Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing
Abstract
:1. Introduction
2. The Physiology of Skin Wound Healing
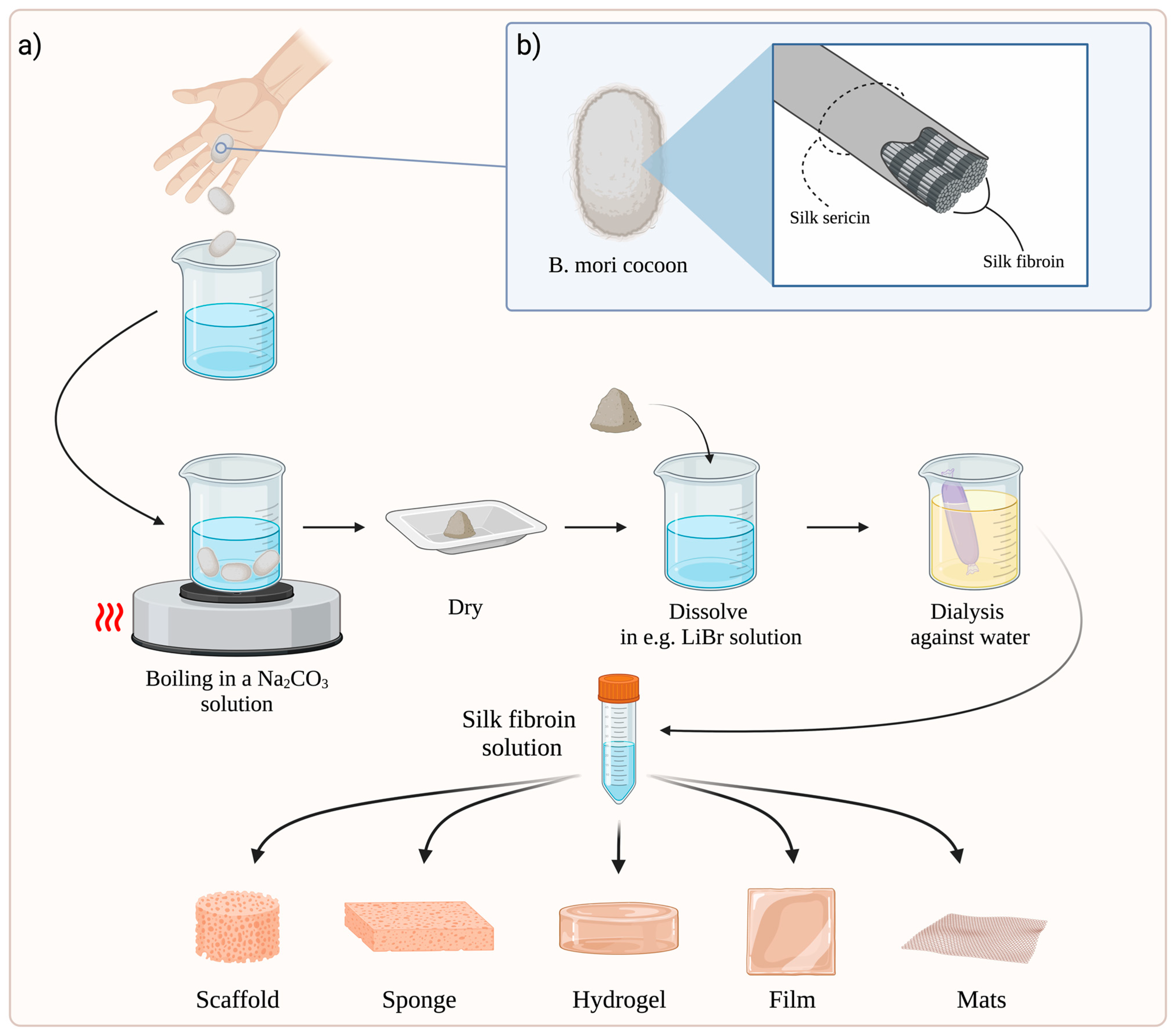
3. Preparation of SF-Based Dressings
4. Molecular Mechanisms of SF Action
5. Antibacterial Properties of Silk Fibres
6. Properties of SF Biomaterials in Skin Wound Healing
6.1. The Role of SF-Based Dressings in the Treatment of Full-Thickness Skin Wounds
6.2. SF-Based Dressings and Their Use in Burn Wound Healing
6.3. SF-Based Dressings and Their Use in Diabetic Wound Healing
6.4. Clinical Study with SF-Based Dressings in Skin Wound Healing
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [Green Version]
- Murphree, R.W. Impairments in Skin Integrity. Nurs. Clin. N. Am. 2017, 52, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Takeo, M.; Lee, W.; Ito, M. Wound healing and skin regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, P.U.; Lona-Ramos, M.C.; Gutiérrez-Verdín, L.D.; Luévano-Colmenero, G.H.; Tenorio-Rocha, F.; García-Contreras, R.; González-García, G.; Rosillo-de la Torre, A.; Delgado, J.; Castellano, L.E.; et al. Gel dressing based on type I collagen modified with oligourethane and silica for skin wound healing. Biomed. Mater. 2022, 17, 045005. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Luo, C.; Qian, B.; Liu, S.; Zeng, Y.; Hou, J.; Deng, B.; Sun, Y.; Yang, J.; et al. N-acetyl cysteine-loaded graphene oxide-collagen hybrid membrane for scarless wound healing. Theranostics 2019, 9, 5839–5853. [Google Scholar] [CrossRef]
- Pallaske, F.; Pallaske, A.; Herklotz, K.; Boese-Landgraf, J. The significance of collagen dressings in wound management: A review. J. Wound Care 2018, 27, 692–702. [Google Scholar] [CrossRef]
- Li, M.N.; Yu, H.P.; Ke, Q.F.; Zhang, C.Q.; Gao, Y.S.; Guo, Y.P. Gelatin methacryloyl hydrogels functionalized with endothelin-1 for angiogenesis and full-thickness wound healing. J. Mater. Chem. B 2021, 9, 4700–4709. [Google Scholar] [CrossRef]
- Jang, H.-J.; Kim, Y.-M.; Yoo, B.-Y.; Seo, Y.-K. Wound-healing effects of human dermal components with gelatin dressing. J. Biomater. Appl. 2018, 32, 716–724. [Google Scholar] [CrossRef] [PubMed]
- İnal, M.; Mülazımoğlu, G. Production and characterization of bactericidal wound dressing material based on gelatin nanofiber. Int. J. Biol. Macromol. 2019, 137, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Czuwara, J.; Kłodzińska, E.; Laskowska, A.K.; Sulejczak, D.; Damps, T.; Zielenkiewicz, U.; Brzozowska, I.; Sureda, A.; Kowalkowski, T.; et al. Evaluation of keratin biomaterial containing silver nanoparticles as a potential wound dressing in full-thickness skin wound model in diabetic mice. J. Tissue Eng. Regen. Med. 2020, 14, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Laskowska, A.K.; Rybka, M.; Kłodzińska, E.; Sulejczak, D.; Schwartz, R.A.; Czuwara, J. Keratin scaffolds containing casomorphin stimulate macrophage infiltration and accelerate full-thickness cutaneous wound healing in diabetic mice. Molecules 2021, 26, 2554. [Google Scholar] [CrossRef]
- Konop, M.; Rybka, M.; Szudzik, M.; Mazurek, Ł.; Laskowska, A.K.; Sulejczak, D.; Ruszczak, Z.; Mazgaj, R.; Cieślik, B.; Schwartz, R.A.; et al. Keratin-Butyrate Scaffolds Promote Skin Wound Healing in Diabetic Rats through Down-Regulation of IL-1β and Up-Regulation of Keratins 16 and 17. J. Nat. Fibers 2023, 20, 2136325. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk biomaterials in wound healing and skin regeneration therapeutics: From bench to bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Chen, J.; Wang, L.; Gui, X.; Ran, J.; Xu, G.; Zhao, H.; Zeng, M.; Ji, J.; et al. Silk Fibroin Biomaterial Shows Safe and Effective Wound Healing in Animal Models and a Randomized Controlled Clinical Trial. Adv. Healthc. Mater. 2017, 6, 1700121. [Google Scholar] [CrossRef]
- Yu, K.; Lu, F.; Li, Q.; Zou, Y.; Xiao, Y.; Lu, B.; Liu, J.; Dai, F.; Wu, D.; Lan, G. Accelerated wound-healing capabilities of a dressing fabricated from silkworm cocoon. Int. J. Biol. Macromol. 2017, 102, 901–913. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Silk fibroin in tissue engineering. Adv. Healthc. Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Moy, R.L.; Lee, A.; Zalka, A. Commonly used suture materials in skin surgery. Am. Fam. Physician 1991, 44, 2123–2128. [Google Scholar] [PubMed]
- Sultan, M.T.; Lee, O.J.; Kim, S.H.; Ju, H.W.; Park, C.H. Silk Fibroin in Wound Healing Process. Adv. Exp. Med. Biol. 2018, 1077, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 6339–6347. [Google Scholar] [CrossRef] [Green Version]
- Ude, A.U.; Eshkoor, R.A.; Zulkifili, R.; Ariffin, A.K.; Dzuraidah, A.W.; Azhari, C.H. Bombyx mori silk fibre and its composite: A review of contemporary developments. Mater. Des. 2014, 57, 298–305. [Google Scholar] [CrossRef]
- Kaplan, D.; Adams, W.W.; Farmer, B.; Viney, C. Silk: Biology, Structure, Properties, and Genetics. In Silk Polymers; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1993; Volume 544, pp. 2–16. [Google Scholar]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sericin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Z.; Song, Y.; Jin, Y.; Zhang, C.; Peng, D.; Chen, Z.; Chang, P.; Kundu, S.C.; Wang, G.; Wang, Z.; et al. In Vivo Characterizations of the Immune Properties of Sericin: An Ancient Material with Emerging Value in Biomedical Applications. Macromol. Biosci. 2017, 17, 1700229. [Google Scholar] [CrossRef]
- Aramwit, P.; Palapinyo, S.; Srichana, T.; Chottanapund, S.; Muangman, P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch. Dermatol. Res. 2013, 305, 585–594. [Google Scholar] [CrossRef]
- Ersel, M.; Uyanikgil, Y.; Karbek Akarca, F.; Ozcete, E.; Altunci, Y.A.; Karabey, F.; Cavusoglu, T.; Meral, A.; Yigitturk, G.; Oyku Cetin, E. Effects of Silk Sericin on Incision Wound Healing in a Dorsal Skin Flap Wound Healing Rat Model. Med. Sci. Monit. 2016, 22, 1064–1078. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Srichana, T. Monitoring of inflammatory mediators induced by silk sericin. J. Biosci. Bioeng. 2009, 107, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, K.; Ma, X.; Jiang, Z.; Huang, W.; Gao, C.; Pu, Y.; Luo, L.; Xu, Y.; Xu, Y. Innate Immunity in Diabetic Wound Healing: Focus on the Mastermind Hidden in Chronic Inflammatory. Front. Pharmacol. 2021, 12, 653940. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [Green Version]
- Stupin, V.; Manturova, N.; Silina, E.; Litvitskiy, P.; Vasin, V.; Artyushkova, E.; Ivanov, A.; Gladchenko, M.; Aliev, S. The Effect of Inflammation on the Healing Process of Acute Skin Wounds Under the Treatment of Wounds with Injections in Rats. J. Exp. Pharmacol. 2020, 12, 409–422. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed. Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruxandra, B.I.; Gupta, S.V.; Octavian, S.; Irinel, C.A.; Jacob, G.; Stina, L.; Teresa, P.; Seppo, Y.-H.; Lorenz, P.; Kerstin, B.; et al. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc. Natl. Acad. Sci. USA 2008, 105, 19426–19431. [Google Scholar] [CrossRef] [Green Version]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Chen, P.; Parks, W.C. Role of matrix metalloproteinases in epithelial migration. J. Cell. Biochem. 2009, 108, 1233–1243. [Google Scholar] [CrossRef]
- Benito-Martínez, S.; Pérez-Köhler, B.; Rodríguez, M.; Izco, J.M.; Recalde, J.I.; Pascual, G. Wound Healing Modulation through the Local Application of Powder Collagen-Derived Treatments in an Excisional Cutaneous Murine Model. Biomedicines 2022, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.A.; Ferguson, M.W.J.; Mason, T.; McGrouther, D.A. Skin tension or skin compression? Small circular wounds are likely to shrink, not gape. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 529–534. [Google Scholar] [CrossRef]
- Wu, M.; Ben Amar, M. Growth and remodelling for profound circular wounds in skin. Biomech. Model. Mechanobiol. 2015, 14, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Chitturi, R.T.; Balasubramaniam, A.M.; Parameswar, R.A.; Kesavan, G.; Haris, K.T.M.; Mohideen, K. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J. Int. Oral Health 2015, 7, 75–80. [Google Scholar] [PubMed]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current Approaches Targeting the Wound Healing Phases to Attenuate Fibrosis and Scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef]
- Sylakowski, K.; Wells, A. ECM-regulation of autophagy: The yin and the yang of autophagy during wound healing. Matrix Biol. 2021, 100–101, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [Green Version]
- Sah, M.K.; Kumar, A.; Pramanik, K. The extraction of fibroin protein from Bombyx mori silk cocoon: Optimization of process parameters. Int. J. Biol. Res. 2010, 2, 33–41. [Google Scholar]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.H.; Le, T.H.; Huynh, V.Q.N.; Vo, D.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.F.P.; Silva, M.M.; de Zea Bermudez, V. Bombyx mori Silk Fibers: An Outstanding Family of Materials. Macromol. Mater. Eng. 2015, 300, 1171–1198. [Google Scholar] [CrossRef]
- Sashina, E.S.; Bochek, A.M.; Novoselov, N.P.; Kirichenko, D.A. Structure and solubility of natural silk fibroin. Russ. J. Appl. Chem. 2006, 79, 869–876. [Google Scholar] [CrossRef]
- Guo, P.; Du, P.; Zhao, P.; Chen, X.; Liu, C.; Du, Y.; Li, J.; Tang, X.; Yang, F.; Lv, G. Regulating the mechanics of silk fibroin scaffolds promotes wound vascularization. Biochem. Biophys. Res. Commun. 2021, 574, 78–84. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Yuan, X.F.; Bayat, A. Electrospun silk fibroin fiber diameter influences in vitro dermal fibroblast behavior and promotes healing of ex vivo wound models. J. Tissue Eng. 2014, 5, 2041731414551661. [Google Scholar] [CrossRef]
- Liang, A.; Zhang, M.; Luo, H.; Niu, L.; Feng, Y.; Li, M. Porous Poly(Hexamethylene Biguanide) Hydrochloride Loaded Silk Fibroin Sponges with Antibacterial Function. Materials 2020, 13, 285. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Wang, D.; Shi, Y.; Li, M.; Gao, M.; Yu, T.; Liu, D.; Zhang, J.; Wang, J.; Zhang, X.; et al. Insulin-Containing Wound Dressing Promotes Diabetic Wound Healing Through Stabilizing HIF-1α. Front. Bioeng. Biotechnol. 2020, 8, 592833. [Google Scholar] [CrossRef]
- Yu, R.; Yang, Y.; He, J.; Li, M.; Guo, B. Novel supramolecular self-healing silk fibroin-based hydrogel via host–guest interaction as wound dressing to enhance wound healing. Chem. Eng. J. 2021, 417, 128278. [Google Scholar] [CrossRef]
- He, X.; Liu, X.; Yang, J.; Du, H.; Chai, N.; Sha, Z.; Geng, M.; Zhou, X.; He, C. Tannic acid-reinforced methacrylated chitosan/methacrylated silk fibroin hydrogels with multifunctionality for accelerating wound healing. Carbohydr. Polym. 2020, 247, 116689. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.S.; Panilaitis, B.; Bellas, E.; Kaplan, D.L. Functionalized silk biomaterials for wound healing. Adv. Healthc. Mater. 2013, 2, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, A.; Sugiura, K.; Morita, H.; Ninagawa, T.; Tubouchi, K.; Tobe, R.; Izumiya, M.; Horio, T.; Abraham, N.G.; Ikehara, S. Promotive effects of a silk film on epidermal recovery from full-thickness skin wounds. Proc. Soc. Exp. Biol. Med. 2000, 225, 58–64. [Google Scholar] [CrossRef]
- Chouhan, D.; Thatikonda, N.; Nilebäck, L.; Widhe, M.; Hedhammar, M.; Mandal, B.B. Recombinant Spider Silk Functionalized Silkworm Silk Matrices as Potential Bioactive Wound Dressings and Skin Grafts. ACS Appl. Mater. Interfaces 2018, 10, 23560–23572. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.W.; Lee, O.J.; Lee, J.M.; Moon, B.M.; Park, H.J.; Park, Y.R.; Lee, M.C.; Kim, S.H.; Chao, J.R.; Ki, C.S.; et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int. J. Biol. Macromol. 2016, 85, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In Vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, P.P.; Reagan, M.R.; Bohara, R.A. Silk fibroin and silk-based biomaterial derivatives for ideal wound dressings. Int. J. Biol. Macromol. 2020, 164, 4613–4627. [Google Scholar] [CrossRef]
- Yun, J.; Lee, J.; Cha, S.-Y.; Maeng, S.; Noh, J.K.; Nam, D.-E.; Stohs, S.J. Mechanistic and Efficacy Study on Effects of Fibroin Enzymatic Hydrolysate (FEH) on Memory and Learning Impairments Induced by Scopolamine in Mice. J. Altern. Complement. Integr. Med. 2021, 7, 160. [Google Scholar] [CrossRef]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Komosinska-Vassev, K.; Wisowski, G.; Mencner, L.; Stojko, J.; Kozma, E.M. Propolis Modulates Fibronectin Expression in the Matrix of Thermal Injury. Biomed. Res. Int. 2014, 2014, 748101. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320–E4327. [Google Scholar] [CrossRef] [Green Version]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The Role of Vascular Endothelial Growth Factor in Wound Healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Chou, K.C.; Chen, C.T.; Cherng, J.H.; Li, M.C.; Wen, C.C.; Hu, S.I.; Wang, Y.W. Cutaneous Regeneration Mechanism of β-Sheet Silk Fibroin in a Rat Burn Wound Healing Model. Polymers 2021, 13, 3537. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mora, C.; Mrowiec, A.; García-Vizcaíno, E.M.; Alcaraz, A.; Cenis, J.L.; Nicolás, F.J. Fibroin and sericin from Bombyx mori silk stimulate cell migration through upregulation and phosphorylation of c-Jun. PLoS ONE 2012, 7, e42271. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.; Shukla, S.; Sayyad, R.; Salunke, S.; Nisal, A.; Venugopalan, P. Silk fibroin and ceramic scaffolds: Comparative in vitro studies for bone regeneration. Bioeng. Transl. Med. 2021, 6, e10221. [Google Scholar] [CrossRef]
- Naserzadeh, P.; Mortazavi, S.A.; Ashtari, K.; Seydi, E.; Pourahmad, J. Evaluation of the Toxicity Effects of Silk Fibroin on Isolated Fibroblast and Huvec Cells. Iran. J. Pharm. Res. 2018, 17, 134–145. [Google Scholar]
- Ghalei, S.; Handa, H. A Review on Antibacterial Silk Fibroin-based Biomaterials: Current State and Prospects. Mater. Today Chem. 2022, 23, 100673. [Google Scholar] [CrossRef]
- Seves, A.; Romanò, M.; Maifreni, T.; Sora, S.; Ciferri, O. The microbial degradation of silk: A laboratory investigation. Int. Biodeterior. Biodegrad. 1998, 42, 203–211. [Google Scholar] [CrossRef]
- Tabei, Y.; Tsutsumi, K.; Ogawa, A.; Era, M.; Morita, H. Application of insoluble fibroin film as conditioning film for biofilm formation. Sens. Mater. 2011, 23, 195–205. [Google Scholar]
- Steinstraesser, L.; Trust, G.; Rittig, A.; Hirsch, T.; Kesting, M.R.; Steinau, H.U.; Jacobsen, F. Colistin-loaded silk membranes against wound infection with Pseudomonas aeruginosa. Plast. Reconstr. Surg. 2011, 127, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, W.; Yao, J.; Shao, Z.; Chen, X. Silk microfibrous mats with long-lasting antimicrobial function. J. Mater. Sci. Technol. 2021, 63, 203–209. [Google Scholar] [CrossRef]
- Patil, S.; George, T.; Mahadik, K. Green synthesized nanosilver loaded silk fibroin gel for enhanced wound healing. J. Drug Deliv. Sci. Technol. 2015, 30, 30–36. [Google Scholar] [CrossRef]
- Pei, Z.; Sun, Q.; Sun, X.; Wang, Y.; Zhao, P. Preparation and characterization of silver nanoparticles on silk fibroin/carboxymethylchitosan composite sponge as anti-bacterial wound dressing. Biomed. Mater. Eng. 2015, 26, S111–S118. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.-X.; Mo, X.-M.; Zhang, K.-H.; Fan, L.-P.; Yin, A.-L.; He, C.-L.; Wang, H.-S. Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-dressing Applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef]
- Guang, S.; An, Y.; Ke, F.; Zhao, D.; Shen, Y.; Xu, H. Chitosan/silk fibroin composite scaffolds for wound dressing. J. Appl. Polym. Sci. 2015, 132, 42503. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lu, L.; Chen, Y.; Wang, J.; Chen, Y.; Mao, C.; Yang, M. Polydopamine modification of silk fibroin membranes significantly promotes their wound healing effect. Biomater. Sci. 2019, 7, 5232–5237. [Google Scholar] [CrossRef]
- Millán-Rivero, J.E.; Martínez, C.M.; Romecín, P.A.; Aznar-Cervantes, S.D.; Carpes-Ruiz, M.; Cenis, J.L.; Moraleda, J.M.; Atucha, N.M.; García-Bernal, D. Silk fibroin scaffolds seeded with Wharton’s jelly mesenchymal stem cells enhance re-epithelialization and reduce formation of scar tissue after cutaneous wound healing. Stem Cell Res. Ther. 2019, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarovart, S.; Sudatis, B.; Meesilpa, P.; Grady, B.P.; Magaraphan, R. The use of sericin as an antioxidant and antimicrobial for polluted air treatment. Rev. Adv. Mater. Sci. 2003, 5, 193–198. [Google Scholar]
- Rajendran, R.; Balakumar, C.; Sivakumar, R.; Amruta, T.; Devaki, N. Extraction and application of natural silk protein sericin from Bombyx mori as antimicrobial finish for cotton fabrics. J. Text. Inst. 2012, 103, 458–462. [Google Scholar] [CrossRef]
- He, S.; Shi, D.; Han, Z.; Dong, Z.; Xie, Y.; Zhang, F.; Zeng, W.; Yi, Q. Heparinized silk fibroin hydrogels loading FGF1 promote the wound healing in rats with full-thickness skin excision. Biomed. Eng. Online 2019, 18, 97. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef]
- Evers, L.H.; Bhavsar, D.; Mailänder, P. The biology of burn injury. Exp. Dermatol. 2010, 19, 777–783. [Google Scholar] [CrossRef]
- Vigani, A.; Culler, C.A. Systemic and Local Management of Burn Wounds. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 1149–1163. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Burn wound healing: Present concepts, treatment strategies and future directions. J. Wound Care 2017, 26, 5–19. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Management of Burns. N. Engl. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Sun, F.; Zhang, X.; Peng, Z.; Jiang, B.; Liang, M.; Wang, Y. Silk fibroin hydrogel promote burn wound healing through regulating TLN1 expression and affecting cell adhesion and migration. J. Mater. Sci. Mater. Med. 2020, 31, 48. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Lohe, T.U.; Samudrala, P.K.; Mandal, B.B. In Situ Forming Injectable Silk Fibroin Hydrogel Promotes Skin Regeneration in Full Thickness Burn Wounds. Adv. Healthc. Mater. 2018, 7, e1801092. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G. Wound healing and diabetes mellitus. Clin. Plast. Surg. 2003, 30, 37–45. [Google Scholar] [CrossRef]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Spec. 2012, 4, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic Wound-Healing Science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef]
- Blakytny, R.; Jude, E.B. Altered Molecular Mechanisms of Diabetic Foot Ulcers. Int. J. Low. Extrem. Wounds 2009, 8, 95–104. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhang, J.; You, R.; Qu, J.; Li, M. Functionalized silk fibroin dressing with topical bioactive insulin release for accelerated chronic wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 394–404. [Google Scholar] [CrossRef]
- Liu, J.; Yan, L.; Yang, W.; Lan, Y.; Zhu, Q.; Xu, H.; Zheng, C.; Guo, R. Controlled-release neurotensin-loaded silk fibroin dressings improve wound healing in diabetic rat model. Bioact. Mater. 2019, 4, 151–159. [Google Scholar] [CrossRef]
- Mouritzen, M.V.; Abourayale, S.; Ejaz, R.; Ardon, C.B.; Carvalho, E.; Dalgaard, L.T.; Roursgaard, M.; Jenssen, H. Neurotensin, substance P, and insulin enhance cell migration. J. Pept. Sci. 2018, 24, e3093. [Google Scholar] [CrossRef] [PubMed]
- Maity, B.; Alam, S.; Samanta, S.; Prakash, R.G.; Govindaraju, T. Antioxidant Silk Fibroin Composite Hydrogel for Rapid Healing of Diabetic Wound. Macromol. Biosci. 2022, 22, 2200097. [Google Scholar] [CrossRef] [PubMed]
- Navone, S.E.; Pascucci, L.; Dossena, M.; Ferri, A.; Invernici, G.; Acerbi, F.; Cristini, S.; Bedini, G.; Tosetti, V.; Ceserani, V.; et al. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res. Ther. 2014, 5, 7. [Google Scholar] [CrossRef] [PubMed]

| Author | Type of SF Dressing | Control Dressing | Dressed Wound | Healing Rate (p-Value) |
|---|---|---|---|---|
| [59] | Scaffold | No dressing | Random SF scaffold (RSS) | p ≤ 0.01 on days 3 and 21, p ≤ 0.05 on day 14, p ≤ 0.001 on day 21 |
| Aligned SF scaffold (ASS) | p ≤ 0.05 on days 3, 7, 14 p ≤ 0.001 on day 21 | |||
| [90] | Electrospun membrane | 3MTM TegadermTM | PDA-modified electrospun SF membrane (PESF) | p < 0.001 |
| Electrospun SF membrane (ESF) | Not significant | |||
| [60] | Electrospun scaffold | No dressing | Electrospun SF scaffold | p < 0.05 |
| [91] | Electrospun scaffold | Linitul® | Electrospun SF scaffolds cellularized with human Wharton’s jelly MSCs (Wj-MSCs-SF) | - |
| [18] | Sol-gel film | No dressing (control group), Mepitel® (positive control) | Silkworm cocoonss ol-gel film (SCSF-90) | p < 0.05 on day 5 p < 0.01 on days 10 and 15, compared to the control group |
| [95] | Hydrogel | 3MTM TegadermTM (control group), commercial chitosan dressing (positive control) | Heparinized SF hydrogel | - |
| Heparinized SF hydrogel with FGF1 (SF + FGF) | p < 0.001 on day 14, compared to the control group | |||
| [88] | Scaffold | - | Chitosan/SF (CS/SF) composite scaffold | - |
| [85] | Hydrogel | Soframycin gel (positive control) | Nanosilver-loaded SF (NSF) gel | p < 0.05 |
| [68] | Electrospun nanomatrix | Medical gauze (control group), Medifoam® (positive control) | Electrospun SF nanomatrix | - |
| [103] | Hydrogel | No dressing (control group), Purilon gel (positive control) | SF hydrogel | p < 0.05 on days 9 and 12 compared to the Purilon gel |
| [104] | Hydrogel | TegadermTM film (control group), Col gel (positive control) | SF hydrogel | p ≤ 0.01 compared to the control group |
| [110] | Sponge | Gauze | SF sponge dressing | - |
| Insulin-loaded SF sponge dressing | p < 0.05 after 1 and 2 weeks compared to the other groups | |||
| [62] | Sponge | SF sponge dressing | Insulin-loaded SF sponge dressing | p < 0.01 on days 5 and 11 |
| [111] | Scaffold | No dressing | SF | - |
| NT/SF | - | |||
| NT/GMs/SF | p < 0.05 on days 14, 21, and 28 | |||
| [113] | Hydrogel | Gauze (control group), SFH (4% SF; positive control) | SFMH (4% SF with 0.01% melanin) | - |
| SFCH (4% SF, 0.01% melanin, and 0.1% of berberine) | - | |||
| [114] | Electrospun scaffold | No dressing (control group), SF scaffold (positive control) | SF patches cellularised with human adipose-derived mesenchymal stromal cells (Ad-MSCs-SF) | p < 0.05, on days 3 and 10 compared to the control group |
| Decellularized (D-Ad-MSCs-SF) | p < 0.05, on days 3 and 10 compared to the control group | |||
| [17], clinical study | Film | Commercial dressing Sidaiyi (SF sponge-silicone, two-layered scaffold) | SF film | p = 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, Ł.; Szudzik, M.; Rybka, M.; Konop, M. Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules 2022, 12, 1852. https://doi.org/10.3390/biom12121852
Mazurek Ł, Szudzik M, Rybka M, Konop M. Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules. 2022; 12(12):1852. https://doi.org/10.3390/biom12121852
Chicago/Turabian StyleMazurek, Łukasz, Mateusz Szudzik, Mateusz Rybka, and Marek Konop. 2022. "Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing" Biomolecules 12, no. 12: 1852. https://doi.org/10.3390/biom12121852
APA StyleMazurek, Ł., Szudzik, M., Rybka, M., & Konop, M. (2022). Silk Fibroin Biomaterials and Their Beneficial Role in Skin Wound Healing. Biomolecules, 12(12), 1852. https://doi.org/10.3390/biom12121852








