Abstract
Breast cancer is the most commonly diagnosed cancer in women. The high incidence of breast cancer, which is continuing to rise, makes treatment a significant challenge. The PI3K–AKT pathway and its downstream targets influence various cellular processes. In recent years, mounting evidence has shown that natural products and synthetic drugs targeting PI3K–AKT signaling have the potential to treat breast cancer. In this review, we discuss the role of the PI3K–AKT signaling pathway in the occurrence and development of breast cancer and highlight PI3K–AKT-targeting natural products and drugs in clinical trials for the treatment of breast cancer.
1. Introduction
Cancer is a leading cause of death worldwide. In 2020, breast cancer surpassed lung cancer as the most common cancer type, accounting for 11.7% (2,261,419 cases) of all newly diagnosed cancer cases and 6.9% (684,996 cases) of all new cancer deaths worldwide [1]. Modern lifestyle has been associated with problems such as decreased fertility and obesity. These factors have contributed to the rising global incidence of breast cancer. The emotional and physical toll that conventional breast cancer treatment takes on survivors can be indelible. Although there currently appears to be no difference in postoperative complications between breast-conserving therapy (BCT) and mastectomy, this may depend on the combination of breast-conserving therapy and postoperative adjuvant therapy [2]. Radiation therapy to the marginal junction after lumpectomy is one of the methods of postoperative adjuvant therapy [3]. However, adjuvant therapy for patients rejected by radiotherapy continues to attract more attention. The phosphoinositide 3-kinase (PI3K)-protein kinase B (AKT) pathway is active in most breast cancers, and 40% of ER+ breast cancers are associated with PIK3CA mutation, which is a common genomic alteration in breast cancer [4]. In addition, the PI3K–AKT pathway, as a receptor tyrosine kinase (RTK) downstream pathway, participates in various cell biological functions such as breast cancer cell proliferation and apoptosis [5]. Targeting the PI3K–AKT pathway in breast cancer may be a potential drug target in the context of endocrine and anti-RTK resistance [6]. However, chemotherapy resistance and drug toxicity have been a concern in neoadjuvant therapy. In recent years, numerous studies have shown that natural products possess anticancer properties. Natural products and their derivatives account for 80% of FDA-approved anticancer drugs [7]. The PI3K–AKT signaling pathway has been associated with various tumorigenic functions in breast cancer, including cell survival, migration, and invasion [8]. In this review, we searched PubMed, Web of Science, clinicalTrials.gov, and China Knowledge Infrastructure (CNKI) for literature and clinical trial studies, and summarized natural products based on the PI3K–AKT pathway in breast cancer treatment, involving flavonoids, polyphenols, alkaloids, and PI3K inhibitors and AKT inhibitors in clinical trials. Finally, we discuss future directions and limitations of natural products.
2. PI3K–AKT Signaling in Breast Cancer
2.1. Overview of the PI3K–AKT Signaling Pathway
The PI3K–AKT cell signaling pathway is associated with the occurrence of various diseases, including neurodegenerative diseases, cancer, and tuberous sclerosis complex. Studies have shown that the binding capacity of PI3K and platelet-derived growth factor (PDGF) receptors affect AKT activation [9,10,11]. It has been demonstrated that AKT is activated by cytokines in a PI3K-dependent manner [12,13,14]. Moreover, AKT was the first effector substrate of PI3K to be identified [15]. PI3K activation is related to the coordinated activation of multiple RTK like human epidermal growth factor receptor 2 (HER2), HER3, and insulin-like growth factor 1 receptor (IGF1R), but calmodulin is also involved [16]. RTKs are high-affinity cell surface receptors for various growth factors, cytokines, and hormones. These RTKs possess an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain [17]. The binding of ligands, such as growth factors, to the extracellular ligand domains of RTKs, triggers the two RTK monomers to dimerize, thereby activating the intracellular tyrosine kinase domain via the autophosphorylation of each monomer [18]. The regulatory subunit of PI3K binds to the intracellular tyrosine kinase domain and recruits the catalytic subunit to form a complex with the activated RTK [19]. The process plays a key role in the regulation of signaling through growth factors and cytokines. Additionally, the catalytic subunit p110 (α, δ, γ) directly binds to the rat sarcoma virus (RAS) family of GTPases to activate PI3K [20]. Activated PI3K catalyzes the phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2), thereby generating the second messenger, phosphatidylinositol 3,4,5-trisphosphate (PIP3) [21]. The activity of class I PI3K is countered by the phosphatase PTEN (phosphatase and tensin homolog), which inhibits PI3K–AKT signaling by dephosphorylating PIP3 [22]. The ablation of PI3K’s catalytic subunit suppresses prostate carcinogenesis that results from PTEN deletion [23]. PIP3 accumulation on the plasma membrane recruits effector proteins such as AKTs. Differences in the functions of the three AKT subtypes stem from their differences in cellular distribution. AKT1 is widely distributed in tissues and plays an important role in cell growth and survival [24]. AKT2 is highly expressed in adipose and skeletal muscle and plays a specific role in the regulation of glucose homeostasis [25]. Compared with the previous two types, the distribution of AKT3 is smaller, mainly in the testis and brain. According to clinical studies, AKT3 mutations cause megalencephaly in many patients [26]. However, its specific pathogenic mechanism is still unclear. AKT1 is recruited to the plasma membrane through the interaction between its pleckstrin homology (PH) domain and PIP3, which leads to AKT1 phosphorylation at T308 by phosphoinositide-dependent protein kinase 1 (PDK1) [15]. This phosphorylation is essential for AKT1 activation and precedes subsequent AKT1 phosphorylation at S473 by mechanistic target of rapamycin (mTOR) complex 2 (mTORC2), to fully activate AKT [27,28,29].
The role of PI3K/AKT signaling in cells is positively correlated with the kinase activity of AKT. Glycogen synthase kinase-3 (GSK-3), the first AKT substrate to be identified, phosphorylates glycogen synthase in response to stimulation by insulin, thereby inactivating it [14]. mTOR is a key PI3K–AKT signaling substrate. AKT activates mTOR by phosphorylating it on S2448. Once activated, mTOR senses cellular energy and nutritional status, and then triggers responses such as autophagy, which generates sources of cellular energy [30]. AKT phosphorylates the protein tyrosine phosphatase (PTP1B), thereby negatively regulating its phosphatase activity and promoting insulin signaling [31]. AKT phosphorylates bcl2-antagonist of cell death (BAD), a member of the B-cell lymphoma 2 (Bcl-2) family, triggering its release from the mitochondrial membrane and preventing its binding to Bcl-2, thereby inhibiting its antiapoptotic function. Thus, by phosphorylating BAD, AKT promotes cell survival [32,33,34]. Studies have also shown that transcription factors, including the Forkhead (FH or FoxO) family of transcription factors and the nuclear transcription factor-kb (NF-κB)/Rel family are regulated by AKT [35]. AKT suppresses the activity of FoxO transcription factors via phosphorylation, thereby influencing the expression of its target genes, including TRADD (TNF receptor type 1-associated death domain) and intracellular apoptotic components such as Bcl-2 interacting mediator of cell death (Bim), and promoting proliferation [36]. NF-κB is a key regulator of immune responses. Tumor necrosis factor-α (TNF-α) activates AKT, which phosphorylates iκB kinase (IKK) (an upstream target of NF-κB) and activates NF-κB [37]. p21 is a cell cycle inhibitory protein (Cip1); the phosphorylation of p21 (T145) by AKT inhibits the nuclear localization of p21, attenuates the cyclin-dependent kinase 2 (Cdk2) inhibitory activity of p21, and promotes endothelial cell proliferation [38]. Figure 1 shows the role of the PI3K–AKT pathway in tumor cells.
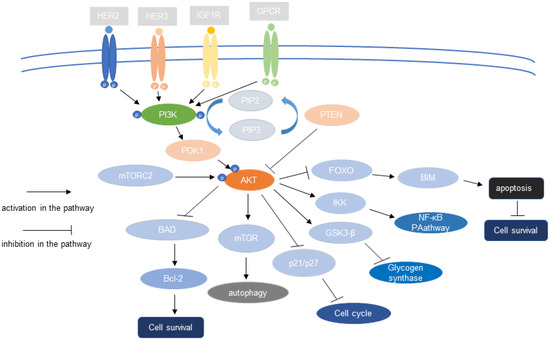
Figure 1.
The PI3K–AKT pathway regulates tumor cell proliferation, cycle, autophagy, metabolism, and other cell biological behaviors through downstream effector molecules.
2.2. The Role of PI3K–AKT Signaling in Breast Cancer
Important risk factors for breast cancer include genetic susceptibility, aging, hormone disorders, family history, and environmental factors. Others risk factors include physiological factors such as early menarche, late menopause, and obesity, as well as personal habits such as drinking and lack of physical activity [39,40]. The main types of breast cancer are lobular carcinoma in situ and ductal carcinoma in situ, with the latter being the most common. Breast cancer falls into five subtypes depending on the presence or absence of estrogen receptors (ER), progesterone receptors (PR), and HER2 receptors. These are the luminal A subtype (ER+, PR±, HER2−, low Ki67), luminal B subtype (ER+, PR±, HER2±, high Ki67), HER2 subtype (ER−, PR−, HER2+), triple negative (ER−, PR−, HER2−), normal-like (ER+, PR±, HER2−, low Ki67) [41,42,43].
The occurrence and development of breast cancer are driven by gene mutation and dysregulation of cell signaling pathways, and PI3K–AKT signaling is the most commonly upregulated pathway in breast cancer [44]. Several studies have implicated PI3K in the development of ER+ breast cancer. For example, the accumulation of PI3K gene mutations in somatic cells also promotes the development of breast cancer and PIK3CA mutations have been found in solid tumors [45]. Additionally, PIK3CA mutations suppress the differentiation of luminal and basal mammary cells, resulting in more cancer cell lineages [46]. John et al. found that mutation of E17K-Akt1 in human breast cancer cells can form a new hydrogen bond between Akt1 and phosphoinositol ligand, thereby activating Akt1 and promoting downstream signaling [47]. A meta-analysis found that breast cancer tissues have a higher rate of PTEN deletion when compared with normal tissues and that these mutations are associated with breast cancer invasiveness and metastatic potential. Patients with such mutations also have poor overall survival (OS) and disease-free survival (DFS) [48].
PI3K–AKT signaling activates ERα in an estrogen-independent manner and AKT overexpression protects breast cancer cells from tamoxifen (anti-estrogenic effect)-induced apoptosis [49]. This shows that inhibiting PI3K can enhance the therapeutic effect against ER+ breast cancer cells. Interestingly, it is reported that the p110α subunit of PI3K promotes apoptosis in estrogen-deprived breast cancer cells and that similar results are obtained using BEZ235, a PI3K inhibitor [50]. A PI3K gene expression signature enrichment analysis of ER+ breast cancer compared the two luminal breast cancer subtypes and found that the luminal B subtype has higher PI3K activity, which may make it less effective for antiestrogen therapy [42,51]. This also suggests that identifying the activation signature of the PI3K pathway can help predict the outcomes of endocrine therapy and identify high-risk ER+ breast cancers [52]. Additionally, it is reported that inhibiting mTOR, which acts downstream of AKT, enhances the antitumor effects of HER2 inhibitors in HER2-overexpressing breast cancer cells [53]. However, HER2/HER3 overexpression attenuates the antitumor effects of PI3K inhibitors [54]. Hence, to treat breast cancer, HER2 antagonists are often used in combination with PI3K inhibitors. DNA-damaging chemotherapy is often used to treat triple-negative breast cancer (TNBC). DNA damage often triggers the DNA-dependent protein kinase-mediated phosphorylation of AKT. Interestingly, several studies indicate that the pro-apoptotic effects of DNA-damaging agents are enhanced by PI3K inhibitors [55,56]. Therefore, PI3K pathway mutations may contribute to TNBC’s resistance to DNA-damaging agents. Glycogen synthase kinase-3β (GSK-3β) knockdown attenuated the apoptotic effect of rapamycin and paclitaxel on breast cancer cells [57]. This indicates that GSK-3β is a tumor suppressor in breast cancer. Taken together, these reports indicate that PI3K–AKT signaling has a key role in the development and treatment of various breast cancer subtypes.
3. Research Status of Inhibitors Targeting PI3K–AKT Pathway in Breast Cancer
Targeted therapy is an important strategy for cancer treatment. Table 1 is a summary of PI3K–AKT-targeting drugs that are in clinical trials (https://clinicaltrials.gov/ct2/home, accessed on 15 May 2022).

Table 1.
Summary of PI3K–AKT-targeting drugs.
3.1. PI3K Inhibitors
PI3Ks fall into three classes based on primary structure and in vitro lipid specificity [81]. Class I PI3K is the most frequently studied class of enzymes that are activated by cell surface receptors and is further divided into class IA and class IB. Class IA PI3Ks are heterodimers of a p110 catalytic subunit and a p85 regulatory subunit. The p110 catalytic subunit is made of three homologous class IA catalytic isomers, p110α, p110β, and p110δ, encoded by PIK3CA, PIK3CB, and PIK3CD genes, respectively. The regulatory subunit of P85 is made of P85α (and its spliced variant P55α), P85β, and P55γ, which are encoded by PIK3R1, PIK3R2, and PIK3R3 genes [20,82]. Class IB PI3Ks are heterodimers of the catalytic subunit p110γ and the regulatory subunit p101 [83]. Class IA PI3Ks (α, β, δ) are activated upon the binding of the SH2 domain of the p85 regulatory subunit to activated RTK receptors or phosphotyrosine residue adaptor proteins, while class IB PI3Ks can be activated by G protein coupled receptors (GPCRs) [84]. Class II PI3Ks consist of a single subunit and are divided into three subtypes, PI3KC2α, PI3KC2β, and PI3KC2γ, which can be activated by various factors, including hormones, growth factors, and calcium ions. However, little is known about their functions [20]. Class III PI3Ks consist of a single subunit, vacuolar protein sorting 34 (VPS34), and are the only PI3Ks that are expressed in all eukaryotic cells. In vivo, Vps34 only phosphorylates phosphatidylinositol, generating phosphatidylinositol (3)-phosphate (PtdIns3P). VPS34 modulates mTOR signaling through its lipid kinase activity, thereby regulating cell growth [85]. VPS34 is also a key modulator of endosomal trafficking and autophagy.
A variety of PI3K inhibitors with the potential to treat breast cancer have been developed. Buparlisib (BKM120) is a potent, highly specific oral pan class I PI3K inhibitor. In clinical trials on the efficacy of BKM120 against advanced triple-negative breast cancer, Ana C et al. found that, alone, it prolonged SD in some patients, and that in combination with other drugs, it should be able to achieve better results [86]. Clinical trials on the efficacy of BKM120 against breast cancer, when combined with other drugs, such as lapatinib (NCT01589861), fulvestrant (NCT01339442), tamoxifen (NCT02404844), and LDE225 (NCT01576666), are ongoing. Interestingly, studies have shown that BKM120 sensitizes BRCA-proficient TNBC to poly ADP-ribose polymerase (PARP) inhibitors by downregulating the expression of BRCA1/2 and Rad51 via the inhibition of PI3K–AKT–NF-κB–c-Myc and the PI3K–AKT–forkhead box m1 (FOXM1)–Exonuclease 1 (Exo1) pathways [87].
Tenalisib is a highly selective, orally active dual PI3K δ/γ inhibitor that also possesses salt-inducible kinase 3 (SIK3) activity. A phase I/Ib study of tenalisib’s maximum tolerated dose (MTD), pharmacokinetics, and efficacy in patients with relapsed/refractory peripheral and cutaneous T-cell lymphoma found that it was clinically safe and tolerable at an MTD of 800 mg/day [88]. Additionally, ongoing studies on the use of tenalisib in combination with romidepsin to treat T-cell lymphoma indicate that tenalisib is well tolerated. [89]. Clinical trials on the use of tenalisib alone to treat patients with metastatic and invasive breast cancer are ongoing (NCT05021900).
Taselisib, in combination with fulvestrant, has previously undergone phase III clinical trials for the treatment of ER+, HER−, PIK3CA-mutated, advanced breast cancer. The results showed that although the taselisib group had a higher incidence of adverse events, INV-PFS (progression-free survival), objective response rate, clinical benefit rate, and duration of objective response after the combination showed a sustained improvement [90]. A phase III study on the use of taselisib in combination with fulvestrant to treat advanced breast cancer during aromatase inhibitor therapy or in relapsed disease is ongoing (NCT02457910).
BYL719 is a selective oral inhibitor of class I PI3K p110α. The combination of BYL719 and letrozole in the treatment of postmenopausal patients with ER+/HER− metastatic breast cancer that was refractory to endocrine therapy revealed that the combination was safe, tolerable, and effective, and phase II clinical trials showed that its MTD and recommended dose was 300 mg/day [91]. A study on the use of BYL719 in combination with paclitaxel to treat HER2− metastatic breast cancer found that patients with tumor/ctDNA mutations experienced better PFS when compared with patients without PIK3CA mutations, and that PFS was also higher in patients with normal metabolism [92]. Phase I clinical trials are ongoing on the use of BYL719 in combination with letrozole in the treatment of postmenopausal women with hormone receptor-positive metastatic breast cancer (NCT01791478).
Pictilisib (GDC-0941) is a potent and highly specific oral PI3K inhibitor (IC50: 3nM [93]. A study on the efficacy of pictilisib when combined with paclitaxel to treat hormone receptor positive, HER2−, locally recurrent, or metastatic breast cancer did not find significant PFS improvement in either the intention-to-treat or the PIK3CA-mutant groups [94]. In the subsequent phase Ib trials of pictilisib combined with paclitaxel, pictilisib + paclitaxel + bevacizumab, and pictilisib + paclitaxel + trastuzumab in the treatment of advanced breast cancer, the results showed that these three combinations had more controllable safety and anticancer activity [70,95].
GDC-0084, a dual PI3K/mTOR inhibitor, exhibited antitumor activity in preclinical models of glioblastoma [96]. In vitro treatment of PIK3CA-mutant breast cancer brain metastases cell lines with GDC-0084 reduced their viability, induced apoptosis, and inhibited the phosphorylation of AKT and p70S6, highlighting its potential as a therapeutic strategy against breast cancer with brain metastases [97]. A phase II clinical trial of the use of GDC-0084 in combination with trastuzumab to treat HER2+ breast cancer with brain metastases is underway (NCT03765983). Gedatolisib (PF-05212384) is a dual PI3K/mTOR inhibitor that also selectively inhibits PI3Kα and PI3Kγ with IC50 values of 0.4 nM and 5.4 nM, respectively [98]. Gedatolisib inhibits the growth of xenografted breast cancer at doses of >10 mg/kg [99]. Clinical trials are ongoing on the use of gedatolisib in combination with docetaxel, cisplatin, and dacomitinib, in triple negative breast cancer (NCT01920061).
AZD8186 is a potent PI3Kβ inhibitor that suppresses PI3Kδ signaling. AZD8186 combined with docetaxel showed a significant inhibitory effect on tumor in PTEN-deficient nude mice [100]. Moreover, AZD8186 is currently used in combination with docetaxel to treat patients with metastatic or unresectable PTEN- or PIK3CB-mutant advanced solid tumors, including breast cancers (NCT03218826).
Serabelisib is a potent, selective, oral PI3Kα inhibitor. A phase Ib study of the use of serabelisib (TAK117) in combination with sapanisertib (TAK228) and paclitaxel, to treat advanced ovarian, endometrial, or breast cancers, found that its dose and timing were well tolerated and that it was clinically active in paclitaxel-resistant patients [101]. Clinical trials on the use of serabelisib in combination with canagliflozin to treat breast cancer are ongoing (NCT04073680).
3.2. AKT Inhibitors
AKT has three highly homologous isoforms, Akt1 (PKBα), Akt2 (PKBβ), and Akt3 (PKBγ). AKT is comprises 480 amino acids and each isoform contains a conserved N-terminal PH domain (ATP binding), a kinase domain (catalytic function), and a C-terminal regulatory disordered tail (C-tail) [102]. Point mutations on the PH domain can limit AKT interaction with PIP3 and PIP2, thereby affecting its upstream kinase recognition and membrane translocation. AKT activation requires the phosphorylation of a threonine residue (AKT1-T308, AKT2-T309, and AKT3-T305) in the kinase domain and the phosphorylation of a serine in the C-terminal hydrophobic motif (AKT1-S473, AKT2-S474, and AKT3-S472). It has been shown that cyclin-dependent kinase 2 can activate AKT1 by phosphorylation of two C-terminal amino acids, S477 and T479 [103,104]. The three AKT isoforms regulate a wide range of functions, including cell growth, survival, proliferation, metabolism, and drug response.
MK-2206 is a selective allosteric inhibitor of AKT. Most PIK3CA-mutant or PTEN-deficient breast cancer cell lines are highly sensitive to MK-2206. A clinical trial found that MK-2206 inhibits AKT phosphorylation in platelets [105]. Results from this phase II clinical trial using MK-2206 alone found that it had limited clinical activity in patients with advanced breast cancer bearing PIK3CA or AKT mutations and PTEN deletion. [106]. When used in combination with anastrozole to treat PIK3CA-mutant, ER+/HER2− breast cancer, MK-2206 was unlikely to increase the efficacy of anastrozole [107]. In the phase I clinical trial involving patients with rectal cancer, ovarian cancer, and metastatic breast cancer, several patients completed dose escalation without experiencing dose-limiting toxicity. Moreover, post-treatment analysis revealed significantly reduced levels of phospho-AKT (S473 and T308), and that the combination was well tolerated [108]. Although the combination of MK-2206 and lapatinib in the treatment of solid tumors was well tolerated, the overlapping toxicity of the two drugs resulted in a high incidence of rash and diarrhea. However, this toxicity can be managed with drugs [109].
The above PI3K inhibitors and AKT inhibitors play an important role in the treatment of breast cancer by targeting the PI3K–AKT pathway. Compared with AKT inhibitors, the types of PI3K inhibitors undergoing clinical trials are more diverse. PI3K/mTOR dual inhibitors can target the kinase pockets of PI3K and mTOR due to their structural similarity [110]. However, mTOR inhibitors may enhance PI3K/PDK1 signaling, thus inhibitors targeting both PI3K and mTOR may have better anticancer activity [111]. Studies have shown that AKT inhibitors such as MK-2206, AZD5363, and GDC-0068 have increased activity in cell lines with altered PIK3CA or PTEN [112,113,114]. This suggests that AKT-specific inhibitors may exhibit greater potency in PTEN-altered tumors. As mentioned above, the occurrence of breast cancer is related to the cascading drive of the PI3K pathway and multiple signaling pathways. Combination RTK inhibitor therapy may be a good approach, for example with inhibitors of EGFR, HER2, or HER3 [115,116] or in combination with MEK inhibitors to overcome parallel induction of the MAPK pathway, a strategy that works well for PI3K pathway gene alterations and KRAS mutations. However, the improved efficacy of this strategy may increase the side effects of chemotherapy [117]. A preclinical study shows that CDK 4/6 inhibitors increase the sensitivity of PIK3CA-mutant breast cancer cells to PI3K inhibitors, and the combination of the two drugs has a synergistic effect on the treatment of breast cancer [118]. In addition, the PI3K/mTOR dual inhibitor gedatolisib combined with immune checkpoint inhibitors (ICI) more significantly inhibited the growth of breast cancer cells and continuously induced the responses of dendritic cells, CD8 + T cells, and NK cells, indicating that the inhibition of PI3K–AKT pathway may enhance breast cancer response to ICIs [119].
4. Natural Products and Synthetic Analogs for PI3K–AKT-Targeting Breast Cancer Treatments
Natural products from plants, mushrooms, and seaweeds are a source of drugs. Moreover, chemical drugs based on the molecular structure and functions of natural products have been developed. About two-thirds of anticancer drugs are derived from natural products and their derivatives [120]. Below, we discuss the research and development of natural compounds based in part on PI3K–AKT signaling targeting breast cancer, with emphasis on those in preclinical development (Table 2). The structures of these natural products and their mechanisms of action are shown in Figure 2 and Figure 3 respectively.

Table 2.
Summary of some natural products for the treatment of breast cancer by inhibiting the PI3K/AKT pathway.
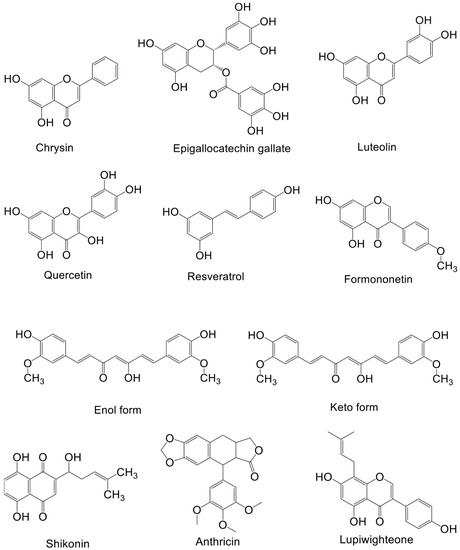
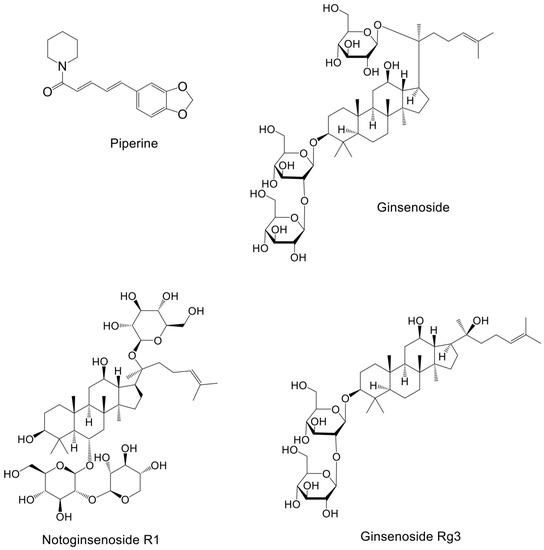
Figure 2.
Structure of natural compounds against breast cancer.
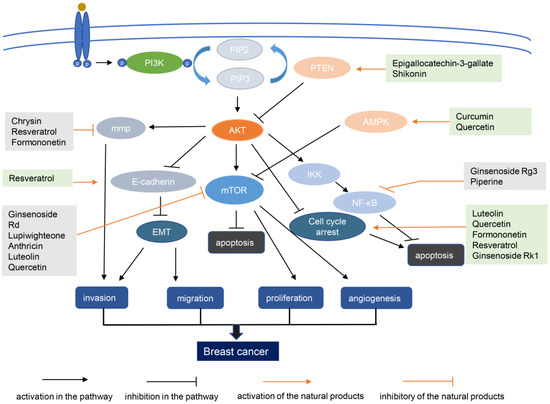
Figure 3.
Mechanism of action diagram of natural products with potential for anti-breast cancer therapy. AMPK, amp-activated protein kinase; EMT, epithelial–mesenchymal transition; MMP, mitochondrial membrane potential; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol (4,5)-disphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PTEN, phosphatase and tensin homolog.
4.1. Flavonoids
4.1.1. Curcumin
Curcumin, the most representative chemical polyphenol extracted from the rhizomes of turmeric, is used to treat various human disorders, including inflammation, metabolic syndromes, neurodegenerative diseases, and cancer. Studies have shown that curcumin inhibits breast cancer cell proliferation by targeting HER2-TK and NF-κB [136,137]. However, PI3K–AKT signaling has increasingly been shown to play an important role in the treatment of breast cancer with curcumin. In MDA-MB-231 breast cancer cells, curcumin-induced AMPK activation is involved in the activation of autophagy and the suppression of AKT levels, thereby inhibiting their proliferation and migration, impairing cellular uninterruptible power supply (UPS) function, accelerating Akt ubiquitination, and reducing Akt aggregation [127]. Additionally, curcumin has also been shown to induce breast cancer cell arrest in the S+G2/M phase and apoptosis, inhibit basic phosphorylation of AKT, and inhibit epidermal growth factor receptor (EGFR) and ERK1/2 phosphorylation induced by EGF pretreatment [128]. Curcumin also modulates the levels of p-Smad2 and β-catenin through transforming growth factor β (TGF-β) and PI3K–AKT signaling, thereby inhibiting doxorubicin-induced EMT [138]. Interestingly, the cytotoxic effects of curcumin on breast cancer cell lines have been shown to depend on their PI3K–AKT signaling status. Compared with MDA-MB-231 cells, a higher curcumin dose and treatment duration is needed against MCF-7 cells to maximize AKT phosphorylation and induce cytotoxicity [139]. Furthermore, Lv et al. found that curcumin can resensitize multidrug-resistant breast cancer to cisplatin via the inhibition of colon cancer associated transcript -1 (CCAT1) and PI3K–AKT signaling [140]. Notably, (2E,6E)-2,6-bis(4-hydroxy-3-methoxybenzylidene) cyclohexanone (BHMC), a curcumin derivative, also exhibits good anti-breast cancer effects in vivo.
4.1.2. Quercetin
Quercetin is widely distributed in nature and is common in fruits and vegetables. Studies have shown that quercetin has a variety of pharmacological effects against human diseases, including antioxidant, anti-inflammatory, and antiproliferative effects [141,142]. Quercetin also inhibits breast cell proliferation and promotes apoptosis. In MCF-7 cells, quercetin has been shown to increase the ratio of bcl2-associated X protein/B-cell lymphoma 2 (Bax/Bcl-2) by reducing the expression of PI3K, AKT, ERα, and cyclin D1. It also significantly inhibits viability, clonal formation, and mammosphere generation in CD44+/CD24− breast cancer stem cells, and significantly suppresses in vivo tumor growth and metastasis of CD44+/CD24 cells [124]. Quercetin-3-methyl ether is reported to upregulate E-cadherin and to downregulate vimentin and matrix metallopeptidase 2 (MMP-2), thereby inhibiting the epithelial–mesenchymal transition (EMT) in breast cancer cells, while downregulating the expression of Notch1, PI3K, AKT, and enhancer of zeste homolog 2 (EZH2), which are important for the activation of breast cancer stem cells [143]. Compared with breast cancer cells treated with a combination of resveratrol, quercetin, and catechin, quercetin alone exhibits more obvious inhibition of proliferation and migration and arrests MDA-MB-231 and MDA-MB-435 cells in the G2/M phase of the cell cycle. Additionally, quercetin also markedly inhibits AKT activity, while enhancing the activity of AMP-activated protein kinase (AMPK), a negative regulator of mTOR. These effects suppress the activity of the downstream mTOR effector proteins, ribosomal protein S6 kinase beta-1 (p70S6K), and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP-1) [144]. Interestingly, quercetin inhibits breast cancer development by suppressing cell migration and sugar degradation through the induction of AKT–mTOR signaling-mediated autophagy [145]. Esfandiar et al. found that the combination of quercetin and docetaxel upregulated p53 and Bax, downregulated Bcl-2, AKT, extracellular signal-regulated kinase 1/2 (ERK1/2), and signal transducer and activator of transcription 3 (STAT3), and enhanced the effect of docetaxel on the proapoptotic effect of MDA-MB-231 cells [146].
4.1.3. Formononetin
Formononetin, a flavonoid compound isolated from Astragalus, has been investigated in recent years for its role in tumors and neurological diseases. Formononetin is a phytoestrogen and of the main components of clover [147]. Formononetin was reported to inhibit the activity of IGF1–PI3K–AKT pathways in a dose-dependent manner. Previously, it was reported that formononetin decreased the expression of cyclin D1, one of the downstream target proteins of AKT, which enhanced the G0/G1 phase in MCF-7 cells thereby decreasing proliferation. Moreover, formononetin treatment also inhibited tumor growth in xenografted human breast cancer cells in vivo [126]. It also significantly suppressed the proliferation of ER-expressing MCF-7 and T47D cells and promoted apoptosis of breast cancer cells by increasing (Ras), rapidly accelerated fibrosarcoma (Raf), and p-p38 expression, as well as the Bax/Bcl-2 ratio [148]. In addition to these effects, formononetin was demonstrated to downregulate the expression of MMP-2 and MMP-9 as well as increase the expression of tissue inhibitors of metalloproteinase-1 (TIMP-1) and TIMP-2. It inhibited the migration and invasion of MDA-MB-231 and 4T1 cells through the inhibition of the PI3K–AKT signaling pathway [149]. In a previous preclinical study, formononetin significantly abolished the Fibroblast growth factor 2 (FGF2)-stimulated human umbilical vein endothelial cell (HUVEC) proliferation. Formononetin inhibits tumor angiogenesis and growth by decreasing the activity of FGF2R, the phosphorylation of PI3K and AKT, and the activity of transcription factor STAT3 [150]. It is evident from the above study that the therapeutic effect of formononetin on breast cancer has clinical potential.
4.1.4. Saponins
Ginsenosides are the main active substances isolated from ginseng. This plant is distributed in Northeast China, Korea, and Japan. American ginseng has also been found to contain ginsenosides. Recent studies have found that ginsenosides possess anti-proliferative, anti-metastatic, pro-apoptotic, anti-angiogenic, anti-multidrug resistance, and autophagy-regulating effects. Moreover, ginsenoside (Rg3) depolarizes mitochondrial membrane potential, releases cytochrome c, increases the expression of cleaved caspase-3 and cleaved PARP, the Bax/Bcl-2 ratio, activates the mitochondrial death pathway, and promotes apoptosis in breast cancer cells [151]. A study by Bo-Min et al. reported that ginsenoside (Rg3) inhibited the DNA-binding ability and transcriptional activity of NF-κB by reducing the expression of mutant p53 (R280K) in MDA-MB-231 cells. This was ascribed to the increased production of reactive oxygen species (ROS), the inhibition of ERK, and Akt phosphorylation by ginsenosides [134]. In addition to this, another ginsenoside (ginsenoside Rd) was found to play a similar role. Studies on MDA-MB-231 cells and HUVECs cells showed that ginsenoside (Rd) abolished vascular endothelial growth factor (VEGF)-induced VEGFR2 activation in HUVECs by decreasing intracellular Akt/mTOR/p70S6K and hypoxia-inducible factor 1-alpha (HIF-1α) activation. Moreover, ginsenoside (Rd) increased the expression of cleaved casepase-3 and other apoptosis proteins to inhibit angiogenesis and tumor growth in vivo and in vitro [133]. It has been reported that ginsenoside (Rk1) can also trigger S-phase cell arrest and induce apoptosis of MCF-7 cells [152]. Interestingly, Liu et al. found that ginsenoside (Rg5) caused less damage to the body compared with the first-line tumor treatment drug (docetaxel). The ginsenoside (Rg5) suppressed the phosphorylation levels of PI3K and AKT, and induced apoptosis and autophagy, thereby inhibiting breast cancer in vitro and in vivo models [153].
4.2. Non-Flavonoid Polyphenols
Resveratrol
Resveratrol is a non-flavonoid polyphenol, mainly found in grapes, peanuts, soybeans, and other plants, and also in Staphylococcus, Penicillium, Mucor, and other fungi. The estrogen receptor (ER) plays an important role in the development of breast cancer. Therefore, breast cancer is mainly treated by inhibiting the production of estrogen or blocking the binding of estrogen to its receptor. For this reason, resveratrol is used as a phytoestrogen to treat breast cancer [154]. Studies have shown that resveratrol inhibits the ERα but not ERβ [155]. In a study published in 2011, the combination of resveratrol and rapamycin increased the sensitivity to rapamycin and inhibited the growth of breast cancer cells by blocking the PI3K–AKT signaling pathway instead of the ERK- Mitogen-activated protein kinases (MAPK) signaling pathway. The downstream target of mTOR p70S6K was also inhibited leading to S-phase cell cycle arrest in breast cancer cells [156]. Interestingly, it has been found that resveratrol downregulated the expression of fatty acid synthase (FASN) and HER2 in a dose-dependent manner, and upregulated the expression of polyoma enhancer activator 3 (PEA3) (targeting the HER2 promoter to downregulate its transcriptional activity), thereby suppressing the proliferation of HER2-overexpressing breast cancer cells [157]. Previously, Kumar-Sinha et al. found that HER2 triggered FASN expression by activating the FASN promoter through the PI3K pathway [158]. Therefore, it is speculated that resveratrol decreases the proliferation of breast cancer cells by inhibiting the PI3K–AKT signaling pathway. Co-treatment of breast cancer cells with TGF-β1 and resveratrol upregulated E-cadherin, downregulated fibronectin, vimentin, Snail1, Slug, and alpha-smooth muscle actin (α-SMA), reversed TGF-β1-induced EMT through inhibition of PI3K–AKT and Smad signaling pathways [125]. In addition, resveratrol did not cause any side effects in mice. Evidence from previous studies also demonstrated that 3,5,4′-trimethoxystilbene (natural methoxylated analog of resveratrol) can reverse EMT by downregulating PI3K–AKT and Wnt/β-catenin signaling pathway cascades to inhibit invasiveness of breast cancer cells [159]. Collectively, these data show that resveratrol is a potential drug for the clinical treatment of breast cancer.
4.3. Others
Anthricin is a natural product isolated from Anthriscus sylvestris (L.) Hoffm. (Apiaceae), the root of which is often used as an antipyretic, cough suppressant, and pain reliever [160]. According to Chang et al., anthricin induces protective autophagy in breast cancer cells but promotes cell death by inhibiting autophagy. These effects are mediated by reduced the phosphorylation of AKT, p70S6K, and mTOR [130]. Piperine is an alkaloid found in black pepper (Piper nigrum L.) and is commonly used in India and China as a treatment for conditions such as intestinal disorders and epilepsy [161]. Studies have shown that piperine downregulates the expression of HER2 in SKBR3 cells. It can also reduce sterol regulatory element-binding transcription factor 1 (SREBP-1) activity by inhibiting the ERK1/2 signaling pathway, thereby decreasing the expression of FAS. Besides, piperine downregulates the transcriptional activity of NF-κB and activating protein-1 (AP-1) by blocking the Akt and MAPK signaling pathways. This abolishes the EGF-induced MMP-9 expression, and inhibits the proliferation and migration ability of breast cancer cells. Furthermore, the co-treatment of breast cancer with piperine and paclitaxel increased the sensitivity of cells to paclitaxel toxicity [132].
5. Limitations of Conventional Breast Cancer Treatment and Potential Biomarkers
The treatment of breast cancer is multidisciplinary, and early diagnosis and treatment may be an effective way to reduce breast cancer mortality [162]. Breast cancer diagnosis includes mammograms, as well as breast tissue biopsies, and multiple mammograms can cause potential radiation damage to the body [163]. Magnetic resonance imaging (MRI) is an effective auxiliary method, and its high sensitivity is helpful for the early diagnosis of breast cancer. However, the toxicity of gadolinium (Gd) metal in the contrast agent to the human body needs to be vigilant [164]. In addition, serological detection of breast cancer tumor markers CA 15-3, carcinoembryonic antigen (CEA), and CA 27-29 is one of the methods for auxiliary diagnosis of metastatic breast cancer. Current conventional treatments for breast cancer mainly include surgery, radiation therapy (RT), chemotherapy (CT), endocrine (hormone) therapy (ET), and targeted therapy [165]. Of these, breast-conserving therapy (BCT) and mastectomy are well-established local therapies for invasive breast cancer [166]. The selection of the treatment method that suits them is something that patients need to carefully consider before treatment. Up to 90% of women with breast cancer have long-term sequelae from treatment, including physical, functional, and psychosocial changes that can greatly affect the quality of life for breast cancer patients [167]. Breast-conserving therapy involves lumpectomy and postoperative radiation therapy, while connective tissue/collagen vascular disease and pregnancy may be contraindications to radiation therapy [166]. In addition, radiation therapy can include some side effects, decreased sensory function in the breast tissue and underarms, and itching, redness, and swelling of the skin at the surgical site [3]. Combining systemic adjuvant therapy may provide better help to patients according to their age, physical condition, and contraindications. For example, endocrine therapy (ET) may be more suitable for ER+ breast cancer, while CT is more recommended for ER− breast cancer and TNBC [165]. In the new adjuvant therapy, chemotherapy is often combined with some targeted drugs to treat breast cancer. The dysregulation of mTOR in TNBC in the PI3K–AKT pathway may increase the sensitivity of mTOR inhibitors in antitumor therapy [168]. Studies have shown that after paclitaxel combined with mTOR inhibitor Afinitor (everolimus) to treat TNBC for 48 h, the expression of mTOR is downregulated, the incidence of adverse events in patients with combined medication is not significantly increased, and the overall safety is good [169]. The role of the androgen receptor AR in breast cancer is gradually being uncovered, and AR is expressed in 90% of primary breast cancers and 75% of metastatic breast cancers [170]. Chia et al. showed that inhibition of AR resulted in decreased levels of phosphorylated Elk1 and c-FOS, as well as decreased ERK target proteins, in xenograft tumor models and patient tumors [171]. In addition, AR exhibits an important role after DNA damage in breast cancer cells, and dsDNA repair is significantly delayed after AR inhibition [172]. A preclinical study showed that AR can promote the invasion and migration of TNBC cells by activating Src signaling [173]. AR may be a potential biomarker in breast cancer treatment. In 15–30% of breast cancers, amplification and overexpression of HER2 occurs [174]. A study showed that HER2 can drive tumorigenesis independently of HER3 by activating the PI3K–AKT pathway [175]. As early as 20 years ago, the use of trastuzumab chemotherapy in HER2+ breast cancer achieved good results [176]. In recent years, new anti-HER2 treatment options have continued to be developed. For example, the novel anti-HER2 drug deruxtecan showed durable antitumor activity in a population of patients with previously treated HER2-positive metastatic breast cancer [177]. In addition, adding tucatinib to trastuzumab-treated metastatic HER2+ breast cancer can significantly prolong the progression-free survival in the trastuzumab and capecitabine combination therapy group [178].
6. Conclusions and Future Perspectives
Evidence from prior studies has demonstrated that impaired PI3K–AKT signaling contributes to the occurrence of many diseases, such as cancer and neurological diseases. The excessive activation of the PI3K–AKT pathway can lead to cancer development, whereas the inhibition of the PI3K–AKT pathway may lead to neuronal or glial cell death. Therefore, researchers should explore how to regulate the PI3K–AKT signaling pathway and its role in the pathogenesis of diseases.
In addition, much research into PI3K–AKT-targeting drugs is based on in vitro experiments or mice; thus, the transformation from basic studies to a clinical application must be screened and achieved. Many drugs or drug combinations have been shown to induce adverse events or toxicity in clinical trials leading to the termination of such trials. Based on the above problems, natural products may be candidates for the treatment of breast cancer. Natural products are widely distributed in vegetables, fruits, and medicinal materials in nature. Human beings have used natural products to treat diseases since ancient times. Diet alone may not be able to achieve the effect of natural products in treating diseases. To improve the applicability of natural products, the first thing we consider is the availability of natural products in the body. Preclinical studies are mainly performed using in vitro and mouse models, which are significantly different from the human body. In addition to screening for appropriate natural products, the optimization of natural products is also an urgent problem to be solved. Researchers have proposed that the use of substances such as turmeric oil, which is more easily absorbed, or combining curcumin with carrier substances such as nanoparticles can improve the efficacy of drugs. For example, combining curcumin with nanoparticles significantly improves the bioavailability of curcumin by increasing intestinal permeability, and stability in the microenvironment, reducing degradation, and extending half-life in blood [179]. Although its applicability needs further proof. In addition, the bioavailability of natural products can be increased through the chemical modification of their structures. For instance, the synthesis of ester derivatives of quercetin to bypass phase II metabolism during absorption potentially increased the systemic levels of quercetin [180]. In a previous study, piperine treatment inhibited breast cancer proliferation and migration, whereas the co-treatment of piperine and paclitaxel increased the sensitivity of breast cancer cells to paclitaxel [132]. A similar effect was reported when quercetin was used [146]. Therefore, drug combinations such as the TH combination (paclitaxel and trastuzumab) are more effective for the treatment of HER2-positive breast cancer. The efficacy of a drug may vary depending on the genotype of patients, and the application of precision clinical therapy is a challenge.
In preclinical experiments, natural products were used to treat breast cancer by targeting the PI3K–AKT pathway, and it was found that ERK, autophagy, UPS, and other signaling pathways were sometimes affected, and these signaling networks crossed each other. The role of natural products is complex. If natural products are further applied to the treatment of breast cancer, more research on their role is needed. Although natural products are promising anti-breast cancer drugs, cancer treatment is a complex process and we still rely on existing anticancer therapies. As mentioned above, natural products play a certain role in enhancing the sensitivity of cisplatin and paclitaxel. However, whether they also play a similar role in most drugs requires further research. It may be a good idea for the treatment of drug-resistant breast cancer. Natural products may play a more important role in tumor therapy by reducing drug toxicity, increasing its therapeutic concentration in the body, and reducing chemotherapeutic drug resistance.
Drug development requires collaborative efforts from many research teams including clinicians, and patients. At present, many studies have shown that natural products can inhibit the growth of breast cancer cells by regulating the PI3K–AKT pathway in mouse models. Most of the natural products discussed in this review have been studied in preclinical research. Their mechanisms in breast cancer treatment need to be further explored.
Author Contributions
Conceptualization, H.L. and Z.Z.; methodology, Y.Y.; Writing—original draft preparation, Y.F. and Y.Y.; Writing—review and editing, Y.Y.; Project administration, B.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (no. 81802952), the Natural Science Foundation of Hunan Province (no. 2020JJ5608), the Scientific Research Project of the Education Department of Hunan Province (no. 21B0411), the Scientific Research Project of Changsha Central Hospital (no. YNKY202201).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Abel, M.K.; Brabham, C.E.; Guo, R.; Fahrner-Scott, K.; Wong, J.; Alvarado, M.; Ewing, C.; Esserman, L.J.; Mukhtar, R.A. Breast conservation therapy versus mastectomy in the surgical management of invasive lobular carcinoma measuring 4 cm or greater. Am. J. Surg. 2020, 221, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Siddiqui, S.A. Breast cancer management: Past, present and evolving. Indian J. Cancer 2012, 49, 277. [Google Scholar] [CrossRef]
- Mollon, L.; Aguilar, A.; Anderson, E.; Dean, J.; Davis, L.; Warholak, T.; Aizer, A.A.; Platt, E.; Bardiya, A.; Tang, D. A systematic literature review of the prevalence of pik3ca mutations and mutation hotspots in hr+/her2-metastatic breast cancer. Cancer Res. 2018, 78, 1207. [Google Scholar] [CrossRef]
- Butti, R.; Das, S.; Gunasekaran, V.P.; Yadav, A.S.; Kumar, D.; Kundu, G.C. Receptor tyrosine kinases (RTKs) in breast cancer: Signaling, therapeutic implications and challenges. Mol. Cancer 2018, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Paplomata, E.; O’Regan, R. The PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- Switon, K.; Kotulska, K.; Janusz-Kaminska, A.; Zmorzynska, J.; Jaworski, J. Tuberous sclerosis complex: From molecular biology to novel therapeutic approaches. IUBMB Life 2016, 68, 955–962. [Google Scholar] [CrossRef]
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 Pathway Involved in Psychiatric Illnesses. Diseases 2019, 7, 22. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Buján, J.; García-Honduvilla, N.; Coca, S. Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/mTOR. J. Oncol. 2020, 2020, 9258396. [Google Scholar] [CrossRef]
- Franke, T.F.; Yang, S.-I.; Chan, T.; Datta, K.; Kazlauskas, A.; Morrison, D.K.; Kaplan, D.R.; Tsichlis, P.N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 1995, 81, 727–736. [Google Scholar] [CrossRef]
- Kohn, A.D.; Kovacina, K.S.; Roth, R.A. Insulin stimulates the kinase activity of RAC-PK, a pleckstrin homology domain containing ser/thr kinase. EMBO J. 1995, 14, 4288–4295. [Google Scholar] [CrossRef]
- Burgering, B.M.T.; Coffer, P.J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 1995, 376, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, P.P.; Cardone, C.; Martini, G.; Ciardiello, D.; Belli, V.; Matrone, N.; Barra, G.; Napolitano, S.; Della Corte, C.; Turano, M.; et al. Receptor tyrosine kinase-dependent PI3K activation is an escape mechanism to vertical suppression of the EGFR/RAS/MAPK pathway in KRAS-mutated human colorectal cancer cell lines. J. Exp. Clin. Cancer Res. 2019, 38, 41. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Akinleye, A.; Avvaru, P.; Furqan, M.; Song, Y.; Liu, D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J. Hematol. Oncol. 2013, 6, 88. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Manning, B.D. Insulin Signaling: Inositol Phosphates Get into the Akt. Cell 2010, 143, 861–863. [Google Scholar] [CrossRef]
- Chalhoub, N.; Baker, S.J. Pten and the pi3-kinase pathway in cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 127–150. [Google Scholar] [CrossRef]
- Jia, S.; Liu, Z.; Zhang, S.; Liu, P.; Zhang, L.; Lee, S.H.; Zhang, J.; Signoretti, S.; Loda, M.; Roberts, T.M. Essential roles of pi (3) k–p110β in cell growth, metabolism and tumorigenesis. Nature 2008, 454, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Duggal, S.; Jailkhani, N.; Midha, M.K.; Agrawal, N.; Rao, K.V.S.; Kumar, A. Defining the Akt1 interactome and its role in regulating the cell cycle. Sci. Rep. 2018, 8, 1303. [Google Scholar] [CrossRef] [PubMed]
- Matheny, R.W., Jr.; Geddis, A.V.; Abdalla, M.N.; Leandry, L.A.; Ford, M.; McClung, H.L.; Pasiakos, S.M. Akt 2 is the predominant akt isoform expressed in human skeletal muscle. Physiol. Rep. 2018, 6, e13652. [Google Scholar] [CrossRef]
- Alcantara, D.; Timms, A.E.; Gripp, K.; Baker, L.; Park, K.; Collins, S.; Cheng, C.; Stewart, F.; Mehta, S.G.; Saggar, A.; et al. Mutations of AKT3 are associated with a wide spectrum of developmental disorders including extreme megalencephaly. Brain 2017, 140, 2610–2622. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [CrossRef]
- Alessi, D.R.; James, S.R.; Downes, C.; Holmes, A.B.; Gaffney, P.R.; Reese, C.B.; Cohen, P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of akt/pkb by the rictor-mtor complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. Mtor at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Ravichandran, L.V.; Chen, H.; Li, Y.; Quon, M.J. Phosphorylation of ptp1b at ser50 by akt impairs its ability to dephosphorylate the insulin receptor. Mol. Endocrinol. 2001, 15, 1768–1780. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. How BAD phosphorylation is good for survival. Nat. Cell Biol. 1999, 1, E33–E35. [Google Scholar] [CrossRef] [PubMed]
- del Peso, L.; González-García, M.; Page, C.; Herrera, R.; Nuñez, G. Interleukin-3-Induced Phosphorylation of BAD Through the Protein Kinase Akt. Science 1997, 278, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt Phosphorylation of BAD Couples Survival Signals to the Cell-Intrinsic Death Machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-mediated regulation of nfκb and the essentialness of nfκb for the oncogenicity of pi3k and akt. Int. J. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef]
- Burgering, B.M.T.; Medema, R.H. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J. Leukoc. Biol. 2003, 73, 689–701. [Google Scholar] [CrossRef]
- Nidai Ozes, O.; Mayo, L.D.; Gustin, J.A.; Pfeffer, S.R.; Pfeffer, L.M.; Donner, D.B. Nf-κb activation by tumour necrosis factor requires the akt serine–threonine kinase. Nature 1999, 401, 82–85. [Google Scholar] [CrossRef]
- Rössig, L.; Jadidi, A.S.; Urbich, C.; Badorff, C.; Zeiher, A.M.; Dimmeler, S. Akt-Dependent Phosphorylation of p21 Cip1 Regulates PCNA Binding and Proliferation of Endothelial Cells. Mol. Cell. Biol. 2001, 21, 5644–5657. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, H.Y. Environmental exposure, and other behavioral risk factors in breast cancer. Curr. Cancer Ther. Rev. 2006, 2, 3–21. [Google Scholar] [CrossRef]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef]
- Abubakar, M.; Figueroa, J.; Ali, H.R.; Blows, F.; Lissowska, J.; Caldas, C.; Easton, D.F.; Sherman, M.E.; Garcia-Closas, M.; Dowsett, M.; et al. Combined quantitative measures of ER, PR, HER2, and KI67 provide more prognostic information than categorical combinations in luminal breast cancer. Mod. Pathol. 2019, 32, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Schnitt, S.J. Will molecular classification replace traditional breast pathology? Int. J. Surg. Pathol. 2010, 18, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Mukohara, T. PI3K mutations in breast cancer: Prognostic and therapeutic implications. Breast Cancer Targets Ther. 2015, 7, 111–123. [Google Scholar] [CrossRef]
- Samuels, Y.; Wang, Z.; Bardelli, A.; Silliman, N.; Ptak, J.; Szabo, S.; Yan, H.; Gazdar, A.; Powell, S.M.; Riggins, G.J.; et al. High Frequency of Mutations of the PIK3CA Gene in Human Cancers. Science 2004, 304, 554. [Google Scholar] [CrossRef]
- Koren, S.; Reavie, L.; Couto, J.P.; De Silva, D.; Stadler, M.B.; Roloff, T.; Britschgi, A.; Eichlisberger, T.; Kohler, H.; Aina, O.; et al. PIK3CAH1047R induces multipotency and multi-lineage mammary tumours. Nature 2015, 525, 114–118. [Google Scholar] [CrossRef]
- Carpten, J.D.; Faber, A.L.; Horn, C.; Donoho, G.P.; Briggs, S.L.; Robbins, C.M.; Hostetter, G.; Boguslawski, S.; Moses, T.Y.; Savage, S.; et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 2007, 448, 439–444. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Wang, M.; Yang, J.; Lv, M.; Li, P.; Chen, Z.; Yang, J. Loss of PTEN expression in breast cancer: Association with clinicopathological characteristics and prognosis. Oncotarget 2017, 8, 32043–32054. [Google Scholar] [CrossRef]
- Campbell, R.A.; Bhat-Nakshatri, P.; Patel, N.M.; Constantinidou, D.; Ali, S.; Nakshatri, H. Phosphatidylinositol 3-kinase/akt-mediated activation of estrogen receptor α: A new model for anti-estrogen resistance. J. Biol. Chem. 2001, 276, 9817–9824. [Google Scholar] [CrossRef]
- Crowder, R.J.; Phommaly, C.; Tao, Y.; Hoog, J.; Luo, J.; Perou, C.M.; Parker, J.S.; Miller, M.A.; Huntsman, D.G.; Lin, L.; et al. PIK3CA and PIK3CB Inhibition Produce Synthetic Lethality when Combined with Estrogen Deprivation in Estrogen Receptor–Positive Breast Cancer. Cancer Res. 2009, 69, 3955–3962. [Google Scholar] [CrossRef]
- Creighton, C.J.; Fu, X.; Hennessy, B.T.; Casa, A.J.; Zhang, Y.; Gonzalez-Angulo, A.M.; Lluch, A.; Gray, J.W.; Brown, P.H.; Hilsenbeck, S.G.; et al. Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER+ breast cancer. Breast Cancer Res. 2010, 12, R40. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Hennessy, B.T.; González-Angulo, A.M.; Fox, E.M.; Mills, G.B.; Chen, H.; Higham, C.; García-Echeverría, C.; Shyr, Y.; Arteaga, C.L. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor–positive human breast cancer. J. Clin. Investig. 2010, 120, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.W.; Forbes, J.T.; Shah, C.; Wyatt, S.K.; Manning, H.C.; Olivares, M.G.; Sanchez, V.; Dugger, T.C.; Granja, N.D.M.; Narasanna, A.; et al. Inhibition of Mammalian Target of Rapamycin Is Required for Optimal Antitumor Effect of HER2 Inhibitors against HER2-Overexpressing Cancer Cells. Clin. Cancer Res. 2009, 15, 7266–7276. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Sánchez, V.; Kuba, M.G.; Rinehart, C.; Arteaga, C.L. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc. Natl. Acad. Sci. USA 2011, 109, 2718–2723. [Google Scholar] [CrossRef]
- Hu, L.; Hofmann, J.; Lu, Y.; Mills, G.B.; Jaffe, R.B. Inhibition of phosphatidylinositol 3’-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002, 62, 1087–1092. [Google Scholar]
- Ssw, N.; Tsao, M.; Chow, S.; Hedley, D. Inhibition of phosphatidylinositide 3-kinase enhances gemcitabine-induced apoptosis in human pancreatic cancer cells. Cancer Res. 2000, 60, 5451–5455. [Google Scholar]
- Dong, J.; Peng, J.; Zhang, H.; Mondesire, W.H.; Jian, W.; Mills, G.B.; Hung, M.-C.; Meric-Bernstam, F. Role of Glycogen Synthase Kinase 3β in Rapamycin-Mediated Cell Cycle Regulation and Chemosensitivity. Cancer Res. 2005, 65, 1961–1972. [Google Scholar] [CrossRef]
- A Trial of bkm120 (a pi3k Inhibitor) in Patients with Triple Negative Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCCT2eTripleI3K&coCancerreast+Cancer&drBreast&raa (accessed on 15 May 2022).
- Safety and Efficacy of Bkm120 and Lapatinib in Her2+/Pi3k-Activated, Trastuzumab-Resistant Advanced Breast Cancer (pikher2). Available online: https://clinicaltrials.gov/ct2/show/NCT01589861?term=PI3K&cond=Breast+Cancer&draw=2&rank=4 (accessed on 15 May 2022).
- Neophoebe: Neoadjuvant Trastuzumab + Bkm120 in Combination with Weekly Paclitaxel in Her2-Positive Primary Breast Cancer (neophoebe). Available online: https://clinicaltrials.gov/ct2/show/NCT01816594?term=PI3K&cond=Breast+Cancer&draw=4&rank=14 (accessed on 15 May 2022).
- Bkm120 and Fulvestrant for Treating Postmenopausal Patients with Estrogen Receptor-Positive Stage Iv Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01339442?term=PI3K&cond=Breast+Cancer&draw=4&rank=17 (accessed on 15 May 2022).
- Trial of Bkm120/Tamoxifen-Combination in Patients with Hr-Pos, Her2-Neg Breast Cancer (Piktam). Available online: https://clinicaltrials.gov/ct2/show/NCT02404844?term=PI3K&cond=Breast+Cancer&draw=4&rank=20 (accessed on 15 May 2022).
- Phase ib, Dose Escalation Study of Oral Lde225 in Combination with Bkm120 in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT01576666?term=PI3K&cond=Breast+Cancer&draw=6&rank=202 (accessed on 15 May 2022).
- Efficacy and Safety of Tenalisib (rp6530), a Pi3k δ/γ and Sik3 Inhibitor, in Patients with Locally Advanced or Metastatic Breast cance. Available online: https://clinicaltrials.gov/ct2/show/NCT05021900?term=PI3K&cond=Breast+Cancer&draw=2&rank=11 (accessed on 15 May 2022).
- Taselisib and Enzalutamide in Treating Patients with Androgen Receptor Positive Triple-Negative Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02457910?term=PI3K&cond=Breast+Cancer&draw=2&rank=51 (accessed on 15 May 2022).
- Phosphatidylinositol 3-Kinase (pi3k) Alpha Inhibition in Advanced Breast Cancer (Piknic). Available online: https://clinicaltrials.gov/ct2/show/NCT02506556?term=PI3K&cond=Breast+Cancer&draw=2&rank=2 (accessed on 15 May 2022).
- Byl719 and Letrozole In Post-Menopausal Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01791478?term=PI3K&cond=Breast+Cancer&draw=2&rank=10 (accessed on 15 May 2022).
- Byl719 and Nab-Paclitaxel in Locally Recurrent or Metastatic Her-2 Negative Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02379247?term=PI3K&cond=Breast+Cancer&draw=2&rank=79 (accessed on 15 May 2022).
- Gdc-0941 and Cisplatin in Treating Patients with Androgen Receptor-Negative Triple Negative Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01918306?term=PI3K&cond=Breast+Cancer&draw=2&rank=32 (accessed on 15 May 2022).
- A Study of Pi3-Kinase Inhibitor gdc-0941 in Combination with Paclitaxel, with and without Bevacizumab or Trastuzumab, and with Letrozole, in Participants with Locally Recurrent or Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00960960?term=PI3K&cond=Breast+Cancer&draw=2 (accessed on 15 May 2022).
- Gdc-0084 in Combination with Trastuzumab for Patients with Her2-Positive Breast Cancer Brain Metastases. Available online: https://clinicaltrials.gov/ct2/show/NCT03765983?term=PI3K&cond=Breast+Cancer&draw=3&rank=143 (accessed on 15 May 2022).
- A study of pf-05212384 in Combination with Other Anti-Tumor Agents and in Combination with Cisplatin in Patients with Triple Negative Breast Cancer in an Expansion Arm (tnbc). Available online: https://clinicaltrials.gov/ct2/show/NCT01920061?term=PI3K&cond=Breast+Cancer&draw=2&rank=74 (accessed on 15 May 2022).
- Dose Finding Study of pf-05212384 with Paclitaxel and Carboplatin in Patients with Advanced Solid Tumor (iosi-ndu-001). Available online: https://clinicaltrials.gov/ct2/show/NCT02069158?term=PI3K&cond=Breast+Cancer&draw=6&rank=221 (accessed on 15 May 2022).
- Pi3kbeta Inhibitor Azd8186 and Docetaxel in Treating Patients Advanced Solid Tumors with Pten or Pik3cb Mutations that Are Metastatic or Cannot Be Removed by Surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT03218826?term=PI3K&cond=Breast+Cancer&draw=3&rank=117 (accessed on 15 May 2022).
- A Phase 1b/2 Study of Serabelisib in Combination with Canagliflozin in Patients with Advanced Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT04073680?term=PI3K&cond=Breast+Cancer&draw=3&rank=148 (accessed on 15 May 2022).
- Ipatasertib + Pertuzumab +Trastuzumab in Advanced Her2+ Pi3kca-Mutant Breast Cancer Patients (Ipather). Available online: https://clinicaltrials.gov/ct2/show/NCT04253561?term=PI3K&cond=Breast+Cancer&draw=3 (accessed on 15 May 2022).
- Akt Inhibitor Mk2206 in Combination with Lapatinib Ditosylate in Patients with Advanced or Metastatic Solid Tumors or Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01245205?term=PI3K&cond=Breast+Cancer&draw=3&rank=184 (accessed on 15 May 2022).
- Mk2206 and Paclitaxel in Treating Patients with Locally Advanced or Metastatic Solid Tumors or Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01263145?term=PI3K&cond=Breast+Cancer&draw=3&rank=165 (accessed on 15 May 2022).
- Akt Inhibitor Mk2206 in Treating Patients with Advanced Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01277757?term=PI3K&cond=Breast+Cancer&draw=3&rank=104 (accessed on 15 May 2022).
- Akt Inhibitor Mk-2206 and Anastrozole with or without Goserelin Acetate in Treating Patients with Stage ii-iii Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01776008?term=PI3K&cond=Breast+Cancer&draw=3 (accessed on 15 May 2022).
- Leevers, S.J.; Vanhaesebroeck, B.; Waterfield, M.D. Signalling Through Phosphoinositide 3-Kinases: The Lipids Take Centre Stage. Curr. Opin. Cell Biol. 1999, 11, 219–225. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-Kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Katso, R.; Okkenhaug, K.; Ahmadi, K.; White, S.; Timms, J.; Waterfield, M.D. Cellular Function of Phosphoinositide 3-Kinases: Implications for Development, Immunity, Homeostasis, and Cancer. Annu. Rev. Cell Dev. Biol. 2001, 17, 615–675. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Backer, J.M. The regulation and function of Class III PI3Ks: Novel roles for Vps34. Biochem. J. 2008, 410, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Castro, A.C.; Saura, C.; Barroso-Sousa, R.; Guo, H.; Ciruelos, E.; Bermejo, B.; Gavilá, J.; Serra, V.; Prat, A.; Paré, L. Phase 2 study of buparlisib (bkm120), a pan-class i pi3k inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2020, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Zhang, W.; Wang, X.; Chen, L.; Wang, S. BKM120 sensitizes BRCA-proficient triple negative breast cancer cells to olaparib through regulating FOXM1 and Exo1 expression. Sci. Rep. 2021, 11, 4774. [Google Scholar] [CrossRef]
- Huen, A.; Haverkos, B.M.; Zain, J.; Radhakrishnan, R.; Lechowicz, M.J.; Devata, S.; Korman, N.J.; Pinter-Brown, L.; Oki, Y.; Barde, P.J.; et al. Phase I/Ib Study of Tenalisib (RP6530), a Dual PI3K δ/γ Inhibitor in Patients with Relapsed/Refractory T-Cell Lymphoma. Cancers 2020, 12, 2293. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy of Tenalisib (Rp6530) in Combination with Romidepsin in Patients with RELAPSED/refractory T-Cell Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03770000 (accessed on 15 May 2022).
- Dent, S.; Cortés, J.; Im, Y.-H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef]
- Mayer, I.A.; Abramson, V.G.; Formisano, L.; Balko, J.M.; Estrada, M.V.; Sanders, M.E.; Juric, D.; Solit, D.; Berger, M.F.; Won, H.H. A phase ib study of alpelisib (byl719), a pi3kα-specific inhibitor, with letrozole in er+/her2− metastatic breast cancer. Clin. Cancer Res. 2017, 23, 26–34. [Google Scholar] [CrossRef]
- Sharma, P.; Abramson, V.G.; O’Dea, A.P.; Nye, L.; Mayer, I.A.; Pathak, H.B.; Hoffmann, M.; Stecklein, S.R.; Elia, M.; Lewis, S.; et al. Clinical and Biomarker Results from Phase I/II Study of PI3K Inhibitor Alpelisib plus Nab-paclitaxel in HER2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2021, 27, 3896–3904. [Google Scholar] [CrossRef]
- Folkes, A.J.; Ahmadi, K.; Alderton, W.K.; Alix, S.; Baker, S.J.; Box, G.; Chuckowree, I.S.; Clarke, P.A.; Depledge, P.; Eccles, S.A. The identification of 2-(1 h-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno [3, 2-d] pyrimidine (gdc-0941) as a potent, selective, orally bioavailable inhibitor of class i pi3 kinase for the treatment of cancer. J. Med. Chem. 2008, 51, 5522–5532. [Google Scholar] [CrossRef]
- Vuylsteke, P.; Huizing, M.; Petrakova, K.; Roylance, R.; Laing, R.; Chan, S.; Abell, F.; Gendreau, S.; Rooney, I.; Apt, D.; et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: Interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann. Oncol. 2016, 27, 2059–2066. [Google Scholar] [CrossRef]
- Schöffski, P.; Cresta, S.; Mayer, I.A.; Wildiers, H.; Damian, S.; Gendreau, S.; Rooney, I.; Morrissey, K.M.; Spoerke, J.M.; Ng, V.W.; et al. A phase Ib study of pictilisib (GDC-0941) in combination with paclitaxel, with and without bevacizumab or trastuzumab, and with letrozole in advanced breast cancer. Breast Cancer Res. 2018, 20, 109. [Google Scholar] [CrossRef]
- Salphati, L.; Alicke, B.; Heffron, T.P.; Shahidi-Latham, S.; Nishimura, M.; Cao, T.; Carano, R.A.; Cheong, J.; Greve, J.; Koeppen, H.; et al. Brain Distribution and Efficacy of the Brain Penetrant PI3K Inhibitor GDC-0084 in Orthotopic Mouse Models of Human Glioblastoma. Drug Metab. Dispos. 2016, 44, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Ippen, F.M.; Alvarez-Breckenridge, C.A.; Kuter, B.M.; Fink, A.L.; Bihun, I.V.; Lastrapes, M.; Penson, T.; Schmidt, S.P.; Wojtkiewicz, G.R.; Ning, J.; et al. The Dual PI3K/mTOR Pathway Inhibitor GDC-0084 Achieves Antitumor Activity in PIK3CA-Mutant Breast Cancer Brain Metastases. Clin. Cancer Res. 2019, 25, 3374–3383. [Google Scholar] [CrossRef]
- Venkatesan, A.M.; Dehnhardt, C.M.; Delos Santos, E.; Chen, Z.; Dos Santos, O.; Ayral-Kaloustian, S.; Khafizova, G.; Brooijmans, N.; Mallon, R.; Hollander, I. Bis (morpholino-1, 3, 5-triazine) derivatives: Potent adenosine 5′-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: Discovery of compound 26 (pki-587), a highly efficacious dual inhibitor. J. Med. Chem. 2010, 53, 2636–2645. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, A.M.; Chen, Z.; Dos Santos, O.; Dehnhardt, C.; Santos, E.D.; Ayral-Kaloustian, S.; Mallon, R.; Hollander, I.; Feldberg, L.; Lucas, J. Pki-179: An orally efficacious dual phosphatidylinositol-3-kinase (pi3k)/mammalian target of rapamycin (mtor) inhibitor. Bioorganic Med. Chem. Lett. 2010, 20, 5869–5873. [Google Scholar] [CrossRef] [PubMed]
- Hancox, U.; Cosulich, S.; Hanson, L.; Trigwell, C.; Lenaghan, C.; Ellston, R.; Dry, H.; Crafter, C.; Barlaam, B.; Fitzek, M.; et al. Inhibition of PI3Kβ Signaling with AZD8186 Inhibits Growth of PTEN-Deficient Breast and Prostate Tumors Alone and in Combination with Docetaxel. Mol. Cancer Ther. 2015, 14, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.B.; Williams, K.A.; Krie, A.K.; De, P.; Dey, N.; Leyland-Jones, B.; Starks, D.; Rojas-Espaillat, L.A. Results of a phase Ib trial evaluating the safety and clinical activity of sapanisertib (TAK 228) in combination with serabelisib (TAK 117) and paclitaxel in patients with advanced ovarian, endometrial, or breast cancer. J. Clin. Oncol. 2020, 38, 3604. [Google Scholar] [CrossRef]
- Chu, N.; Viennet, T.; Bae, H.; Salguero, A.; Boeszoermenyi, A.; Arthanari, H.; Cole, P.A. The structural determinants of PH domain-mediated regulation of Akt revealed by segmental labeling. Elife 2020, 9, e59151. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Z.; Wei, W. Phosphorylation of Akt at the C-terminal tail triggers Akt Activation. Cell Cycle 2014, 13, 2162–2164. [Google Scholar] [CrossRef]
- Liu, P.; Begley, M.; Michowski, W.; Inuzuka, H.; Ginzberg, M.; Gao, D.; Tsou, P.; Gan, W.; Papa, A.; Kim, B.M.; et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature 2014, 508, 541–545. [Google Scholar] [CrossRef]
- Fruman, D.A.; Rommel, C. Pi3k and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Lin, N.U.; Maurer, M.A.; Chen, H.; Mahvash, A.; Sahin, A.; Akcakanat, A.; Li, Y.; Abramson, V.; Litton, J.; et al. Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Res. 2019, 21, 78. [Google Scholar] [CrossRef]
- Ma, C.X.; Suman, V.; Goetz, M.P.; Northfelt, D.; Burkard, M.E.; Ademuyiwa, F.; Naughton, M.; Margenthaler, J.; Aft, R.; Gray, R. A phase ii trial of neoadjuvant mk-2206, an akt inhibitor, with anastrozole in clinical stage ii or iii pik3ca-mutant er-positive and her2-negative breast cancer. Clin. Cancer Res. 2017, 23, 6823–6832. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Angulo, A.M.; Krop, I.; Akcakanat, A.; Chen, H.; Liu, S.; Li, Y.; Culotta, K.S.; Tarco, E.; Piha-Paul, S.; Moulder-Thompson, S.; et al. SU2C Phase Ib Study of Paclitaxel and MK-2206 in Advanced Solid Tumors and Metastatic Breast Cancer. JNCI J. Natl. Cancer Inst. 2015, 107, dju493. [Google Scholar] [CrossRef]
- Wisinski, K.B.; Tevaarwerk, A.J.; Burkard, M.E.; Rampurwala, M.; Eickhoff, J.; Bell, M.C.; Kolesar, J.M.; Flynn, C.; Liu, G. Phase I Study of an AKT Inhibitor (MK-2206) Combined with Lapatinib in Adult Solid Tumors Followed by Dose Expansion in Advanced HER2+ Breast Cancer. Clin. Cancer Res. 2016, 22, 2659–2667. [Google Scholar] [CrossRef]
- Takeda, T.; Wang, Y.; Bryant, S.H. Structural insights of a PI3K/mTOR dual inhibitor with the morpholino-triazine scaffold. J. Comput.-Aided Mol. Des. 2016, 30, 323–330. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mtor for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Sangai, T.; Akcakanat, A.; Chen, H.; Tarco, E.; Wu, Y.; Do, K.-A.; Miller, T.W.; Arteaga, C.L.; Mills, G.B.; Gonzalez-Angulo, A.M. Biomarkers of response to akt inhibitor mk-2206 in breast cancerantitumor activity of mk-2206. Clin. Cancer Res. 2012, 18, 5816–5828. [Google Scholar] [CrossRef]
- Davies, B.R.; Greenwood, H.; Dudley, P.; Crafter, C.; Yu, D.-H.; Zhang, J.; Li, J.; Gao, B.; Ji, Q.; Maynard, J. Preclinical pharmacology of azd5363, an inhibitor of akt: Pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic backgroundazd5363, an oral inhibitor of akt. Mol. Cancer Ther. 2012, 11, 873–887. [Google Scholar] [CrossRef]
- Lin, J.; Sampath, D.; Nannini, M.A.; Lee, B.B.; Degtyarev, M.; Oeh, J.; Savage, H.; Guan, Z.; Hong, R.; Kassees, R. Targeting activated akt with gdc-0068, a novel selective akt inhibitor that is efficacious in multiple tumor modelsgdc-0068, a novel selective atp-competitive akt inhibitor. Clin. Cancer Res. 2013, 19, 1760–1772. [Google Scholar] [CrossRef]
- Han, H.S.; Swanton, C.; Janjigian, Y.Y.; Sutherland, S.C.; Chandarlapaty, S.; Lehman, R.; Hamilton, N.; Knowles, J.; Lee, R.; Yan, L.; et al. A phase I study of the AKT inhibitor (MK-2206) with concurrent trastuzumab and lapatinib in patients with HER2-positive solid tumors. J. Clin. Oncol. 2011, 29, 3028. [Google Scholar] [CrossRef]
- Krop, I.E.; Saura, C.; Ahnert, J.R.; Becerra, C.; Britten, C.D.; Isakoff, S.J.; Demanse, D.; Hackl, W.; Quadt, C.; Silva, A.P.; et al. A phase I/IB dose-escalation study of BEZ235 in combination with trastuzumab in patients with PI3-kinase or PTEN altered HER2+ metastatic breast cancer. J. Clin. Oncol. 2012, 30, 508. [Google Scholar] [CrossRef]
- Shimizu, T.; Tolcher, A.W.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Smith, L.S.; Gunn, S.; Smetzer, L.; Mays, T.A.; Kaiser, B. The clinical effect of the dual-targeting strategy involving pi3k/akt/mtor and ras/mek/erk pathways in patients with advanced cancerclinical effect of dual pi3k and mapk pathways inhibitions. Clin. Cancer Res. 2012, 18, 2316–2325. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.R.; Juric, D.; Kim, N.; Mino-Kenudson, M.; Huynh, T.; Costa, C.; Lockerman, E.L.; Pollack, S.F.; Liu, M.; Li, X. Cdk 4/6 inhibitors sensitize pik3ca mutant breast cancer to pi3k inhibitors. Cancer Cell 2014, 26, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, J.; Saleh, N.; Chen, S.-C.; Ayers, G.; Abramson, V.; Mayer, I.; Richmond, A. Inhibition of the PI3K/mTOR Pathway in Breast Cancer to Enhance Response to Immune Checkpoint Inhibitors in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 5207. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2015, 25, 41–59. [Google Scholar] [CrossRef]
- Yang, B.; Huang, J.; Xiang, T.; Yin, X.; Luo, X.; Huang, J.; Luo, F.; Li, H.; Li, H.; Ren, G. Chrysin inhibits metastatic potential of human triple-negative breast cancer cells by modulating matrix metalloproteinase-10, epithelial to mesenchymal transition, and pi3k/akt signaling pathway. J. Appl. Toxicol. 2014, 34, 105–112. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef]
- Wu, H.-T.; Liu, Y.-E.; Hsu, K.-W.; Wang, Y.-F.; Chan, Y.-C.; Chen, Y.; Chen, D.-R. MLL3 Induced by Luteolin Causes Apoptosis in Tamoxifen-Resistant Breast Cancer Cells through H3K4 Monomethylation and Suppression of the PI3K/AKT/mTOR Pathway. Am. J. Chin. Med. 2020, 48, 1221–1241. [Google Scholar] [CrossRef]
- Li, X.; Zhou, N.; Wang, J.; Liu, Z.; Wang, X.; Zhang, Q.; Liu, Q.; Gao, L.; Wang, R. Quercetin suppresses breast cancer stem cells (cd44+/cd24−) by inhibiting the pi3k/akt/mtor-signaling pathway. Life Sci. 2018, 196, 56–62. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.-M.; Lu, Y.-Y.; Zhang, H.; Chen, Q.-L.; Zhao, M.; Su, S.-B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-β1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zeng, J.; Xin, M.; Huang, W.; Chen, X. Formononetin induces cell cycle arrest of human breast cancer cells via igf1/pi3k/akt pathways in vitro and in vivo. Horm. Metab. Res. 2011, 43, 681–686. [Google Scholar] [CrossRef]
- Guan, F.; Ding, Y.; Zhang, Y.; Zhou, Y.; Li, M.; Wang, C. Curcumin Suppresses Proliferation and Migration of MDA-MB-231 Breast Cancer Cells through Autophagy-Dependent Akt Degradation. PLoS ONE 2016, 11, e0146553. [Google Scholar] [CrossRef]
- Squires, M.S.; Hudson, E.; Howells, L.; Sale, S.; Houghton, C.E.; Jones, J.; Fox, L.H.; Dickens, M.; Prigent, S.A.; Manson, M.M. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem. Pharmacol. 2002, 65, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Liu, T.; Qian, L.; Xiao, C.; Zhou, X.; Ai, H.; Wang, J.; Fan, W.; Pan, J. Shikonin inhibits migration and invasion of triple-negative breast cancer cells by suppressing epithelial-mesenchymal transition via miR-17-5p/PTEN/Akt pathway. J. Cancer 2021, 12, 76–88. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, H.; Ahn, J.; Jung, S.K.; Um, M.Y.; Son, K.-H.; Kim, T.W.; Ha, T.Y. Anthricin Isolated from Anthriscus sylvestris (L.) Hoffm. Inhibits the Growth of Breast Cancer Cells by Inhibiting Akt/mTOR Signaling, and Its Apoptotic Effects Are Enhanced by Autophagy Inhibition. Evidence-Based Complement. Altern. Med. 2013, 2013, 385219. [Google Scholar] [CrossRef]
- Won, Y.-S.; Seo, K.-I. Lupiwighteone induces caspase-dependent and -independent apoptosis on human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway. Food Chem. Toxicol. 2020, 135, 110863. [Google Scholar] [CrossRef]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Khanal, T.; Park, B.H.; Tran, T.P.; Jeong, T.C.; Jeong, H.G. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013, 141, 2591–2599. [Google Scholar] [CrossRef]
- Zhang, E.; Shi, H.; Yang, L.; Wu, X.; Wang, Z. Ginsenoside Rd regulates the Akt/mTOR/p70S6K signaling cascade and suppresses angiogenesis and breast tumor growth. Oncol. Rep. 2017, 38, 359–367. [Google Scholar] [CrossRef]
- Kim, B.-M.; Kim, D.-H.; Park, J.-H.; Surh, Y.-J.; Na, H.-K. Ginsenoside rg3 inhibits constitutive activation of nf-κb signaling in human breast cancer (mda-mb-231) cells: Erk and akt as potential upstream targets. J. Cancer Prev. 2014, 19, 23. [Google Scholar] [CrossRef]
- Qin, H.-L.; Wang, X.-J.; Yang, B.-X.; Du, B.; Yun, X.-L. Notoginsenoside R1 attenuates breast cancer progression by targeting CCND2 and YBX3. Chin. Med. J. 2021, 134, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Yim-Im, W.; Sawatdichaikul, O.; Semsri, S.; Horata, N.; Mokmak, W.; Tongsima, S.; Suksamrarn, A.; Choowongkomon, K. Computational analyses of curcuminoid analogs against kinase domain of HER2. BMC Bioinform. 2014, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Loo, W.T.; Sze, S.; Tong, Y. Curcumin inhibits cell proliferation of mda-mb-231 and bt-483 breast cancer cells mediated by down-regulation of nfκb, cyclind and mmp-1 transcription. Phytomedicine 2009, 16, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Lai, Y.-A.; Lin, Y.-C.; Ma, J.-W.; Huang, L.-F.; Yang, N.-S.; Ho, C.-T.; Kuo, S.-C.; Way, T.-D. Curcumin Suppresses Doxorubicin-Induced Epithelial–Mesenchymal Transition via the Inhibition of TGF-β and PI3K/AKT Signaling Pathways in Triple-Negative Breast Cancer Cells. J. Agric. Food Chem. 2013, 61, 11817–11824. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, L.; Duan, Y.; Zhang, M.; Wang, G.; Zhang, J.; Zhao, Z. The differential susceptibilities of MCF-7 and MDA-MB-231 cells to the cytotoxic effects of curcumin are associated with the PI3K/Akt-SKP2-Cip/Kips pathway. Cancer Cell Int. 2014, 14, 126. [Google Scholar] [CrossRef]
- Xiao-Ai, L.; Bei, W.; Xiao-Hong, X.; Lei, P.; Bin, W.; Xiao-Xue, D.; Chen-Hui, Z.; Qi-Wei, D. Curcumin re-sensitizes multidrug resistant (MDR) breast cancer to cisplatin through inducing autophagy by decreasing CCAT1 expression. RSC Adv. 2017, 7, 33572–33579. [Google Scholar] [CrossRef]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and Its Derivatives: Synthesis, Pharmacological Uses with Special Emphasis on Anti-Tumor Properties and Prodrug with Enhanced Bio-Availability. Anti-Cancer Agents Med. Chem. 2009, 9, 138–161. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Cao, L.; Yang, Y.; Ye, Z.; Lin, B.; Zeng, J.; Li, C.; Liang, T.; Zhou, K.; Li, J. Quercetin-3-methyl ether suppresses human breast cancer stem cell formation by inhibiting the Notch1 and PI3K/Akt signaling pathways. Int. J. Mol. Med. 2018, 42, 1625–1636. [Google Scholar] [CrossRef]
- Rivera Rivera, A.; Castillo-Pichardo, L.; Gerena, Y.; Dharmawardhane, S. Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Cascade. PLoS ONE 2016, 11, e0157251. [Google Scholar] [CrossRef]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through akt-mtor pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef]
- Safi, A.; Heidarian, E.; Ahmadi, R. Quercetin Synergistically Enhances the Anticancer Efficacy of Docetaxel through Induction of Apoptosis and Modulation of PI3K/AKT, MAPK/ERK, and JAK/STAT3 Signaling Pathways in MDA-MB-231 Breast Cancer Cell Line. Int. J. Mol. Cell. Med. 2021, 10, 11–22. [Google Scholar] [CrossRef]
- LEMEŽIENĖ, N.; Padarauskas, A.; BUTKUTĖ, B.; CESEVIČIENĖ, J.; Taujenis, L.; NORKEVIČIENĖ, E.; MIKALIŪNIENĖ, J. The concentration of isolavones in red clover (trifolium pratense l.) at lowering stage. Zemdirb. -Agric. 2015, 102, 443–448. [Google Scholar] [CrossRef]
- Chen, J.; Sun, L. Formononetin-induced Apoptosis by Activation of Ras/p38 Mitogen-activated Protein Kinase in Estrogen Receptor-positive Human Breast Cancer Cells. Horm. Metab. Res. 2012, 44, 943–948. [Google Scholar] [CrossRef]
- Zhou, R.; Xu, L.; Ye, M.; Liao, M.; Du, H.; Chen, H. Formononetin Inhibits Migration and Invasion of MDA-MB-231 and 4T1 Breast Cancer Cells by Suppressing MMP-2 and MMP-9 Through PI3K/AKT Signaling Pathways. Horm. Metab. Res. 2014, 46, 753–760. [Google Scholar] [CrossRef]
- Wu, X.Y.; Xu, H.; Wu, Z.F.; Chen, C.; Liu, J.Y.; Wu, G.N.; Yao, X.Q.; Liu, F.K.; Li, G.; Shen, L. Formononetin, a novel FGFR2 inhibitor, potently inhibits angiogenesis and tumor growth in preclinical models. Oncotarget 2015, 6, 44563–44578. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-M.; Kim, D.-H.; Park, J.-H.; Na, H.-K.; Surh, Y.-J. Ginsenoside Rg3 Induces Apoptosis of Human Breast Cancer (MDA-MB-231) Cells. J. Cancer Prev. 2013, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Fan, D. Ginsenoside Rk1 induces cell death through ROS-mediated PTEN/PI3K/Akt/mTOR signaling pathway in MCF-7 cells. J. Funct. Foods 2019, 57, 255–265. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, D. Ginsenoside Rg5 induces apoptosis and autophagy via the inhibition of the PI3K/Akt pathway against breast cancer in a mouse model. Food Funct. 2018, 9, 5513–5527. [Google Scholar] [CrossRef]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for Breast Cancer Prevention and Therapy: Preclinical Evidence and Molecular Mechanisms; Academic Press: Cambridge, MA, USA, 2016; Volume 40–41, pp. 209–232. [Google Scholar] [CrossRef]
- Park, M.-A.; Hwang, K.-A.; Choi, K.-C. Diverse animal models to examine potential role (s) and mechanism of endocrine disrupting chemicals on the tumor progression and prevention: Do they have tumorigenic or anti-tumorigenic property? Lab. Anim. Res. 2011, 27, 265–273. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Zhu, J.; Orloff, M.; Eng, C. Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett. 2011, 301, 168–176. [Google Scholar] [CrossRef]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Faisal, S.M.; Khan, M.A. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014, 38, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Sinha, C.; Ignatoski, K.; Lippman, M.E.; Ethier, S.P.; Chinnaiyan, A.M. Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res. 2003, 63, 132–139. [Google Scholar] [PubMed]
- Tsai, J.-H.; Hsu, L.-S.; Lin, C.-L.; Hong, H.-M.; Pan, M.-H.; Way, T.-D.; Chen, W.-J. 3, 5, 4′-trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of pi3k/akt and wnt/β-catenin signaling cascades and reversal of epithelial–mesenchymal transition. Toxicol. Appl. Pharmacol. 2013, 272, 746–756. [Google Scholar]
- Hendrawati, O.; Woerdenbag, H.J.; Hille, J.; Quax, W.J.; Kayser, O. Seasonal Variations in the Deoxypodophyllotoxin Content and Yield of Anthriscus sylvestris L. (Hoffm.) Grown in the Field and under Controlled Conditions. J. Agric. Food Chem. 2011, 59, 8132–8139. [Google Scholar] [CrossRef] [PubMed]
- Alli, P.M.; Pinn, M.L.; Jaffee, E.M.; McFadden, J.M.; Kuhajda, F.P. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene 2004, 24, 39–46. [Google Scholar] [CrossRef]
- Malhotra, G.K.; Zhao, X.; Band, H.; Band, V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol. Ther. 2010, 10, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Kerlikowske, K.; Hubbard, R.A.; Miglioretti, D.L.; Geller, B.M.; Yankaskas, B.C.; Lehman, C.D.; Taplin, S.H.; Sickles, E.A.; Consortium, B.C.S. Comparative effectiveness of digital versus film-screen mammography in community practice in the united states: A cohort study. Ann. Intern. Med. 2011, 155, 493–502. [Google Scholar] [CrossRef]
- Do, C.; DeAguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-Based Contrast Agent Use, Their Safety, and Practice Evolution. Kidney360 2020, 1, 561–568. [Google Scholar] [CrossRef]
- Dhankhar, R.; Vyas, S.P.; Jain, A.K.; Arora, S.; Rath, G.; Goyal, A.K. Advances in Novel Drug Delivery Strategies for Breast Cancer Therapy. Artif. Cells Blood Substit. Biotechnol. 2010, 38, 230–249. [Google Scholar] [CrossRef]
- Moo, T.-A.; Sanford, R.; Dang, C.; Morrow, M. Overview of Breast Cancer Therapy. PET Clin. 2018, 13, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, D.L.; McDaniel, L.R.; Golden, D. Long-term effects of breast cancer surgery, treatment, and survivor care. J. Midwifery Women’s Health 2019, 64, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Mancini, P.; Angeloni, A.; Risi, E.; Orsi, E.; Mezi, S. Standard of Care and Promising New Agents for Triple Negative Metastatic Breast Cancer. Cancers 2014, 6, 2187–2223. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.; Akcakanat, A.; Liu, S.; Green, M.; Murray, J.; Chen, H.; Palla, S.; Koenig, K.; Brewster, A.; Valero, V.; et al. Open-label randomized clinical trial of standard neoadjuvant chemotherapy with paclitaxel followed by FEC versus the combination of paclitaxel and everolimus followed by FEC in women with triple receptor-negative breast cancer. Ann. Oncol. 2014, 25, 1122–1127. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Bianco-Miotto, T.; Jindal, S.; Butler, L.M.; Leung, S.; McNeil, C.M.; O’Toole, S.A.; Ebrahimie, E.; Millar, E.K.; Sakko, A.J. The magnitude of androgen receptor positivity in breast cancer is critical for reliable prediction of disease outcomear robustly predicts outcome in breast cancer. Clin. Cancer Res. 2018, 24, 2328–2341. [Google Scholar] [CrossRef]
- Chia, K.M.; Liu, J.; Francis, G.D.; Naderi, A. A Feedback Loop between Androgen Receptor and ERK Signaling in Estrogen Receptor-Negative Breast Cancer. Neoplasia 2011, 13, 154–166. [Google Scholar] [CrossRef]
- Yard, B.D.; Adams, D.J.; Chie, E.K.; Tamayo, P.; Battaglia, J.S.; Gopal, P.; Rogacki, K.; Pearson, B.E.; Phillips, J.; Raymond, D.P.; et al. A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat. Commun. 2016, 7, 11428. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Auricchio, F.; Castoria, G.; Migliaccio, A. Androgens Induce Invasiveness of Triple Negative Breast Cancer Cells Through AR/Src/PI3-K Complex Assembly. Sci. Rep. 2019, 9, 4490. [Google Scholar] [CrossRef]
- Burstein, H.J. The Distinctive Nature of HER2-Positive Breast Cancers. N. Engl. J. Med. 2005, 353, 1652–1654. [Google Scholar] [CrossRef]
- Ruiz-Saenz, A.; Dreyer, C.; Campbell, M.R.; Steri, V.; Gulizia, N.; Moasser, M.M. Her2 amplification in tumors activates pi3k/akt signaling independent of her3her2-amplified tumors overcome the requirement for her3. Cancer Res. 2018, 78, 3645–3658. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Biasutto, L.; Marotta, E.; De Marchi, U.; Zoratti, M.; Paradisi, C. Ester-Based Precursors to Increase the Bioavailability of Quercetin. J. Med. Chem. 2006, 50, 241–253. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).