Acyl-Lipid Δ6-Desaturase May Act as a First FAD in Cyanobacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cyanobacterial and Bacterial Strains
2.2. Genetic Constructions
2.3. FA Analysis
2.3.1. Synthesis of Fatty Acid Methyl Esters (FAMEs)
2.3.2. Pre-Concentration of Minor FA
2.3.3. 3-Pyridylcarbinol Esters Synthesis
2.4. GC/MS Parameters
3. Results
3.1. Expression of the desD Gene in S. elongatus PCC 7942
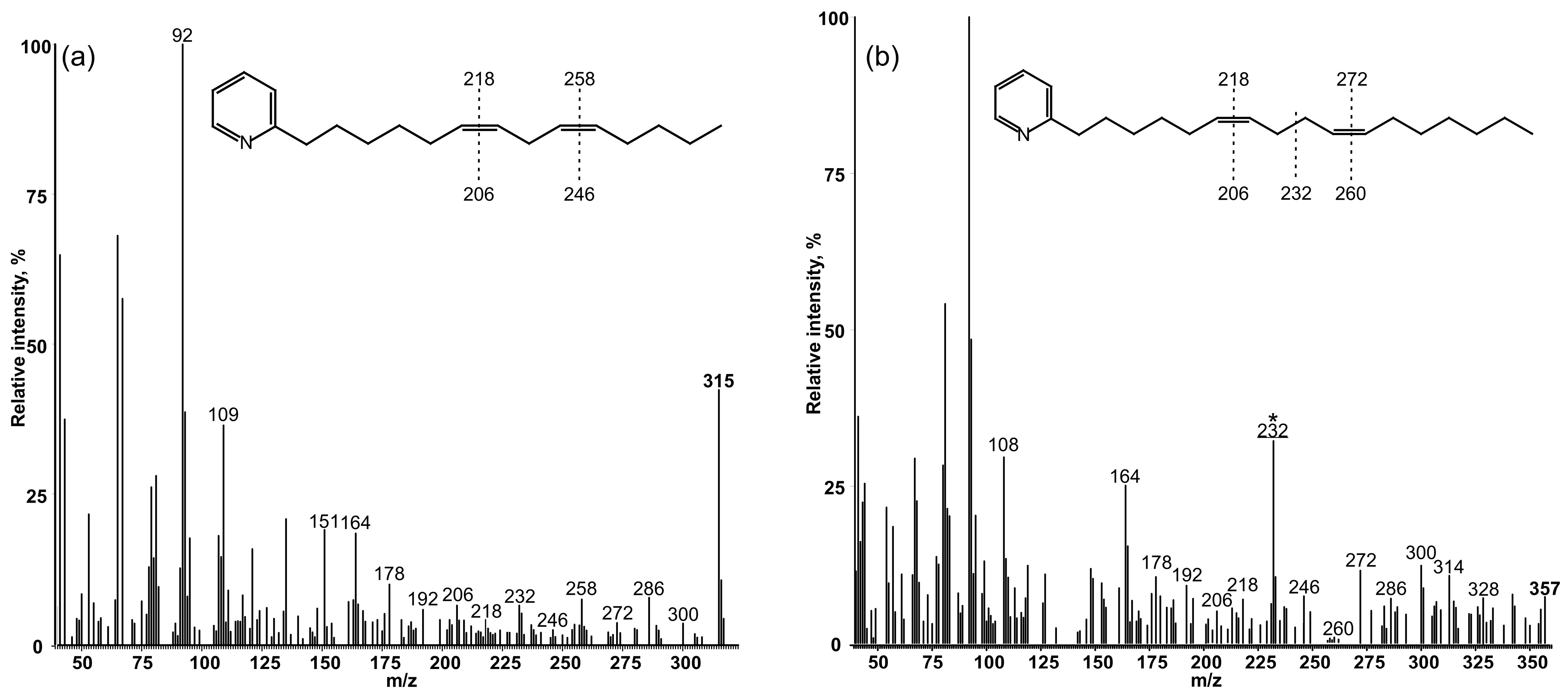
3.2. Determination of the Position of Double Bonds in FAs
3.3. Determination of the Position of the Second Double Bond in Fas
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meesapyodsuk, D.; Qiu, X. The front-end desaturase: Structure, function, evolution and biotechnological use. Lipids 2012, 47, 227–237. [Google Scholar] [CrossRef]
- Sayanova, O.; Smith, M.A.; Lapinskas, P.; Stobart, A.K.; Dobson, G.; Christie, W.W.; Shewry, P.R.; Napier, J.A. Expression of a borage desaturase cDNA containing an N-terminal cytochrome b5 domain results in the accumulation of high levels of Δ6-desaturated fatty acids in transgenic tobacco. Proc. Natl. Acad. Sci. USA 1997, 94, 4211–4216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napier, J.A.; Hey, S.J.; Lacey, D.J.; Shewry, P.R. Identification of a Caenorhabditis elegans Δ6-fatty-acid-desaturase by heterologous expression in Saccharomyces cerevisiae. Biochem. J. 1998, 330, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Los, D.A.; Ray, M.K.; Murata, N. Differences in the control of the temperature-dependent expression of four genes for desaturases in Synechocystis sp. PCC 6803. Mol. Microbiol. 1997, 25, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Nuccio, M.L.; Gross, L.M.; Thomas, T.L. Isolation of a Δ6-desaturase gene from the cyanobacterium Synechocystis sp. strain PCC 6803 by gain-of-function expression in Anabaena sp. strain PCC 7120. Plant Mol. Biol. 1993, 22, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Deshnium, P.; Tasaka, Y. Biosynthesis of γ-linolenic acid in the cyanobacterium Spirulina platensis. In γ-Linolenic Acid: Metabolism and Its Roles in Nutrition and Medicine; Huang, Y.S., Mill, D.E., Eds.; AOCS Press: Urbana, IL, USA, 1996; pp. 22–32. [Google Scholar]
- Higashi, S.; Murata, N. An in vivo study of substrate specificities of acyl-lipid desaturases and acyltransferases in lipid synthesis in Synechocystis PCC6803. Plant Physiol. 1993, 102, 1275–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanklin, J.; Cahoon, E.B. Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 611–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starikov, A.Y.; Sidorov, R.A.; Mironov, K.S.; Goriainov, S.V.; Los, D.A. Delta or Omega? Δ12 (ω6) fatty acid desaturases count 3C after the pre-existing double bond. Biochimie 2020, 179, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R. Isolation and purification of cyanobacteria. Methods Enzymol. 1988, 167, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.R.; Tsinoremas, N.F.; Shelton, J.; Lebedeva, N.V.; Yarrow, J.; Min, H.; Golden, S.S. Application of bioluminescence to the study of circadian rhythms in cyanobacteria. Methods Enzymol. 2000, 305, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.G.K. Construction of specific mutations in photosystem II photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Methods Enzymol. 1988, 167, 766–778. [Google Scholar] [CrossRef]
- Garwin, J.L.; Klages, A.L.; Cronan, J.E. β-Ketoacyl-acyl carrier protein synthase II of Escherichia coli. Evidence for function in the thermal regulation of fatty acid synthesis. J. Biol. Chem. 1980, 255, 3263–3265. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Moorman, R.; Vanhercke, T.; Petrie, J.; Singh, S.; Jackson, C.J. Classification and substrate head-group specificity of membrane fatty acid desaturases. Comput. Struct. Biotechnol. J. 2016, 14, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahoon, E.B.; Ohlrogge, J.B. Metabolic evidence for the involvement of a Δ4-palmitoyl-acyl carrier protein desaturase in the synthesis of petroselinic acid in coriander endosperm and transgenic tobacco cells. Plant Physiol. 1994, 104, 827–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, K.J. Palmitoleate formation by soybean stearoyl-acyl carrier protein desaturase. Biochim. Biophys. Acta 1993, 1169, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Cerone, M.; Smith, T.K. Desaturases: Structural and mechanistic insights into the biosynthesis of unsaturated fatty acids. IUBMB Life 2022, 74, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Hongthong, A.; Paithoonrangsarid, K.; Prapugrangkul, P.; Deshnium, P.; Sirijuntarut, M.; Subhudhi, S.; Cheevadhanarak, S.; Morakot, T. The expression of three desaturase genes of Spirulina platensis in Escherichia coli DH5α. Heterologous expression of Spirulina-desaturase genes. Mol. Biol. Rep. 2004, 31, 177–189. [Google Scholar] [CrossRef]
- Kurdrid, P.; Subudhi, S.; Hongsthong, A.; Ruengjitchatchawalya, M.; Tanticharoen, M. Functional expression of Spirulina-Δ6 desaturase gene in yeast, Saccharomyces cerevisiae. Mol. Biol. Rep. 2005, 32, 215–226. [Google Scholar] [CrossRef]
- Wada, H.; Murata, N. Synechocystis PCC6803 mutants defective in desaturation of fatty acids. Plant Cell Physiol. 1989, 30, 971–978. [Google Scholar] [CrossRef]
- Wada, H.; Murata, N. Temperature-induced changes in the fatty acid composition of the cyanobacterium, Synechocystis PCC6803. Plant Physiol. 1990, 92, 1062–1069. [Google Scholar] [CrossRef]

| FA | WT | desD |
|---|---|---|
| 14:0 | 0.6 | 1.3 |
| 14:1Δ6 | nd | 0.5 |
| 14:1Δ9 | 1.1 | 1.1 |
| 16:0 | 55.6 | 48.7 |
| 16:1Δ6 | nd | 9.7 |
| 16:1Δ9 | 35.8 | 21.0 |
| 16:2Δ6,9 | nd | 7.4 |
| 18:0 | 1.4 | 3.5 |
| 18:1Δ6 | nd | 1.5 |
| 18:1Δ9 | 4.1 | 3.5 |
| 18:1Δ9,11 | 1.4 | 0.4 |
| 18:2Δ6,9 | nd | 1.4 |
| FA | RT min | RRT18:0 | M+ | 12 amu Gap | 40 amu Gap |
|---|---|---|---|---|---|
| 14:0 | 10.276 | 0.602 | |||
| 14:1Δ6 | 10.926 | 0.640 | 317 | 206–218 | 178–218 |
| 14:1Δ9 | 11.136 | 0.653 | 317 | 248–260 | 234–274 |
| 14:2Δ6,9 | 12.32 | 0.722 | 315 | 206–218, 246–258 | 178–218, 218–258 |
| 16:0 | 12.96 | 0.759 | |||
| 16:1Δ6 | 13.821 | 0.810 | 345 | 206–218 | 192–232 |
| 16:1Δ9 | 14.006 | 0.821 | 345 | 248–260 | 234–274 |
| 17:1Δ10 | 14.758 | 0.865 | 359 | 262–274 | 248–288 |
| 16:2Δ6,9 | 15.276 | 0.895 | 343 | 206–218, 246–258 | 232–272, 192–232 |
| 17:2Δ6,10 | 16.956 | 0.994 | 357 | 206–218, 260–272 | 178–218, 232–272 |
| 18:0 | 17.064 | 1.000 | |||
| 18:1Δ6 | 18.159 | 1.064 | 373 | 220–232 | 206–246 |
| 18:1Δ9 | 18.264 | 1.070 | 373 | 248–260 | 234–274 |
| 18:1Δ11 | 18.465 | 1.082 | 373 | 276–288 | 248–288 |
| 18:2Δ6,9 | 19.615 | 1.149 | 371 | 206–218, 246–258 | 178–218, 232–272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starikov, A.Y.; Sidorov, R.A.; Goriainov, S.V.; Los, D.A. Acyl-Lipid Δ6-Desaturase May Act as a First FAD in Cyanobacteria. Biomolecules 2022, 12, 1795. https://doi.org/10.3390/biom12121795
Starikov AY, Sidorov RA, Goriainov SV, Los DA. Acyl-Lipid Δ6-Desaturase May Act as a First FAD in Cyanobacteria. Biomolecules. 2022; 12(12):1795. https://doi.org/10.3390/biom12121795
Chicago/Turabian StyleStarikov, Alexander Y., Roman A. Sidorov, Sergei V. Goriainov, and Dmitry A. Los. 2022. "Acyl-Lipid Δ6-Desaturase May Act as a First FAD in Cyanobacteria" Biomolecules 12, no. 12: 1795. https://doi.org/10.3390/biom12121795
APA StyleStarikov, A. Y., Sidorov, R. A., Goriainov, S. V., & Los, D. A. (2022). Acyl-Lipid Δ6-Desaturase May Act as a First FAD in Cyanobacteria. Biomolecules, 12(12), 1795. https://doi.org/10.3390/biom12121795







