Potential and Limitations of Induced Pluripotent Stem Cells-Derived Mesenchymal Stem Cells in Musculoskeletal Disorders Treatment
Abstract
1. Introduction
2. iPSC Sources to Derive MSC
3. Methods to Derive iMSC from iPSC
4. iMSC Therapeutic Potential—Advantages and Disadvantages
5. Preclinical and Clinical Studies in MSK Disorders Using iMSC
6. Concluding Remarks and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 10 April 2023).
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What Low Back Pain Is and Why We Need to Pay Attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Williams, A.; Kamper, S.J.; Wiggers, J.H.; O’Brien, K.M.; Lee, H.; Wolfenden, L.; Yoong, S.L.; Robson, E.; McAuley, J.H.; Hartvigsen, J.; et al. Musculoskeletal Conditions May Increase the Risk of Chronic Disease: A Systematic Review and Meta-Analysis of Cohort Studies. BMC Med. 2018, 16, 167. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global Estimates of the Need for Rehabilitation Based on the Global Burden of Disease Study 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms Involved in the Therapeutic Properties of Mesenchymal Stem Cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. The MSC: An Injury Drugstore. Cell Stem Cell 2011, 9, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal Stem Cells: Environmentally Responsive Therapeutics for Regenerative Medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [PubMed]
- Čamernik, K.; Barlič, A.; Drobnič, M.; Marc, J.; Jeras, M.; Zupan, J. Mesenchymal Stem Cells in the Musculoskeletal System: From Animal Models to Human Tissue Regeneration? Stem Cell Rev. Rep. 2018, 14, 346–369. [Google Scholar] [CrossRef]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal Stem Cells: Amazing Remedies for Bone and Cartilage Defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef]
- Ferro, F.; Spelat, R.; Baheney, C.S. Dental Pulp Stem Cell (DPSC) Isolation, Characterization, and Differentiation. In Stem Cells and Tissue Repair; Kioussi, C., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; Volume 1210, pp. 91–115. ISBN 978-1-4939-1434-0. [Google Scholar]
- Shi, S.; Gronthos, S. Perivascular Niche of Postnatal Mesenchymal Stem Cells in Human Bone Marrow and Dental Pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.-H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef]
- Vahedi, P.; Moghaddamshahabi, R.; Webster, T.J.; Calikoglu Koyuncu, A.C.; Ahmadian, E.; Khan, W.S.; Jimale Mohamed, A.; Eftekhari, A. The Use of Infrapatellar Fat Pad-Derived Mesenchymal Stem Cells in Articular Cartilage Regeneration: A Review. Int. J. Mol. Sci. 2021, 22, 9215. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent Mesenchymal Stem Cells from Adult Human Synovial Membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Dellavalle, A.; Sampaolesi, M.; Tonlorenzi, R.; Tagliafico, E.; Sacchetti, B.; Perani, L.; Innocenzi, A.; Galvez, B.G.; Messina, G.; Morosetti, R.; et al. Pericytes of Human Skeletal Muscle Are Myogenic Precursors Distinct from Satellite Cells. Nat. Cell Biol. 2007, 9, 255–267. [Google Scholar] [CrossRef]
- Rojewski, M.T.; Weber, B.M.; Schrezenmeier, H. Phenotypic Characterization of Mesenchymal Stem Cells from Various Tissues. Transfus. Med. Hemotherapy 2008, 35, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, H.; Kaneda, M.; Metwally, E.; Shimada, K.; Tanaka, T.; Tanaka, R. A Comparative Study of the Effect of Anatomical Site on Multiple Differentiation of Adipose-Derived Stem Cells in Rats. Cells 2021, 10, 2469. [Google Scholar] [CrossRef]
- Hua, J.; Gong, J.; Meng, H.; Xu, B.; Yao, L.; Qian, M.; He, Z.; Zou, S.; Zhou, B.; Song, Z. Comparison of Different Methods for the Isolation of Mesenchymal Stem Cells from Umbilical Cord Matrix: Proliferation and Multilineage Differentiation as Compared to Mesenchymal Stem Cells from Umbilical Cord Blood and Bone Marrow. Cell Biol. Int. 2014, 38, 198–210. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Alves, L.; Bogalho, I.; Cabral, J.M.S.; Da Silva, C.L. Impact of Donor Age on the Osteogenic Supportive Capacity of Mesenchymal Stromal Cell-Derived Extracellular Matrix. Front. Cell Dev. Biol. 2021, 9, 747521. [Google Scholar] [CrossRef]
- Bruder, S.P.; Jaiswal, N.; Haynesworth, S.E. Growth Kinetics, Self-Renewal, and the Osteogenic Potential of Purified Human Mesenchymal Stem Cells during Extensive Subcultivation and Following Cryopreservation. J. Cell. Biochem. 1997, 64, 278–294. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Čamernik, K.; Mihelič, A.; Mihalič, R.; Haring, G.; Herman, S.; Marolt Presen, D.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Comprehensive Analysis of Skeletal Muscle- and Bone-Derived Mesenchymal Stem/Stromal Cells in Patients with Osteoarthritis and Femoral Neck Fracture. Stem Cell Res. Ther. 2020, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.A.; Eiro, N.; Fraile, M.; Gonzalez, L.O.; Saá, J.; Garcia-Portabella, P.; Vega, B.; Schneider, J.; Vizoso, F.J. Functional Heterogeneity of Mesenchymal Stem Cells from Natural Niches to Culture Conditions: Implications for Further Clinical Uses. Cell. Mol. Life Sci. 2021, 78, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Luzzani, C.D.; Miriuka, S.G. Pluripotent Stem Cells as a Robust Source of Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2017, 13, 68–78. [Google Scholar] [CrossRef]
- McGrath, M.; Tam, E.; Sladkova, M.; AlManaie, A.; Zimmer, M.; De Peppo, G.M. GMP-Compatible and Xeno-Free Cultivation of Mesenchymal Progenitors Derived from Human-Induced Pluripotent Stem Cells. Stem Cell Res. Ther. 2019, 10, 11. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Karagiannis, P.; Takahashi, K.; Saito, M.; Yoshida, Y.; Okita, K.; Watanabe, A.; Inoue, H.; Yamashita, J.K.; Todani, M.; Nakagawa, M.; et al. Induced Pluripotent Stem Cells and Their Use in Human Models of Disease and Development. Physiol. Rev. 2019, 99, 79–114. [Google Scholar] [CrossRef]
- Kim, K.; Doi, A.; Wen, B.; Ng, K.; Zhao, R.; Cahan, P.; Kim, J.; Aryee, M.J.; Ji, H.; Ehrlich, L.I.R.; et al. Epigenetic Memory in Induced Pluripotent Stem Cells. Nature 2010, 467, 285–290. [Google Scholar] [CrossRef]
- Ye, L.; Muench, M.O.; Fusaki, N.; Beyer, A.I.; Wang, J.; Qi, Z.; Yu, J.; Kan, Y.W. Blood Cell-Derived Induced Pluripotent Stem Cells Free of Reprogramming Factors Generated by Sendai Viral Vectors. Stem Cells Transl. Med. 2013, 2, 558–566. [Google Scholar] [CrossRef]
- Streckfuss-Bömeke, K.; Wolf, F.; Azizian, A.; Stauske, M.; Tiburcy, M.; Wagner, S.; Hübscher, D.; Dressel, R.; Chen, S.; Jende, J.; et al. Comparative Study of Human-Induced Pluripotent Stem Cells Derived from Bone Marrow Cells, Hair Keratinocytes, and Skin Fibroblasts. Eur. Heart J. 2013, 34, 2618–2629. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine. Stem Cells 2022, 40, 546–555. [Google Scholar] [CrossRef]
- Hynes, K.; Menicanin, D.; Mrozik, K.; Gronthos, S.; Bartold, P.M. Generation of Functional Mesenchymal Stem Cells from Different Induced Pluripotent Stem Cell Lines. Stem Cells Dev. 2014, 23, 1084–1096. [Google Scholar] [CrossRef]
- Ray, A.; Joshi, J.M.; Sundaravadivelu, P.K.; Raina, K.; Lenka, N.; Kaveeshwar, V.; Thummer, R.P. An Overview on Promising Somatic Cell Sources Utilized for the Efficient Generation of Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2021, 17, 1954–1974. [Google Scholar] [CrossRef]
- Gao, R.; Ye, T.; Zhu, Z.; Li, Q.; Zhang, J.; Yuan, J.; Zhao, B.; Xie, Z.; Wang, Y. Small Extracellular Vesicles from IPSC-Derived Mesenchymal Stem Cells Ameliorate Tendinopathy Pain by Inhibiting Mast Cell Activation. Nanomedicine 2022, 17, 513–529. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, R.; Ye, T.; Feng, K.; Zhang, J.; Chen, Y.; Xie, Z.; Wang, Y. The Therapeutic Effect of IMSC-Derived Small Extracellular Vesicles on Tendinopathy Related Pain Through Alleviating Inflammation: An in Vivo and in Vitro Study. J. Inflamm. Res. 2022, 15, 1421–1436. [Google Scholar] [CrossRef]
- Ye, T.; Chen, Z.; Zhang, J.; Luo, L.; Gao, R.; Gong, L.; Du, Y.; Xie, Z.; Zhao, B.; Li, Q.; et al. Large Extracellular Vesicles Secreted by Human IPSC-Derived MSCs Ameliorate Tendinopathy via Regulating Macrophage Heterogeneity. Bioact. Mater. 2023, 21, 194–208. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Li, X. Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Deliver Exogenous MiR-105-5p via Small Extracellular Vesicles to Rejuvenate Senescent Nucleus Pulposus Cells and Attenuate Intervertebral Disc Degeneration. Stem Cell Res. Ther. 2021, 12, 286. [Google Scholar] [CrossRef]
- Sheyn, D.; Ben-David, S.; Shapiro, G.; De Mel, S.; Bez, M.; Ornelas, L.; Sahabian, A.; Sareen, D.; Da, X.; Pelled, G.; et al. Human Induced Pluripotent Stem Cells Differentiate into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl. Med. 2016, 5, 1447–1460. [Google Scholar] [CrossRef]
- Villa-Diaz, L.G.; Brown, S.E.; Liu, Y.; Ross, A.M.; Lahann, J.; Parent, J.M.; Krebsbach, P.H. Derivation of Mesenchymal Stem Cells from Human Induced Pluripotent Stem Cells Cultured on Synthetic Substrates. Stem Cells 2012, 30, 1174–1181. [Google Scholar] [CrossRef]
- Eto, S.; Goto, M.; Soga, M.; Kaneko, Y.; Uehara, Y.; Mizuta, H.; Era, T. Mesenchymal Stem Cells Derived from Human IPS Cells via Mesoderm and Neuroepithelium Have Different Features and Therapeutic Potentials. PLoS ONE 2018, 13, e0200790. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, P.; Spitzhorn, L.-S.; Grassmann, J.; Tanner, S.; Latz, D.; Rahman, M.S.; Bohndorf, M.; Wruck, W.; Sager, M.; Grotheer, V.; et al. Human IPSC-Derived IMSCs Improve Bone Regeneration in Mini-Pigs. Bone Res. 2019, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, X.; Zhao, L.; Weir, M.D.; Sun, J.; Chen, W.; Man, Y.; Xu, H.H.K. Bone Tissue Engineering via Human Induced Pluripotent, Umbilical Cord and Bone Marrow Mesenchymal Stem Cells in Rat Cranium. Acta Biomater. 2015, 18, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, W.; Zhang, C.; Thein-Han, W.; Hu, K.; Reynolds, M.A.; Bao, C.; Wang, P.; Zhao, L.; Xu, H.H.K. Co-Seeding Human Endothelial Cells with Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells on Calcium Phosphate Scaffold Enhances Osteogenesis and Vascularization in Rats. Tissue Eng. Part A 2017, 23, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, X.; Chen, Q.; Bao, C.; Zhao, L.; Zhu, Z.; Xu, H.H.K. Angiogenic and Osteogenic Regeneration in Rats via Calcium Phosphate Scaffold and Endothelial Cell Co-Culture with Human Bone Marrow Mesenchymal Stem Cells (MSCs), Human Umbilical Cord MSCs, Human Induced Pluripotent Stem Cell-Derived MSCs and Human Embry: Co-Culture Stem Cells and Angiogenic and Osteogenic Regeneration in Rats. J. Tissue Eng. Regen. Med. 2018, 12, 191–203. [Google Scholar] [CrossRef]
- Zhou, M.; Xi, J.; Cheng, Y.; Sun, D.; Shu, P.; Chi, S.; Tian, S.; Ye, S. Reprogrammed Mesenchymal Stem Cells Derived from IPSCs Promote Bone Repair in Steroid-Associated Osteonecrosis of the Femoral Head. Stem Cell Res. Ther. 2021, 12, 175. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of Exosomes Secreted by Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells and Synovial Membrane-Derived Mesenchymal Stem Cells for the Treatment of Osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Chung, M.-J.; Park, S.; Son, J.-Y.; Lee, J.-Y.; Yun, H.H.; Lee, E.-J.; Lee, E.M.; Cho, G.-J.; Lee, S.; Park, H.-S.; et al. Differentiation of Equine Induced Pluripotent Stem Cells into Mesenchymal Lineage for Therapeutic Use. Cell Cycle 2019, 18, 2954–2971. [Google Scholar] [CrossRef]
- Jeong, J.; Shin, K.; Lee, S.B.; Lee, D.R.; Kwon, H. Patient-Tailored Application for Duchene Muscular Dystrophy on Mdx Mice Based Induced Mesenchymal Stem Cells. Exp. Mol. Pathol. 2014, 97, 253–258. [Google Scholar] [CrossRef]
- Arakura, M.; Lee, S.Y.; Fukui, T.; Oe, K.; Takahara, S.; Matsumoto, T.; Hayashi, S.; Matsushita, T.; Kuroda, R.; Niikura, T. Endochondral Bone Tissue Engineering Using Human Induced Pluripotent Stem Cells. Tissue Eng. Part A 2022, 28, 184–195. [Google Scholar] [CrossRef]
- Najar, M.; Raicevic, G.; André, T.; Fayyad-Kazan, H.; Pieters, K.; Bron, D.; Toungouz, M.; Lagneaux, L. Mesenchymal Stromal Cells from the Foreskin: Tissue Isolation, Cell Characterization and Immunobiological Properties. Cytotherapy 2016, 18, 320–335. [Google Scholar] [CrossRef]
- Skrzypczyk, A.; Giri, S.; Bader, A. Generation of Induced Pluripotent Stem Cell Line from Foreskin Fibroblasts. Stem Cell Res. 2016, 17, 572–575. [Google Scholar] [CrossRef][Green Version]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically Defined Conditions for Human IPSC Derivation and Culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef]
- Tang, M.; Chen, W.; Liu, J.; Weir, M.D.; Cheng, L.; Xu, H.H.K. Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cell Seeding on Calcium Phosphate Scaffold for Bone Regeneration. Tissue Eng. Part A 2014, 20, 1295–1305. [Google Scholar] [CrossRef]
- Dupuis, V.; Oltra, E. Methods to Produce Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells: Mesenchymal Stem Cells from Induced Pluripotent Stem Cells. World J. Stem Cells 2021, 13, 1094–1111. [Google Scholar] [CrossRef]
- Estrada, J.C.; Torres, Y.; Benguría, A.; Dopazo, A.; Roche, E.; Carrera-Quintanar, L.; Pérez, R.A.; Enríquez, J.A.; Torres, R.; Ramírez, J.C.; et al. Human Mesenchymal Stem Cell-Replicative Senescence and Oxidative Stress Are Closely Linked to Aneuploidy. Cell Death Dis. 2013, 4, e691. [Google Scholar] [CrossRef]
- Muraglia, A.; Cancedda, R.; Quarto, R. Clonal Mesenchymal Progenitors from Human Bone Marrow Differentiate in Vitro According to a Hierarchical Model. J. Cell Sci. 2000, 113, 1161–1166. [Google Scholar] [CrossRef]
- Wruck, W.; Graffmann, N.; Spitzhorn, L.-S.; Adjaye, J. Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Acquire Rejuvenation and Reduced Heterogeneity. Front. Cell Dev. Biol. 2021, 9, 717772. [Google Scholar] [CrossRef]
- Lian, Q.; Zhang, Y.; Zhang, J.; Zhang, H.K.; Wu, X.; Zhang, Y.; Lam, F.F.-Y.; Kang, S.; Xia, J.C.; Lai, W.-H.; et al. Functional Mesenchymal Stem Cells Derived from Human Induced Pluripotent Stem Cells Attenuate Limb Ischemia in Mice. Circulation 2010, 121, 1113–1123. [Google Scholar] [CrossRef]
- Lee, H.-R.; Kim, S.; Shin, S.; Jeong, S.-Y.; Lee, D.-W.; Lim, S.-U.; Kang, J.Y.; Son, M.-Y.; Lee, C.; Yu, K.-R.; et al. IPSC-Derived MSCs Are a Distinct Entity of MSCs with Higher Therapeutic Potential than Their Donor-Matched Parental MSCs. Int. J. Mol. Sci. 2023, 24, 881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, L.; Wang, Z.; Gao, W.; Chen, W.; Zhang, H.; Jing, B.; Zhu, X.; Chen, L.; Zheng, C.; et al. Eradication of Specific Donor-Dependent Variations of Mesenchymal Stem Cells in Immunomodulation to Enhance Therapeutic Values. Cell Death Dis. 2021, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.M.; Kameishi, S.; Grainger, D.W.; Okano, T. Strategies to Address Mesenchymal Stem/Stromal Cell Heterogeneity in Immunomodulatory Profiles to Improve Cell-Based Therapies. Acta Biomater. 2021, 133, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Liao, J.; Zhang, F.; Zhou, G. Donor Genetic Backgrounds Contribute to the Functional Heterogeneity of Stem Cells and Clinical Outcomes. Stem Cells Transl. Med. 2020, 9, 1495–1499. [Google Scholar] [CrossRef]
- Spitzhorn, L.-S.; Megges, M.; Wruck, W.; Rahman, M.S.; Otte, J.; Degistirici, Ö.; Meisel, R.; Sorg, R.V.; Oreffo, R.O.C.; Adjaye, J. Human IPSC-Derived MSCs (IMSCs) from Aged Individuals Acquire a Rejuvenation Signature. Stem Cell Res. Ther. 2019, 10, 100. [Google Scholar] [CrossRef]
- Zhao, C.; Ikeya, M. Generation and Applications of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells. Stem Cells Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Joswig, A.-J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R.; Watts, A.E. Repeated Intra-Articular Injection of Allogeneic Mesenchymal Stem Cells Causes an Adverse Response Compared to Autologous Cells in the Equine Model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef]
- Berglund, A.K.; Fortier, L.A.; Antczak, D.F.; Schnabel, L.V. Immunoprivileged No More: Measuring the Immunogenicity of Allogeneic Adult Mesenchymal Stem Cells. Stem Cell Res. Ther. 2017, 8, 288. [Google Scholar] [CrossRef]
- Araki, R.; Uda, M.; Hoki, Y.; Sunayama, M.; Nakamura, M.; Ando, S.; Sugiura, M.; Ideno, H.; Shimada, A.; Nifuji, A.; et al. Negligible Immunogenicity of Terminally Differentiated Cells Derived from Induced Pluripotent or Embryonic Stem Cells. Nature 2013, 494, 100–104. [Google Scholar] [CrossRef]
- Ng, J.; Hynes, K.; White, G.; Sivanathan, K.N.; Vandyke, K.; Bartold, P.M.; Gronthos, S. Immunomodulatory Properties of Induced Pluripotent Stem Cell-Derived Mesenchymal Cells. J. Cell. Biochem. 2016, 117, 2844–2853. [Google Scholar] [CrossRef]
- Shahsavari, A.; Weeratunga, P.; Ovchinnikov, D.A.; Whitworth, D.J. Pluripotency and Immunomodulatory Signatures of Canine Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells Are Similar to Harvested Mesenchymal Stromal Cells. Sci. Rep. 2021, 11, 3486. [Google Scholar] [CrossRef]
- Giuliani, M.; Oudrhiri, N.; Noman, Z.M.; Vernochet, A.; Chouaib, S.; Azzarone, B.; Durrbach, A.; Bennaceur-Griscelli, A. Human Mesenchymal Stem Cells Derived from Induced Pluripotent Stem Cells Down-Regulate NK-Cell Cytolytic Machinery. Blood 2011, 118, 3254–3262. [Google Scholar] [CrossRef]
- Kang, R.; Luo, Y.; Zou, L.; Xie, L.; Lysdahl, H.; Jiang, X.; Chen, C.; Bolund, L.; Chen, M.; Besenbacher, F.; et al. Osteogenesis of Human Induced Pluripotent Stem Cells Derived Mesenchymal Stem Cells on Hydroxyapatite Contained Nanofibers. RSC Adv. 2014, 4, 5734. [Google Scholar] [CrossRef]
- Zou, L.; Luo, Y.; Chen, M.; Wang, G.; Ding, M.; Petersen, C.C.; Kang, R.; Dagnaes-Hansen, F.; Zeng, Y.; Lv, N.; et al. A Simple Method for Deriving Functional MSCs and Applied for Osteogenesis in 3D Scaffolds. Sci. Rep. 2013, 3, 2243. [Google Scholar] [CrossRef]
- Liu, T.M.; Yildirim, E.D.; Li, P.; Fang, H.T.; Denslin, V.; Kumar, V.; Loh, Y.H.; Lee, E.H.; Cool, S.M.; Teh, B.T.; et al. Ascorbate and Iron Are Required for the Specification and Long-Term Self-Renewal of Human Skeletal Mesenchymal Stromal Cells. Stem Cell Rep. 2020, 14, 210–225. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.M. Generation of Mesenchymal Stem-like Cells for Producing Extracellular Vesicles. World J. Stem Cells 2019, 11, 270–280. [Google Scholar] [CrossRef]
- Bloor, A.J.C.; Patel, A.; Griffin, J.E.; Gilleece, M.H.; Radia, R.; Yeung, D.T.; Drier, D.; Larson, L.S.; Uenishi, G.I.; Hei, D.; et al. Production, Safety and Efficacy of IPSC-Derived Mesenchymal Stromal Cells in Acute Steroid-Resistant Graft versus Host Disease: A Phase I, Multicenter, Open-Label, Dose-Escalation Study. Nat. Med. 2020, 26, 1720–1725. [Google Scholar] [CrossRef]
- Sakurai, H.; Sakaguchi, Y.; Shoji, E.; Nishino, T.; Maki, I.; Sakai, H.; Hanaoka, K.; Kakizuka, A.; Sehara-Fujisawa, A. In Vitro Modeling of Paraxial Mesodermal Progenitors Derived from Induced Pluripotent Stem Cells. PLoS ONE 2012, 7, e47078. [Google Scholar] [CrossRef]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of Tumorigenicity in Mesenchymal Stromal Cell–Based Therapies—Bridging Scientific Observations and Regulatory Viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef]
- Neofytou, E.; O’Brien, C.G.; Couture, L.A.; Wu, J.C. Hurdles to Clinical Translation of Human Induced Pluripotent Stem Cells. J. Clin. Investig. 2015, 125, 2551–2557. [Google Scholar] [CrossRef]
- Liu, X.; Robbins, S.; Wang, X.; Virk, S.; Schuck, K.; Deveza, L.A.; Oo, W.M.; Carmichael, K.; Antony, B.; Eckstein, F.; et al. Efficacy and Cost-Effectiveness of Stem Cell Injections for Symptomatic Relief and StrUctural Improvement in People with Tibiofemoral Knee OsteoaRthritis: Protocol for a Randomised Placebo-Controlled Trial (the SCUlpTOR Trial). BMJ Open 2021, 11, e056382. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hong, Y.; Zhang, H.; Li, X. Mesenchymal Stem Cell Senescence and Rejuvenation: Current Status and Challenges. Front. Cell Dev. Biol. 2020, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, J.D.; Bao, X.; Kaneda, G.; Avalos, P.; Behrens, P.; Salehi, K.; Da, X.; Chen, A.; Castaneda, C.; Nakielski, P.; et al. IPSC-Neural Crest Derived Cells Embedded in 3D Printable Bio-Ink Promote Cranial Bone Defect Repair. Sci. Rep. 2022, 12, 18701. [Google Scholar] [CrossRef] [PubMed]
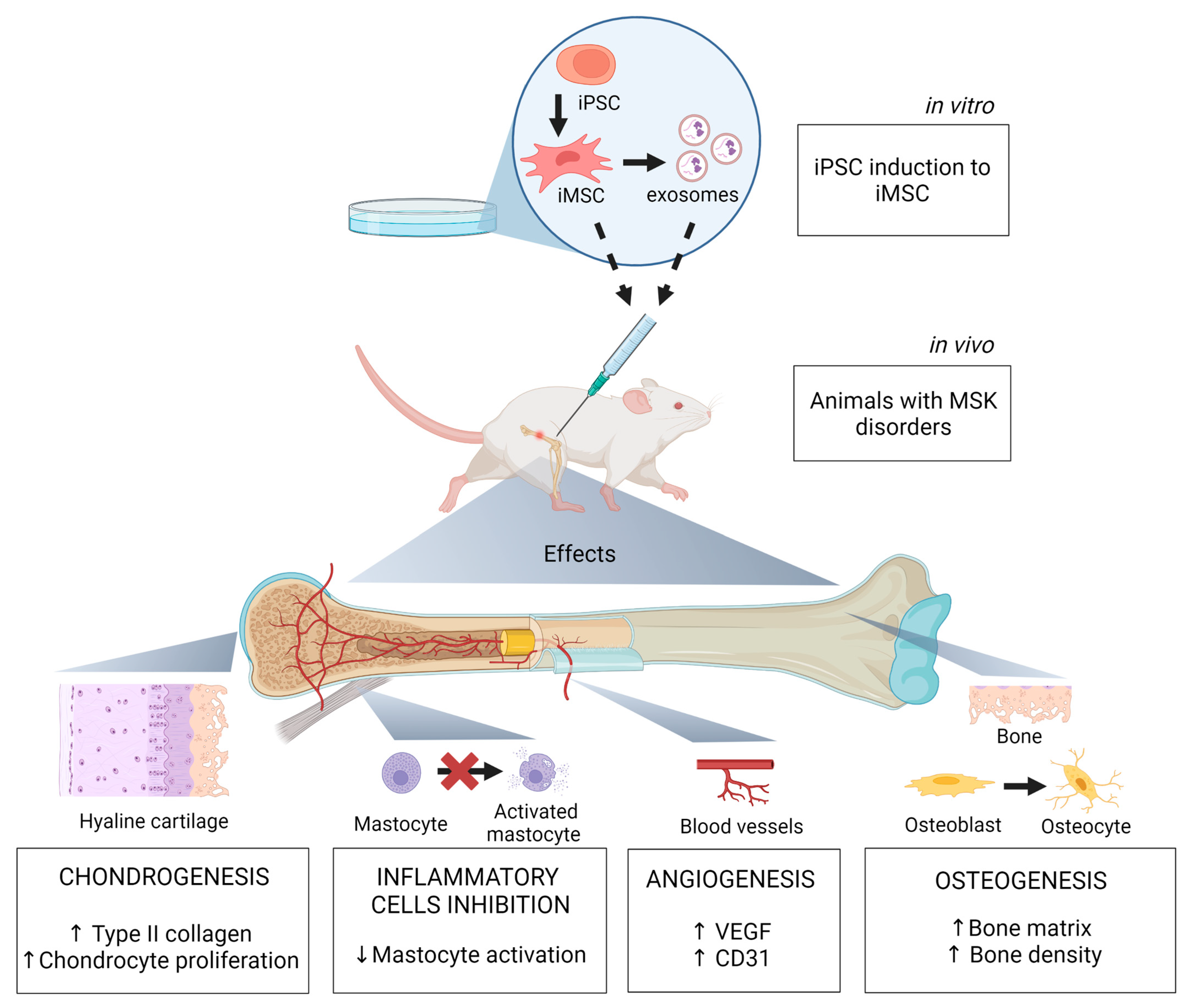
| References | iPSC Source | iMSC Differentiation Method | Animal Model | MSK Disorder | Effects/Mechanisms |
|---|---|---|---|---|---|
| [37] | Fibroblast | MSC Switch | Sprague Dawley Rats injected with 4% carrageenan | Tendinopathy | iMSC exosomes inhibit mast cells activation and their interaction with nerve fibers via the HIF-1 signaling pathway |
| [38] | Fibroblast | MSC Switch | Sprague Dawley Rats injected with 4% carrageenan | Tendinopathy | iMSC-EV alleviate inflammation (reduced proinflammatory cytokines) and inhibit capillary proliferation |
| [39] | Fibroblast | MSC Switch | Sprague Dawley Rats injected with 4% carrageenan | Tendinopathy | iMSC-lEV attenuate pain and inflammation by regulating the p38 MAPK signaling pathway |
| [40] | Fibroblast | MSC Switch | Sprague Dawley Rats with intervertebral caudal disc puncture | Intervertebral disc degeneration | iMSC-sEV rejuvenate nucleus pulposus cells and attenuate intervertebral disc degeneration |
| [41] | Fibroblast | Embryoid Body Formation | NOD/SCID mice | Nonunion radial fracture | iMSC transfected with BMP-6 induced ectopic bone formation by increasing bone volume density |
| [42] | Fibroblast | Embryoid Body formation | SCID mice | Craniofacial bone defect | iMSC promoted new bone formation in mice with calvarial defects |
| [43] | Fibroblast | Specific Differentiation | NOJ male mice with anterior cruciate ligament transection | Osteoarthritis | iMSC generated by mesodermal and neuroepithelium differentiation suppressed the degeneration of knee cartilage |
| [44] | Fibroblast | Pathway inhibitor and MSC switch | Goettingen mini-pigs | Critical-sized defect of proximal tibia | iMSC loaded on calcium phosphate granules promote new bone formation on the central defect area |
| [45,46,47] | Adult bone marrow CD 34+ | Embryoid Body Formation | Athymic nude rats | Critical-sized cranial defect | iMSC seeded on CPC scaffolds increased blood vessel density and promoted de novo bone formation; effects were increased with co-seeded endothelial cells. |
| [48] | HEK293T | Embryoid body formation | Steroid-induced ONFH Sprague Dawley Rats | Osteonecrosis of femoral head (ONFH) | iMSC promote bone repair by forming new dense trabecular bones and increase angiogenesis by elevating VEGF and CD3 expression in the femoral head |
| [49] | - | MSC Switch | Collagen-induced osteoarthritis in C57B/L10 mice | Osteoarthritis | iMSC exosomes stimulate chondrocyte migration and proliferation; increased expression of collagen type II in superficial and deep zones of articular cartilage |
| [50] | - | MSC Switch | Bilateral ovariectomy Sprague Dawley Rats | Critical-sized bone defects in osteoporosis | iMSC exosomes associated with β-TCP scaffolds improve bone regeneration through osteogenesis and angiogenesis |
| [51] | Adipose derived Stem Cell | MSC Switch | Thoroughbreds horses | Fracture, arthritis, tendonitis, and osteochondritis | iMSC reduce lameness, fever, and fracture lines |
| [52] | - | Embryoid Body Formation | C57BL/10 mdx mice (injured tibialis anterior skeletal muscle) | Duchenne Muscular Dystrophy | iMSC diminish cellular stress related to reactive oxygen species and restor dystrophin expression in muscle cells |
| [53] | - | - | Nude mice | Radial bone defect | Chondrogenic pellets derived from iMSC promote high rate of bone union and transition from hypertrophic cartilage to newly formed woven bone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, I.X.; Cordeiro, A.; Guimarães, J.A.M.; Silva, K.R. Potential and Limitations of Induced Pluripotent Stem Cells-Derived Mesenchymal Stem Cells in Musculoskeletal Disorders Treatment. Biomolecules 2023, 13, 1342. https://doi.org/10.3390/biom13091342
Dias IX, Cordeiro A, Guimarães JAM, Silva KR. Potential and Limitations of Induced Pluripotent Stem Cells-Derived Mesenchymal Stem Cells in Musculoskeletal Disorders Treatment. Biomolecules. 2023; 13(9):1342. https://doi.org/10.3390/biom13091342
Chicago/Turabian StyleDias, Isabelle Xavier, Aline Cordeiro, João Antonio Matheus Guimarães, and Karina Ribeiro Silva. 2023. "Potential and Limitations of Induced Pluripotent Stem Cells-Derived Mesenchymal Stem Cells in Musculoskeletal Disorders Treatment" Biomolecules 13, no. 9: 1342. https://doi.org/10.3390/biom13091342
APA StyleDias, I. X., Cordeiro, A., Guimarães, J. A. M., & Silva, K. R. (2023). Potential and Limitations of Induced Pluripotent Stem Cells-Derived Mesenchymal Stem Cells in Musculoskeletal Disorders Treatment. Biomolecules, 13(9), 1342. https://doi.org/10.3390/biom13091342






