Increasing Cellular Uptake and Permeation of Curcumin Using a Novel Polymer-Surfactant Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Formulations A and B
2.2.2. Solubility Study
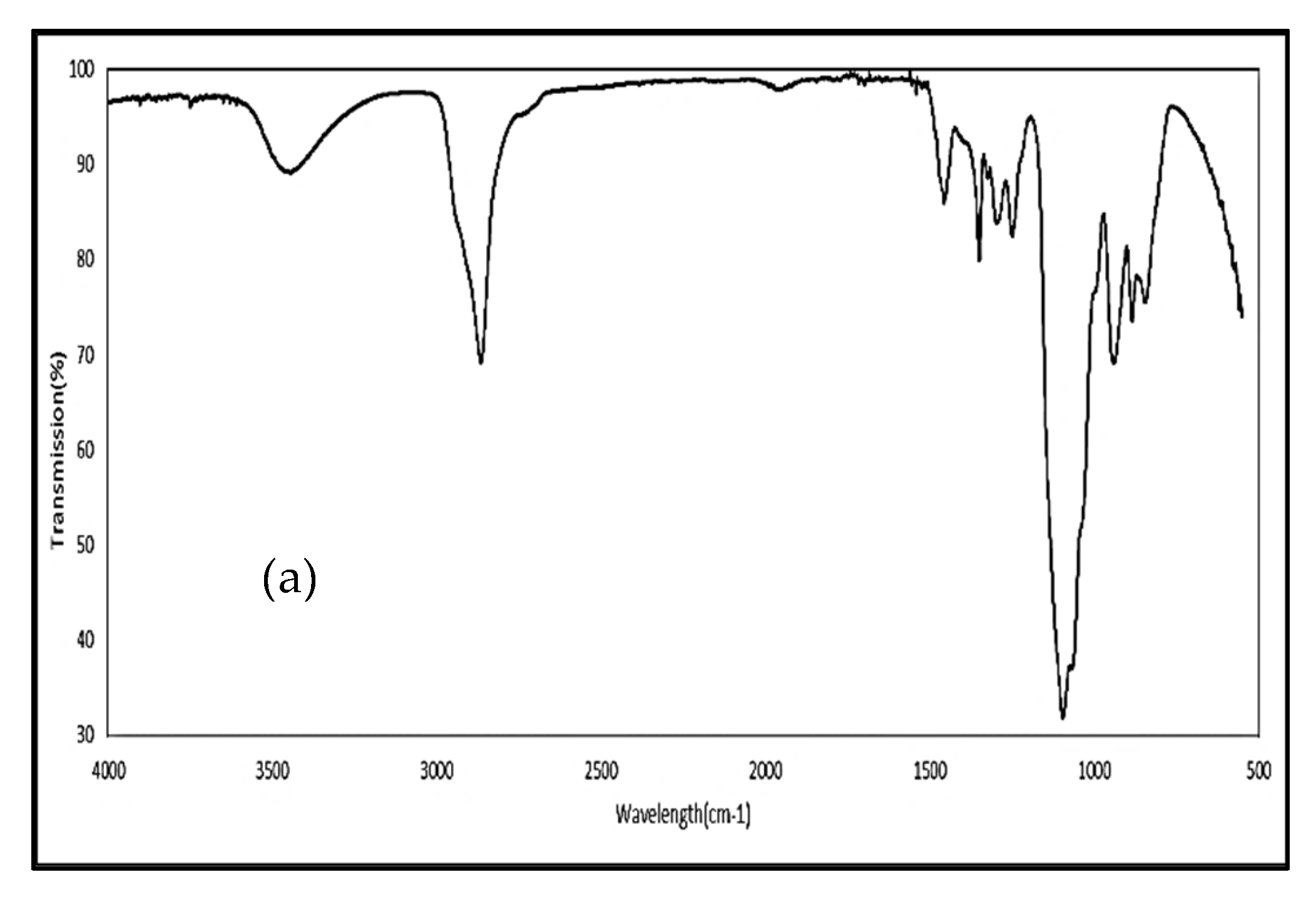
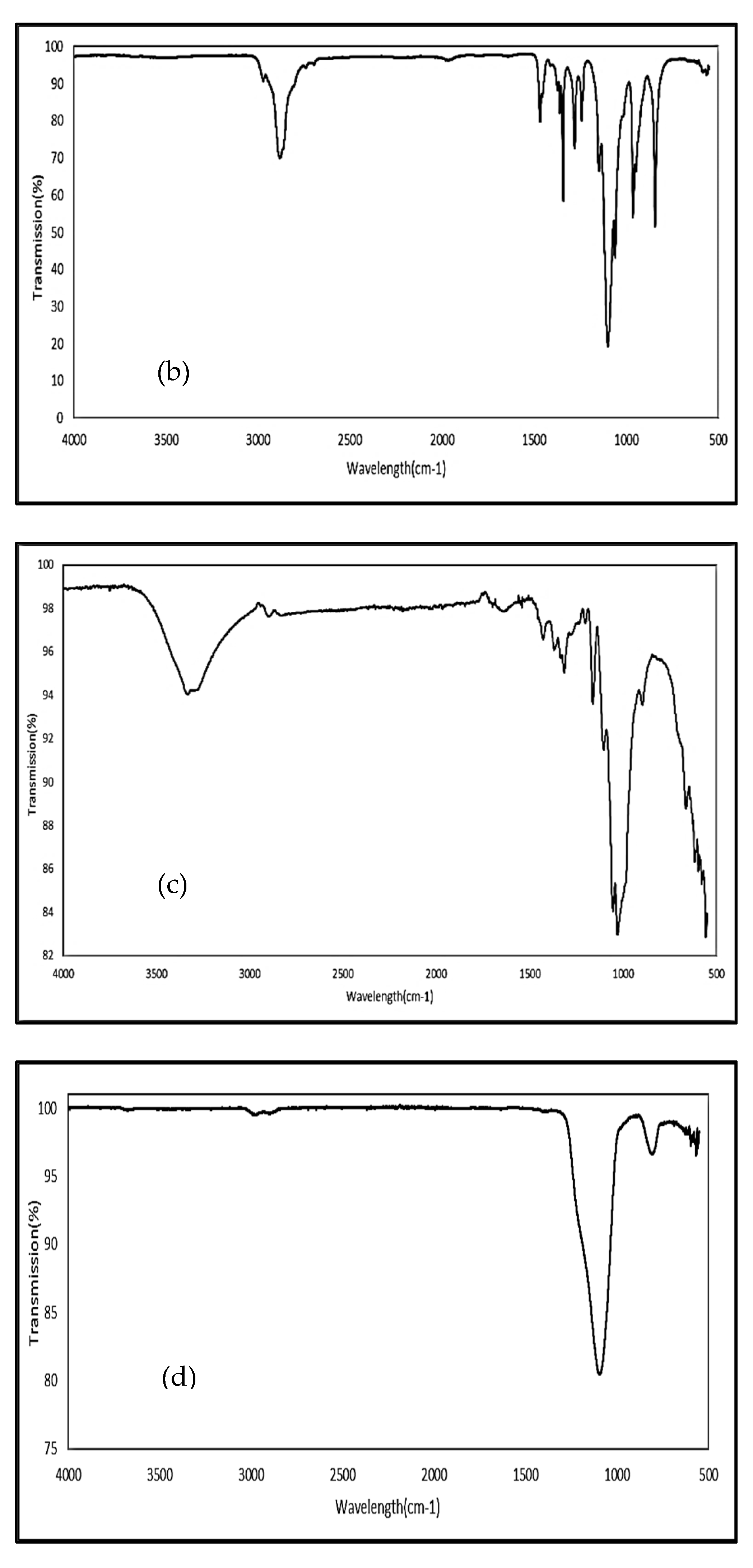
2.2.3. Fourier Transform Infrared (FTIR) Spectroscopy
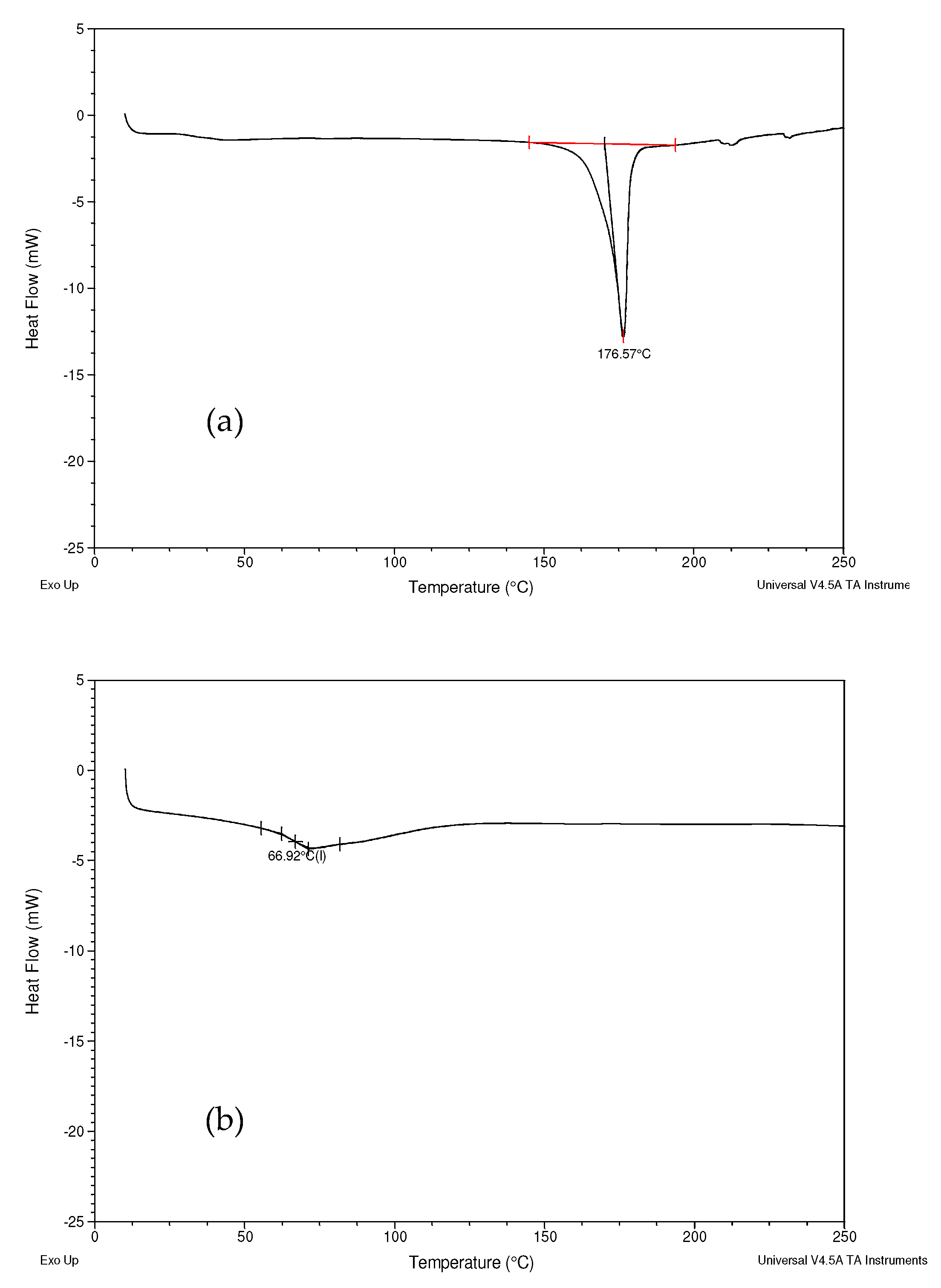
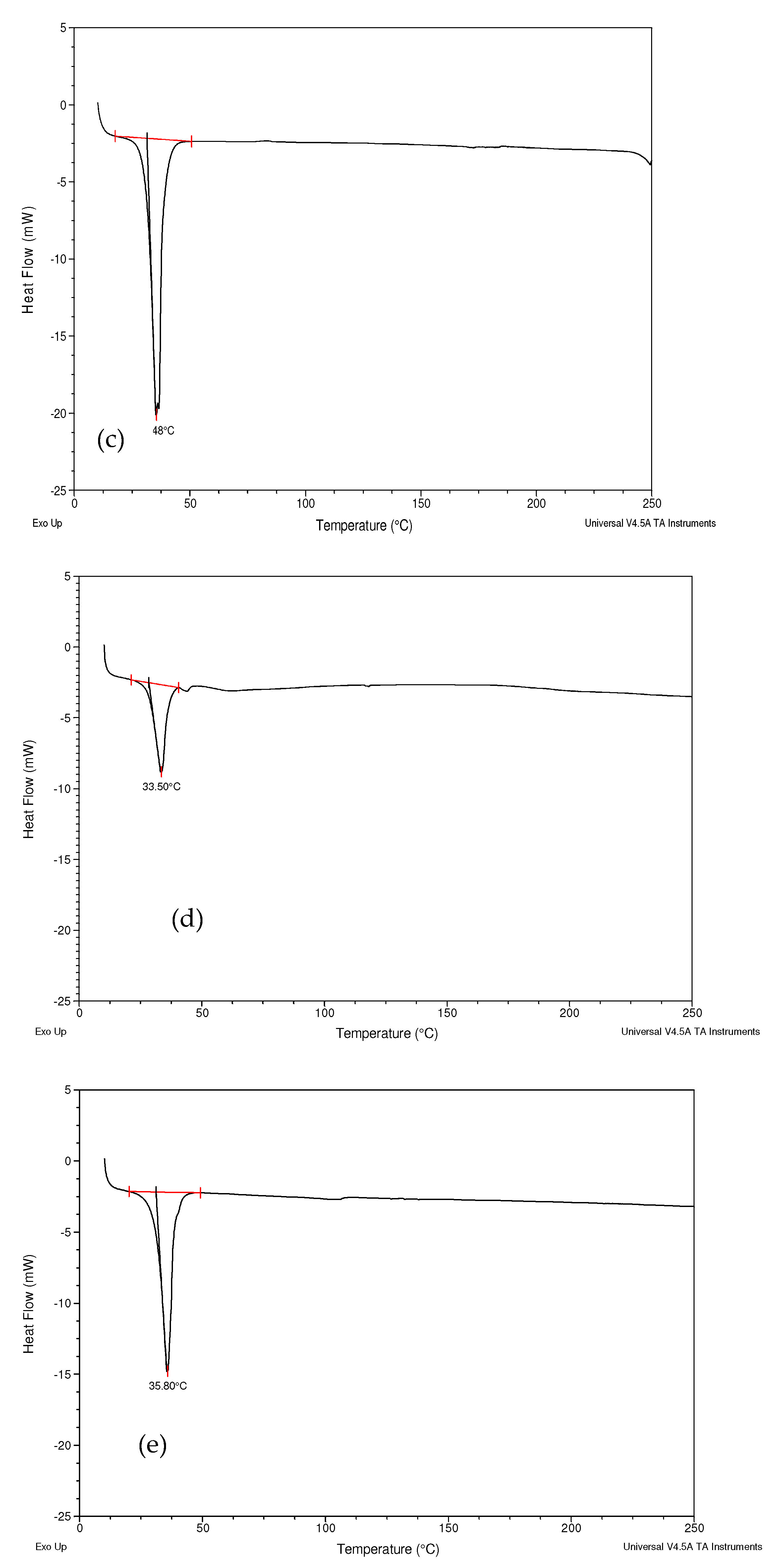
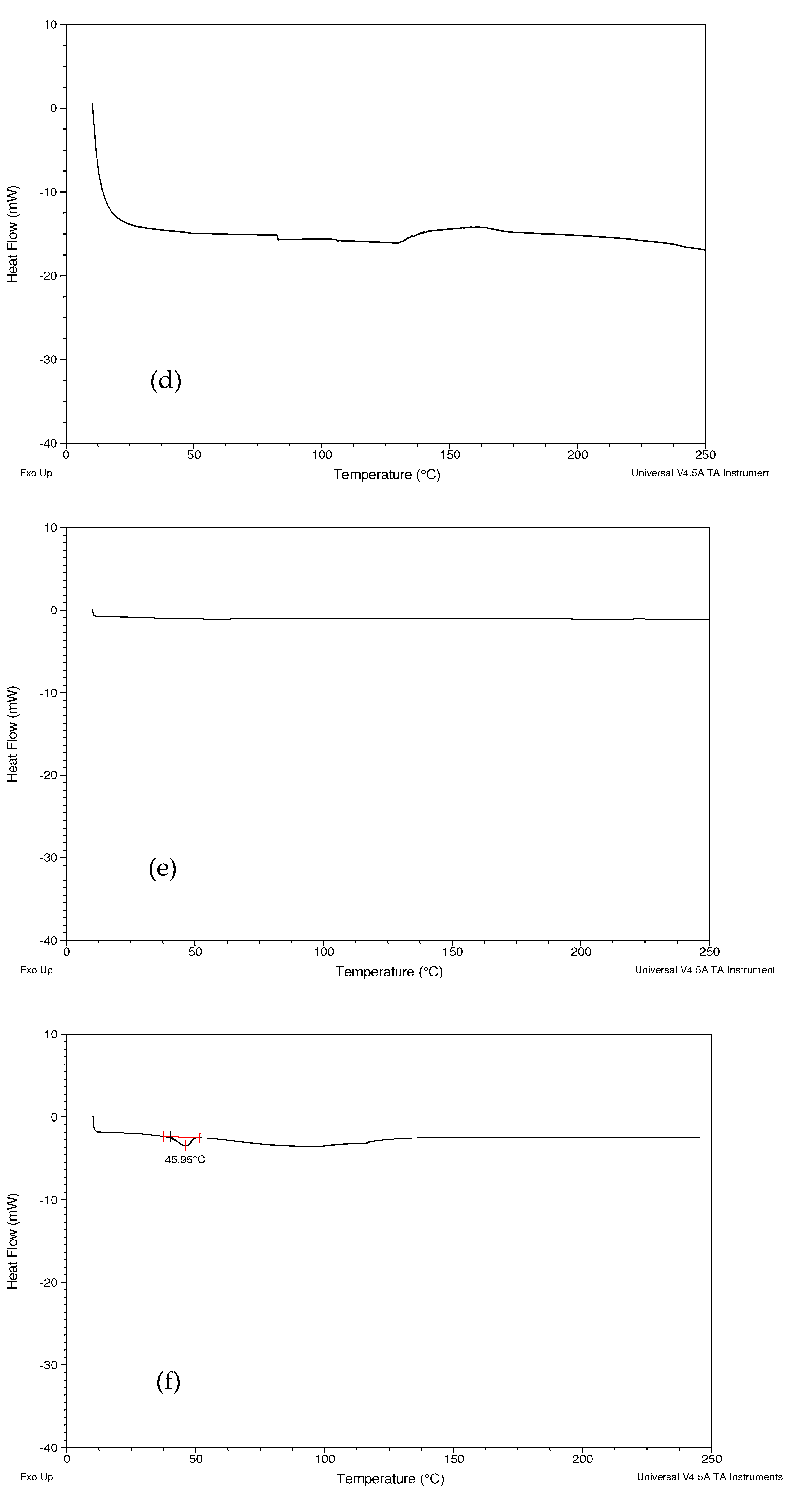
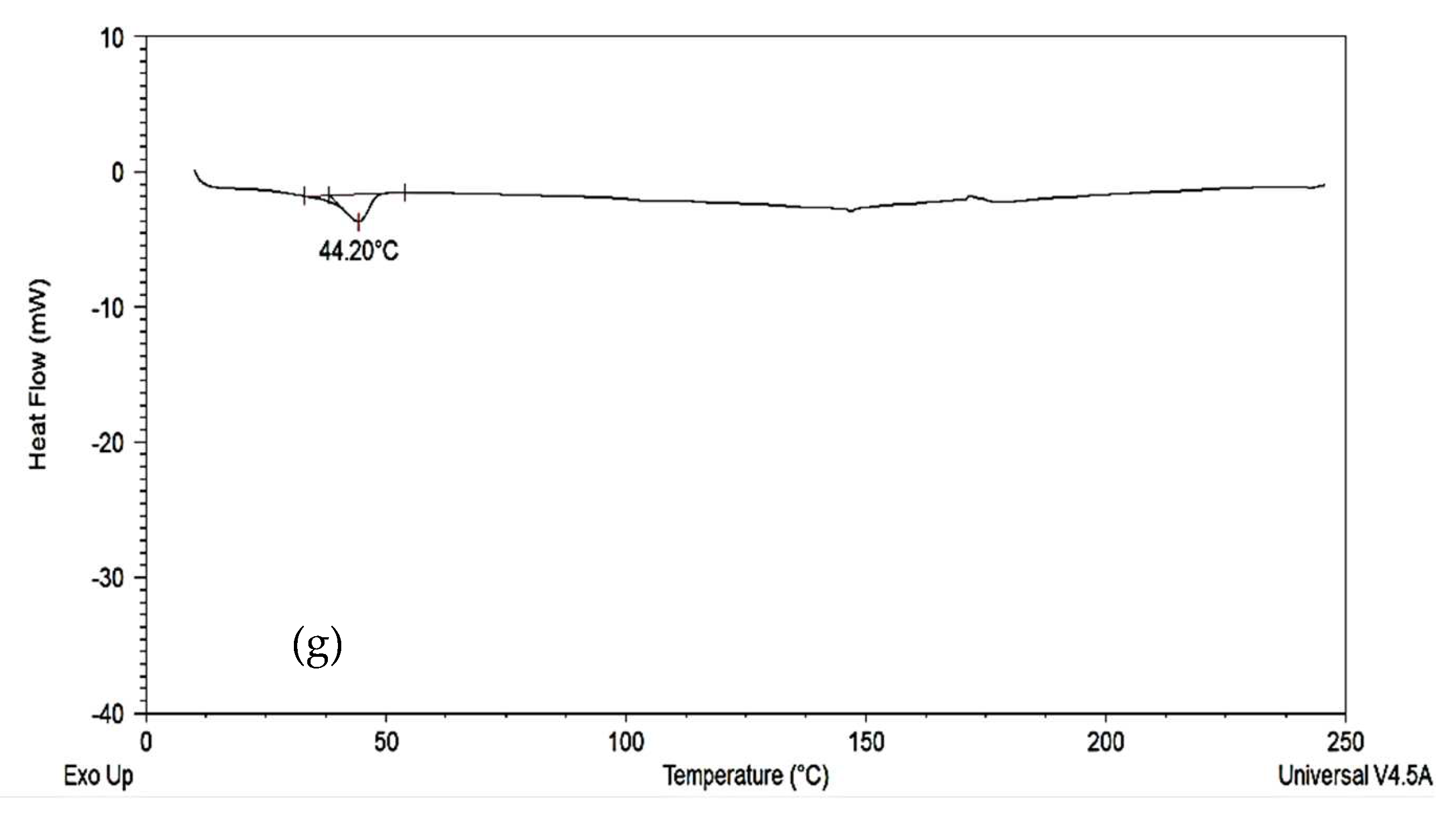
2.2.4. Differential Scanning Calorimetry (DSC)
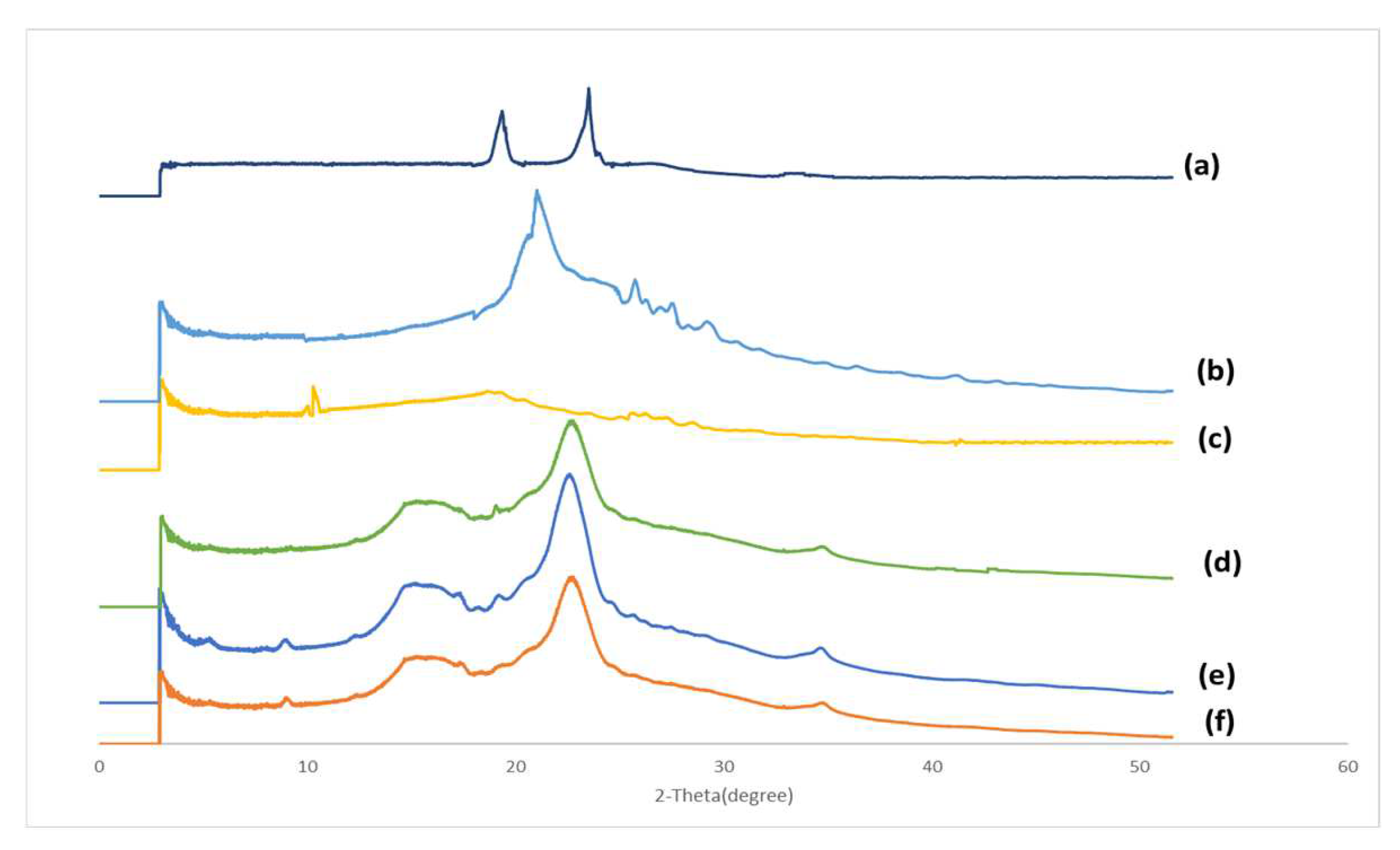
2.2.5. X-ray Powder Diffraction (XRD)
2.2.6. Determining Curcumin Content in Commercial Curcumin Powder, Formulation A, Formulation B, Longvida® and Nacumin®
2.2.7. Caco-2 Cell Culture
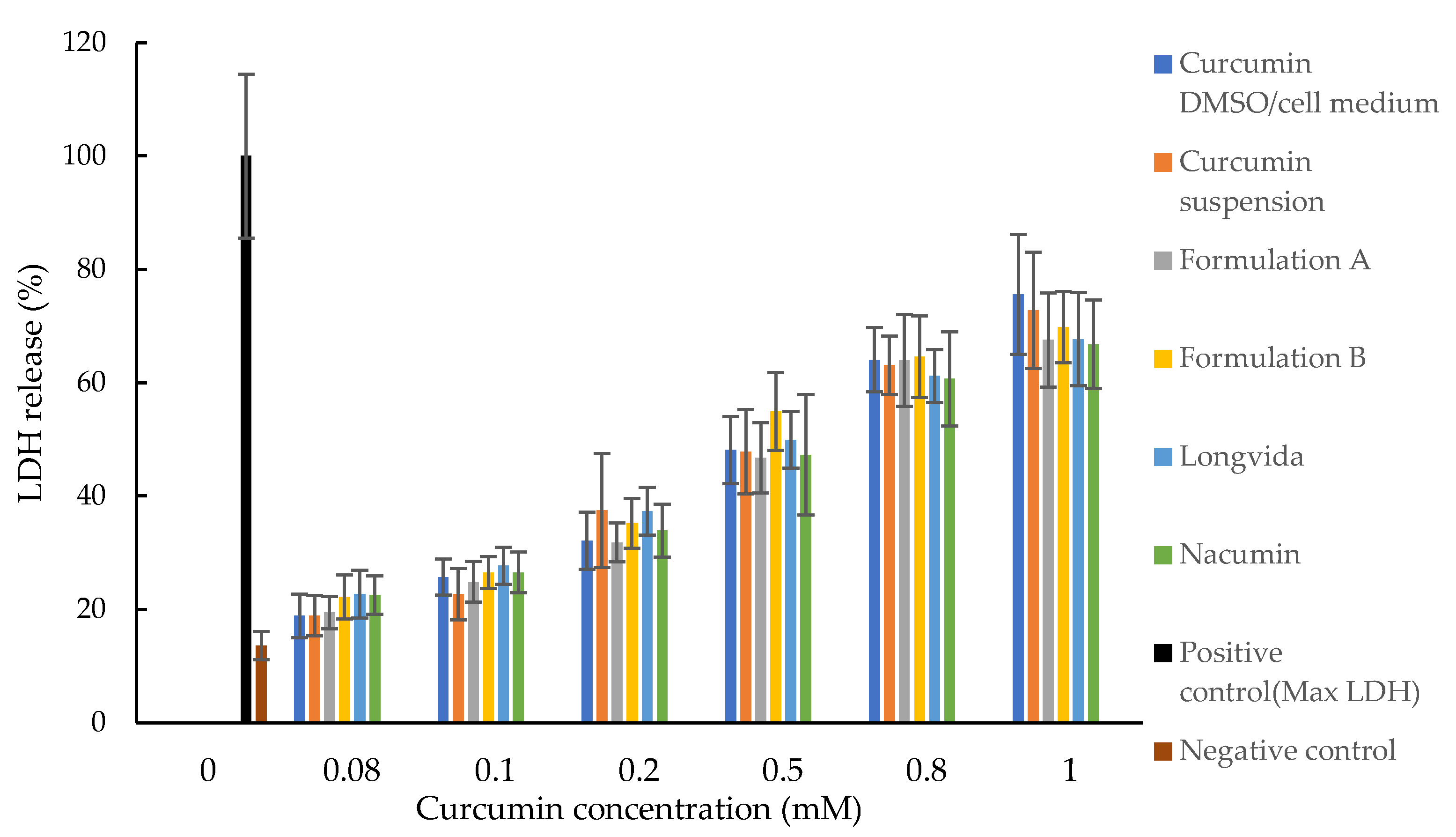
2.2.8. Cytotoxicity Tests (MTT and LDH Assays)
MTT Assay
LDH Assay
2.2.9. Measurement of Transepithelial Electrical Resistance (TEER)
2.2.10. Determination of In Vitro Drug Transport
2.2.11. Cellular Uptake Study
2.3. Statistical Analysis
3. Results
3.1. Solubility Study
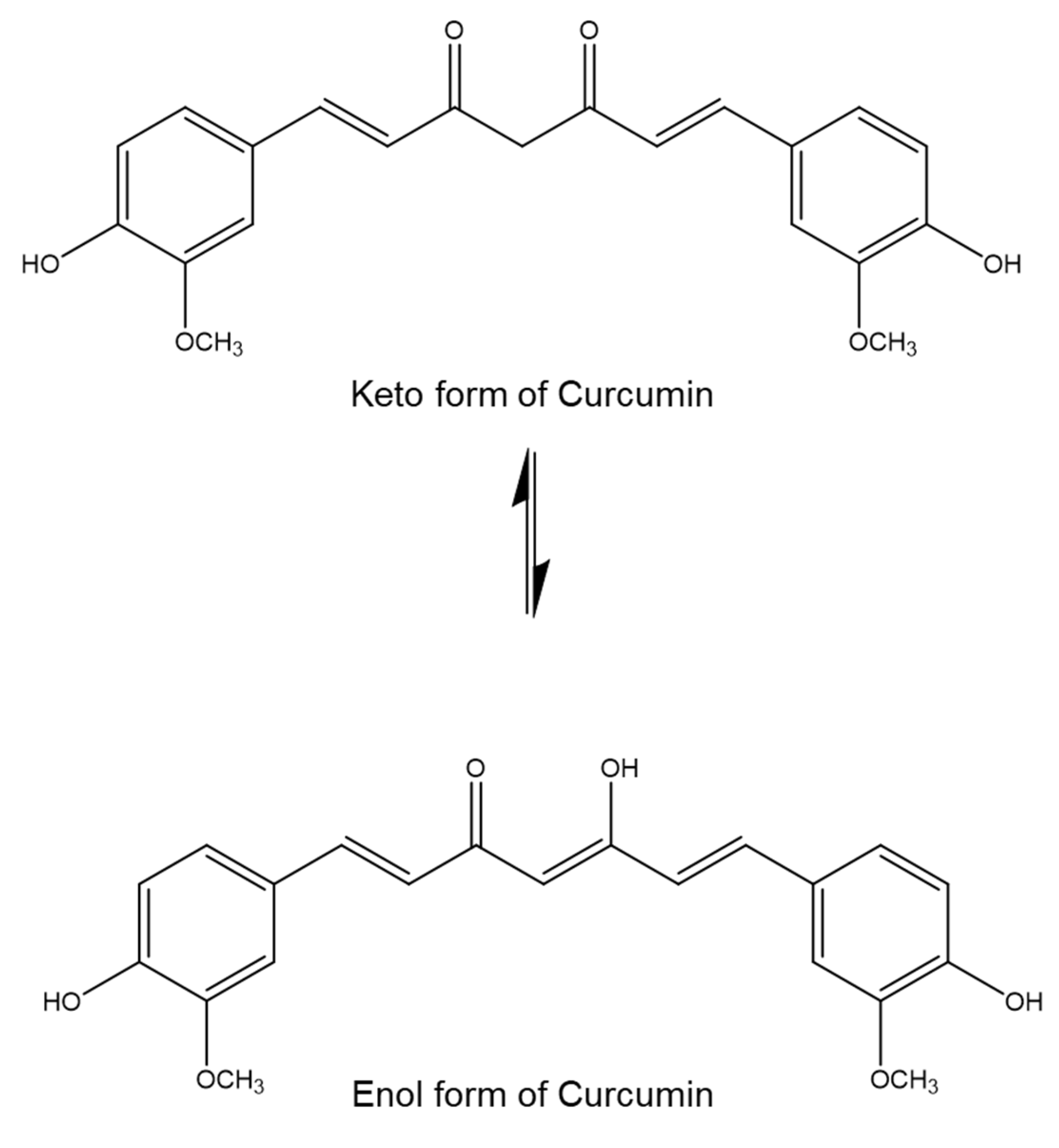
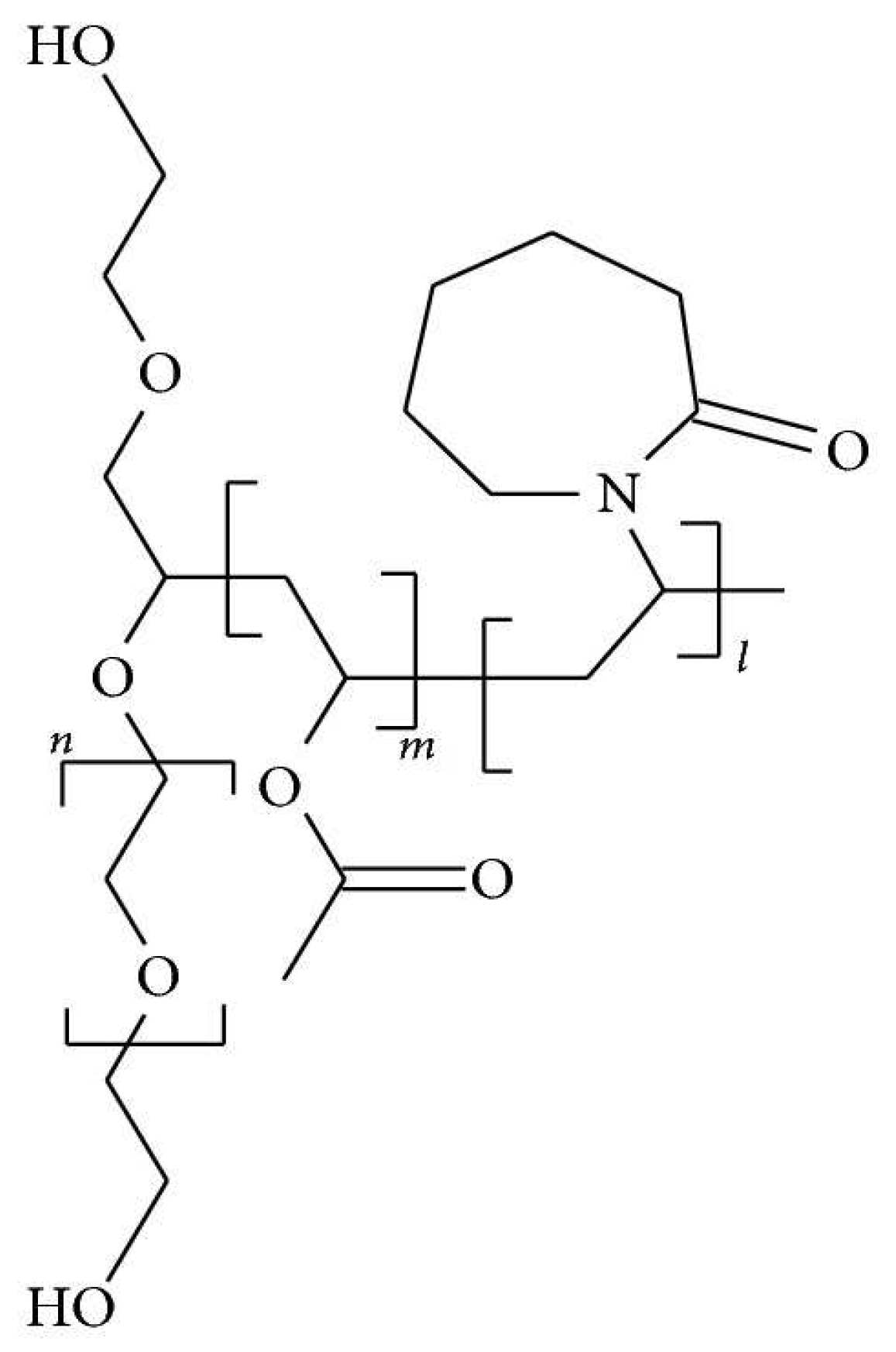
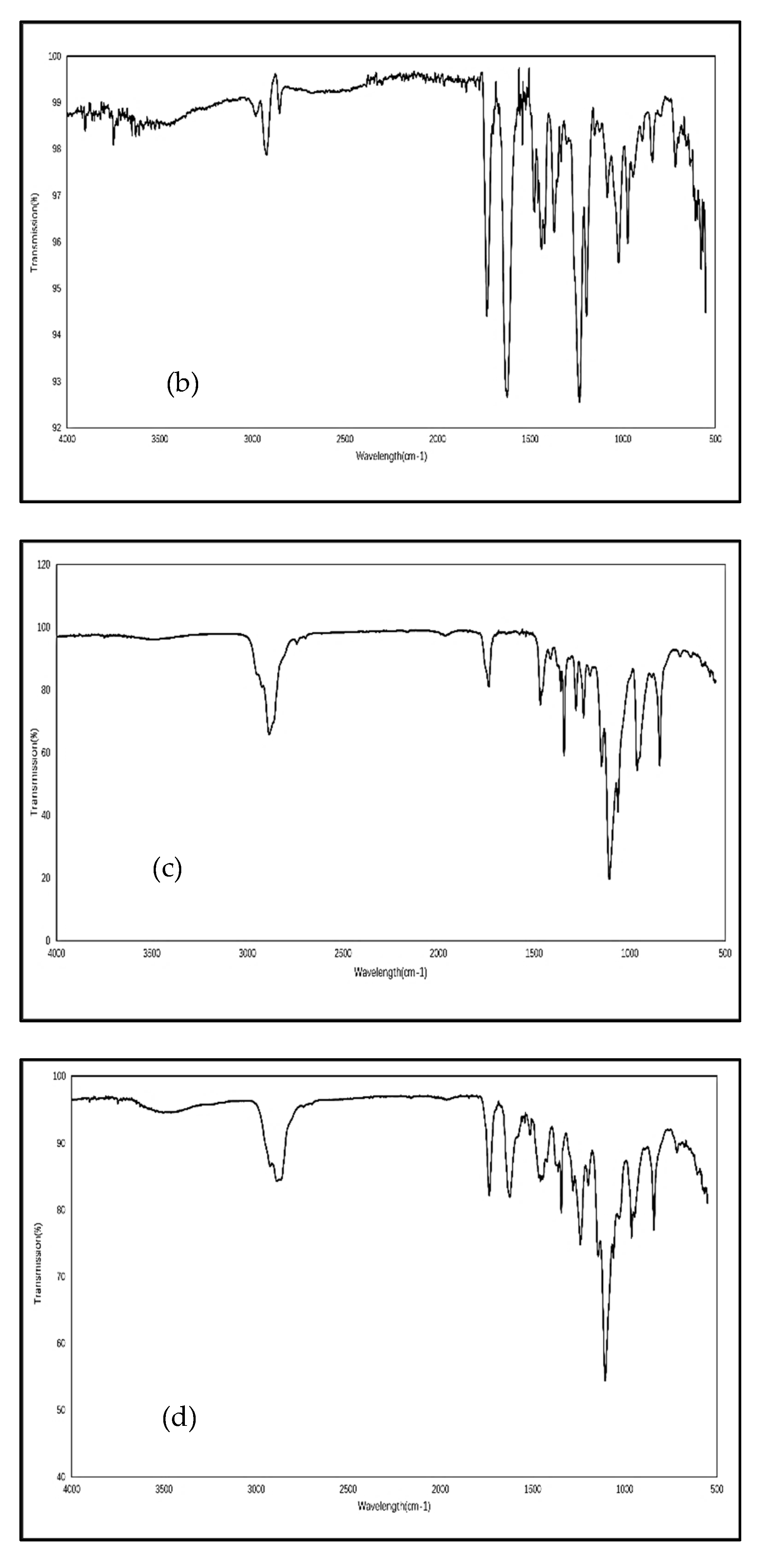
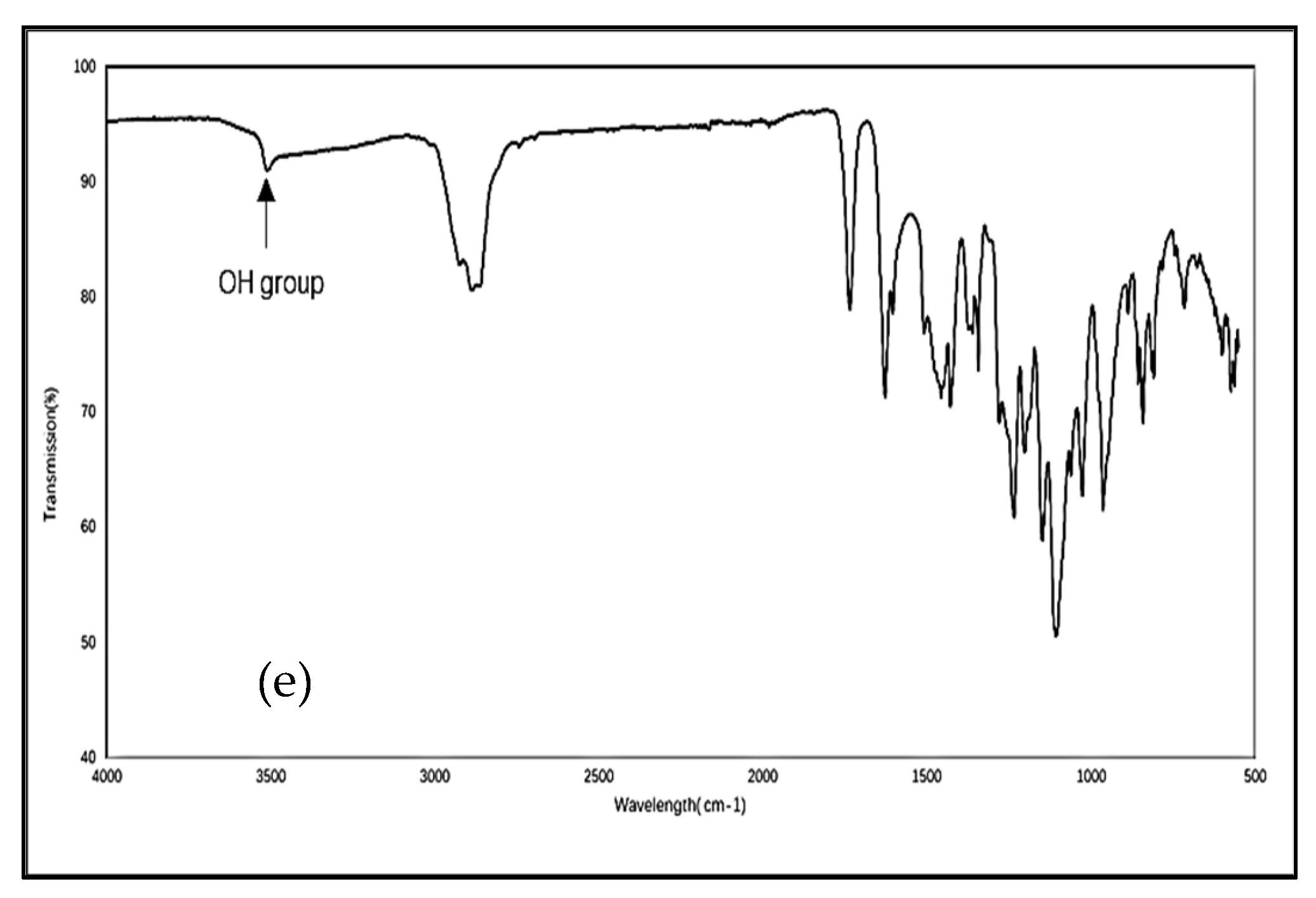
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Differential Scanning Calorimetry (DSC)
3.4. X-ray Powder Diffraction (XRD)
3.5. Determining the Curcumin Content in Commercial Curcumin Powder, Formulation A, Formulation B, Longvida® and Nacumin®
3.6. Cytotoxicity Tests (MTT and LDH Assays)
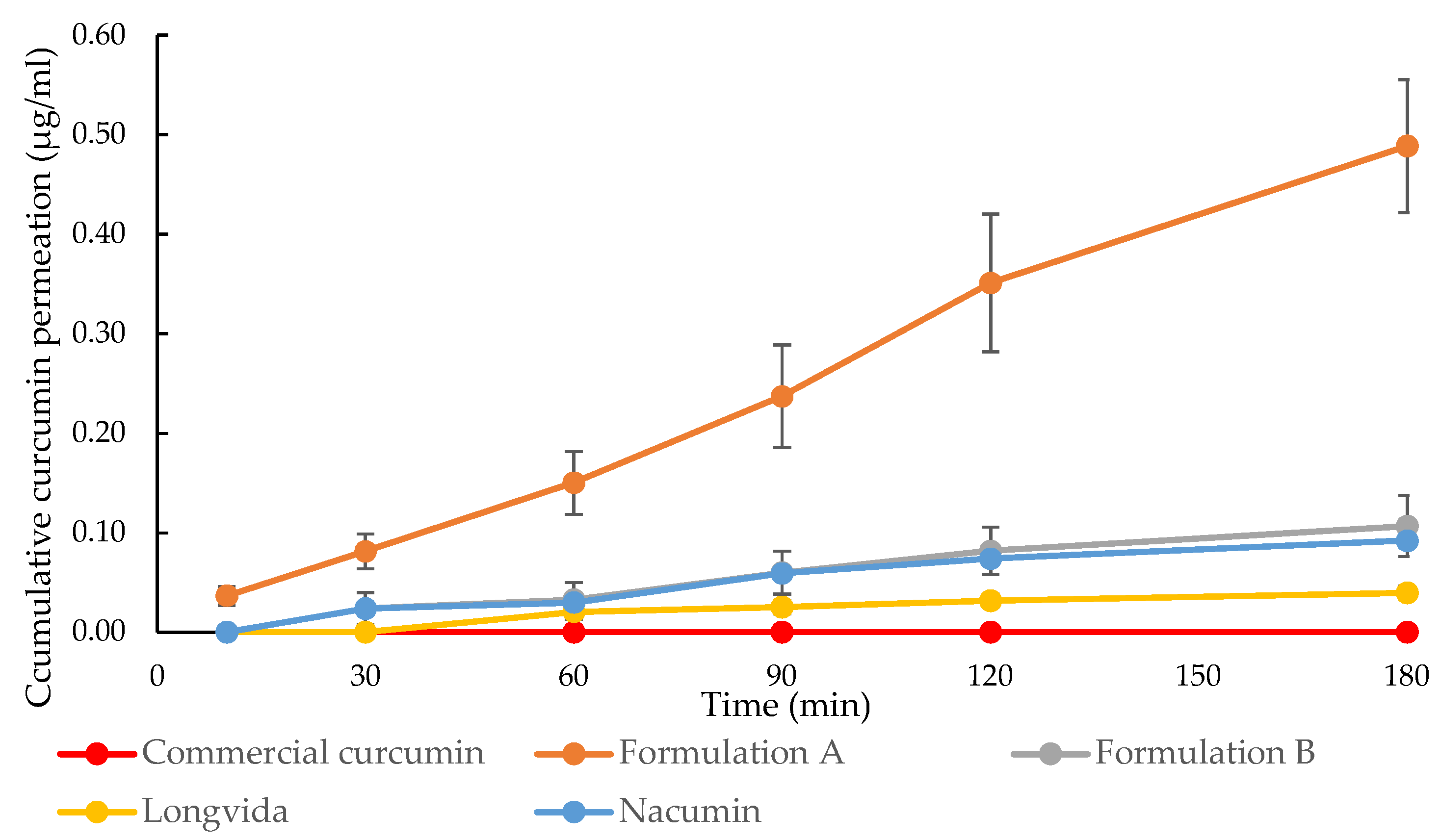
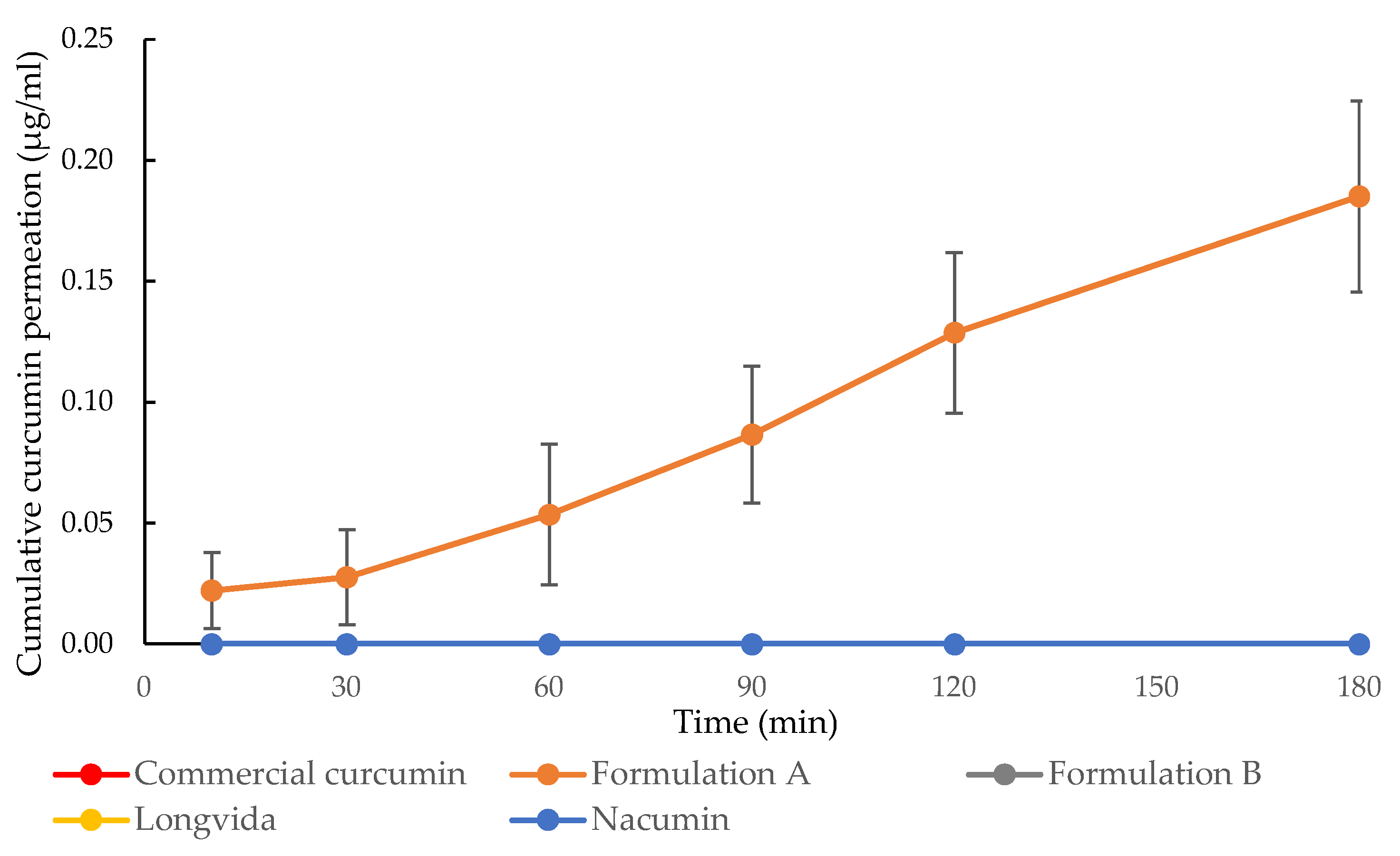
3.7. In Vitro Drug Transport Experiments and the Measurement of Transepithelial Electrical Resistance (TEER)
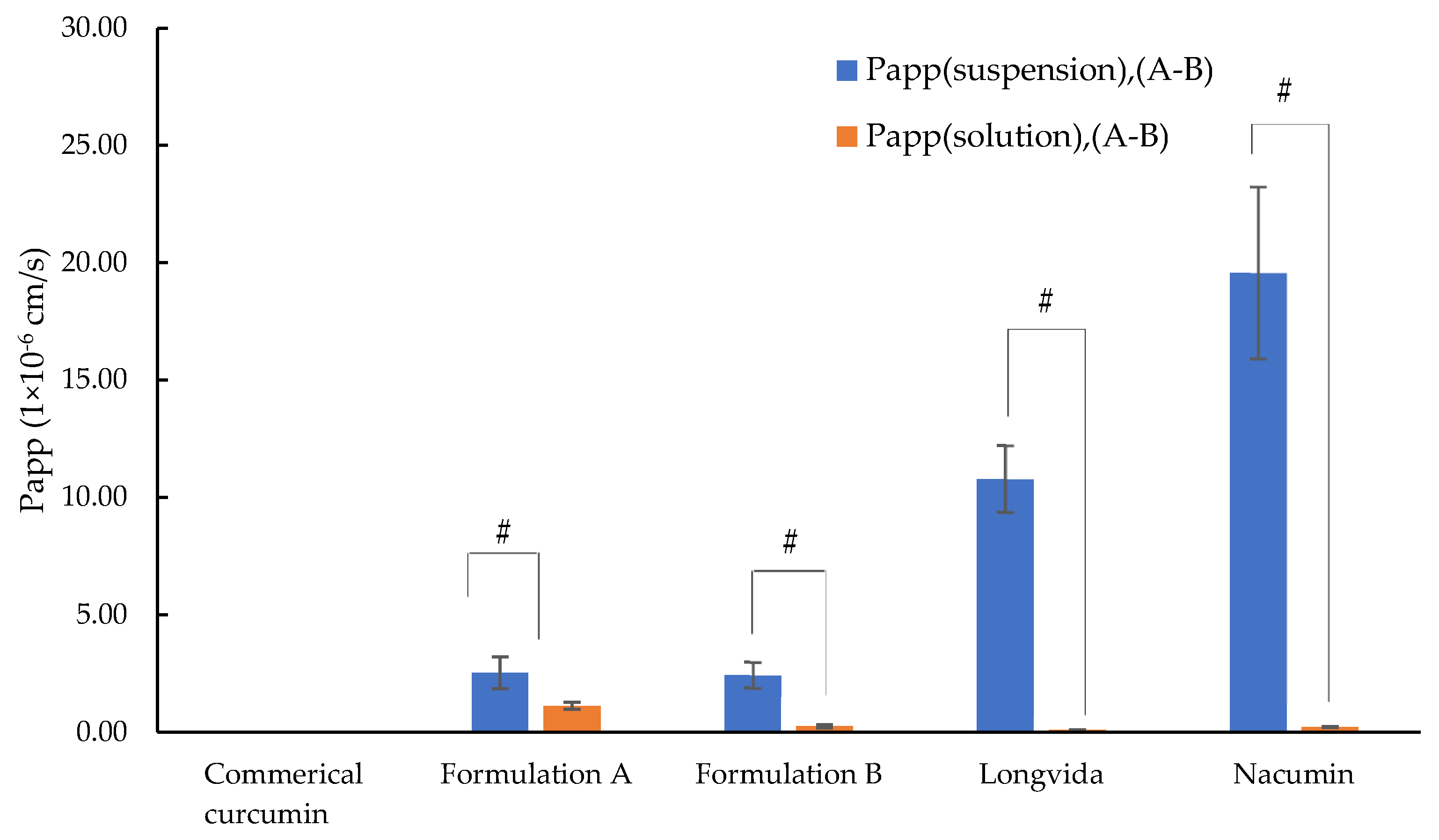
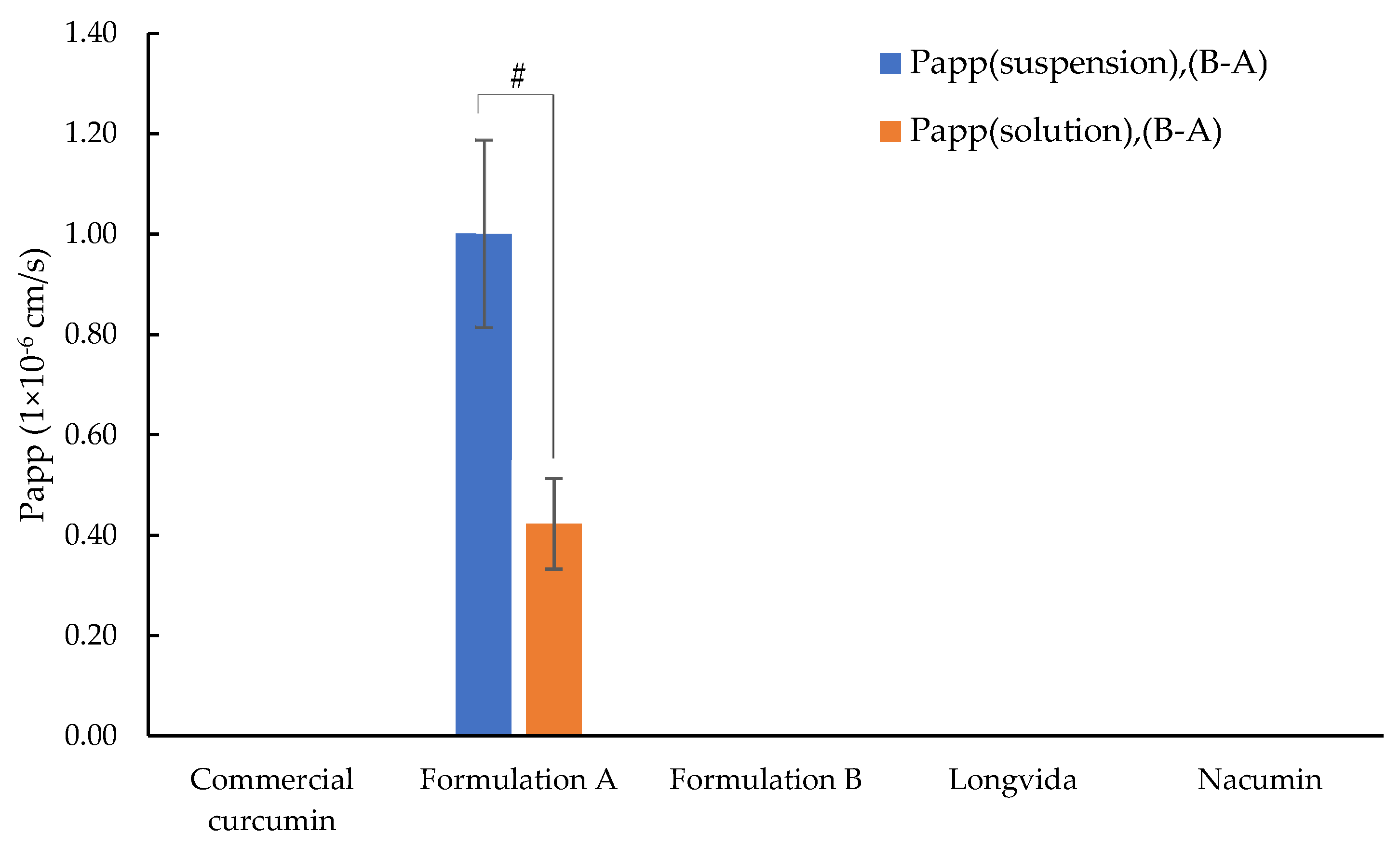
3.8. Determination of Permeability Coefficient (Papp)
- Papp (suspension) was calculated based on C0 which represents the initial concentration of curcumin dissolved in the suspension measured in the apical chamber. Here C0 is referred to as C0 (suspension). C0 (suspension) for each test sample is listed in Table 6.
- Papp (solution) was calculated based on C0 which represents the total applied dose of curcumin for each test sample. Here C0 is referred to as C0 (solution).
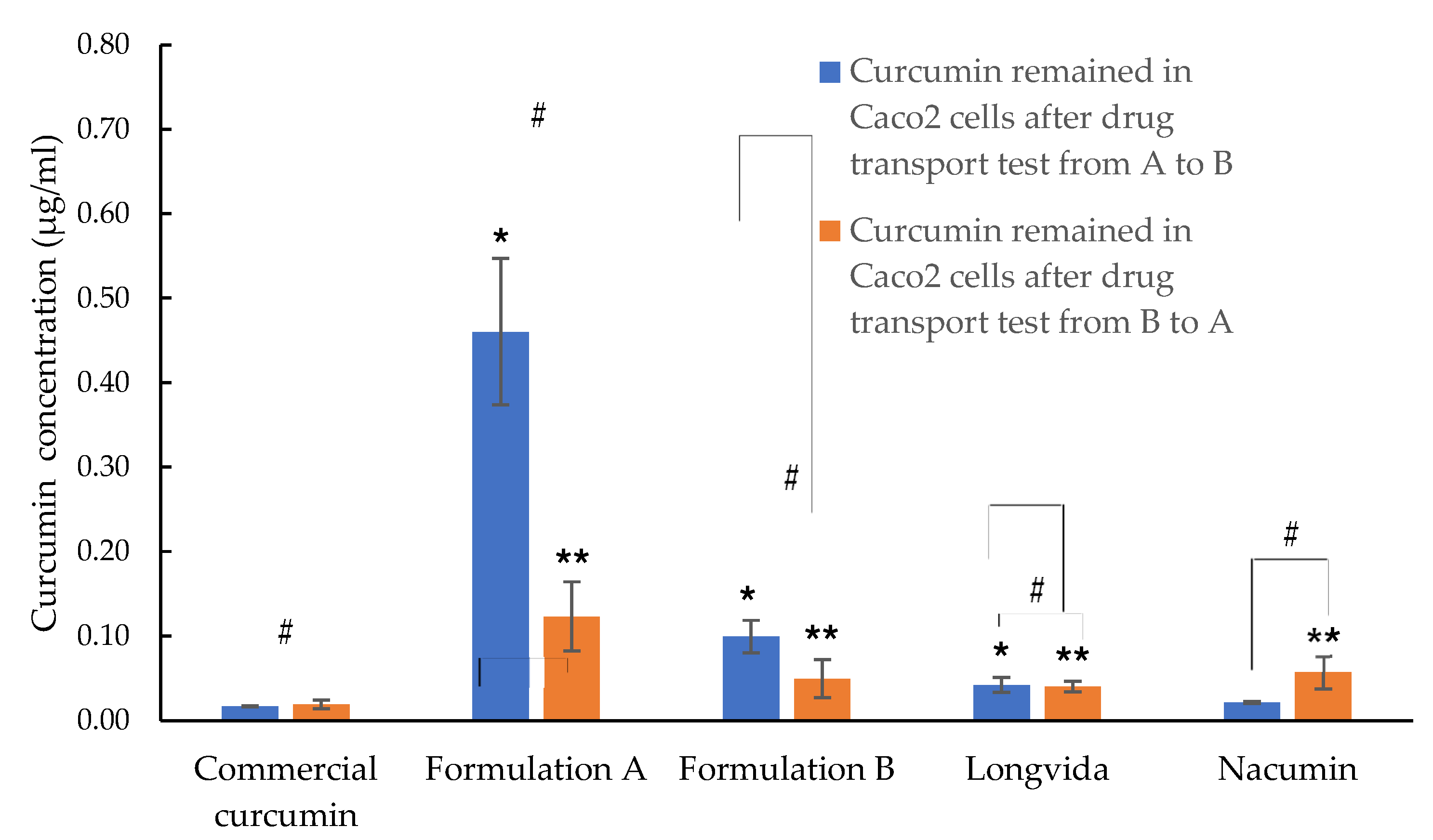
3.9. Cellular Uptake Study
4. Discussion
4.1. Solubility Study
4.2. Fourier Transform Infrared Spectroscopy (FTIR)
4.3. Differential Scanning Calorimetry (DSC)
4.4. X-ray Powder Diffraction (XRD)
4.5. Determining Curcumin Content in Commercial Curcumin Powder, Formulation A, Formulation B, Longvida® and Nacumin®
4.6. Determination of Cytotoxicity (MTT and LDH Assays)
4.7. Measurement of Transepithelial Electrical Resistance (TEER)
4.8. In Vitro Drug Transport Study
4.9. Cellular Uptake Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Smart, J.D.; Pannala, A.S. Recent developments in formulation design for improving oral bioavailability of curcumin: A review. J. Drug Deliv. Sci. Technol. 2020, 60, 102082. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahlström, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. 1978, 43, 86–92. [Google Scholar] [CrossRef]
- Srimal, R.C.; Dhawan, B.N. Pharmacology of diferuloyl methane (curcumin), a non-steroidal anti-inflammatory agent. J. Pharm. Pharmacol. 1973, 25, 447–452. [Google Scholar] [CrossRef]
- Kim, M.K.; Choi, G.J.; Lee, H.S. Fungicidal property of Curcuma longa L. rhizome-derived curcumin against phytopathogenic fungi in a greenhouse. J. Agric. Food Chem. 2003, 51, 1578–1581. [Google Scholar] [CrossRef] [PubMed]
- Senft, C.; Polacin, M.; Priester, M.; Seifert, V.; Kögel, D.; Weissenberger, J. The nontoxic natural compound Curcumin exerts anti-proliferative, anti-migratory, and anti-invasive properties against malignant gliomas. BMC Cancer 2010, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2018, 65, 1631–1652. [Google Scholar] [CrossRef] [Green Version]
- Gullapalli, R.P.; Mazzitelli, C.L. Polyethylene glycols in oral and parenteral formulations—A critical review. Int. J. Pharm. 2015, 496, 219–239. [Google Scholar] [CrossRef]
- Linn, M.; Collnot, E.M.; Djuric, D.; Hempel, K.; Fabian, E.; Kolter, K.; Lehr, C.M. Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur. J. Pharm. Sci. 2012, 45, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kang, W.S.; Piao, J.; Yoon, I.S.; Kim, D.D.; Cho, H.J. Soluplus®/TPGS-based solid dispersions prepared by hot-melt extrusion equipped with twin-screw systems for enhancing oral bioavailability of valsartan. Drug Des. Devel. Ther. 2015, 9, 2745–2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tønsberg, H.; Holm, R.; Bisgaard, J.; Jacobsen, J.; Müllertz, A. Effects of polysorbate 80 on the in-vitro precipitation and oral bioavailability of halofantrine from polyethylene glycol 400 formulations in rats. J. Pharm. Pharmacol. 2010, 62, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tønsberg, H.; Holm, R.; Mu, H.; Boll, J.B.; Jacobsen, J.; Müllertz, A. Effect of bile on the oral absorption of halofantrine in polyethylene glycol 400 and polysorbate 80 formulations dosed to bile duct cannulated rats. J. Pharm. Pharmacol. 2011, 63, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Nandi, I.; Bateson, M.; Bari, M.; Joshi, H.N. Synergistic effect of PEG-400 and cyclodextrin to enhance solubility of progesterone. AAPS PharmSciTech 2003, 4, E1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barmpalexis, P.; Karagianni, A.; Kachrimanis, K. Molecular simulations for amorphous drug formulation: Polymeric matrix properties relevant to hot-melt extrusion. Eur. J. Pharm. Sci. 2018, 119, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.Q.; Lai, H.W.; Zhang, Y.; Feng, B.; Xiao, X.; Zhang, H.M.; Li, Z.Q.; Qi, X.R. On the inherent properties of Soluplus® and its application in ibuprofen solid dispersions generated by microwave-quench cooling technology. Pharm. Dev. Technol. 2018, 23, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.Q.; Zhang, Y.; Li, Y.; Lai, H.W.; Xiao, X.; Feng, B.; Qi, X.R. Self-micellizing solid dispersions enhance the properties and therapeutic potential of fenofibrate: Advantages, profiles and mechanisms. Int. J. Pharm. 2017, 528, 563–577. [Google Scholar] [CrossRef]
- Solanki, N.; Gupta, S.S.; Serajuddin, A. Rheological analysis of itraconazole-polymer mixtures to determine optimal melt extrusion temperature for development of amorphous solid dispersion. Eur. J. Pharm. Sci. 2018, 111, 482–491. [Google Scholar] [CrossRef]
- Parikh, A.; Kathawala, K.; Song, Y.; Zhou, X.; Garg, S. Curcumin-loaded self-nanomicellizing solid dispersion system: Part I: Development, optimization, characterization, and oral bioavailability. Drug Deliv. Transl. Res. 2018, 8, 1389–1405. [Google Scholar] [CrossRef]
- Wang, L.L.; He, D.D.; Wang, S.X.; Dai, Y.H.; Ju, J.M.; Zhao, C.L. Preparation and evaluation of curcumin-loaded self-assembled micelles. Drug Dev. Ind. Pharm. 2018, 44, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Al-Akayleh, F.; Al-Naji, I.; Adwan, S.; Al-Remawi, M.; Shubair, M. Enhancement of curcumin solubility using a novel solubilizing polymer soluplus®. J. Pharm. Innov. 2022, 17, 142–154. [Google Scholar] [CrossRef]
- Krasavage, W.J.; Terhaar, C.J. d-alpha-Tocopheryl poly(ethylene glycol) 1000 succinate. Acute toxicity, subchronic feeding, reproduction, and teratologic studies in the rat. J. Agric. Food Chem. 1977, 25, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Goddeeris, C.; Willems, T.; Houthoofd, K.; Martens, J.A.; Van den Mooter, G. Dissolution enhancement of the anti-HIV drug UC 781 by formulation in a ternary solid dispersion with TPGS 1000 and Eudragit E100. Eur. J. Pharm. Biopharm. 2008, 70, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Goddeeris, C.; Willems, T.; Van den Mooter, G. Formulation of fast disintegrating tablets of ternary solid dispersions consisting of TPGS 1000 and HPMC 2910 or PVPVA 64 to improve the dissolution of the anti-HIV drug UC 781. Eur. J. Pharm. Biopharm. 2008, 34, 293–302. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Q. Investigation of the absorption mechanism of solubilized curcumin using Caco-2 cell monolayers. J. Agric. Food Chem. 2011, 59, 9120–9126. [Google Scholar] [CrossRef]
- Gupte, P.A.; Giramkar, S.A.; Harke, S.M.; Kulkarni, S.K.; Deshmukh, A.P.; Hingorani, L.L.; Mahajan, M.P.; Bhalerao, S.S. Evaluation of the efficacy and safety of Capsule Longvida® Optimized Curcumin (solid lipid curcumin particles) in knee osteoarthritis: A pilot clinical study. J. Inflamm. Res. 2019, 12, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Longvida® Vs-Corp. Available online: https://www.vs-corp.com/Longvida (accessed on 29 August 2020).
- Jamwal, R. Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef]
- McFarlin, B.K.; Venable, A.S.; Henning, A.L.; Sampson, J.N.B.; Pennel, K.; Vingren, J.L.; Hill, D.W. Reduced inflammatory and muscle damage biomarkers following oral supplementation with bioavailable curcumin. BBA Clin. 2016, 5, 72–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, K.H.; White, D.J.; Pipingas, A.; Poorun, K.; Scholey, A. Further evidence of benefits to mood and working memory from lipidated curcumin in healthy older people: A 12-week, double-blind, placebo-controlled, partial replication study. Nutrients 2020, 12, 1678. [Google Scholar] [CrossRef] [PubMed]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 2017, 9, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nacumin®. Techbifarm. Available online: http://www.techbifarm.com.vn/nacumin (accessed on 29 August 2020).
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavra, Z.M.M.; Pereira de Santana, D.; Ré, M.I. Solubility and dissolution performances of spray-dried solid dispersion of Efavirenz in Soluplus. Drug Dev. Ind. Pharm. 2017, 43, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Moneghini, M.; De Zordi, N.; Solinas, D.; Macchiavelli, S.; Princivalle, F. Characterization of solid dispersions of itraconazole and vitamin E TPGS prepared by microwave technology. Future Med. Chem. 2010, 2, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, A.; Güngör, N.; Ece, O.; Atici, O. The rheological properties and characterization of bentonite dispersions in the presence of non-ionic polymer PEG. J. Mater. Sci. 2005, 40, 171–177. [Google Scholar] [CrossRef]
- Lin, B.; Zhou, S. Poly (ethylene glycol)-grafted silica nanoparticles for highly hydrophilic acrylic-based polyurethane coatings. Prog. Org. Coat. 2017, 106, 145–154. [Google Scholar] [CrossRef]
- Karolewicz, B.; Gajda, M.; Górniak, A.; Owczarek, A.; Mucha, I. Pluronic F127 as a suitable carrier for preparing the imatinib base solid dispersions and its potential in development of a modified release dosage forms. J. Therm. Anal. Calorim. 2017, 130, 383–390. [Google Scholar] [CrossRef]
- Jia, N.; Li, S.-M.; Ma, M.-G.; Zhu, J.-F.; Sun, R.-C. Synthesis and characterization of cellulose-silica composite fiber in ethanol/water mixed solvents. BioResources 2011, 6, 1186–1195. [Google Scholar] [CrossRef]
- Parvinzadeh, M.; Moradian, S.; Rashidi, A.; Yazdanshenas, M.-E. Surface characterization of polyethylene terephthalate/silica nanocomposites. Appl. Surf. Sci. 2010, 256, 2792–2802. [Google Scholar] [CrossRef]
- Stulzer, H.; Tagliari, M.; Cruz, A.; Silva, M.; Laranjeira, M. Compatibility studies between piroxicam and pharmaceutical excipients used in solid dosage forms. Pharm. Chem. J. 2008, 42, 215–219. [Google Scholar] [CrossRef]
- Sayyar, Z.; Jafarizadeh-Malmiri, H. Temperature effects on thermodynamic parameters and solubility of curcumin O/W nanodispersions using different thermodynamic models. Int. J. Food Eng. 2019, 15, 20180311. [Google Scholar] [CrossRef]
- Clas, S.-D.; Dalton, C.R.; Hancock, B.C. Differential scanning calorimetry: Applications in drug development. Pharm. Sci. Tech. Today 1999, 2, 311–320. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Neau, S.H. Investigation of the in vitro performance difference of drug-Soluplus® and drug-PEG 6000 dispersions when prepared using spray drying or lyophilization. Saudi Pharm. J. 2017, 25, 419–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newa, M.; Bhandari, K.H.; Oh, D.H.; Kim, Y.R.; Sung, J.H.; Kim, J.O.; Woo, J.S.; Choi, H.G.; Yong, C.S. Enhanced dissolution of ibuprofen using solid dispersion with poloxamer 407. Arch. Pharmacol. Res. 2008, 31, 1497–1507. [Google Scholar] [CrossRef]
- Haware, R.V.; Vinjamuri, B.P.; Sarkar, A.; Stefik, M.; Stagner, W.C. Deciphering magnesium stearate thermotropic behavior. Int. J. Pharm. 2018, 548, 314–324. [Google Scholar] [CrossRef]
- Barboza, F.; Vecchia, D.D.; Tagliari, M.P.; Silva, M.; Stulzer, H. Differential scanning calorimetry as a screening technique in compatibility studies of acyclovir extended release formulations. Pharm. Chem. J. 2009, 43, 363–368. [Google Scholar] [CrossRef]
- Delaney, S.P.; Nethercott, M.J.; Mays, C.J.; Winquist, N.T.; Arthur, D.; Calahan, J.L.; Sethi, M.; Pardue, D.S.; Kim, J.; Amidon, G. Characterization of synthesized and commercial forms of magnesium stearate using differential scanning calorimetry, thermogravimetric analysis, powder X-ray diffraction, and solid-state NMR spectroscopy. J. Pharm. Sci. 2017, 106, 338–347. [Google Scholar] [CrossRef]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Pade, V.; Stavchansky, S. Link between drug absorption solubility and permeability measurements in Caco-2 cells. J. Pharm Sci. 1998, 87, 1604–1607. [Google Scholar] [CrossRef] [PubMed]
- Coltescu, A.-R.; Butnariu, M.; Sarac, I. The importance of solubility for new drug molecules. Biomed. Pharmacol. J. 2020, 13, 577–583. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O. Therapeutic potential of quercetin: New insights and perspectives for human health. Acs Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. Int. Sch. Res. Not. 2012, 2012, 195727. [Google Scholar] [CrossRef] [Green Version]
- Vo, C.L.-N.; Park, C.; Lee, B.-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef]
- Rangel-Yagui, C.O.; Pessoa, A., Jr.; Tavares, L.C. Micellar solubilization of drugs. J. Pharm. Pharm. Sci. 2005, 8, 147–163. [Google Scholar]
- Rong, W.-T.; Lu, Y.-P.; Tao, Q.; Guo, M.; Lu, Y.; Ren, Y.; Yu, S.-Q. Hydroxypropyl-sulfobutyl-β-cyclodextrin improves the oral bioavailability of edaravone by modulating drug efflux pump of enterocytes. J. Pharm. Sci. 2014, 103, 730–742. [Google Scholar] [CrossRef]
- Sharma, V.; Pathak, K. Effect of hydrogen bond formation/replacement on solubility characteristics, gastric permeation and pharmacokinetics of curcumin by application of powder solution technology. Acta Pharm. Sin. B 2016, 6, 600–613. [Google Scholar] [CrossRef] [Green Version]
- Song, I.S.; Cha, J.S.; Choi, M.K. Characterization, in vivo and in vitro Evaluation of Solid Dispersion of Curcumin Containing d-α-Tocopheryl Polyethylene Glycol 1000 Succinate and Mannitol. Molecules 2016, 21, 1386. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Cazzador, F.; Grønlien, K.G.; Tønnesen, H.H. Effect of curcumin and cosolvents on the micellization of Pluronic F127 in aqueous solution. Colloids Surf. B Biointerfaces 2020, 195, 111250. [Google Scholar] [CrossRef] [PubMed]
- Cullity, B.D. Elements of X-ray Diffraction, 3rd ed.; Addison-Wesley Publishing: Boston, MA, USA, 1956; pp. 272–295. [Google Scholar]
- Aditya, N.; Yang, H.; Kim, S.; Ko, S. Fabrication of amorphous curcumin nanosuspensions using β-lactoglobulin to enhance solubility, stability, and bioavailability. Colloids Surf. B Biointerfaces 2015, 127, 114–121. [Google Scholar] [CrossRef]
- Pawar, Y.B.; Shete, G.; Popat, D.; Bansal, A.K. Phase behavior and oral bioavailability of amorphous Curcumin. Eur. J. Pharm. Sci. 2012, 47, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Indianapolis, IN, USA, 2013. [Google Scholar]
- Zeng, Z.; Shen, Z.L.; Zhai, S.; Xu, J.L.; Liang, H.; Shen, Q.; Li, Q.Y. Transport of curcumin derivatives in Caco-2 cell monolayers. Eur. J. Pharm. Biopharm. 2017, 117, 123–131. [Google Scholar] [CrossRef]
- Faralli, A.; Shekarforoush, E.; Ajalloueian, F.; Mendes, A.C.; Chronakis, I.S. In vitro permeability enhancement of curcumin across Caco-2 cells monolayers using electrospun xanthan-chitosan nanofibers. Carbohydr. Polym. 2019, 206, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. SLAS Technol. 2015, 20, 107–126. [Google Scholar] [CrossRef] [Green Version]
- Duizer, E.; Gilde, A.J.; Versantvoort, C.H.; Groten, J.P. Effects of cadmium chloride on the paracellular barrier function of intestinal epithelial cell lines. Toxicol. Appl. Pharmacol. 1999, 155, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Meindl, C.; Stranzinger, S.; Dzidic, N.; Salar-Behzadi, S.; Mohr, S.; Zimmer, A.; Fröhlich, E. Permeation of Therapeutic Drugs in Different Formulations across the Airway Epithelium In vitro. PLoS ONE 2015, 10, e0135690. [Google Scholar] [CrossRef] [Green Version]
- Narai, A.; Arai, S.; Shimizu, M. Rapid decrease in transepithelial electrical resistance of human intestinal Caco-2 cell monolayers by cytotoxic membrane perturbents. Toxicol. Vitr. 1997, 11, 347–354. [Google Scholar] [CrossRef]
- Davis, S.; Hardy, J.; Fara, J. Transit of pharmaceutical dosage forms through the small intestine. Gut 1986, 27, 886–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Y.; Zhang, W.; Huang, Y.; Hein, K.; Hidalgo, I.J. The role of a basolateral transporter in rosuvastatin transport and its interplay with apical breast cancer resistance protein in polarized cell monolayer systems. Drug Metab. Dispos. 2012, 40, 2102–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zastre, J.; Dowd, C.; Bruckner, J.; Popovici, A. Lack of P-glycoprotein-mediated efflux and the potential involvement of an influx transport process contributing to the intestinal uptake of deltamethrin, cis-permethrin, and trans-permethrin. Toxicol. Sci. 2013, 136, 284–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Cheng, Y.; Xu, L.; Zhang, L. Study of the intestinal absorption characteristics of curcumin in vivo and in vitro. J. Appl. Pharm. 2017, 9, 1000246. [Google Scholar] [CrossRef]
- Brodin, B.; Steffansen, B.; Nielsen, C.U. Passive diffusion of drug substances: The concepts of flux and permeability. In Molecular Biopharmaceutics: Aspects of Drug Characterisation, Drug Delivery and Dosage Form Evaluation, 1st ed.; Pharmaceutical Press: London, UK, 2010; pp. 135–152. [Google Scholar]
- Di, L.; Kerns, E. Chapter 7: Solubility. In Drug-Like Properties: Concepts, Structure Design and Methods from ADME to Toxicity Optimization, 1st ed.; Academic Press: London, UK, 2008; pp. 56–82. [Google Scholar]
- Chanburee, S.; Tiyaboonchai, W. Enhanced intestinal absorption of curcumin in Caco-2 cell monolayer using mucoadhesive nanostructured lipid carriers. JBMR Part B Appl. Biomater. 2018, 106, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Schiborr, C.; Kocher, A.; Meins, J.; Behnam, D.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Transepithelial transport of curcumin in Caco-2 cells is significantly enhanced by micellar solubilisation. Plant Foods Hum. Nutr. 2017, 72, 48–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, K.; Stella, L.; Jones, D.S.; Andrews, G.P.; Du, H.; Tian, Y. Drug-Rich Phases Induced by Amorphous Solid Dispersion: Arbitrary or Intentional Goal in Oral Drug Delivery? Pharmaceutics 2021, 13, 889. [Google Scholar] [CrossRef]
- den Mooter, G.V.D. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Derakhshandeh, K.; Hochhaus, G.; Dadashzadeh, S. In-vitro Cellular Uptake and Transport Study of 9-Nitrocamptothecin PLGA Nanoparticles Across Caco-2 Cell Monolayer Model. Iran. J. Pharm. Res. IJPR 2011, 10, 425–434. [Google Scholar] [PubMed]
- Joshi, G.; Kumar, A.; Sawant, K. Bioavailability enhancement, Caco-2 cells uptake and intestinal transport of orally administered lopinavir-loaded PLGA nanoparticles. Drug Deliv. 2016, 23, 3492–3504. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, S.; Alayoubi, A.; Nazzal, S.; Sylvester, P.W.; Kaddoumi, A. Enhanced solubility and oral bioavailability of γ-Tocotrienol using a self-emulsifying drug delivery system (SEDDS). Lipids 2014, 49, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Tubtimsri, S.; Weerapol, Y. Improvement in solubility and absorption of nifedipine using solid solution: Correlations between surface free energy and drug dissolution. Polymers 2021, 13, 2963. [Google Scholar] [CrossRef] [PubMed]





| Ingredient | Amount/mL |
|---|---|
| L-lactate | 2.5 mg |
| ß-NAD | 2.5 mg |
| MTT | 0.25 mg |
| MPMS | 0.034 mg |
| Tris-HCL | 0.9 ml |
| 1% (v/v) Triton-X100 | 0.1 ml |
| Curcumin Solubility (μg/mL) | |
|---|---|
| Formulation A | 70.33 ± 10.26 |
| Formulation B | 25.00 ± 0.60 |
| Commercial curcumin | 0.08 ± 0.01 |
| Curcumin (% w/w) | |
|---|---|
| Commercial curcumin powder | 81.46 ± 2.19 |
| Formulation A | 6.74 ± 0.45 |
| Formulation B | 8.91 ± 0.85 |
| Longvida® | 30.67 ± 1.33 |
| Nacumin® | 7.15 ± 0.15 |
| Sample Concentration (μg/mL) | |
|---|---|
| Commercial curcumin powder | 45.22 |
| Formulation A | 545.59 |
| Formulation B | 413.46 |
| Longvida® | 120.12 |
| Nacumin® | 515.24 |
| At t = 0 min | After t = 180 min | |
|---|---|---|
| Curcumin | 328 ± 23 | 271 ± 23 |
| Formulation A | 330 ± 28 | 270 ± 28 |
| Formulation B | 316 ± 56 | 270 ± 50 |
| Longvida | 313 ± 48 | 257 ± 35 |
| Nacumin | 330 ± 57 | 271 ± 58 |
| C0 (Suspension), (A-B) (μg/mL) | C0 (Suspension), (B-A) (μg/mL) | |
|---|---|---|
| Curcumin | 0.07 ± 0.01 | 0.04 ± 0.01 |
| Formulation A | 17.00 ± 2.95 | 15.51 ± 1.46 |
| Formulation B | 3.72 ± 0.71 | 1.88 ± 0.13 |
| Longvida | 0.31 ± 0.03 | 0.23 ± 0.04 |
| Nacumin | 0.40 ± 0.05 | 0.31 ± 0.03 |
| Papp (Suspension), (A-B) (cm/s) | Papp ( Solution), (A-B) (cm/s) | |
|---|---|---|
| 1st | Nacumin® (19.58 ± 3.66 × 10−6) | Formulation A (1.12 ± 0.15 × 10−6) |
| 2nd | Longvida® (10.79 ± 1.42 × 10−6) | Formulation B (0.24 ± 0.07 × 10−6) |
| 3rd | Formulation A (2.52 ± 0.68 × 10−6) | Nacumin® (0.21 ± 0.03 × 10−6) |
| 4th | Formulation B (2.43 ± 0.55 × 10−6) | Longvida® (0.09 ± 0.01 × 10−6) |
| 5th | Commercial curcumin (0) | Commercial Curcumin (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Lansley, A.B.; Duong, T.N.; Smart, J.D.; Pannala, A.S. Increasing Cellular Uptake and Permeation of Curcumin Using a Novel Polymer-Surfactant Formulation. Biomolecules 2022, 12, 1739. https://doi.org/10.3390/biom12121739
Liu Z, Lansley AB, Duong TN, Smart JD, Pannala AS. Increasing Cellular Uptake and Permeation of Curcumin Using a Novel Polymer-Surfactant Formulation. Biomolecules. 2022; 12(12):1739. https://doi.org/10.3390/biom12121739
Chicago/Turabian StyleLiu, Zhenqi, Alison B. Lansley, Tu Ngoc Duong, John D. Smart, and Ananth S. Pannala. 2022. "Increasing Cellular Uptake and Permeation of Curcumin Using a Novel Polymer-Surfactant Formulation" Biomolecules 12, no. 12: 1739. https://doi.org/10.3390/biom12121739
APA StyleLiu, Z., Lansley, A. B., Duong, T. N., Smart, J. D., & Pannala, A. S. (2022). Increasing Cellular Uptake and Permeation of Curcumin Using a Novel Polymer-Surfactant Formulation. Biomolecules, 12(12), 1739. https://doi.org/10.3390/biom12121739







