The Potential Roles of Post-Translational Modifications of PPARγ in Treating Diabetes
Abstract
1. Introduction
2. Domain Structure of PPARγ
3. PPARγ Agonists and T2DM
4. Post-Translational Modifications (PTMs) of PPARγ in Diabetes
4.1. Phosphorylation
4.2. Acetylation
4.3. Ubiquitination
4.4. SUMOylation
4.5. O-GlcNAcylation
4.6. S-Nitrosylation
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chan, J.C.N.; Lim, L.-L.; Wareham, N.J.; Shaw, J.E.; Orchard, T.J.; Zhang, P.; Lau, E.S.H.; Eliasson, B.; Kong, A.P.S.; Ezzati, M.; et al. The Lancet Commission on diabetes: Using data to transform diabetes care and patient lives. Lancet 2021, 396, 2019–2082. [Google Scholar] [CrossRef]
- Diabetes around the World in 2021; IDF Diabetes Atlas 10th Edition and other resources; International Diabetes Federation: Brussels, Belgium, 2022.
- AAmerican Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 89, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Barnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections from 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gu, Z. The Role of Peroxisome Proliferator-Activated Receptors in Kidney Diseases. Front. Pharm. 2022, 13, 832732. [Google Scholar] [CrossRef] [PubMed]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; de Bosscher, K. Molecular Actions of PPARalpha in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- RSoccio, E.; Chen, E.R.; Lazar, M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014, 20, 573–591. [Google Scholar]
- Ha, J.; Choi, D.W.; Kim, K.Y.; Nam, C.M.; Kim, E. Pioglitazone use associated with reduced risk of the first attack of ischemic stroke in patients with newly onset type 2 diabetes: A nationwide nested case-control study. Cardiovasc. Diabetol 2021, 20, 152. [Google Scholar] [CrossRef]
- Opazo-Rios, L.; Mas, S.; Marin-Royo, G.; Mezzano, S.; Gomez-Guerrero, C.; Moreno, J.A.; Egido, J. Lipotoxicity and Diabetic Nephropathy: Novel Mechanistic Insights and Therapeutic Opportunities. Int. J. Mol. Sci. 2020, 21, 2632. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Zheng, W.; Zhang, L.; Gui, L.; Liang, G.; Zhang, Y.; Hu, L.; Li, X.; Zhong, Y.; et al. Action mechanism of hypoglycemic principle 9-(R)-HODE isolated from cortex lycii based on a metabolomics approach. Front. Pharmacol. 2022, 13, 1011608. [Google Scholar] [CrossRef]
- Yin, L.; Wang, L.; Shi, Z.; Ji, X.; Liu, L. The Role of Peroxisome Proliferator-Activated Receptor Gamma and Atherosclerosis: Post-translational Modification and Selective Modulators. Front. Physiol. 2022, 13, 826811. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Liang, Z.; Zhao, K.; Luo, C. Drug design targeting active posttranslational modification protein isoforms. Med. Res. Rev. 2021, 41, 1701–1750. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Park, J.; Choi, J.H. Revisiting PPARgamma as a target for the treatment of metabolic disorders. BMB Rep. 2014, 47, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Recarte, D.; Barroso, E.; Guma, A.; Pizarro-Delgado, J.; Pena, L.; Ruart, M.; Palomer, X.; Wahli, W.; Vazquez-Carrera, M. GDF15 mediates the metabolic effects of PPARbeta/delta by activating AMPK. Cell Rep. 2021, 36, 109501. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Liu, S.N.; Liu, Q.; Li, L.Y.; Huan, Y.; Sun, S.J.; Shen, Z.F. Long-term fenofibrate treatment impaired glucose-stimulated insulin secretion and up-regulated pancreatic NF-kappa B and iNOS expression in monosodium glutamate-induced obese rats: Is that a latent disadvantage? J. Transl. Med. 2011, 9, 176. [Google Scholar] [CrossRef]
- Beamer, B.A.; Negri, C.; Yen, C.J.; Gavrilova, O.; Rumberger, J.M.; Durcan, M.J.; Yarnall, D.P.; Hawkins, A.L.; Griffin, C.A.; Burns, D.K.; et al. Chromosomal localization and partial genomic structure of the human peroxisome proliferator activated receptor-gamma (hPPAR gamma) gene. Biochem. Biophys. Res. Commun. 1997, 233, 756–759. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, C.; Korenberg, J.R.; Chen, X.N.; Noya, D.; Rao, M.S.; Reddy, J.K. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: Alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc. Natl. Acad. Sci. USA 1995, 92, 7921–7925. [Google Scholar] [CrossRef]
- Wyles, C.C.; Paradise, C.R.; Houdek, M.T.; Slager, S.L.; Terzic, A.; Behfar, A.; van Wijnen, A.J.; Sierra, R.J. CORR® ORS Richard A. Brand Award: Disruption in Peroxisome Proliferator-Activated Receptor-γ (PPARG) Increases Osteonecrosis Risk Through Genetic Variance and Pharmacologic Modulation. Clin. Orthop. Relat. Res. 2019, 477, 1800–1812. [Google Scholar] [CrossRef]
- Kokeny, G.; Calvier, L.; Hansmann, G. PPARgamma and TGFbeta-Major Regulators of Metabolism, Inflammation, and Fibrosis in the Lungs and Kidneys. Int. J. Mol. Sci. 2021, 22, 12955. [Google Scholar] [CrossRef]
- Gavin, K.M.; Sullivan, T.M.; Maltzahn, J.K.; Jackman, M.R.; Libby, A.E.; MacLean, P.S.; Kohrt, W.M.; Majka, S.M.; Klemm, D.J. Hematopoietic Stem Cell-Derived Adipocytes Modulate Adipose Tissue Cellularity, Leptin Production and Insulin Responsiveness in Female Mice. Front. Endocrinol 2022, 13, 844877. [Google Scholar] [CrossRef] [PubMed]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef]
- Hu, W.; Jiang, C.; Kim, M.; Xiao, Y.; Richter, H.J.; Guan, D.; Zhu, K.; Krusen, B.M.; Roberts, A.N.; Miller, J.; et al. Isoform-specific functions of PPARgamma in gene regulation and metabolism. Genes Dev. 2022, 36, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Jang, D.M.; Park, S.C.; An, S.; Shin, J.; Han, B.W.; Noh, M. Cyclin-Dependent Kinase 5 Inhibitor Butyrolactone I Elicits a Partial Agonist Activity of Peroxisome Proliferator-Activated Receptor gamma. Biomolecules 2020, 10, 275. [Google Scholar] [CrossRef]
- Nyland, J.E.; Raja-Khan, N.T.; Bettermann, K.; Haouzi, P.A.; Leslie, D.L.; Kraschnewski, J.L.; Parent, L.J.; Grigson, P.S. Diabetes, Drug Treatment, and Mortality in COVID-19: A Multinational Retrospective Cohort Study. Diabetes 2021, 70, 2903–2916. [Google Scholar] [CrossRef] [PubMed]
- Gilardi, F.; Winkler, C.; Quignodon, L.; Diserens, J.G.; Toffoli, B.; Schiffrin, M.; Sardella, C.; Preitner, F.; Desvergne, B. Systemic PPARgamma deletion in mice provokes lipoatrophy, organomegaly, severe type 2 diabetes and metabolic inflexibility. Metabolism 2019, 95, 8–20. [Google Scholar] [CrossRef]
- Tang, W.; Zeve, D.; Suh, J.M.; Bosnakovski, D.; Kyba, M.; Hammer, R.E.; Tallquist, M.D.; Graff, J.M. White fat progenitor cells reside in the adipose vasculature. Science 2008, 322, 583–586. [Google Scholar] [CrossRef]
- Imai, T.; Takakuwa, R.; Marchand, S.; Dentz, E.; Bornert, J.M.; Messaddeq, N.; Wendling, O.; Mark, M.; Desvergne, B.; Wahli, W.; et al. Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc. Natl. Acad. Sci. USA 2004, 101, 4543–4547. [Google Scholar] [CrossRef]
- Guo, Z.; Ali, Q.; Abaidullah, M.; Gao, Z.; Diao, X.; Liu, B.; Wang, Z.; Zhu, X.; Cui, Y.; Li, D.; et al. High fat diet-induced hyperlipidemia and tissue steatosis in rabbits through modulating ileal microbiota. Appl. Microbiol. Biotechnol. 2022, 106, 7187–7207. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Abbas, A.; Blandon, J.; Rude, J.; Elfar, A.; Mukherjee, D. PPAR- gamma agonist in treatment of diabetes: Cardiovascular safety considerations. Cardiovasc. Hematol. Agents Med. Chem. 2012, 10, 124–134. [Google Scholar] [CrossRef]
- de Sa, P.M.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. Genes Dev. 2017, 7, 635–674. [Google Scholar]
- Brunmeir, R.; Xu, F. Functional Regulation of PPARs through Post-Translational Modifications. Int. J. Mol. Sci. 2018, 19, 1738. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Banks, A.S.; Estall, J.L.; Kajimura, S.; Boström, P.; Laznik, D.; Ruas, J.L.; Chalmers, M.J.; Kamenecka, T.M.; Blüher, M.; et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 2010, 466, 451–456. [Google Scholar] [CrossRef]
- Kim, S.; Rabhi, N.; Blum, B.C.; Hekman, R.; Wynne, K.; Emili, A.; Farmer, S.; Schlezinger, J.J. Triphenyl phosphate is a selective PPARgamma modulator that does not induce brite adipogenesis in vitro and in vivo. Arch. Toxicol. 2020, 94, 3087–3103. [Google Scholar] [CrossRef]
- Hall, J.A.; Ramachandran, D.; Roh, H.C.; Di Spirito, J.R.; Belchior, T.; Zushin, P.H.; Palmer, C.; Hong, S.; Mina, A.I.; Liu, B.; et al. Obesity-Linked PPARgamma S273 Phosphorylation Promotes Insulin Resistance through Growth Differentiation Factor 3. Cell Metab. 2020, 32, 665–675.e6. [Google Scholar] [CrossRef]
- Shao, M.; Hepler, C.; Zhang, Q.; Shan, B.; Vishvanath, L.; Henry, G.H.; Zhao, S.; An, Y.A.; Wu, Y.; Strand, D.W.; et al. Pathologic HIF1alpha signaling drives adipose progenitor dysfunction in obesity. Cell Stem. Cell 2021, 28, 685–701.e7. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, E.S.; Koh, M.; Lee, S.J.; Lim, D.; Yang, Y.R.; Jang, H.J.; Seo, K.A.; Min, S.H.; Lee, I.H.; et al. A novel non-agonist peroxisome proliferator-activated receptor gamma (PPARgamma) ligand UHC1 blocks PPARgamma phosphorylation by cyclin-dependent kinase 5 (CDK5) and improves insulin sensitivity. J. Biol. Chem. 2014, 289, 26618–26629. [Google Scholar] [CrossRef]
- Camp, H.S.; Tafuri, S.R.; Leff, T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology 1999, 140, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R.; et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef]
- Kraakman, M.J.; Liu, Q.; Postigo-Fernandez, J.; Ji, R.; Kon, N.; Larrea, D.; Namwanje, M.; Fan, L.; Chan, M.; Area-Gomez, E.; et al. PPARgamma deacetylation dissociates thiazolidinedione’s metabolic benefits from its adverse effects. J. Clin. Investig. 2018, 128, 2600–2612. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, L.; Chan, M.; Kraakman, M.J.; Yang, J.; Fan, Y.; Aaron, N.; Wan, Q.; Carrillo-Sepulveda, M.A.; Tall, A.R.; et al. PPARgamma Deacetylation Confers the Antiatherogenic Effect and Improves Endothelial Function in Diabetes Treatment. Diabetes 2020, 69, 1793–1803. [Google Scholar] [CrossRef]
- Norris, K.L.; Lee, J.Y.; Yao, T.P. Acetylation goes global: The emergence of acetylation biology. Sci. Signal. 2009, 2, pe76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spange, S.; Wagner, T.; Heinzel, T.; Kramer, O.H. Acetylation of non-histone proteins modulates cellular signalling at multiple levels. Int. J. Biochem. Cell Biol. 2009, 41, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Kwak, S.H.; Koo, Y.D.; Cho, Y.K.; Lee, H.M.; Jung, H.S.; Cho, Y.M.; Park, Y.J.; Chung, S.S.; Park, K.S. F-box only protein 9 is an E3 ubiquitin ligase of PPARgamma. Exp. Mol. Med. 2016, 48, e234. [Google Scholar] [CrossRef]
- Lee, K.W.; Cho, J.G.; Kim, C.M.; Kang, A.Y.; Kim, M.; Ahn, B.Y.; Chung, S.S.; Lim, K.H.; Baek, K.H.; Sung, J.H.; et al. Herpesvirus-associated ubiquitin-specific protease (HAUSP) modulates peroxisome proliferator-activated receptor gamma (PPARgamma) stability through its deubiquitinating activity. J. Biol. Chem. 2013, 288, 32886–32896. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, K.W.; Lee, E.W.; Jang, W.S.; Seo, J.; Shin, S.; Hwang, K.A.; Song, J. Suppression of PPARgamma through MKRN1-mediated ubiquitination and degradation prevents adipocyte differentiation. Cell Death Differ. 2014, 21, 594–603. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, R.; Lama, R.; Wang, X.; Floyd, Z.E.; Park, E.A.; Liao, F.F. Ubiquitin Ligase NEDD4 Regulates PPARgamma Stability and Adipocyte Differentiation in 3T3-L1 Cells. Sci. Rep. 2016, 6, 38550. [Google Scholar] [CrossRef]
- He, J.; Quintana, M.T.; Sullivan, J.; Parry, T.; Grevengoed, T.; Schisler, J.C.; Hill, J.A.; Yates, C.C.; Mapanga, R.F.; Essop, M.F.; et al. MuRF2 regulates PPARgamma1 activity to protect against diabetic cardiomyopathy and enhance weight gain induced by a high fat diet. Cardiovasc. Diabetol. 2015, 14, 97. [Google Scholar] [CrossRef]
- Peng, J.; Li, Y.; Wang, X.; Deng, S.; Holland, J.; Yates, E.; Chen, J.; Gu, H.; Essandoh, K.; Mu, X.; et al. An Hsp20-FBXO4 Axis Regulates Adipocyte Function through Modulating PPARgamma Ubiquitination. Cell Rep. 2018, 23, 3607–3620. [Google Scholar] [CrossRef]
- Flotho, A.; Melchior, F. Sumoylation: A regulatory protein modification in health and disease. Annu. Rev. Biochem. 2013, 82, 357–385. [Google Scholar] [CrossRef]
- Rott, R.; Szargel, R.; Shani, V.; Hamza, H.; Savyon, M.; Elghani, F.A.; Bandopadhyay, R.; Engelender, S. SUMOylation and ubiquitination reciprocally regulate alpha-synuclein degradation and pathological aggregation. Proc. Natl. Acad. Sci. USA 2017, 114, 13176–13181. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Ma, C.; Zhou, Y.; Wang, M.; Zeng, G.; Huang, Q. Negative regulation of eNOS-NO signaling by over-SUMOylation of PPARgamma contributes to insulin resistance and dysfunction of vascular endothelium in rats. Vasc. Pharmacol. 2019, 123, 106597. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, T.; Mei, W.; Li, D.; Cai, R.; Zuo, Y.; Cheng, J. Small ubiquitin-like modifier (SUMO) protein-specific protease 1 de-SUMOylates Sharp-1 protein and controls adipocyte differentiation. J. Biol. Chem. 2014, 289, 22358–22364. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cao, Y.; Chen, Y.; Wang, J.; Fan, Q.; Huang, X.; Wang, Y.; Wang, T.; Wang, X.; Ma, J.; et al. Senp2 regulates adipose lipid storage by de-SUMOylation of Setdb1. J. Mol. Cell Biol. 2018, 10, 258–266. [Google Scholar] [CrossRef]
- Dutchak, P.A.; Katafuchi, T.; Bookout, A.L.; Choi, J.H.; Yu, R.T.; Mangelsdorf, D.J.; Kliewer, S. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell 2012, 148, 556–567. [Google Scholar] [CrossRef]
- Haschemi, A.; Chin, B.Y.; Jeitler, M.; Esterbauer, H.; Wagner, O.; Bilban, M.; Otterbein, L.E. Carbon monoxide induced PPARgamma SUMOylation and UCP2 block inflammatory gene expression in macrophages. PLoS ONE 2011, 6, e26376. [Google Scholar] [CrossRef]
- Pascual, G.; Fong, A.L.; Ogawa, S.; Gamliel, A.; Li, A.C.; Perissi, V.; Rose, D.W.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 2005, 437, 759–763. [Google Scholar] [CrossRef]
- Ji, S.; Park, S.Y.; Roth, J.; Kim, H.S.; Cho, J.W. O-GlcNAc modification of PPARgamma reduces its transcriptional activity. Biochem. Biophys. Res. Commun. 2012, 417, 1158–1163. [Google Scholar] [CrossRef]
- Chung, S.S.; Kim, J.H.; Park, H.S.; Choi, H.H.; Lee, K.W.; Cho, Y.M.; Lee, H.K.; Park, K.S. Activation of PPARgamma negatively regulates O-GlcNAcylation of Sp1. Biochem. Biophys. Res. Commun. 2008, 372, 713–718. [Google Scholar] [CrossRef]
- Liu, Q.; Gu, T.; Su, L.Y.; Jiao, L.; Qiao, X.; Xu, M.; Xie, T.; Yang, L.X.; Yu, D.; Xu, L.; et al. GSNOR facilitates antiviral innate immunity by restricting TBK1 cysteine S-nitrosation. Redox. Biol. 2021, 47, 102172. [Google Scholar] [CrossRef]
- Yin, R.; Fang, L.; Li, Y.; Xue, P.; Li, Y.; Guan, Y.; Chang, Y.; Chen, C.; Wang, N. Pro-inflammatory Macrophages suppress PPARgamma activity in Adipocytes via S-nitrosylation. Free. Radic. Biol. Med. 2015, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gomes, S.A.; Rangel, E.B.; Paulino, E.C.; Fonseca, T.L.; Li, J.; Teixeira, M.B.; Gouveia, C.H.; Bianco, A.C.; Kapiloff, M.S.; et al. S-nitrosoglutathione reductase-dependent PPARgamma denitrosylation participates in MSC-derived adipogenesis and osteogenesis. J. Clin. Investig. 2015, 125, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.A.; Heuvel, J.P.V. Modulation of PPAR activity via phosphorylation. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 952–960. [Google Scholar] [CrossRef]
- Yang, N.; Wang, Y.; Tian, Q.; Wang, Q.; Lu, Y.; Sun, L.; Wang, S.; Bei, Y.; Ji, J.; Zhou, H.; et al. Blockage of PPARgamma T166 phosphorylation enhances the inducibility of beige adipocytes and improves metabolic dysfunctions. Cell Death Differ. 2022, 1–13. [Google Scholar] [CrossRef]
- Minakhina, S.; De Oliveira, V.; Kim, S.Y.; Zheng, H.; Wondisford, F.E. Thyroid hormone receptor phosphorylation regulates acute fasting-induced suppression of the hypothalamic-pituitary-thyroid axis. Proc. Natl. Acad. Sci. USA 2021, 118, e2107943118. [Google Scholar] [CrossRef]
- Hu, E.; Kim, J.B.; Sarraf, P.; Spiegelman, B.M. Spiegelman, Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science 1996, 274, 2100–2103. [Google Scholar] [CrossRef]
- Montanari, R.; Capelli, D.; Yamamoto, K.; Awaishima, H.; Nishikata, K.; Barendregt, A.; Heck, A.J.R.; Loiodice, F.; Altieri, F.; Paiardini, A.; et al. Insights into PPARgamma Phosphorylation and Its Inhibition Mechanism. J. Med. Chem. 2020, 63, 4811–4823. [Google Scholar] [CrossRef]
- Choi, S.; Jung, J.E.; Yang, Y.R.; Kim, E.S.; Jang, H.J.; Kim, E.K.; Kim, I.S.; Lee, J.Y.; Kim, J.K.; Seo, J.K.; et al. Novel phosphorylation of PPARgamma ameliorates obesity-induced adipose tissue inflammation and improves insulin sensitivity. Cell. Signal. 2015, 27, 2488–2495. [Google Scholar] [CrossRef]
- Peralta, G.M.; Serra, E.; Alonso, V.L. Update on the Biological Relevance of Lysine Acetylation as a Novel Drug Target in Trypanosomatids. Curr. Med. Chem. 2022, 29, 3638–3659. [Google Scholar] [CrossRef]
- Williams, R.V.; Huang, C.; McDermott, C.; Ahmed, T.; Columbus, L.; Moremen, K.W.; Prestegard, J.H.; Amster, I.J. Site-to-site cross-talk in OST-B glycosylation of hCEACAM1-IgV. Proc. Natl. Acad. Sci. USA 2022, 119, e2202992119. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.S.; Geras-Raaka, E.; Gershengorn, M.C. Heritability of fat accumulation in white adipocytes. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E335–E344. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Luo, Z.Q. Post-translational regulation of ubiquitin signaling. J. Cell Biol. 2019, 218, 1776–1786. [Google Scholar] [CrossRef]
- Zheng, N.; Shabek, N. Ubiquitin Ligases: Structure, Function, and Regulation. Annu. Rev. Biochem. 2017, 86, 129–157. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, J.K.; Ko, K.Y.; Jin, Y.; Ham, M.; Kang, H.; Kim, I.Y. Degradation of selenoprotein S and selenoprotein K through PPARgamma-mediated ubiquitination is required for adipocyte differentiation. Cell Death Differ. 2019, 26, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Moreau, F.; Chadee, K. PPARgamma is an E3 ligase that induces the degradation of NFkappaB/p65. Nat. Commun. 2012, 3, 1300. [Google Scholar] [CrossRef]
- Li, P.; Song, Y.; Zan, W.; Qin, L.; Han, S.; Jiang, B.; Dou, H.; Shao, C.; Gong, Y. Lack of CUL4B in Adipocytes Promotes PPARgamma-Mediated Adipose Tissue Expansion and Insulin Sensitivity. Diabetes 2017, 66, 300–313. [Google Scholar] [CrossRef] [PubMed]
- MPotthoff, J.; Kliewer, S.A.; Mangelsdorf, D. Endocrine fibroblast growth factors 15/19 and 21: From feast to famine. Genes Dev. 2012, 26, 312–324. [Google Scholar] [CrossRef]
- Chang, H.M.; Yeh, E.T.H. SUMO: From Bench to Bedside. Physiol. Rev. 2020, 100, 1599–1619. [Google Scholar] [CrossRef]
- Diezko, R.; Suske, G. Ligand binding reduces SUMOylation of the peroxisome proliferator-activated receptor gamma (PPARgamma) activation function 1 (AF1) domain. PLoS ONE 2013, 8, e66947. [Google Scholar] [CrossRef]
- Katafuchi, T.; Holland, W.L.; Kollipara, R.K.; Kittler, R.; Mangelsdorf, D.J.; Kliewer, S.A. PPARgamma-K107 SUMOylation regulates insulin sensitivity but not adiposity in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12102–12111. [Google Scholar] [CrossRef]
- Leibler, C.; John, S.; Elsner, R.A.; Thomas, K.B.; Smita, S.; Joachim, S.; Levack, R.C.; Callahan, D.J.; Gordon, R.A.; Bastacky, S.; et al. Genetic dissection of TLR9 reveals complex regulatory and cryptic proinflammatory roles in mouse lupus. Nat. Immunol. 2022, 23, 1457–1469. [Google Scholar] [CrossRef]
- Peterson, S.B.; Hart, G.W. New insights: A role for O-GlcNAcylation in diabetic complications. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hart, G.W. Protein O-GlcNAcylation in diabetes and diabetic complications. Expert Rev. Proteom. 2013, 10, 365–380. [Google Scholar] [CrossRef]
- Saha, A.; Bello, D.; Fernandez-Tejada, A. Advances in chemical probing of protein O-GlcNAc glycosylation: Structural role and molecular mechanisms. Chem. Soc. Rev. 2021, 50, 10451–10485. [Google Scholar] [CrossRef]
- Bond, M.R.; Hanover, J.A. O-GlcNAc cycling: A link between metabolism and chronic disease. Annu. Rev. Nutr. 2013, 33, 205–229. [Google Scholar] [CrossRef]
- Gall, T.; Nagy, P.; Garai, D.; Potor, L.; Balla, G.J.; Balla, G.; Balla, J. Overview on hydrogen sulfide-mediated suppression of vascular calcification and hemoglobin/heme-mediated vascular damage in atherosclerosis. Redox. Biol. 2022, 57, 102504. [Google Scholar] [CrossRef]
- Philipson, L.H. Harnessing heterogeneity in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 79–80. [Google Scholar] [CrossRef]
- Chung, W.K.; Erion, K.; Florez, J.C.; Hattersley, A.T.; Hivert, M.F.; Lee, C.G.; McCarthy, M.I.; Nolan, J.J.; Norris, J.M.; Pearson, E.R.; et al. Precision Medicine in Diabetes: A Consensus Report From the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 1617–1635. [Google Scholar] [CrossRef]
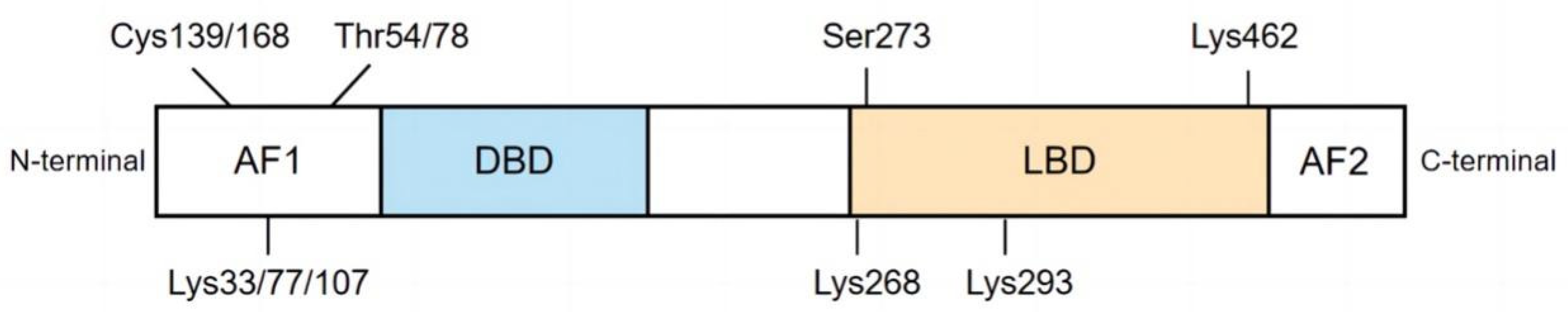

| Modification Type | Site | Enzyme | Domain | Function in Diabetes | Refs. |
|---|---|---|---|---|---|
| Phosphorylation | Ser273, Ser112, Tyr78, | MAPK | N-A/B | ↓insulin sensitivity, obesity ↑insulin resistance and proinflammatory genes in adipose tissue | [35,36,37,38,39,40] |
| Acetylation | Lys268, Lys293 | p300, CBP, HDACs | C-LBD | ↓insulin sensitivity, energy expenditure, differentiation of preadipocytes into adipocytes ↑fat accumulation, bone loss, edema, and congestive heart failure | [41,42,43,44,45] |
| Ubiquitination | Lys107, Lys462 | FBXO9 | N-AF1, C-LBD | ↓glucose tolerance, adipocyte insulin sensitivity, stability of endogenous and exogenous PPARγ, ↑insulin resistance, blood glucose and triglyceride levels, glucose and fatty acid uptake diabetic cardiomyopathy | [46,47,48,49,50,51] |
| SUMOylation | Lys107, Lys33, Lys77 | E1, E2, E3 | N-AF1, C-LBD | ↑insulin resistance, lipogenesis, obese, adipose tissue accumulation, inflammation, vascular endothelial dysfunction, | [52,53,54,55,56,57,58,59] |
| O-GlcNAcylation | Thr54 | OGT, OGA | N-AF1 | ↑PPARγ transcriptional activity, adipocyte differentiation, hyperglycemia-induced transcriptional activation of multiple genes | [60,61] |
| S-nitrosylation | Cys168, Cys139 | GSNOR | N-AF1 | ↓adiponectin expression, transcriptional activity, protein stability, insulin sensitivity, adipogenic differentiation of BMSCs ↑adipocyte generation | [62,63,64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Zhang, W.; Yin, L.; Shi, Z.; Luan, J.; Chen, L.; Liu, L. The Potential Roles of Post-Translational Modifications of PPARγ in Treating Diabetes. Biomolecules 2022, 12, 1832. https://doi.org/10.3390/biom12121832
Ji X, Zhang W, Yin L, Shi Z, Luan J, Chen L, Liu L. The Potential Roles of Post-Translational Modifications of PPARγ in Treating Diabetes. Biomolecules. 2022; 12(12):1832. https://doi.org/10.3390/biom12121832
Chicago/Turabian StyleJi, Xiaohui, Wenqian Zhang, Liqin Yin, Zunhan Shi, Jinwen Luan, Linshan Chen, and Longhua Liu. 2022. "The Potential Roles of Post-Translational Modifications of PPARγ in Treating Diabetes" Biomolecules 12, no. 12: 1832. https://doi.org/10.3390/biom12121832
APA StyleJi, X., Zhang, W., Yin, L., Shi, Z., Luan, J., Chen, L., & Liu, L. (2022). The Potential Roles of Post-Translational Modifications of PPARγ in Treating Diabetes. Biomolecules, 12(12), 1832. https://doi.org/10.3390/biom12121832







