Evaluation of Biointegration and Inflammatory Response to Blood Vessels Produced by Tissue Engineering—Experimental Model in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Handling Conditions and Tissue Acquisition
2.2. Scaffolds Production
2.3. Obtaining Adipose-Derived Stem Cells (ASCs)
2.4. Experimental Groups
2.5. Biological Scaffold and ASCs Seeding
2.6. Surgical Experimentation
2.7. Analysis of Interleukins and Oxidative Stress
2.8. Histomorphology
2.9. Statistical Analysis
3. Results
3.1. Animals
3.2. Vein Decellularization, ASC Characterization, and In Vivo Experimentation
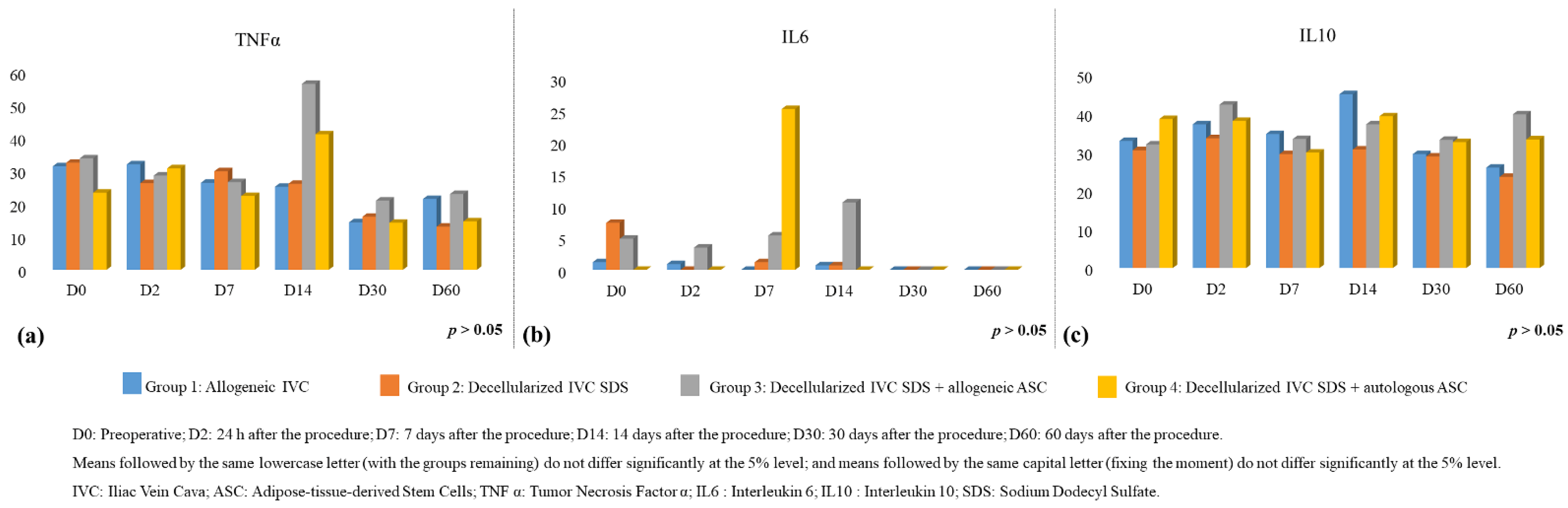
3.3. Interleukin Analysis
3.3.1. Plasma
3.3.2. Frozen Explant Tissue Homogenate
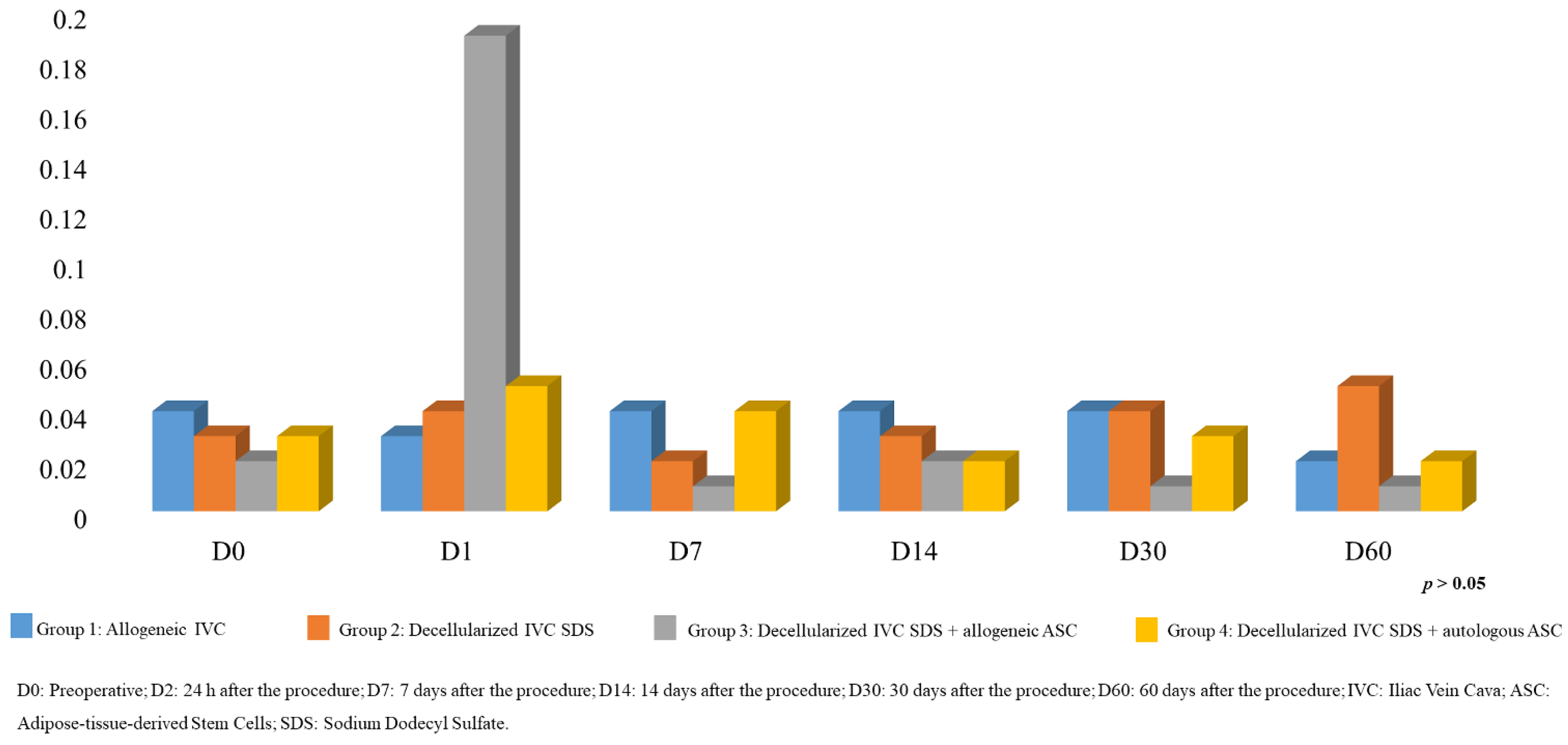
3.3.3. Oxidative Stress by Plasma MDA Analysis
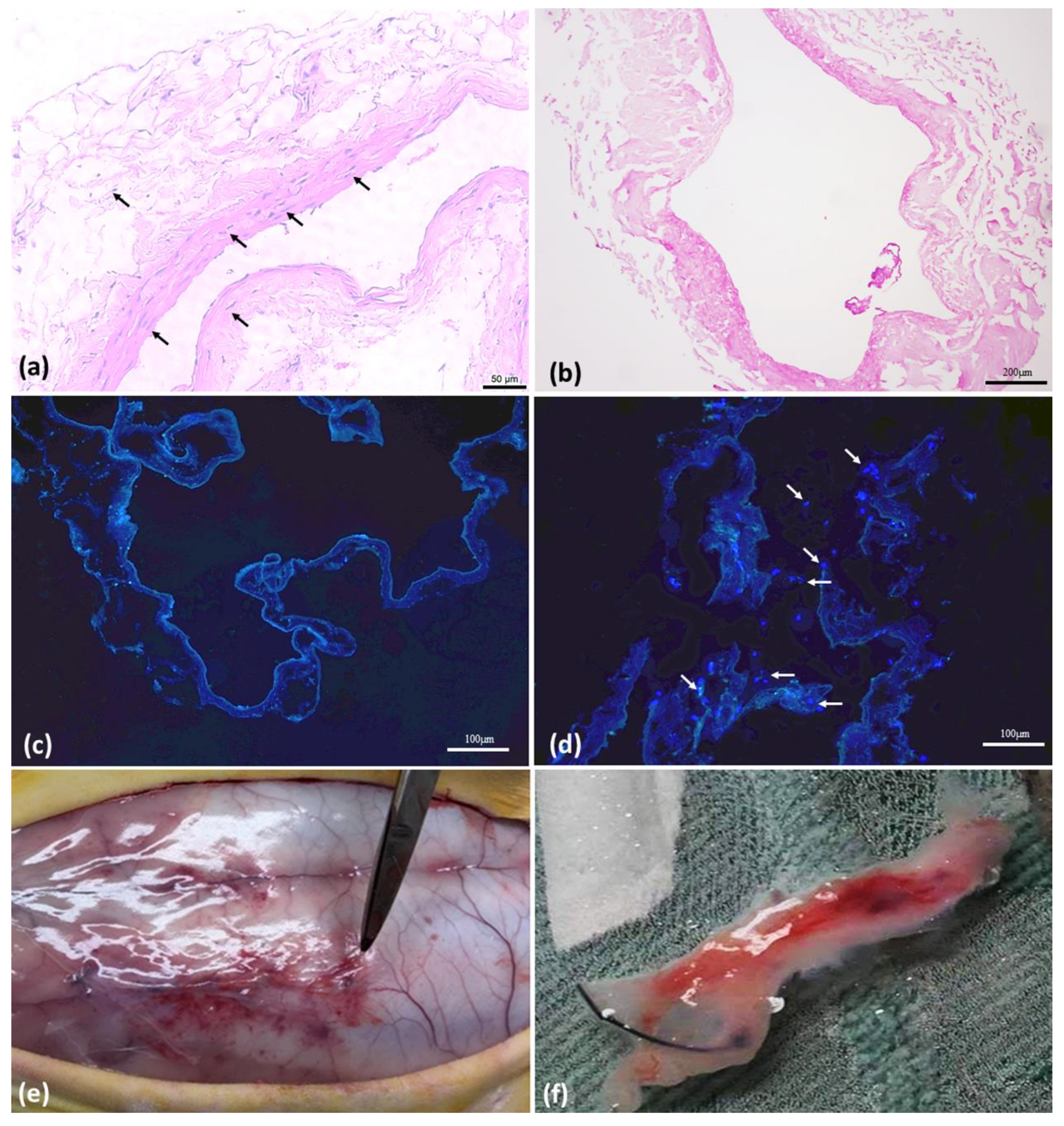
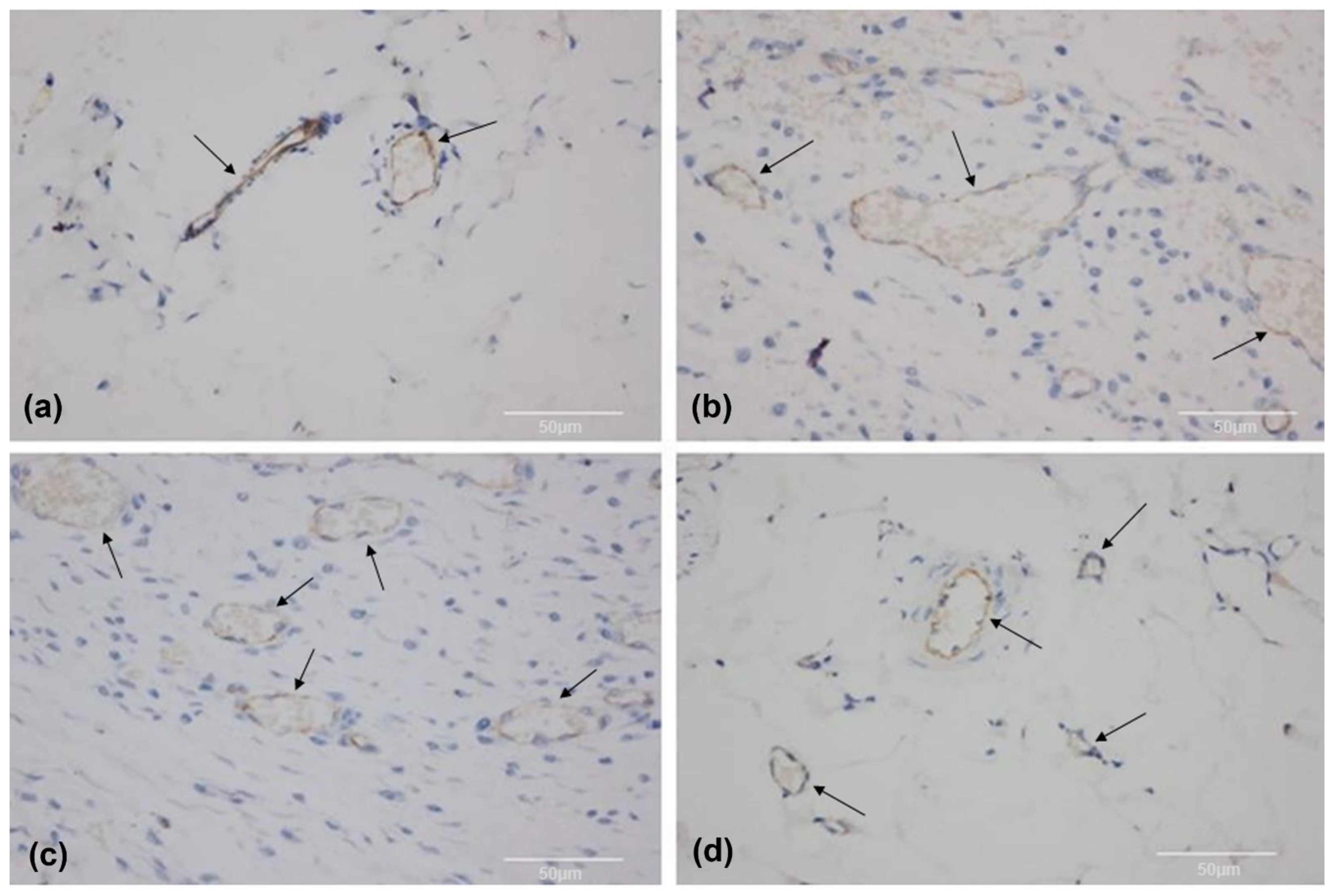
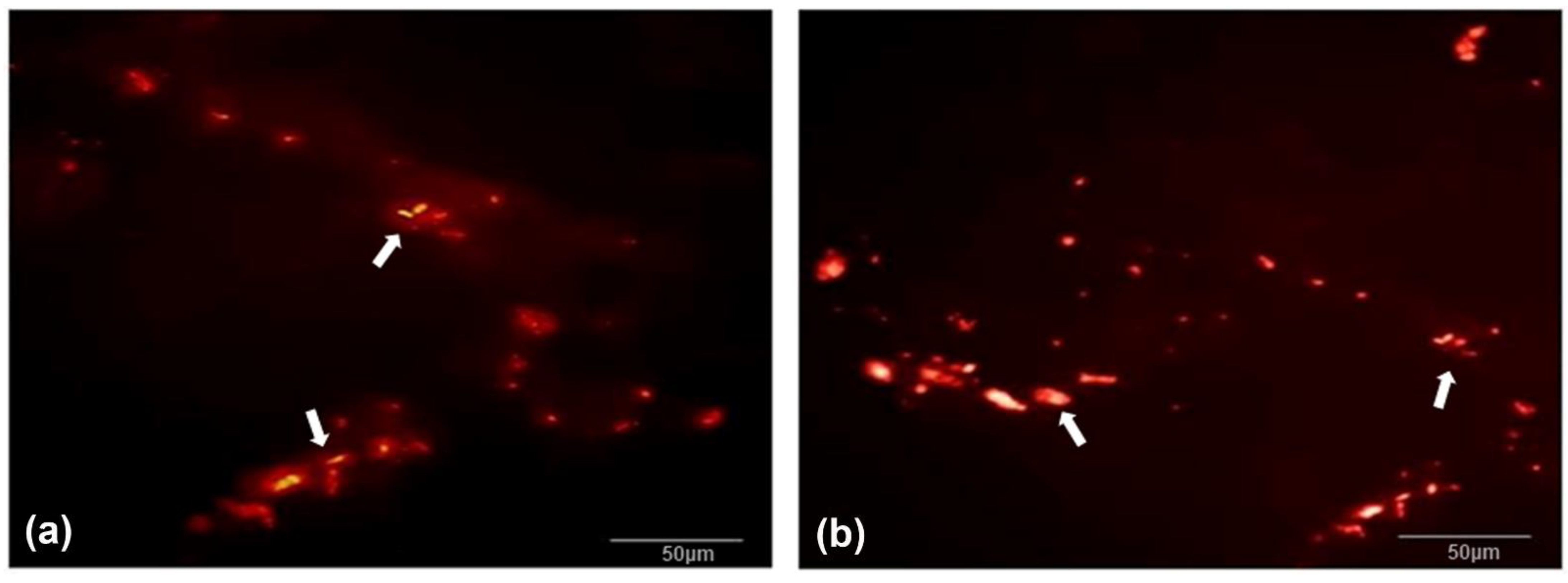
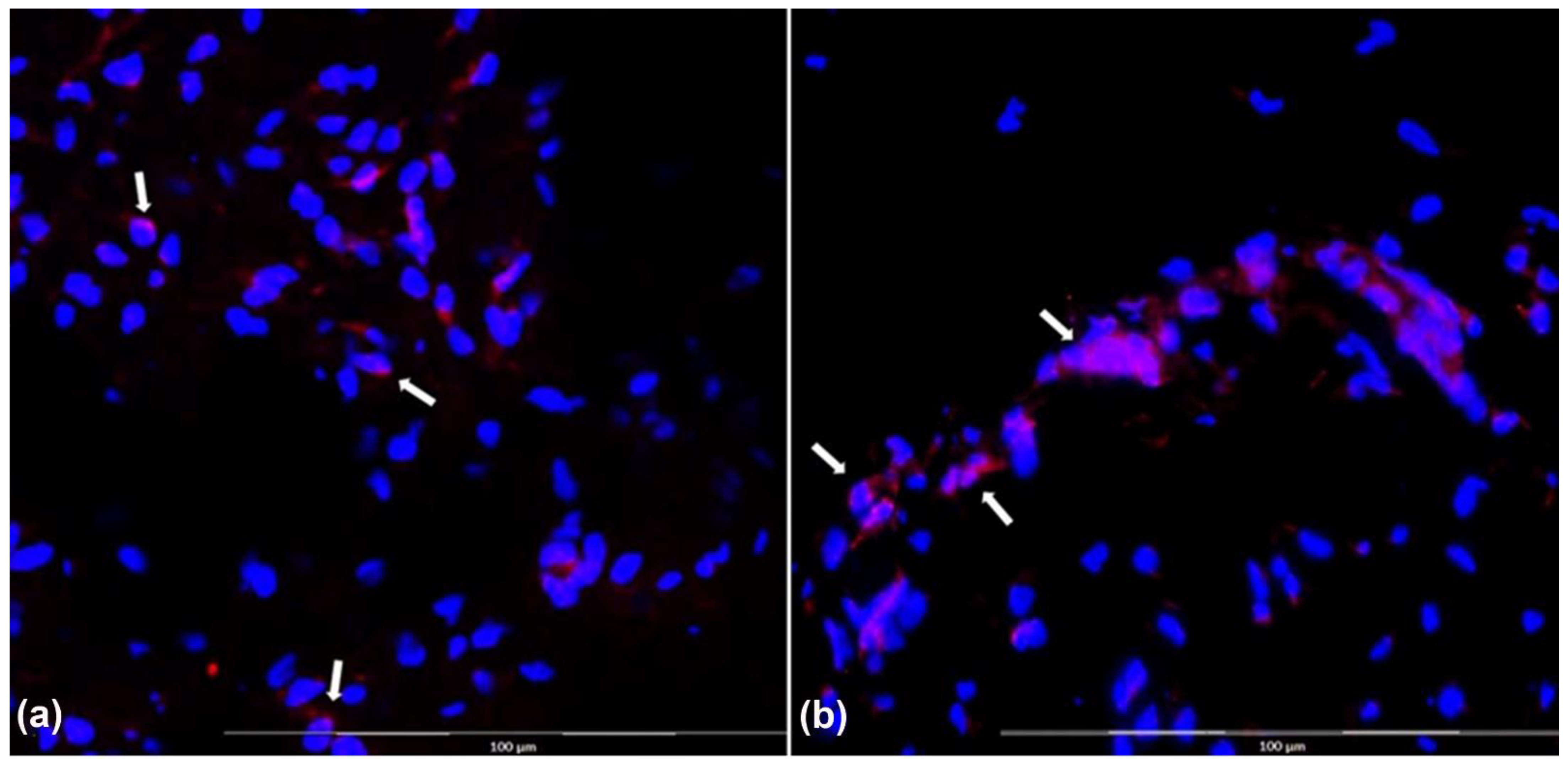
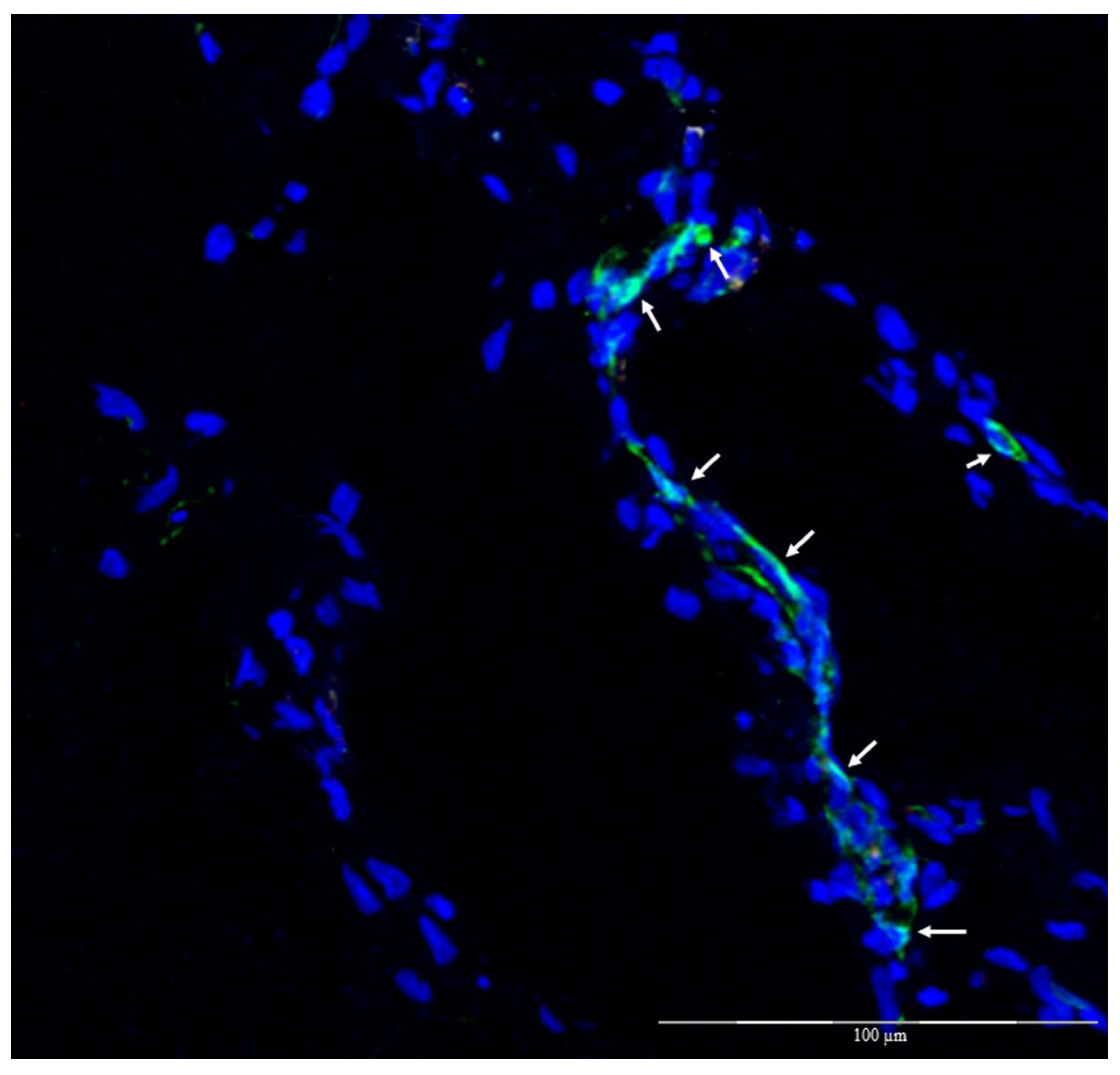
3.4. Histomorphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Deb, S.; Wijeysundera, H.C.; Ko, D.T.; Tsubota, H.; Hill, S.; Fremes, S.E. Coronary artery bypass graft surgery vs percutaneous interventions in coronary revascularization: A systematic review. JAMA 2013, 310, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Halliday, A.; Bax, J.J. The 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 301–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharamti, A.; Kanafani, Z.A. Vascular Graft Infections: An update. Infect. Dis. Clin. N. Am. 2018, 32, 789–809. [Google Scholar] [CrossRef] [PubMed]
- Lejay, A.; Vento, V.; Kuntz, S.; Steinmetz, L.; Georg, Y.; Thaveau, F.; Heim, F.; Chakfé, N. Current status on vascular substitutes. J. Cardiovasc. Surg. 2020, 61, 538–543. [Google Scholar] [CrossRef]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [Green Version]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [Green Version]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [Green Version]
- Bertanha, M.; Moroz, A.; Jaldin, R.G.; Silva, R.A.; Rinaldi, J.C.; Golim, M.A.; Felisbino, S.L.; Domingues, M.A.; Sobreira, M.L.; Reis, P.P.; et al. Morphofunctional characterization of decellularized vena cava as tissue engineering scaffolds. Exp. Cell Res. 2014, 326, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Bertanha, M.; Sobreira, M.L.; Bovolato, A.L.C.; Rinaldi, J.D.C.; Reis, P.P.; Moroz, A.; de Moraes, L.N.; Deffune, E. Ultrastructural analysis and residual DNA evaluation of rabbit vein arcabouço. Acta Cir. Bras. 2017, 32, 706–711. [Google Scholar] [CrossRef]
- Vemuri, M.C.; Chase, L.G.; Rao, M.S. Mesenchymal stem cell assays and applications. Methods Mol. Biol. 2011, 698, 3–8. [Google Scholar] [PubMed]
- Fang, S.; Ellman, D.G.; Andersen, D.C. Review: Tissue Engineering of Small-Diameter Vascular Grafts and Their In Vivo Evaluation in Large Animals and Humans. Cells 2021, 10, 713. [Google Scholar] [CrossRef]
- Bertanha, M.; Moroz, A.; Almeida, R.; Alves, F.C.; Valério, M.J.A.; Moura, R.; Domingues, M.A.C.; Sobreira, M.L.; Deffune, E. Tissue-engineered blood vessel substitute by reconstruction of endothelium using mesenchymal stem cells induced by platelet growth factors. J. Vasc. Surg. 2014, 59, 1677–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertanha, M. Perspectivas de uso de células-tronco em cirurgia vascular. J. Vasc. Bras. 2016, 15, 173–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, L.D.S.; Bovolato, A.L.C.; Silva, B.E.; Chizzolini, L.V.; da Cruz, B.L.; de Toledo Moraes, M.P.; de Arruda Lourenção, P.L.T.; Bertanha, M. Quantification of adhesion of mesenchymal stem cells spread on decellularized vein scaffold. Acta Cir. Bras. 2021, 36, e361001. [Google Scholar] [CrossRef]
- Elias, D.B.D.; Freitas, R.M.D.; Gonçalves, R.P.; Magalhães, H.Y.F.; De Sousa, J.H.; Magalhães, S.M.M. Evaluation of the concentration of malondialdehyde and nitrite in patients with sickle cell anemia treated or not with hydroxyurea. Einstein 2010, 8, 414–418. [Google Scholar] [CrossRef]
- Harasymiak-Krzyżanowska, I.; Niedojadło, A.; Karwat, J.; Kotuła, L.; Gil-Kulik, P.; Sawiuk, M.; Kocki, J. Adipose tissue-derived stem cells show considerable promise for regenerative medicine applications. Cell Mol. Biol. Lett. 2013, 18, 479–493. [Google Scholar] [CrossRef]
- Baksh, D.; Song, L.; Tuan, R.S. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell Mol. Med. 2004, 8, 301–316. [Google Scholar] [CrossRef]
- Song, H.G.; Rumma, R.T.; Osaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular tissue engineering: Progress, challenges, and clinical promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef] [Green Version]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef]
- Afra, S.; Matin, M.M. Potential of mesenchymal stem cells for bioengineered blood vessels in comparison with other eligible cell sources. Cell Tissue Res. 2020, 380, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vollkommer, T.; Henningsen, A.; Friedrich, R.E.; Felthaus, O.H.; Eder, F.; Morsczeck, C.; Smeets, R.; Gehmert, S.; Gosau, M. Extent of inflammation and foreign body reaction to porous polyethylene in vitro and in vivo. In Vivo 2019, 33, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariotti, J.; Penack, O.; Castagna, L. Acute graft-versus-host-disease other than typical targets: Between myths and facts. Transplant Cell Ther. 2021, 27, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Lange, C.; Schulz, U.; Sviland, L.; Eissner, G.; Oliver, K.M.; Jackson, G.H.; Holler, E.; Dickinson, A.M. Interleukin-10 modulation of alloreactivity and graft-versus-host reactions. Transplantation 2002, 74, 772–778. [Google Scholar] [CrossRef]
- Steen-Louws, C.; Boross, P.; Prado, J.; Meeldijk, J.; Langenhorst, J.B.; Huitema, A.D.R.; Hartog, M.T.D.; Boon, L.; Lafeber, F.P.J.G.; Hack, C.E.; et al. Sialic acid-engineered IL4-10 fusion protein is bioactive and rapidly cleared from the circulation. Pharm. Res. 2019, 37, 17. [Google Scholar] [CrossRef] [Green Version]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.C.; Yu, K.R. Impact of mesenchymal stem cell senescence on inflammaging. BMB Rep. 2020, 53, 65–73. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secondo, M.T.S.; Rodrigues, L.d.S.; Ramos, L.P.M.; Bovolato, A.L.C.; Rodriguez-Sanchez, D.N.; Sobreira, M.L.; Moraes, M.P.d.T.; Bertanha, M. Evaluation of Biointegration and Inflammatory Response to Blood Vessels Produced by Tissue Engineering—Experimental Model in Rabbits. Biomolecules 2022, 12, 1776. https://doi.org/10.3390/biom12121776
Secondo MTS, Rodrigues LdS, Ramos LPM, Bovolato ALC, Rodriguez-Sanchez DN, Sobreira ML, Moraes MPdT, Bertanha M. Evaluation of Biointegration and Inflammatory Response to Blood Vessels Produced by Tissue Engineering—Experimental Model in Rabbits. Biomolecules. 2022; 12(12):1776. https://doi.org/10.3390/biom12121776
Chicago/Turabian StyleSecondo, Mariana Thaís Silva, Lenize da Silva Rodrigues, Leandro Pereira Miranda Ramos, Ana Lívia Carvalho Bovolato, Diego Noé Rodriguez-Sanchez, Marcone Lima Sobreira, Marcelo Padovani de Toledo Moraes, and Matheus Bertanha. 2022. "Evaluation of Biointegration and Inflammatory Response to Blood Vessels Produced by Tissue Engineering—Experimental Model in Rabbits" Biomolecules 12, no. 12: 1776. https://doi.org/10.3390/biom12121776
APA StyleSecondo, M. T. S., Rodrigues, L. d. S., Ramos, L. P. M., Bovolato, A. L. C., Rodriguez-Sanchez, D. N., Sobreira, M. L., Moraes, M. P. d. T., & Bertanha, M. (2022). Evaluation of Biointegration and Inflammatory Response to Blood Vessels Produced by Tissue Engineering—Experimental Model in Rabbits. Biomolecules, 12(12), 1776. https://doi.org/10.3390/biom12121776






