Anti-Inflammatory Effects of Encapsulated Human Mesenchymal Stromal/Stem Cells and a Method to Scale-Up Cell Encapsulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Encapsulated Human MSC (eMSC)
2.2. Spinal Cord Injury (SCI) and Capsule Injection
2.3. ELISA and Multiplex Assays
2.4. qRT-PCR
2.5. Rat Endotoxemia Model
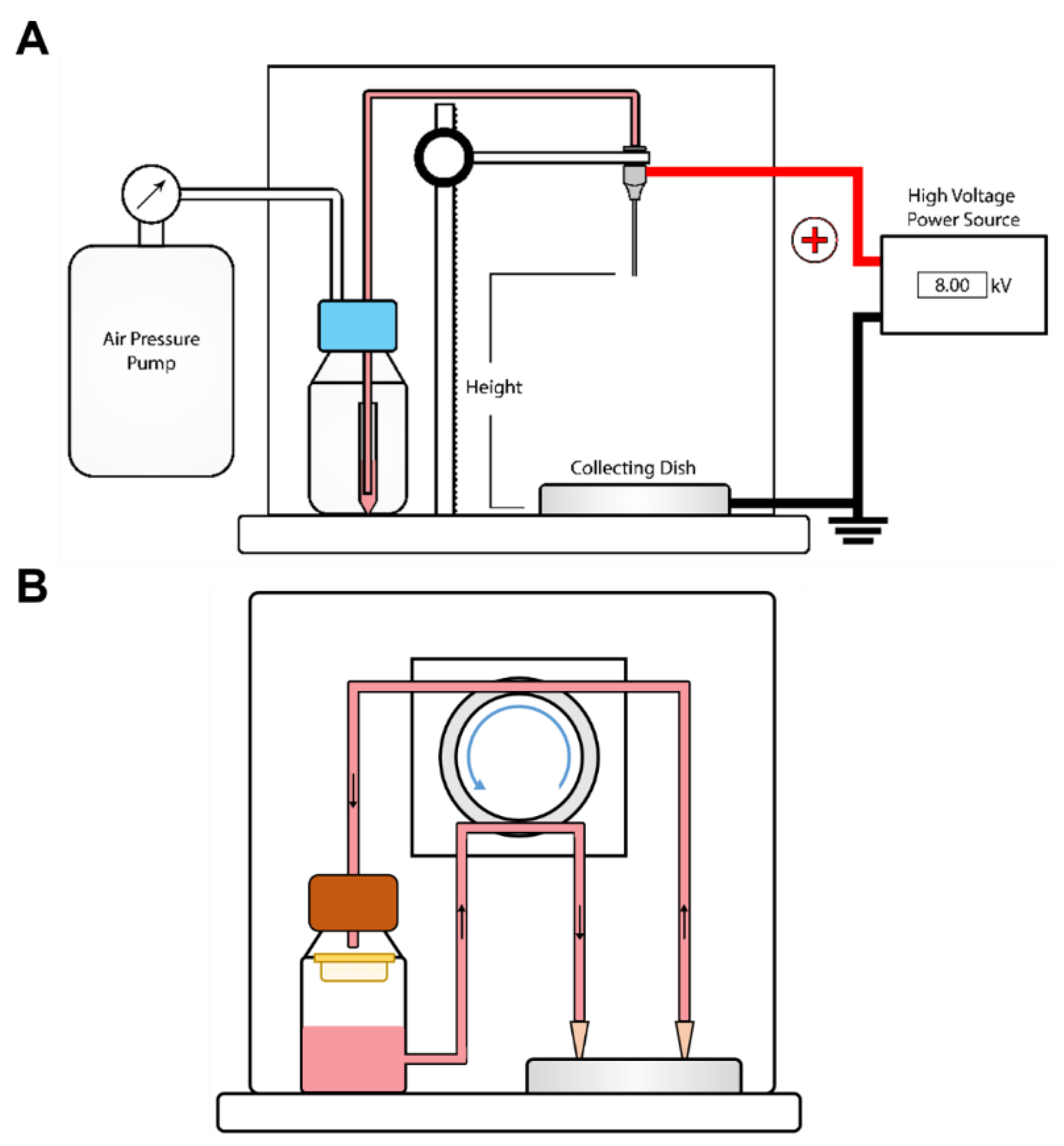
2.6. Rapid Capsule Collection System (RaCCS)
2.7. Statistical Analysis
3. Results
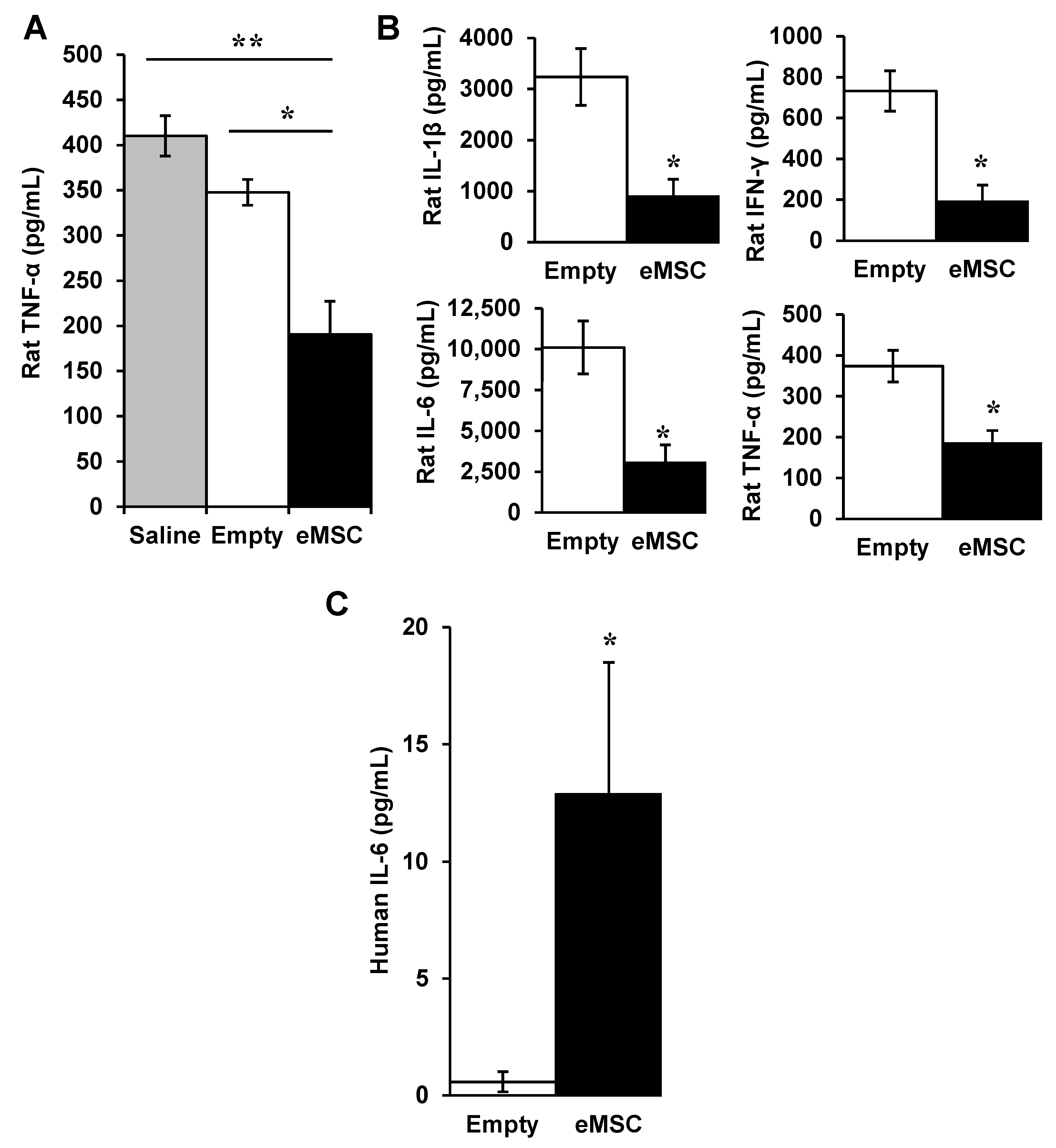
3.1. Effects of Encapsulated MSC In Vivo
3.1.1. Encapsulated MSC in SCI
3.1.2. eMSC in Rat Endotoxemia
3.1.3. Viability of MSC in Unpolymerized Alginate
3.2. A Rapid Capsule Collection System (RaCCS) for Scaling-Up Cell Encapsulation
3.2.1. A Rapid Capsules Collection System (RaCCS)
3.2.2. Properties of Encapsulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Matthay, M.A.; Pati, S.; Lee, J.-W. Concise Review: Mesenchymal Stem (Stromal) Cells: Biology and Preclinical Evidence for Therapeutic Potential for Organ Dysfunction Following Trauma or Sepsis. Stem Cells 2017, 35, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J.; Kota, D.J.; Bazhanov, N.; Reger, R.L. Evolving Paradigms for Repair of Tissues by Adult Stem/Progenitor Cells (MSCs). J. Cell Mol. Med. 2010, 14, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J. The Exciting Prospects of New Therapies with Mesenchymal Stromal Cells. Cytotherapy 2017, 19, 1–8. [Google Scholar] [CrossRef]
- de Almeida, D.C.; Donizetti-Oliveira, C.; Barbosa-Costa, P.; Origassa, C.S.; Câmara, N.O. In Search of Mechanisms Associated with Mesenchymal Stem Cell-Based Therapies for Acute Kidney Injury. Clin. Biochem. Rev. 2013, 34, 131–144. [Google Scholar] [PubMed]
- Maron-Gutierrez, T.; Laffey, J.G.; Pelosi, P.; Rocco, P.R.M. Cell-Based Therapies for the Acute Respiratory Distress Syndrome. Curr. Opin. Crit. Care 2014, 20, 122–131. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Belizna, C.; Selva-O’Callaghan, A.; Pardos-Gea, J.; Quintana, A.; Mekinian, A.; Anunciacion-Llunell, A.; Miró-Mur, F. Immunomodulatory Therapy for the Management of Severe COVID-19. Beyond the Anti-Viral Therapy: A Comprehensive Review. Autoimmun. Rev. 2020, 19, 102569. [Google Scholar] [CrossRef]
- Cavaillon, J.-M. Exotoxins and Endotoxins: Inducers of Inflammatory Cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef]
- Huet, O.; Chin-Dusting, J.P.F. Septic Shock: Desperately Seeking Treatment. Clin. Sci. 2014, 126, 31–39. [Google Scholar] [CrossRef]
- Sakr, Y.; Jaschinski, U.; Wittebole, X.; Szakmany, T.; Lipman, J.; Ñamendys-Silva, S.A.; Martin-Loeches, I.; Leone, M.; Lupu, M.-N.; Vincent, J.-L.; et al. Sepsis in Intensive Care Unit Patients: Worldwide Data from the Intensive Care over Nations Audit. Open Forum Infect. Dis. 2018, 5, ofy313. [Google Scholar] [CrossRef]
- Lombardo, E.; van der Poll, T.; DelaRosa, O.; Dalemans, W. Mesenchymal Stem Cells as a Therapeutic Tool to Treat Sepsis. World J. Stem Cells 2015, 7, 368–379. [Google Scholar] [CrossRef]
- Prockop, D.J.; Oh, J.Y. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Strand, B.L.; Mørch, Y.; Lacík, I.; Wang, Y.; Salehi, P.; Barbaro, B.; Gangemi, A.; Kuechle, J.; Romagnoli, T.; et al. Encapsulation of Human Islets in Novel Inhomogeneous Alginate-Ca2+/Ba2+ Microbeads: In Vitro and in Vivo Function. Artif. Cells Blood Substit. Immobil. Biotechnol. 2008, 36, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Paredes Juárez, G.A.; Spasojevic, M.; Faas, M.M.; de Vos, P. Immunological and Technical Considerations in Application of Alginate-Based Microencapsulation Systems. Front. Bioeng. Biotechnol. 2014, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Vegas, A.J.; Veiseh, O.; Doloff, J.C.; Ma, M.; Tam, H.H.; Bratlie, K.; Li, J.; Bader, A.R.; Langan, E.; Olejnik, K.; et al. Combinatorial Hydrogel Library Enables Identification of Materials That Mitigate the Foreign Body Response in Primates. Nat. Biotechnol. 2016, 34, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Gurruchaga, H.; Saenz del Burgo, L.; Ciriza, J.; Orive, G.; Hernández, R.M.; Pedraz, J.L. Advances in Cell Encapsulation Technology and Its Application in Drug Delivery. Expert Opin Drug Deliv. 2015, 12, 1251–1267. [Google Scholar] [CrossRef]
- Levit, R.D.; Landázuri, N.; Phelps, E.A.; Brown, M.E.; García, A.J.; Davis, M.E.; Joseph, G.; Long, R.; Safley, S.A.; Suever, J.D.; et al. Cellular Encapsulation Enhances Cardiac Repair. J. Am. Heart Assoc. 2013, 2, e000367. [Google Scholar] [CrossRef]
- Landázuri, N.; Levit, R.D.; Joseph, G.; Ortega-Legaspi, J.M.; Flores, C.A.; Weiss, D.; Sambanis, A.; Weber, C.J.; Safley, S.A.; Taylor, W.R. Alginate Microencapsulation of Human Mesenchymal Stem Cells as a Strategy to Enhance Paracrine-Mediated Vascular Recovery after Hindlimb Ischaemia. J. Tissue Eng. Regen. Med. 2016, 10, 222–232. [Google Scholar] [CrossRef]
- Barminko, J.; Kim, J.H.; Otsuka, S.; Gray, A.; Schloss, R.; Grumet, M.; Yarmush, M.L. Encapsulated Mesenchymal Stromal Cells for in Vivo Transplantation. Biotechnol. Bioeng. 2011, 108, 2747–2758. [Google Scholar] [CrossRef]
- Kumar, S.; Babiarz, J.; Basak, S.; Kim, J.H.; Barminko, J.; Gray, A.; Mendapara, P.; Schloss, R.; Yarmush, M.L.; Grumet, M. Sizes and Sufficient Quantities of MSC Microspheres for Intrathecal Injection to Modulate Inflammation in Spinal Cord Injury. Nano Life 2015, 5, 1550004. [Google Scholar] [CrossRef]
- Tobias, C.A.; Han, S.S.W.; Shumsky, J.S.; Kim, D.; Tumolo, M.; Dhoot, N.O.; Wheatley, M.A.; Fischer, I.; Tessler, A.; Murray, M. Alginate Encapsulated BDNF-Producing Fibroblast Grafts Permit Recovery of Function after Spinal Cord Injury in the Absence of Immune Suppression. J. Neurotrauma 2005, 22, 138–156. [Google Scholar] [CrossRef]
- Grandoso, L.; Ponce, S.; Manuel, I.; Arrúe, A.; Ruiz-Ortega, J.A.; Ulibarri, I.; Orive, G.; Hernández, R.M.; Rodríguez, A.; Rodríguez-Puertas, R.; et al. Long-Term Survival of Encapsulated GDNF Secreting Cells Implanted within the Striatum of Parkinsonized Rats. Int. J. Pharm. 2007, 343, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Goren, A.; Dahan, N.; Goren, E.; Baruch, L.; Machluf, M. Encapsulated Human Mesenchymal Stem Cells: A Unique Hypoimmunogenic Platform for Long-Term Cellular Therapy. FASEB J. 2010, 24, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Klinge, P.M.; Harmening, K.; Miller, M.C.; Heile, A.; Wallrapp, C.; Geigle, P.; Brinker, T. Encapsulated Native and Glucagon-like Peptide-1 Transfected Human Mesenchymal Stem Cells in a Transgenic Mouse Model of Alzheimer’s Disease. Neurosci. Lett. 2011, 497, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Detante, O.; Moisan, A.; Dimastromatteo, J.; Richard, M.-J.; Riou, L.; Grillon, E.; Barbier, E.; Desruet, M.-D.; De Fraipont, F.; Segebarth, C.; et al. Intravenous Administration of 99mTc-HMPAO-Labeled Human Mesenchymal Stem Cells after Stroke: In Vivo Imaging and Biodistribution. Cell Transpl. 2009, 18, 1369–1379. [Google Scholar] [CrossRef]
- Mørch, Y.A.; Donati, I.; Strand, B.L.; Skjåk-Braek, G. Effect of Ca2+, Ba2+, and Sr2+ on Alginate Microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Friedlander, D.R.; Brittis, P.A.; Sakurai, T.; Shif, B.; Wirchansky, W.; Fishell, G.; Grumet, M. Generation of a Radial-like Glial Cell Line. J. Neurobiol. 1998, 37, 291–304. [Google Scholar] [CrossRef]
- Moran, D.M.; Koniaris, L.G.; Jablonski, E.M.; Cahill, P.A.; Halberstadt, C.R.; McKillop, I.H. Microencapsulation of Engineered Cells to Deliver Sustained High Circulating Levels of Interleukin-6 to Study Hepatocellular Carcinoma Progression. Cell Transplant 2006, 15, 785–798. [Google Scholar] [CrossRef]
- Hasegawa, K.; Chang, Y.-W.; Li, H.; Berlin, Y.; Ikeda, O.; Kane-Goldsmith, N.; Grumet, M. Embryonic Radial Glia Bridge Spinal Cord Lesions and Promote Functional Recovery Following Spinal Cord Injury. Exp. Neurol. 2005, 193, 394–410. [Google Scholar] [CrossRef]
- Yagi, H.; Soto-Gutierrez, A.; Parekkadan, B.; Kitagawa, Y.; Tompkins, R.G.; Kobayashi, N.; Yarmush, M.L. Mesenchymal Stem Cells: Mechanisms of Immunomodulation and Homing. Cell Transpl. 2010, 19, 667–679. [Google Scholar] [CrossRef]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A New Look at Cerebrospinal Fluid Circulation. Fluids Barriers CNS 2014, 11, 10. [Google Scholar] [CrossRef]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human Mesenchymal Stem/Stromal Cells Cultured as Spheroids Are Self-Activated to Produce Prostaglandin E2 That Directs Stimulated Macrophages into an Anti-Inflammatory Phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef] [PubMed]
- Gansau, J.; Kelly, L.; Buckley, C.T. Influence of Key Processing Parameters and Seeding Density Effects of Microencapsulated Chondrocytes Fabricated Using Electrohydrodynamic Spraying. Biofabrication 2018, 10, 035011. [Google Scholar] [CrossRef] [PubMed]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in Mesenchymal Stem Cell Clinical Trials 2004-2018: Is Efficacy Optimal in a Narrow Dose Range? Stem Cells Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Grumet, M.; Sherman, J.; Dorf, B.S. Efficacy of MSC in Severe COVID-19 Patients: A Review and a Case Study. Stem Cells Transl. Med 2022, 11, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, M.A.; Veiseh, O.; Vegas, A.J.; McGarrigle, J.J.; Qi, M.; Marchese, E.; Omami, M.; Doloff, J.C.; Mendoza-Elias, J.; Nourmohammadzadeh, M.; et al. Alginate Encapsulation as Long-Term Immune Protection of Allogeneic Pancreatic Islet Cells Transplanted into the Omental Bursa of Macaques. Nat. Biomed. Eng. 2018, 2, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Henschler, R. Fate of Intravenously Injected Mesenchymal Stem Cells and Significance for Clinical Application. Adv Biochem. Eng. Biotechnol. 2013, 130, 19–37. [Google Scholar] [CrossRef]
- Cheung, T.S.; Bertolino, G.M.; Giacomini, C.; Bornhäuser, M.; Dazzi, F.; Galleu, A. Mesenchymal Stromal Cells for Graft Versus Host Disease: Mechanism-Based Biomarkers. Front Immunol. 2020, 11, 1338. [Google Scholar] [CrossRef]
- Bellingan, G.; Jacono, F.; Bannard-Smith, J.; Brealey, D.; Meyer, N.; Thickett, D.; Young, D.; Bentley, A.; McVerry, B.J.; Wunderink, R.G.; et al. Safety and Efficacy of Multipotent Adult Progenitor Cells in Acute Respiratory Distress Syndrome (MUST-ARDS): A Multicentre, Randomised, Double-Blind, Placebo-Controlled Phase 1/2 Trial. Intensive Care Med. 2022, 48, 36–44. [Google Scholar] [CrossRef]
- Gryshkov, O.; Pogozhykh, D.; Zernetsch, H.; Hofmann, N.; Mueller, T.; Glasmacher, B. Process Engineering of High Voltage Alginate Encapsulation of Mesenchymal Stem Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 36, 77–83. [Google Scholar] [CrossRef]
- Huang, H.; Sun, M.; Heisler-Taylor, T.; Kiourti, A.; Volakis, J.; Lafyatis, G.; He, X. Stiffness-Independent Highly Efficient On-Chip Extraction of Cell-Laden Hydrogel Microcapsules from Oil Emulsion into Aqueous Solution by Dielectrophoresis. Small 2015, 11, 5369–5374. [Google Scholar] [CrossRef]
- Orive, G.; Santos, E.; Pedraz, J.L.; Hernández, R.M. Application of Cell Encapsulation for Controlled Delivery of Biological Therapeutics. Adv. Drug Deliv. Rev. 2014, 67–68, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Nedović, V.A.; Obradović, B.; Leskošek-Čukalović, I.; Trifunović, O.; Pešić, R.; Bugarski, B. Electrostatic Generation of Alginate Microbeads Loaded with Brewing Yeast. Process Biochem. 2001, 37, 17–22. [Google Scholar] [CrossRef]
- Rahman, K.; Ko, J.-B.; Khan, S.; Kim, D.-S.; Choi, K.-H. Simulation of Droplet Generation through Electrostatic Forces. J. Mech. Sci. Technol. 2010, 24, 307–310. [Google Scholar] [CrossRef]
- Lee, S.-H.; Nguyen, X.H.; Ko, H.S. Study on Droplet Formation with Surface Tension for Electrohydrodynamic Inkjet Nozzle. J. Mech. Sci. Technol. 2012, 26, 1403–1408. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Kabat, M.; Basak, S.; Babiarz, J.; Berthiaume, F.; Grumet, M. Anti-Inflammatory Effects of Encapsulated Human Mesenchymal Stromal/Stem Cells and a Method to Scale-Up Cell Encapsulation. Biomolecules 2022, 12, 1803. https://doi.org/10.3390/biom12121803
Kumar S, Kabat M, Basak S, Babiarz J, Berthiaume F, Grumet M. Anti-Inflammatory Effects of Encapsulated Human Mesenchymal Stromal/Stem Cells and a Method to Scale-Up Cell Encapsulation. Biomolecules. 2022; 12(12):1803. https://doi.org/10.3390/biom12121803
Chicago/Turabian StyleKumar, Suneel, Maciej Kabat, Sayantani Basak, Joanne Babiarz, Francois Berthiaume, and Martin Grumet. 2022. "Anti-Inflammatory Effects of Encapsulated Human Mesenchymal Stromal/Stem Cells and a Method to Scale-Up Cell Encapsulation" Biomolecules 12, no. 12: 1803. https://doi.org/10.3390/biom12121803
APA StyleKumar, S., Kabat, M., Basak, S., Babiarz, J., Berthiaume, F., & Grumet, M. (2022). Anti-Inflammatory Effects of Encapsulated Human Mesenchymal Stromal/Stem Cells and a Method to Scale-Up Cell Encapsulation. Biomolecules, 12(12), 1803. https://doi.org/10.3390/biom12121803






