Weil’s Disease—Immunopathogenesis, Multiple Organ Failure, and Potential Role of Gut Microbiota
Abstract
:1. Introduction
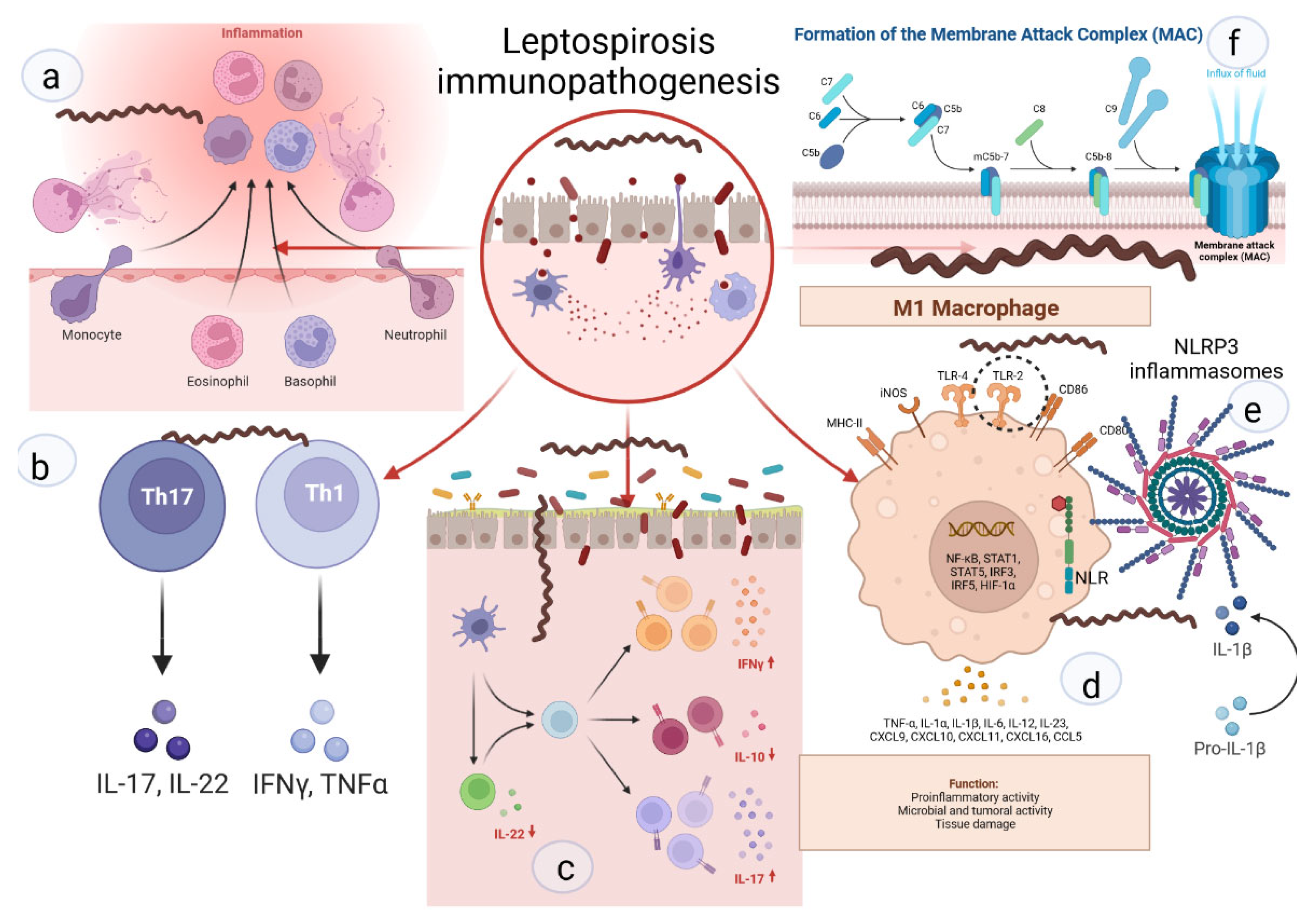
2. Leptospira Immunity
3. Cytokines, Chemokines, Genetics, and Their Role in Severe Leptospirosis
4. Immune Evasion in Leptospirosis
4.1. Neutrophils Are Poor Phagocytes
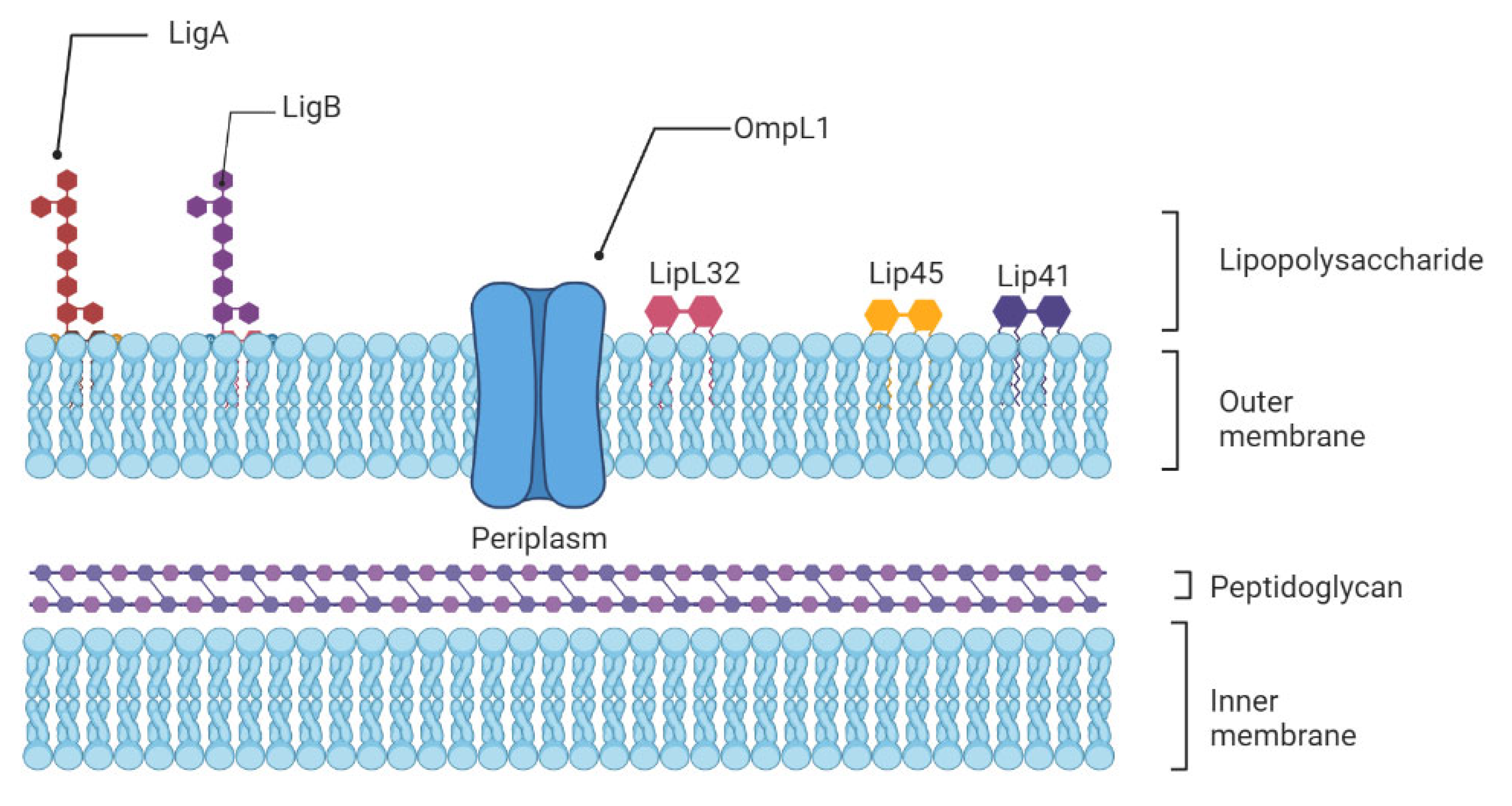
4.2. Leptospiral Complement Evasion
5. Immunological Aspects of Damage to the Kidneys, Liver, and Pulmonary Bleeding as the Basis of Weil’s Syndrome
5.1. Immunological Aspects of Renal Damage
5.2. Pulmonary Involvement in Leptospirosis
5.3. Pathogenesis of Leptospirosis Liver Injury
5.4. Pathogenesis of Pancreatic Involvement in Leptospirosis
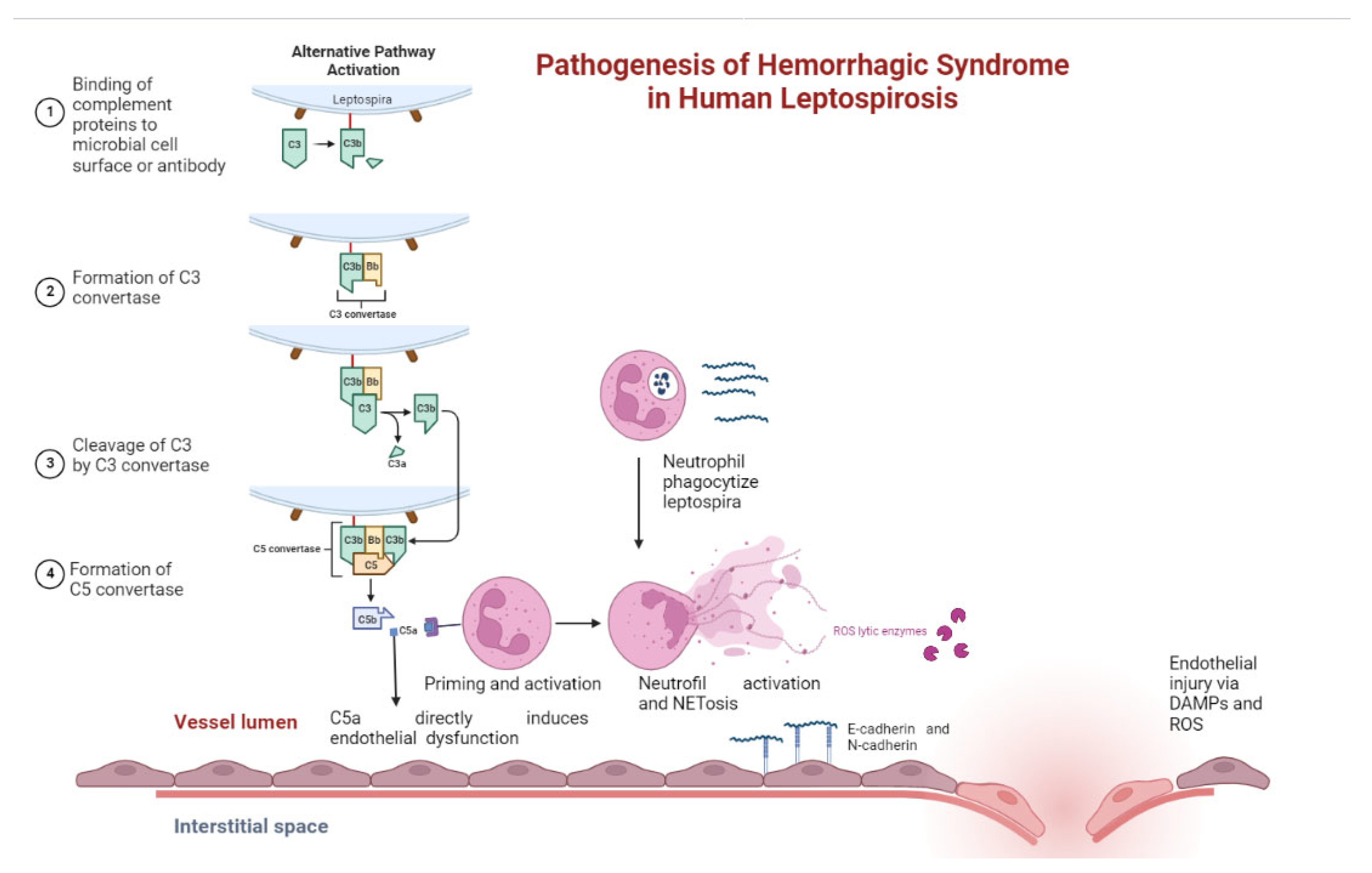
5.5. Pathogenesis of Bleeding in Leptospirosis
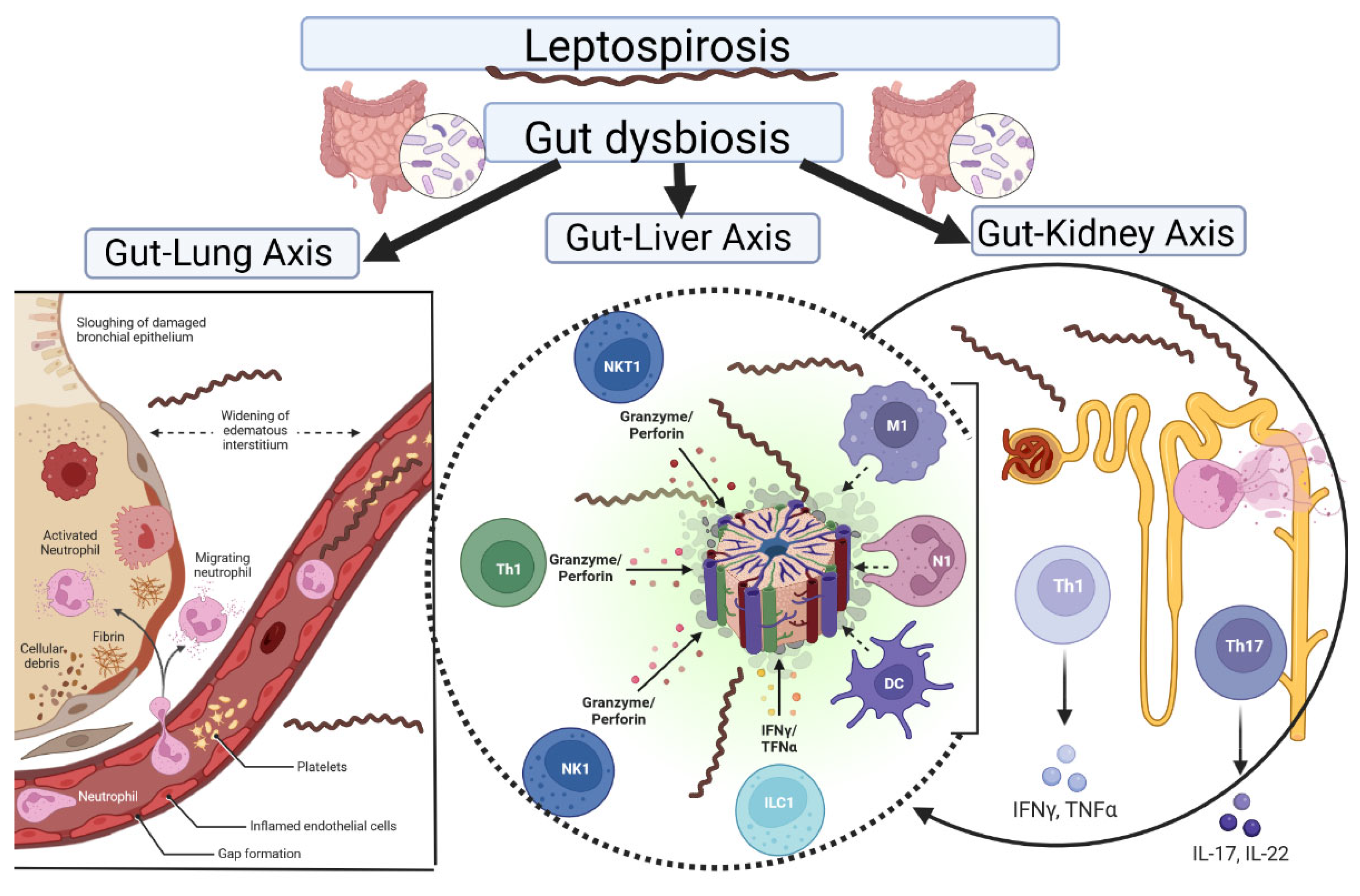
6. Gut Microbiota Involved in Leptospirosis
6.1. Gut–Kidney Axis
6.2. Gut–Liver Axis
6.3. Gut–Lung Axis
6.4. Gut Microbiota and Leptospiral Infections
7. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Govindan, P.; Pitchaikani, S.; Kandasamy, S.; Rajan, M.; Shakila, H.; Eed, E.M.; Elfasakhany, A.; Pugazhendhi, A. Biomacromolecules of chitosan—Bacopa saponin based LipL32 gene delivery system for leptospirosis therapy. Environ. Res. 2021, 202, 111699. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.I.; Goarant, C.; Picardeau, M. Leptospira: The dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat. Rev. Microbiol. 2009, 7, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Latchoumi, T.; Reddy, M.S.; Balamurugan, K. Applied machine learning predictive analytics to SQL injection attack detection and prevention. Eur. J. Mol. Clin. Med. 2020, 7, 2020. [Google Scholar]
- Abdullah, M.; Chaubey, K.K.; Namdev, R.; Sharma, R. Leptospira: A Review on Pathogenesis and Host Immune Response. Ann. Rom. Soc. Cell Biol. 2021, 25, 18686–18694. [Google Scholar]
- Haake, D.A.; Levett, P.N. Leptospirosis in Humans. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limothai, U.; Lumlertgul, N.; Sirivongrangson, P.; Kulvichit, W.; Tachaboon, S.; Dinhuzen, J.; Chaisuriyong, W.; Peerapornratana, S.; Chirathaworn, C.; Praditpornsilpa, K.; et al. The role of leptospiremia and specific immune response in severe leptospirosis. Sci. Rep. 2021, 11, 14630. [Google Scholar] [CrossRef] [PubMed]
- Petakh, P.; Isevych, V.; Griga, V.; Kamyshnyi, A. The risk factors of severe leptospirosis in the Transcarpathian region of Ukraine–search for “red flags”. Arch. Balk Med. Union 2022, 57, 231–237. [Google Scholar] [CrossRef]
- Jiménez, J.I.S.; Marroquin, J.L.H.; Richards, G.A.; Amin, P. Leptospirosis: Report from the task force on tropical diseases by the World Federation of Societies of Intensive and Critical Care Medicine. J. Crit. Care 2018, 43, 361–365. [Google Scholar] [CrossRef]
- Fraga, T.R.; Barbosa, A.S.; Isaac, L. Leptospirosis: Aspects of Innate Immunity, Immunopathogenesis and Immune Evasion From the Complement System. Scand. J. Immunol. 2011, 73, 408–419. [Google Scholar] [CrossRef]
- Reis, E.A.G.; Hagan, J.; Ribeiro, G.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Montgomery, R.; Shaw, A.C.; Ko, A.; Reis, M.G. Cytokine Response Signatures in Disease Progression and Development of Severe Clinical Outcomes for Leptospirosis. PLoS Negl. Trop. Dis. 2013, 7, e2457. [Google Scholar] [CrossRef] [Green Version]
- Mikulski, M.; Boisier, P.; Lacassin, F.; Soupé-Gilbert, M.-E.; Mauron, C.; Bruyère-Ostells, L.; Bonte, D.; Barguil, Y.; Gourinat, A.-C.; Matsui, M.; et al. Severity markers in severe leptospirosis: A cohort study. Eur. J. Clin. Microbiol. 2015, 34, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Kyriakidis, I.; Samara, P.; Papa, A. Serum TNF-α, sTNFR1, IL-6, IL-8 and IL-10 levels in Weil’s syndrome. Cytokine 2011, 54, 117–120. [Google Scholar] [CrossRef]
- Xie, X.; Liu, J.; Chen, X.; Zhang, S.; Tang, R.; Wu, X.; Zhang, W.; Cao, Y. Gut microbiota involved in leptospiral infections. ISME J. 2022, 16, 764–773. [Google Scholar] [CrossRef]
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut–lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2017, 43, 81–95. [Google Scholar] [CrossRef]
- Bruning, J.; Chapp, A.; Kaurala, G.A.; Wang, R.; Techtmann, S.; Chen, Q.-H. Gut Microbiota and Short Chain Fatty Acids: Influence on the Autonomic Nervous System. Neurosci. Bull. 2020, 36, 91–95. [Google Scholar] [CrossRef]
- Li, M.; Van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.; Debelius, J.; Brenner, D.A.; Karin, M.; Loomba, R.; Schnabl, B.; Knight, R. The gut–liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 397–411. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell. Microbiol. 2006, 124, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Miller, S.I.; Ernst, R.; Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [Google Scholar] [CrossRef]
- Werts, C.; Tapping, R.I.; Mathison, J.C.; Chuang, T.-H.; Kravchenko, V.V.; Girons, I.S.; Haake, D.A.; Godowski, P.J.; Hayashi, F.; Ozinsky, A.; et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2001, 2, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Que-Gewirth, N.L.; Ribeiro, A.A.; Kalb, S.R.; Cotter, R.J.; Bulach, D.M.; Adler, B.; Saint Girons, I.; Werts, C.; Raetz, C.R. A methylated phosphate group and four amide-linked acyl chains in Leptospira interrogans lipid A: The membrane anchor of an unusual lipopolysaccharide that activates TLR2. J. Biol. Chem. 2004, 279, 25420–25429. [Google Scholar] [CrossRef] [PubMed]
- Nahori, M.-A.; Fournié-Amazouz, E.; Que-Gewirth, N.S.; Balloy, V.; Chignard, M.; Raetz, C.R.H.; Girons, I.S.; Werts, C. Differential TLR Recognition of Leptospiral Lipid A and Lipopolysaccharide in Murine and Human Cells. J. Immunol. 2005, 175, 6022–6031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geijtenbeek, T.B.H.; Van Kooyk, Y. Pathogens target DC-SIGN to influence their fate DC-SIGN functions as a pathogen receptor with broad specificity. APMIS 2003, 111, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Lourido, S.; Zychlinsky, A. How do microbes evade neutrophil killing? Cell. Microbiol. 2006, 8, 1687–1696. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell Host Microbe 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef] [Green Version]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [Green Version]
- Meri, T.; Murgia, R.; Stefanel, P.; Meri, S.; Cinco, M. Regulation of complement activation at the C3-level by serum resistant leptospires. Microb. Pathog. 2005, 39, 139–147. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Abreu, P.A.E.; Vasconcellos, S.A.; Morais, Z.M.; Gonçales, A.P.; Silva, A.S.; Daha, M.R.; Isaac, L. Immune Evasion of Leptospira Species by Acquisition of Human Complement Regulator C4BP. Infect. Immun. 2009, 77, 1137–1143. [Google Scholar] [CrossRef]
- Gaudart, N.; Ekpo, P.; Pattanapanyasat, K.; Van Kooyk, Y.; Engering, A. Leptospira interrogans is recognized through DC-SIGN and induces maturation and cytokine production by human dendritic cells. FEMS Immunol. Med. Microbiol. 2008, 53, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.-Q.; Li, W.-M.; Lu, Z.-Q.; Sheng, Z.-Y.; Yao, Y.-M. The Growing Spectrum of Anti-Inflammatory Interleukins and Their Potential Roles in the Development of Sepsis. J. Interf. Cytokine Res. 2015, 35, 242–251. [Google Scholar] [CrossRef]
- Ben, A.; de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar]
- Estavoyer, J.M.; Racadot, E.; Couetdic, G.; Leroy, J.; Grosperrin, L. Tumor Necrosis Factor in Patients with Leptospirosis. Rev. Infect. Dis. 1991, 13, 1245. [Google Scholar] [CrossRef]
- Tajiki, M.H.; Salomão, R. Association of plasma levels of tumor necrosis factor α with severity of disease and mortality among patients with leptospirosis. Clin. Infect. Dis. 1996, 23, 1177–1178. [Google Scholar] [CrossRef] [Green Version]
- Chirathaworn, C.; Supputtamongkol, Y.; Lertmaharit, S.; Poovorawan, Y. Cytokine levels as biomarkers for leptospirosis patients. Cytokine 2016, 85, 80–82. [Google Scholar] [CrossRef]
- Wagenaar, J.F.P.; Gasem, M.H.; Goris, M.G.A.; Leeflang, M.; Hartskeerl, R.A.; van der Poll, T.; Veer, C.V.; van Gorp, E.C.M. Soluble ST2 Levels Are Associated with Bleeding in Patients with Severe Leptospirosis. PLoS Negl. Trop. Dis. 2009, 3, e453. [Google Scholar] [CrossRef] [Green Version]
- Opal, S.M.; DePalo, V.A. Anti-inflammatory cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef] [Green Version]
- Fialho, R.N.; Martins, L.; Pinheiro, J.P.; Bettencourt, B.F.; Couto, A.R.; Santos, M.R.; Peixoto, M.J.; Garrett, F.; Leal, J.; Tomás, A.M.; et al. Role of human leukocyte antigen, killer-cell immunoglobulin-like receptors, and cytokine gene polymorphisms in leptospirosis. Hum. Immunol. 2009, 70, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Roche, L.; Geroult, S.; Soupé-Gilbert, M.-E.; Monchy, D.; Huerre, M.; Goarant, C. Cytokine and Chemokine Expression in Kidneys during Chronic Leptospirosis in Reservoir and Susceptible Animal Models. PLoS ONE 2016, 11, e0156084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, F.D.C.; Ctenas, B.; da Silva, L.F.F.; Nicodemo, A.C.; Saldiva, P.H.N.; Dolhnikoff, M.; Mauad, T. Immune receptors and adhesion molecules in human pulmonary leptospirosis. Hum. Pathol. 2012, 43, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, J.F.; Goris, M.G.; Gasem, M.H.; Isbandrio, B.; Moalli, F.; Mantovani, A.; Boer, K.R.; Hartskeerl, R.A.; Garlanda, C.; van Gorp, E.C. Long pentraxin PTX3 is associated with mortality and disease severity in severe Leptospirosis. J. Infect. 2009, 58, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Chin, V.; Lee, T.; Lim, W.; Ywy, W.S.; Syafinaz, A.; Zamberi, S.; Maha, A. Leptospirosis in human: Biomarkers in host immune responses. Microbiol. Res. 2018, 207, 108–115. [Google Scholar]
- Zhang, C.; Xu, J.; Zhang, T.; Qiu, H.; Li, Z.; Zhang, E.; Li, S.; Chang, Y.-F.; Guo, X.; Jiang, X.; et al. Genetic characteristics of pathogenic Leptospira in wild small animals and livestock in Jiangxi Province, China, 2002–2015. PLoS Negl. Trop. Dis. 2019, 13, e0007513. [Google Scholar] [CrossRef]
- Esteves, L.; Bulhões, S.; Branco, C.C.; Mota, F.M.; Paiva, C.; Cabral, R.; Vieira, M.L.; Mota-Vieira, L. Human Leptospirosis: Seroreactivity and Genetic Susceptibility in the Population of São Miguel Island (Azores, Portugal). PLoS ONE 2014, 9, e108534. [Google Scholar] [CrossRef]
- Cédola, M.; Chiani, Y.; Pretre, G.; Alberdi, L.; Vanasco, B.; Gómez, R.M. Association of Toll-like receptor 2 Arg753Gln and Toll-like receptor 1 Ile602Ser single-nucleotide polymorphisms with leptospirosis in an argentine population. Acta Trop. 2015, 146, 73–80. [Google Scholar] [CrossRef]
- Charo, N.; Scharrig, E.; Ferrer, M.F.; Sanjuan, N.; Silva, E.A.C.; Schattner, M.; Gómez, R.M. Leptospira species promote a pro-inflammatory phenotype in human neutrophils. Cell. Microbiol. 2019, 21, e12990. [Google Scholar] [CrossRef]
- Murgia, R.; Garcia, R.; Cinco, M. Leptospires Are Killed In Vitro by Both Oxygen-Dependent and -Independent Reactions. Infect. Immun. 2002, 70, 7172–7175. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.L.; Teixeira, A.F.; Pidde, G.; Ching, A.T.C.; Tambourgi, D.V.; Nascimento, A.L.T.O.; Herwald, H. Leptospira interrogans outer membrane protein LipL21 is a potent inhibitor of neutrophil myeloperoxidase. Virulence 2018, 9, 414–425. [Google Scholar] [CrossRef] [Green Version]
- Santecchia, I.; Ferrer, M.F.; Vieira, M.L.; Gómez, R.M.; Werts, C. Phagocyte Escape of Leptospira: The Role of TLRs and NLRs. Front. Immunol. 2020, 11, 571816. [Google Scholar] [CrossRef]
- Scharrig, E.; Carestia, A.; Ferrer, M.F.; Cédola, M.; Pretre, G.; Drut, R.; Picardeau, M.; Schattner, M.; Gomez, R. Neutrophil Extracellular Traps are Involved in the Innate Immune Response to Infection with Leptospira. PLoS Negl. Trop. Dis. 2015, 9, e0003927. [Google Scholar] [CrossRef] [Green Version]
- Zipfel, P.F.; Würzner, R.; Skerka, C. Complement evasion of pathogens: Common strategies are shared by diverse organisms. Mol. Immunol. 2007, 44, 3850–3857. [Google Scholar] [CrossRef]
- Choy, H.A. Multiple Activities of LigB Potentiate Virulence of Leptospira interrogans: Inhibition of Alternative and Classical Pathways of Complement. PLoS ONE 2012, 7, e41566. [Google Scholar] [CrossRef] [Green Version]
- Souza, N.M.; Vieira, M.L.; Alves, I.J.; de Morais, Z.M.; Vasconcellos, S.A.; Nascimento, A.L. Lsa30, a novel adhesin of Leptospira interrogans binds human plasminogen and the complement regulator C4bp. Microb. Pathog. 2012, 53, 125–134. [Google Scholar] [CrossRef]
- Domingos, R.F.; Vieira, M.L.; Romero, E.C.; Gonçales, A.P.; de Morais, Z.M.; A Vasconcellos, S.; O Nascimento, A.L.T. Features of two proteins of Leptospira interrogans with potential role in host-pathogen interactions. BMC Microbiol. 2012, 12, 50. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, A.S.; Monaris, D.; Silva, L.B.; Morais, Z.M.; Vasconcellos, S.A.; Cianciarullo, A.M.; Isaac, L.; Abreu, P.A.E. Functional Characterization of LcpA, a Surface-Exposed Protein of Leptospira spp. That Binds the Human Complement Regulator C4BP. Infect. Immun. 2010, 78, 3207–3216. [Google Scholar] [CrossRef] [Green Version]
- Marshall, R.B. The route of entry of leptospires into the kidney tubule (Plates X and XI). J. Med Microbiol. 1976, 9, 149–152. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-W.; Wu, M.-S.; Pan, M.-J.; Hsieh, W.-J.; Vandewalle, A.; Huang, C.-C. The Leptospira Outer Membrane Protein LipL32 Induces Tubulointerstitial Nephritis-Mediated Gene Expression in Mouse Proximal Tubule Cells. J. Am. Soc. Nephrol. 2002, 13, 2037–2045. [Google Scholar] [CrossRef] [Green Version]
- Ondee, T.; Gillen, J.; Visitchanakun, P.; Somparn, P.; Issara-Amphorn, J.; Phi, C.D.; Chancharoenthana, W.; Gurusamy, D.; Nita-Lazar, A.; Leelahavanichkul, A. Lipocalin-2 (Lcn-2) Attenuates Polymicrobial Sepsis with LPS Preconditioning (LPS Tolerance) in FcGRIIb Deficient Lupus Mice. Cells 2019, 8, 1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-W.; Wu, M.-S.; Pan, M.-J.; Hong, J.-J.; Yu, C.-C.; Vandewalle, A.; Huang, C.C. Leptospira outer membrane protein activates NF-κB and downstream genes expressed in medullary thick ascending limb cells. J. Am. Soc. Nephrol. 2000, 11, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Haake, D.A.; Chao, G.; Zuerner, R.L.; Barnett, J.K.; Barnett, D.; Mazel, M.; Matsunaga, J.; Levett, P.N.; Bolin, C.A. The Leptospiral Major Outer Membrane Protein LipL32 Is a Lipoprotein Expressed during Mammalian Infection. Infect. Immun. 2000, 68, 2276–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Kim, K.A.; Park, Y.G.; Seong, I.W.; Kim, M.J.; Lee, Y.J. Identification and partial characterization of a novel hemolysin from Leptospira interrogans serovar lai. Gene 2000, 254, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-W.; Hung, C.-C.; Wu, M.-S.; Tian, Y.-C.; Chang, C.-T.; Pan, M.-J.; Vandewalle, A. Toll-like receptor 2 mediates early inflammation by leptospiral outer membrane proteins in proximal tubule cells. Kidney Int. 2006, 69, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, M.; Ojcius, D.M.; Zhou, B.; Hu, W.; Liu, Y.; Ma, Q.; Tang, G.; Wang, D.; Yan, J. Leptospira interrogans infection leads to IL-1β and IL-18 secretion from a human macrophage cell line through reactive oxygen species and cathepsin B mediated-NLRP3 inflammasome activation. Microbes Infect. 2018, 20, 254–260, Corrigendum in 2021, 23, 104756. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Ojcius, D.M.; Yang, X.F.; Zhang, C.; Ding, S.; Lin, X.A.; Yan, J. Leptospiral hemolysins induce proinflammatory cytokines through Toll-like receptor 2-and 4-mediated JNK and NF-κB signaling pathways. PLoS ONE 2012, 7, e42266. [Google Scholar] [CrossRef]
- Yang, C.-W. Leptospirosis renal disease: Understanding the initiation by Toll-like receptors. Kidney Int. 2007, 72, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Segura, E.R.; Ganoza, C.; Campos, K.; Ricaldi, J.; Torres, S.; Silva, H.; Cespedes, M.J.; Matthias, M.A.; Swancutt, M.A.; Linan, R.L.; et al. Clinical Spectrum of Pulmonary Involvement in Leptospirosis in a Region of Endemicity, with Quantification of Leptospiral Burden. Clin. Infect. Dis. 2005, 40, 343–351. [Google Scholar] [CrossRef]
- Gouveia, E.L.; Metcalfe, J.; De Carvalho, A.L.F.; Aires, T.S.; Bisneto, J.C.V.; Queirroz, A.; Santos, A.C.; Salgado, K.; Reis, M.G.; Ko, A. Leptospirosis-associated Severe Pulmonary Hemorrhagic Syndrome, Salvador, Brazil. Emerg. Infect. Dis. 2008, 14, 505–508. [Google Scholar] [CrossRef]
- Miller, N.G.; Allen, J.E.; Wilson, R.B. The pathogenesis of hemorrhage in the lung of the hamster during acute leptospirosis. Med. Microbiol. Immunol. 1974, 160, 269–278. [Google Scholar] [CrossRef]
- Ren, S.-X.; Fu, G.; Jiang, X.-G.; Zeng, R.; Miao, Y.-G.; Xu, H.; Zhang, Y.-X.; Xiong, H.; Lu, G.; Lu, L.-F.; et al. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nat. Rev. Gastroenterol. 2003, 422, 888–893. [Google Scholar] [CrossRef] [Green Version]
- Bulach, D.M.; Zuerner, R.L.; Wilson, P.; Seemann, T.; McGrath, A.; Cullen, P.A.; Davis, J.; Johnson, M.; Kuczek, E.; Alt, D.P.; et al. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc. Natl. Acad. Sci. USA 2006, 103, 14560–14565. [Google Scholar] [CrossRef] [Green Version]
- Nally, J.E.; Chantranuwat, C.; Wu, X.-Y.; Fishbein, M.C.; Pereira, M.M.; da Silva, J.J.P.; Blanco, D.R.; Lovett, M.A. Alveolar Septal Deposition of Immunoglobulin and Complement Parallels Pulmonary Hemorrhage in a Guinea Pig Model of Severe Pulmonary Leptospirosis. Am. J. Pathol. 2004, 164, 1115–1127. [Google Scholar] [CrossRef]
- Silva, J.J.P.; Dalston, M.O.; Carvalho, J.E.M.; Setúbal, S.; Oliveira, J.M.C.; Pereira, M.M. Clinicopathological and immunohistochemical featuresof the severe pulmonary form of leptospirosis. Rev. Soc. Bras. Med. Trop. 2002, 35, 395–399. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.-G.; Hsu, Y.-H. Nitric oxide production and immunoglobulin deposition in leptospiral hemorrhagic respiratory failure. J. Formos. Med. Assoc. 2005, 104, 759–763. [Google Scholar]
- Seguro, A.C.; Andrade, L. Pathophysiology of Leptospirosis. Shock 2013, 39, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Andrade, L.; Rodrigues, A.C., Jr.; Sanches, T.R.; Souza, R.B.; Seguro, A.C. Leptospirosis leads to dysregulation of sodium transporters in the kidney and lung. Am. J. Physiol.-Ren. Physiol. 2007, 292, F586–F592. [Google Scholar] [CrossRef]
- Taylor, A.J.; Paris, D.H.; Newton, P. A Systematic Review of the Mortality from Untreated Leptospirosis. PLoS Negl. Trop. Dis. 2015, 9, e0003866. [Google Scholar] [CrossRef]
- De Brito, T.; Da Silva, A.M.G.; Abreu, P.A.E. Pathology and pathogenesis of human leptospirosis: A commented review. Rev. Inst. Med. Trop. São Paulo 2018, 60, e23. [Google Scholar] [CrossRef]
- Brito, T.; Machado, M.M.; Montans, S.D.; Hoshino, S. Liver biopsy in human leptospirosis: A light and electron microscopy study. Virchows Arch. 1967, 342, 61–69. [Google Scholar] [CrossRef] [PubMed]
- De Brito, T.; Prado, M.J.; A Negreiros, V.; Nicastri, A.L.; E Sakata, E.; Yasuda, P.H.; Santos, R.T.; A Alves, V. Detection of leptospiral antigen (L. interrogans serovar copenhageni serogroup Icterohaemorrhagiae) by immunoelectron microscopy in the liver and kidney of experimentally infected guinea-pigs. Int. J. Exp. Pathol. 1992, 73, 633–642. [Google Scholar] [PubMed]
- Alves, V.; Gayotto, L.; De Brito, T.; Santos, R.T.M.; Wakamatsu, A.; Vianna, M.; Sakata, E. Leptospiral antigens in the liver of experimentally infected guinea pig and their relation to the morphogenesis of liver damage. Exp. Toxicol. Pathol. 1992, 44, 425–434. [Google Scholar] [CrossRef] [PubMed]
- De Brito, T.; Freymüller, E.; Hoshino, S.; Penna, D.O. Pathology of the kidney and liver in the experimental leptospirosis of the guinea-pig. Virchows Arch. 1966, 341, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, S.; Saito, M.; Kanemaru, T.; Villanueva, S.Y.; Gloriani, N.G.; Yoshida, S.I. Destruction of the hepatocyte junction by intercellular invasion of Leptospira causes jaundice in a hamster model of Weil’s disease. Int. J. Exp. Pathol. 2014, 95, 271–281. [Google Scholar] [CrossRef]
- De, T.B.; O Penna, D.; Hoshino, S.; Pereira, V.G.; Caldas, A.C.; Rothstein, W. Cholestasis in human leptospirosis: A clinical, histochemical, biochemical and electron microscopy study based on liver biopsies. Beitr. Pathol. Anat. Allg. Pathol. 1970, 140, 345–361. [Google Scholar]
- Daher, E.D.F.; Brunetta, D.M.; Júnior, G.B.D.S.; Puster, R.A.; Patrocínio, R.M.D.S.V. Pancreatic involvement in fatal human leptospirosis: Clinical and histopathological features. Rev. Inst. Med. Trop. São Paulo 2003, 45, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Herath, N.J.; Kamburapola, C.J.; Agampodi, S.B. Severe leptospirosis and pancreatitis; A case series from a leptospirosis outbreak in Anuradhapura district, Sri Lanka. BMC Infect. Dis. 2016, 16, 644. [Google Scholar] [CrossRef] [Green Version]
- Kaya, E.; Dervisoglu, A.; Eroglu, C.; Polat, C.; Sunbul, M.; Ozkan, K. Acute pancreatitis caused by leptospirosis: Report of two cases. World J. Gastroenterol. 2005, 11, 4447–4449. [Google Scholar] [CrossRef]
- Panagopoulos, P.; Terzi, I.; Karanikas, M.; Galanopoulos, N.; Maltezos, E. Myocarditis, pancreatitis, polyarthritis, mononeuritis multiplex and vasculitis with symmetrical peripheral gangrene of the lower extremities as a rare presentation of leptospirosis: A case report and review of the literature. J. Med. Case Rep. 2014, 8, 150. [Google Scholar] [CrossRef] [Green Version]
- Tapper, H.; Herwald, H. Modulation of hemostatic mechanisms in bacterial infectious diseases. Blood 2000, 96, 2329–2337. [Google Scholar] [CrossRef]
- Wagenaar, J.F.P.; Goris, M.G.A.; Partiningrum, D.L.; Isbandrio, B.; Hartskeerl, R.A.; Brandjes, D.P.M.; Meijers, J.C.M.; Gasem, M.H.; van Gorp, E.C.M. Coagulation disorders in patients with severe leptospirosis are associated with severe bleeding and mortality. Trop. Med. Int. Health 2010, 15, 152–159. [Google Scholar] [CrossRef]
- Vieira, M.L.; Naudin, C.; Mörgelin, M.; Romero, E.C.; Nascimento, A.L.T.O.; Herwald, H. Modulation of Hemostatic and Inflammatory Responses by Leptospira spp. PLoS Negl. Trop. Dis. 2016, 10, e0004713. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, K.; Franco, R.; Schwab, A.; Coburn, J. Leptospira interrogans Binds to Cadherins. PLoS Negl. Trop. Dis. 2014, 8, e2672. [Google Scholar] [CrossRef]
- Kochi, L.T.; Fernandes, L.G.; Souza, G.O.; Vasconcellos, S.A.; Heinemann, M.B.; Romero, E.C.; Kirchgatter, K.; Nascimento, A.L. The interaction of two novel putative proteins of Leptospira interrogans with E-cadherin, plasminogen and complement components with potential role in bacterial infection. Virulence 2019, 10, 734–753. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.L.; Fernandes, L.G.V.; Domingos, R.F.; Oliveira, R.; Siqueira, G.H.; Souza, N.M.; Teixeira, A.R.; Atzingen, M.V.; Nascimento, A.L. Leptospiral extracellular matrix adhesins as mediators of pathogen-host interactions. FEMS Microbiol. Lett. 2014, 352, 129–139. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Jones, G.W.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef] [Green Version]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell. Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; LeVan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell Metab. 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bingula, R.; Filaire, M.; Radosevic-Robin, N.; Bey, M.; Berthon, J.-Y.; Bernalier-Donadille, A.; Vasson, M.-P.; Filaire, E. Desired Turbulence? Gut-Lung Axis, Immunity, and Lung Cancer. J. Oncol. 2017, 2017, 5035371. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Romanidou, G.; Voidarou, C.; Bezirtzoglou, E. Focus on the Gut–Kidney Axis in Health and Disease. Front. Med. 2021, 7, 620102. [Google Scholar] [CrossRef] [PubMed]
- Allali, I.; Bakri, Y.; Amzazi, S.; Ghazal, H. Gut-Lung Axis in COVID-19. Interdiscip. Perspect. Infect. Dis. 2021, 2021, 6655380. [Google Scholar] [CrossRef] [PubMed]
- Hobby, G.P.; Karaduta, O.; Dusio, G.F.; Singh, M.; Zybailov, B.L.; Arthur, J.M. Chronic kidney disease and the gut microbiome. Am. J. Physiol. Physiol. 2019, 316, F1211–F1217. [Google Scholar] [CrossRef]
- Al Khodor, S.; Shatat, I.F. Gut microbiome and kidney disease: A bidirectional relationship. Pediatr. Nephrol. 2017, 32, 921–931. [Google Scholar] [CrossRef]
- Lin, C.-Y. Leptospirosis-Associated Acute Kidney Injury. In Leptospirosis and the Kidney, 7th ed.; Karger Publishers: Basel, Switzerland, 2019; pp. 20–26. [Google Scholar] [CrossRef]
- De Francesco Daher, E.; Soares de Abreu, K.L.; Bezerra da Silva, G., Jr. Leptospirosis-associated acute kidney injury. J. Bras. Nefrol. 2010, 32, 408–415. [Google Scholar]
- Jo, S.; Yarishkin, O.; Hwang, Y.J.; Chun, Y.E.; Park, M.; Woo, D.H.; Bae, J.Y.; Kim, T.; Lee, J.; Chun, H.; et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014, 20, 886–896. [Google Scholar] [CrossRef]
- Chou, Y.-T.; Kan, W.-C.; Shiao, C.-C. Acute Kidney Injury and Gut Dysbiosis: A Narrative Review Focus on Pathophysiology and Treatment. Int. J. Mol. Sci. 2022, 23, 3658. [Google Scholar] [CrossRef]
- Ohtani, N.; Kawada, N. Role of the Gut-Liver Axis in Liver Inflammation, Fibrosis, and Cancer: A Special Focus on the Gut Microbiota Relationship. Hepatol. Commun. 2019, 3, 456–470. [Google Scholar] [CrossRef] [Green Version]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, J.S.; Khoruts, A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J. Hepatol. 2020, 72, 1003–1027. [Google Scholar] [CrossRef] [Green Version]
- Tokuhara, D. Role of the Gut Microbiota in Regulating Non-alcoholic Fatty Liver Disease in Children and Adolescents. Front. Nutr. 2021, 8, 700058. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, B. The Gut-Liver Axis in Health and Disease: The Role of Gut Microbiota-Derived Signals in Liver Injury and Regeneration. Front. Immunol. 2021, 12, 775526. [Google Scholar] [CrossRef]
- Cornell, R.P. Gut-derived endotoxin elicits hepatotrophic factor secretion for liver regeneration. Am. J. Physiol. Integr. Comp. Physiol. 1985, 249, R551–R562. [Google Scholar] [CrossRef]
- Cornell, R.P.; Liljequist, B.L.; Bartizal, K.F. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatol. Commun. 1990, 11, 916–922. [Google Scholar] [CrossRef]
- Garrett, W.S. Immune recognition of microbial metabolites. Nat. Rev. Immunol. 2020, 20, 91–92. [Google Scholar] [CrossRef]
- Nolan, J.P. The role of intestinal endotoxin in liver injury: A long and evolving history. Hepatol. Commun. 2010, 52, 1829–1835. [Google Scholar] [CrossRef]
- Taub, R. Liver regeneration: From myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Liu, B.; Qian, J.; Wang, Q.; Wang, F.; Ma, Z.; Qiao, Y. Butyrate Protects Rat Liver against Total Hepatic Ischemia Reperfusion Injury with Bowel Congestion. PLoS ONE 2014, 9, e106184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.L.; Wesselingh, S.; Rogers, G.B. Host-microbiome interactions in acute and chronic respiratory infections. Cell. Microbiol. 2016, 18, 652–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauguet, S.; D’Ortona, S.; Ahnger-Pier, K.; Duan, B.; Surana, N.K.; Lu, R.; Cywes-Bentley, C.; Gadjeva, M.; Shan, Q.; Priebe, G.P.; et al. Intestinal Microbiota of Mice Influences Resistance to Staphylococcus aureus Pneumonia. Infect. Immun. 2015, 83, 4003–4014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruzzese, E.; Callegari, M.L.; Raia, V.; Viscovo, S.; Scotto, R.; Ferrari, S.; Morelli, L.; Buccigrossi, V.; Vecchio, A.L.; Ruberto, E.; et al. Disrupted Intestinal Microbiota and Intestinal Inflammation in Children with Cystic Fibrosis and Its Restoration with Lactobacillus GG: A Randomised Clinical Trial. PLoS ONE 2014, 9, e87796. [Google Scholar] [CrossRef] [Green Version]
- Ranucci, G.; Buccigrossi, V.; De Freitas, M.B.; Guarino, A.; Giannattasio, A. Early-Life Intestine Microbiota and Lung Health in Children. J. Immunol. Res. 2017, 2017, 8450496. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Q.; Liu, H. Coronavirus disease 2019 and the gut–lung axis. Int. J. Infect. Dis. 2021, 113, 300–307. [Google Scholar] [CrossRef]
- Becattini, S.; Sorbara, M.T.; Kim, S.G.; Littmann, E.L.; Dong, Q.; Walsh, G.; Wright, R.; Amoretti, L.; Fontana, E.; Hohl, T.M.; et al. Rapid transcriptional and metabolic adaptation of intestinal microbes to host immune activation. Cell Host Microbe 2021, 29, 378–393. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Agostini, D.; Piccoli, G.; Stocchi, V.; Sestili, P. Gut microbiota status in COVID-19: An unrecognized player? Front. Cell. Infect. Microbiol. 2020, 10, 576551. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Feng, J.; Hu, X.-T.; Wang, T.; Gong, W.-X.; Huang, K.; Guo, Y.-X.; Zou, Z.; Lin, X.; et al. Influenza infection elicits an expansion of gut population of endogenous Bifidobacterium animalis which protects mice against infection. Genome Biol. 2020, 21, 99. [Google Scholar] [CrossRef]
- Petakh, P.; Kamyshna, I.; Nykyforuk, A.; Yao, R.; Imbery, J.F.; Oksenych, V.; Korda, M.; Kamyshnyi, A. Immunoregulatory Intestinal Microbiota and COVID-19 in Patients with Type Two Diabetes: A Double-Edged Sword. Viruses 2022, 14, 477. [Google Scholar] [CrossRef]
- Winglee, K.; Eloe-Fadrosh, E.; Gupta, S.; Guo, H.; Fraser, C.; Bishai, W. Aerosol Mycobacterium tuberculosis Infection Causes Rapid Loss of Diversity in Gut Microbiota. PLoS ONE 2014, 9, e97048. [Google Scholar] [CrossRef]
- Ejtahed, H.-S.; Hasani-Ranjbar, S.; Siadat, S.D.; Larijani, B. The most important challenges ahead of microbiome pattern in the post era of the COVID-19 pandemic. J. Diabetes Metab. Disord. 2020, 19, 2031–2033. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955. [Google Scholar] [CrossRef]
- Chen, C.-J.; Wu, G.-H.; Kuo, R.-L.; Shih, S.-R. Role of the intestinal microbiota in the immunomodulation of influenza virus infection. Microbes Infect. 2017, 19, 570–579. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. eBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.W.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-K.; Lee, M.-H.; Chen, Y.-C.; Hsueh, P.-R.; Chang, S.-C. Factors associated with severity and mortality in patients with confirmed leptospirosis at a regional hospital in northern Taiwan. J. Microbiol. Immunol. Infect. 2020, 53, 307–314. [Google Scholar] [CrossRef]
- Abraham, G.; Thirumurthi, K.; Mathew, M.; Reddy, Y.N.; Subrahmanian, P.S. Reversible acute kidney injury due to bilateral papillary necrosis in a patient with leptospirosis and diabetes mellitus. Indian J. Nephrol. 2012, 22, 392–394. [Google Scholar] [CrossRef]
- Tubiana, S.; Mikulski, M.; Becam, J.; Lacassin, F.; Lefèvre, P.; Gourinat, A.-C.; Goarant, C.; D’Ortenzio, E. Risk Factors and Predictors of Severe Leptospirosis in New Caledonia. PLoS Negl. Trop. Dis. 2013, 7, e1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petakh, P.; Isevych, V.; Kamyshnyi, A.; Oksenych, V. Weil’s Disease—Immunopathogenesis, Multiple Organ Failure, and Potential Role of Gut Microbiota. Biomolecules 2022, 12, 1830. https://doi.org/10.3390/biom12121830
Petakh P, Isevych V, Kamyshnyi A, Oksenych V. Weil’s Disease—Immunopathogenesis, Multiple Organ Failure, and Potential Role of Gut Microbiota. Biomolecules. 2022; 12(12):1830. https://doi.org/10.3390/biom12121830
Chicago/Turabian StylePetakh, Pavlo, Vitaliia Isevych, Aleksandr Kamyshnyi, and Valentyn Oksenych. 2022. "Weil’s Disease—Immunopathogenesis, Multiple Organ Failure, and Potential Role of Gut Microbiota" Biomolecules 12, no. 12: 1830. https://doi.org/10.3390/biom12121830
APA StylePetakh, P., Isevych, V., Kamyshnyi, A., & Oksenych, V. (2022). Weil’s Disease—Immunopathogenesis, Multiple Organ Failure, and Potential Role of Gut Microbiota. Biomolecules, 12(12), 1830. https://doi.org/10.3390/biom12121830







