Hydrogel-Encapsulated Heterogenous Mesoporous Resin Catalyst for In Situ Anti-Cancer Agent Production under Biological Conditions
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis and Characterization of Mesoporous Catalyst
2.2. Procedure for In Vitro Pro Drug Synthesis and Assessment of Efficacy
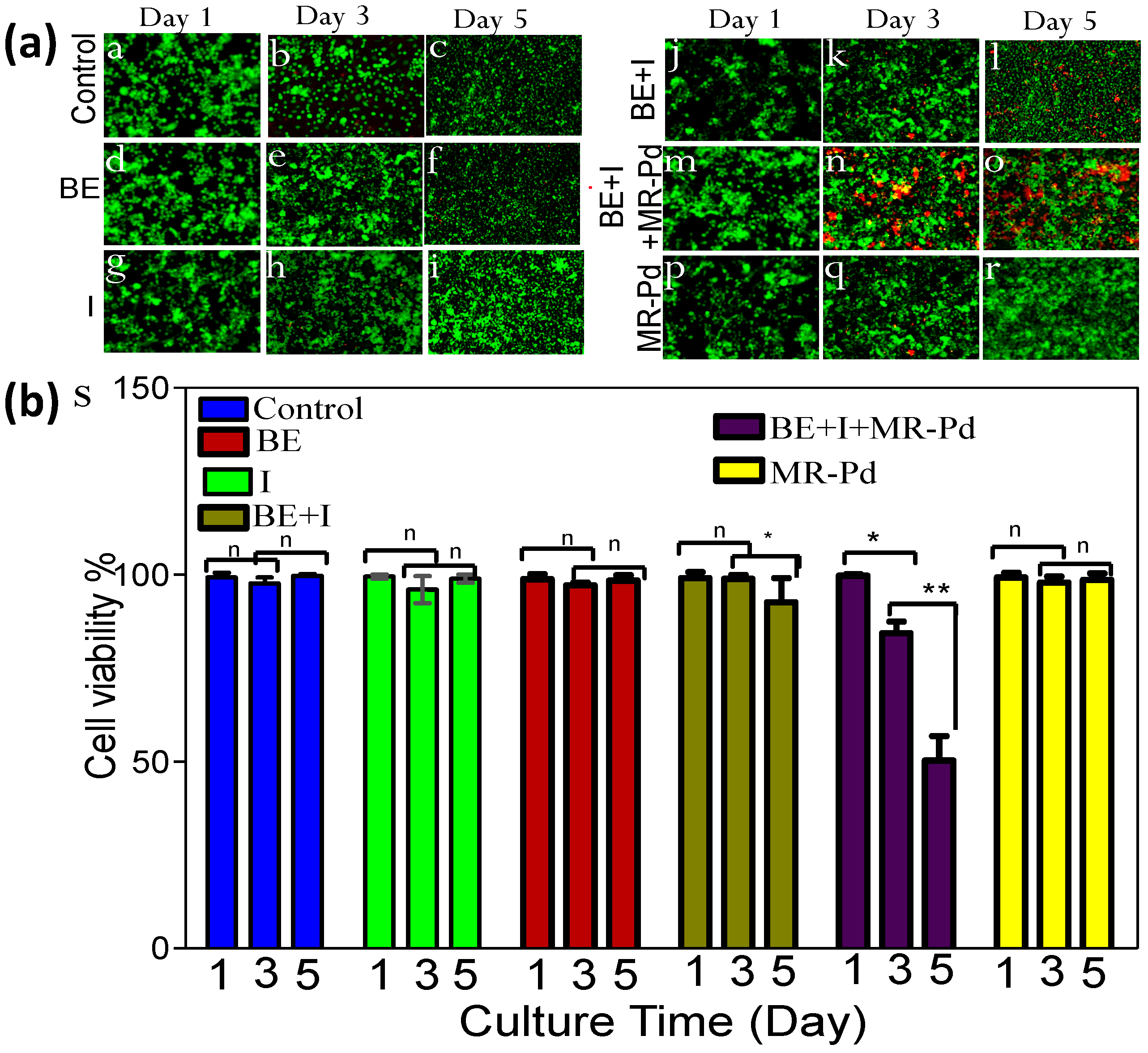
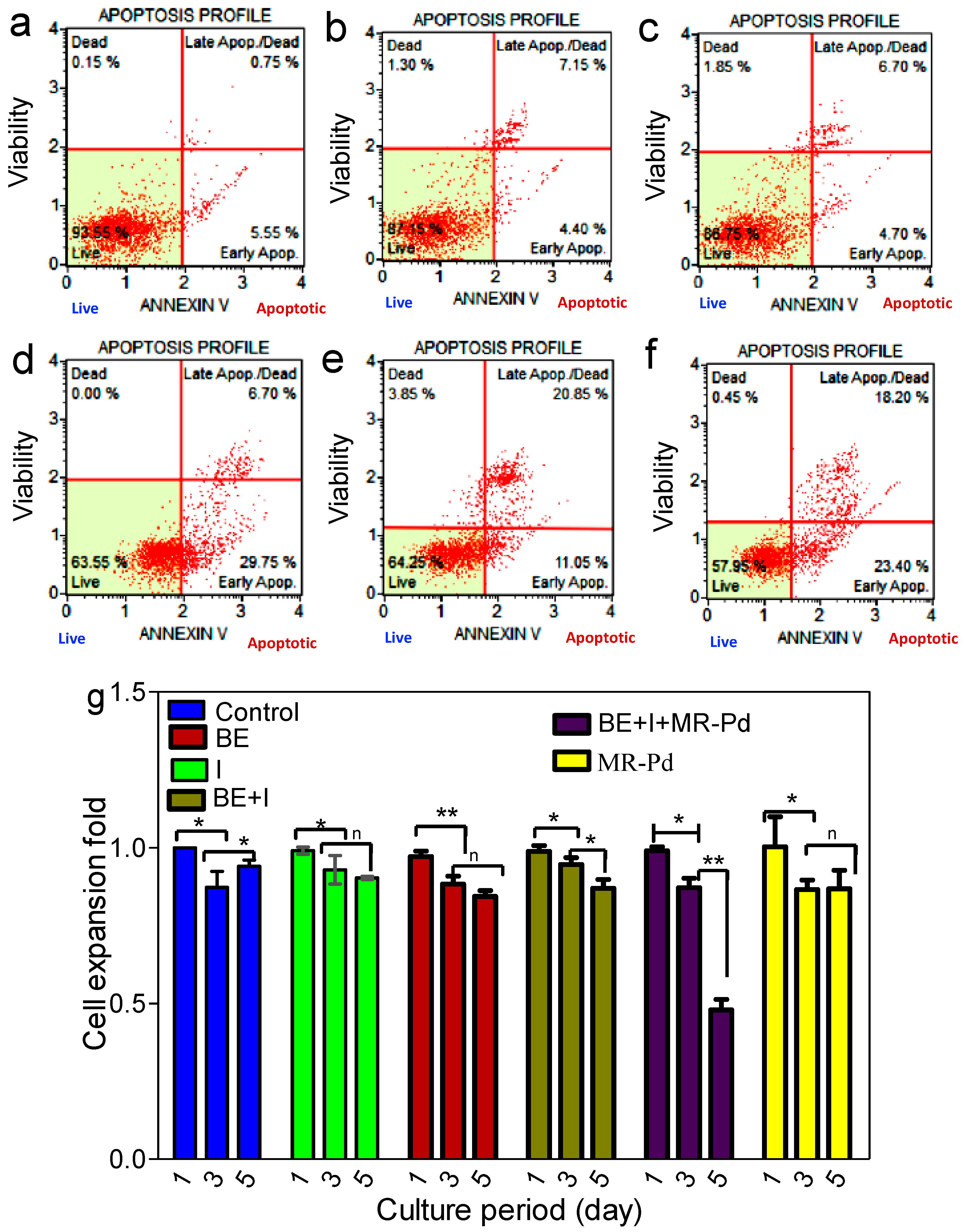
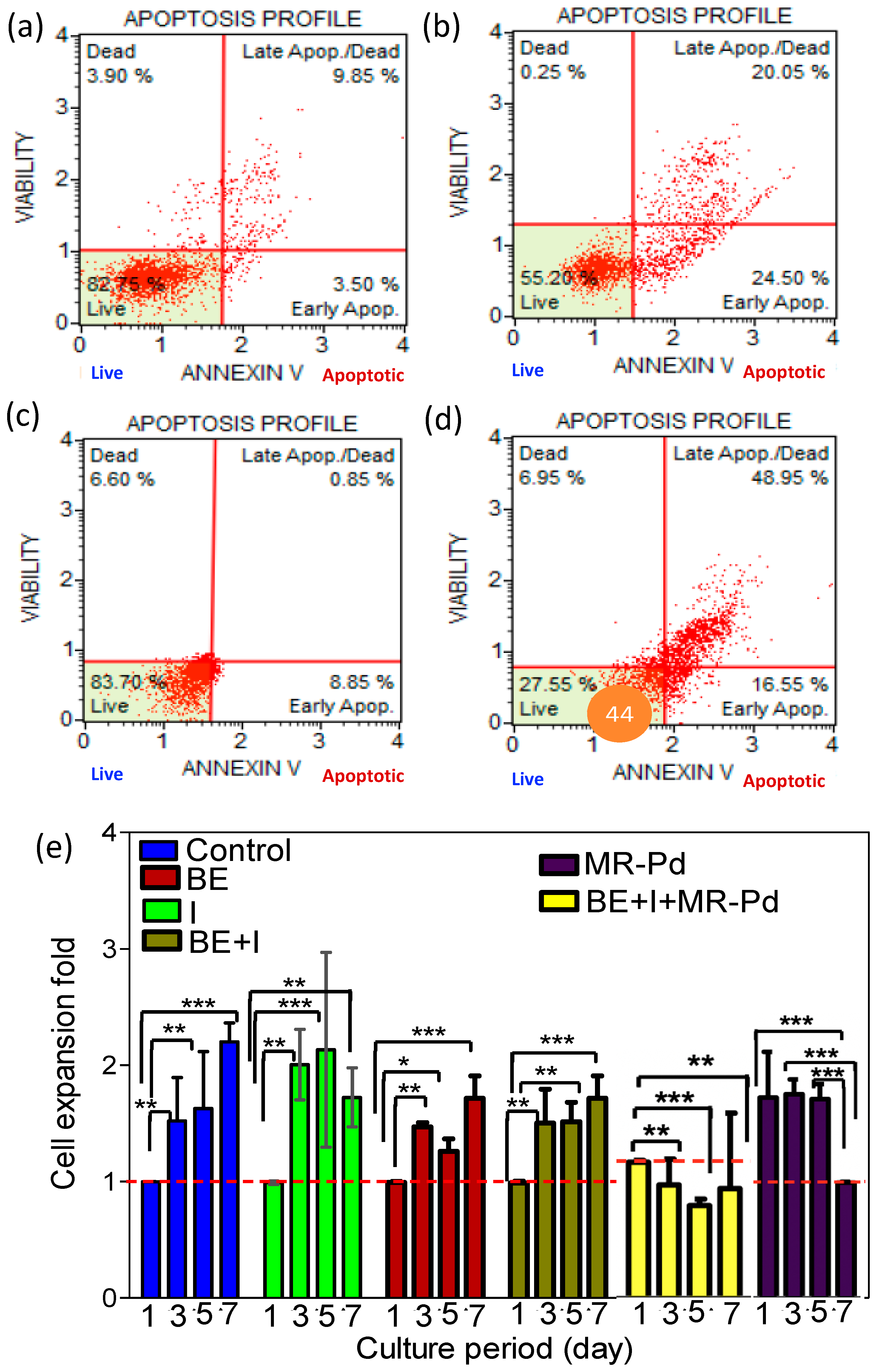
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmermann, S.; Dziadziuszko, R.; Peters, S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat. Rev. 2014, 40, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, B.; Hojjat-Farsangi, M.; Mohammadi, H.; Anvari, E.; Ghalamfarsa, G.; Yousefi, M.; Jadidi-Niaragh, F. Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 2017, 190, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Sun, J.; Sun, B.; He, Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol. Sci. 2014, 35, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Compain, G.; Oumata, N.; Clarhaut, J.; Péraudeau, E.; Renoux, B.; Galons, H.; Papot, S. A β-glucuronidase-responsive albumin-binding prodrug for potential selective kinase inhibitor-based cancer chemotherapy. Eur. J. Med. Chem. 2018, 158, 1–6. [Google Scholar] [CrossRef]
- Hedley, D.; Ogilvie, L.; Springer, C. Carboxypeptidase G2-based gene-directed enzyme–prodrug therapy: A new weapon in the GDEPT armoury. Nat. Rev. Cancer 2007, 7, 870–879. [Google Scholar] [CrossRef]
- Roy, P.; Waxman, D.J. Activation of oxazaphosphorines by cytochrome P450: Application to gene-directed enzyme prodrug therapy for cancer. Toxicol. Vitr. 2006, 20, 176–186. [Google Scholar] [CrossRef]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef]
- Oliveira, B.L.; Stenton, B.J.; Unnikrishnan, V.B.; de Almeida, C.R.; Conde, J.; Negrão, M.; Schneider, F.S.; Cordeiro, C.; Ferreira, M.G.; Caramori, G.F.; et al. Platinum-triggered bond-cleavage of pentynoyl amide and N-propargyl handles for drug-activation. J. Am. Chem. Soc. 2020, 142, 10869–10880. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Zhao, J.; Wang, J.; Zheng, S.; Lin, S.; Chen, L.; Yang, M.; Jia, S.; Zhang, X.; et al. Palladium-triggered deprotection chemistry for protein activation in living cells. Nat. Chem. 2014, 6, 352–361. [Google Scholar] [CrossRef]
- Weiss, J.T.; Dawson, J.C.; Macleod, K.G.; Rybski, W.; Fraser, C.; Torres-Sánchez, C.; Patton, E.E.; Bradley, M.; Carragher, N.O.; Unciti-Broceta, A. Extracellular palladium-catalysed dealkylation of 5-fluoro-1-propargyl-uracil as a bioorthogonally activated prodrug approach. Nat. Commun. 2014, 5, 3277. [Google Scholar] [CrossRef]
- Weiss, J.T.; Dawson, J.C.; Fraser, C.; Rybski, W.; Torres-Sánchez, C.; Bradley, M.; Patton, E.E.; Carragher, N.O.; Unciti-Broceta, A. Development and bioorthogonal activation of palladium-labile prodrugs of gemcitabine. J. Med. Chem. 2014, 57, 5395–5404. [Google Scholar] [CrossRef] [PubMed]
- Busacca, C.A.; Fandrick, D.R.; Song, J.J.; Senanayake, C.H. The growing impact of catalysis in the pharmaceutical industry. Adv. Synth. Catal. 2011, 353, 1825–1864. [Google Scholar] [CrossRef]
- Anutrasakda, W.; Eiamsantipaisarn, K.; Jiraroj, D.; Phasuk, A.; Tuntulani, T.; Liu, H.; Tungasmita, D.N. One-pot catalytic conversion of cellobiose to sorbitol over nickel phosphides supported on MCM-41 and Al-MCM-41. Catalysts 2019, 9, 92. [Google Scholar] [CrossRef] [Green Version]
- Krishnadoss, V.; Melillo, A.; Kanjilal, B.; Hannah, T.; Ellis, E.; Kapetanakis, A.; Hazelton, J.; San Roman, J.; Masoumi, A.; Leijten, J.; et al. Bioionic liquid conjugation as universal approach to engineer hemostatic bioadhesives. ACS Appl. Mater. Interfaces 2019, 11, 38373–38384. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Xie, C.; Yang, Z.; Suo, Y.; Zhu, X.; Wei, D.; Weng, X.; Wei, X.; Gu, Z. Monitoring circulating prostate cancer cells by in vivo flow cytometry assesses androgen deprivation therapy on metastasis. Cytom. Part A 2018, 93, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Che, H.Y.; Guo, H.Y.; Si, X.W.; You, Q.Y.; Lou, W.Y. PP121, a dual inhibitor of tyrosine and phosphoinositide kinases, inhibits anaplastic thyroid carcinoma cell proliferation and migration. Tumor Biol. 2014, 35, 8659–8664. [Google Scholar] [CrossRef]
- Smit, T.; Calitz, C.; Willers, C.; Svitina, H.; Hamman, J.; Fey, S.J.; Gouws, C.; Wrzesinski, K. Characterization of an alginate encapsulated LS180 spheroid model for anti-colorectal cancer compound screening. ACS Med. Chem. Lett. 2020, 11, 1014–1021. [Google Scholar] [CrossRef]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D cell culture in alginate hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef]
- Lan, S.F.; Starly, B. Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicol. Appl. Pharmacol. 2011, 256, 62–72. [Google Scholar] [CrossRef]
- Alkhursani, S.A.; Ghobashy, M.M.; Al-Gahtany, S.A.; Meganid, A.S.; Abd El-Halim, S.M.; Ahmad, Z.; Khan, F.S.; Atia, G.A.; Cavalu, S. Application of Nano-Inspired Scaffolds-Based Biopolymer Hydrogel for Bone and Periodontal Tissue Regeneration. Polymers 2022, 14, 3791. [Google Scholar] [CrossRef]
- Tewari, A.K.; Upadhyay, S.C.; Kumar, M.; Pathak, K.; Kaushik, D.; Verma, R.; Bhatt, S.; Massoud, E.E.; Rahman, M.H.; Cavalu, S. Insights on Development Aspects of Polymeric Nanocarriers: The Translation from Bench to Clinic. Polymers 2022, 14, 3545. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabavinia, M.; Kanjilal, B.; Pandey, M.; Jonnalagadda, S.; Hesketh, R.; Martins-Green, M.; Noshadi, I. Hydrogel-Encapsulated Heterogenous Mesoporous Resin Catalyst for In Situ Anti-Cancer Agent Production under Biological Conditions. Biomolecules 2022, 12, 1796. https://doi.org/10.3390/biom12121796
Nabavinia M, Kanjilal B, Pandey M, Jonnalagadda S, Hesketh R, Martins-Green M, Noshadi I. Hydrogel-Encapsulated Heterogenous Mesoporous Resin Catalyst for In Situ Anti-Cancer Agent Production under Biological Conditions. Biomolecules. 2022; 12(12):1796. https://doi.org/10.3390/biom12121796
Chicago/Turabian StyleNabavinia, Mahboubeh, Baishali Kanjilal, Manoj Pandey, Subash Jonnalagadda, Robert Hesketh, Manuela Martins-Green, and Iman Noshadi. 2022. "Hydrogel-Encapsulated Heterogenous Mesoporous Resin Catalyst for In Situ Anti-Cancer Agent Production under Biological Conditions" Biomolecules 12, no. 12: 1796. https://doi.org/10.3390/biom12121796
APA StyleNabavinia, M., Kanjilal, B., Pandey, M., Jonnalagadda, S., Hesketh, R., Martins-Green, M., & Noshadi, I. (2022). Hydrogel-Encapsulated Heterogenous Mesoporous Resin Catalyst for In Situ Anti-Cancer Agent Production under Biological Conditions. Biomolecules, 12(12), 1796. https://doi.org/10.3390/biom12121796






