CTNNB1 | TBPL1 | ATF7 | POU2F1 | JUN | ATF7 | BATF3 | |
| GSK3B | DDX39A | FOS | CEBPA | BATF | DR1 | CEBPA | |
| DBP BATF MAFG | RUNX1 | ENO2 | CTNNB1 | FOS | MAFG | DLD | ATF3 |
| SERPINH1 | ACAT2 | CEBPG | JUN | BATF3 | ETS1 | ATF5 | |
| TCF12 | MVK | ATF3 | MAFF | MAFK | FOS | MAFK | |
| BATF3 MAFK HIF1A | KDM5B | ZNF281 | ETS1 | ETS1 | ATF2 | NFIL3 | DBP |
| HSPD1 | LDHA | DDIT3 | KMT2A | ATF7 | POU2F1 | FOS | |
| NMI | GAPDH | BACH1 | RARA | FOSL1 | MTF2 | FOSL1 | |
| HDAC9 CEBPE NFATC2 | CREB1 | TPI1 | BATF | NR3C2 | FOS | RFX3 | NFIL3 |
| CDK6 | HSPH1 | JUNB | HSPD1 | ETS1 | ZNF436 | JUN | |
| SMAD4 FOS ETS1 | CXCR4 | NR4A1 | SERPINH1 | ZNF92 | |||
| TOP2A | PLOD2 | SMAD4 | |||||
| NFYC | CASP7 | ||||||
| ATF7 ATF2 FOSL1 | SMARCC1 | ACLY | CANX | ||||
| HSPH1 | ALDOA | CD44 | |||||
| PIM1 | NFYA | ||||||
| ZNF281 | |||||||
| CCT6A | HSPH1 | ||||||
| HSP90AA1 CEBPA CEBPG | ETV3 | DLD | |||||
| IDH3B | HSPD1 | ||||||
| CTNNB1 GSK3B RUNX1 | GCDH | ALDOA | |||||
| ACOT8 | TPI1 | ||||||
| IKBKB NR3C1 STAT1 | ZNF121 | LDHA | |||||
| NUP205 | GAPDH | ||||||
| POU2F1 CXCR4 TOP2A | PTPN11 | S100A10 | |||||
| IDH3G | ACADVL | ||||||
| SSR1 | |||||||
| LGALS1 | |||||||
| NFYA MYC CREB1 | ERCC3 | ||||||
| PLK1 | |||||||
| GCLM TCF4 BTG1 | ADNP | ||||||
| RBPJ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, M.W.; Chow, N.; Checco, A.; Kunar, B.; Redmond, D.; Rafii, S.; Rabbany, S.Y. Systems Biology Analysis of Temporal Dynamics That Govern Endothelial Response to Cyclic Stretch. Biomolecules 2022, 12, 1837. https://doi.org/10.3390/biom12121837
Lai MW, Chow N, Checco A, Kunar B, Redmond D, Rafii S, Rabbany SY. Systems Biology Analysis of Temporal Dynamics That Govern Endothelial Response to Cyclic Stretch. Biomolecules. 2022; 12(12):1837. https://doi.org/10.3390/biom12121837
Chicago/Turabian StyleLai, Michael W., Nathan Chow, Antonio Checco, Balvir Kunar, David Redmond, Shahin Rafii, and Sina Y. Rabbany. 2022. "Systems Biology Analysis of Temporal Dynamics That Govern Endothelial Response to Cyclic Stretch" Biomolecules 12, no. 12: 1837. https://doi.org/10.3390/biom12121837
APA StyleLai, M. W., Chow, N., Checco, A., Kunar, B., Redmond, D., Rafii, S., & Rabbany, S. Y. (2022). Systems Biology Analysis of Temporal Dynamics That Govern Endothelial Response to Cyclic Stretch. Biomolecules, 12(12), 1837. https://doi.org/10.3390/biom12121837







