Osteoglycin: An ECM Factor Regulating Fibrosis and Tumorigenesis
Abstract
1. Introduction
2. OGN—Structure and Functions
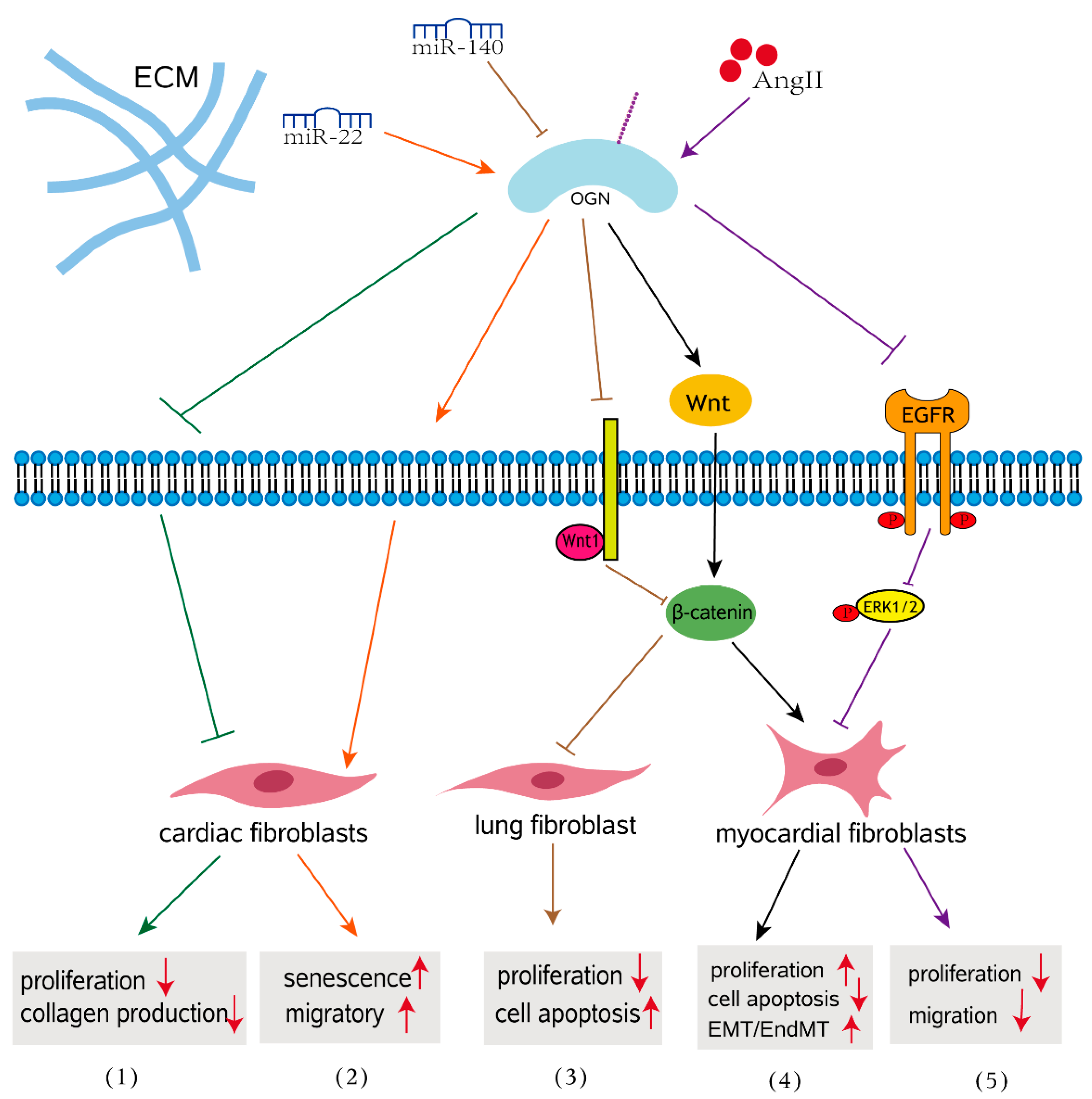
3. OGN as a Regulator of Fibrosis
3.1. Role of OGN in Cardiac Fibrosis
3.2. Impact of OGN on Fibrosis in Other Organs
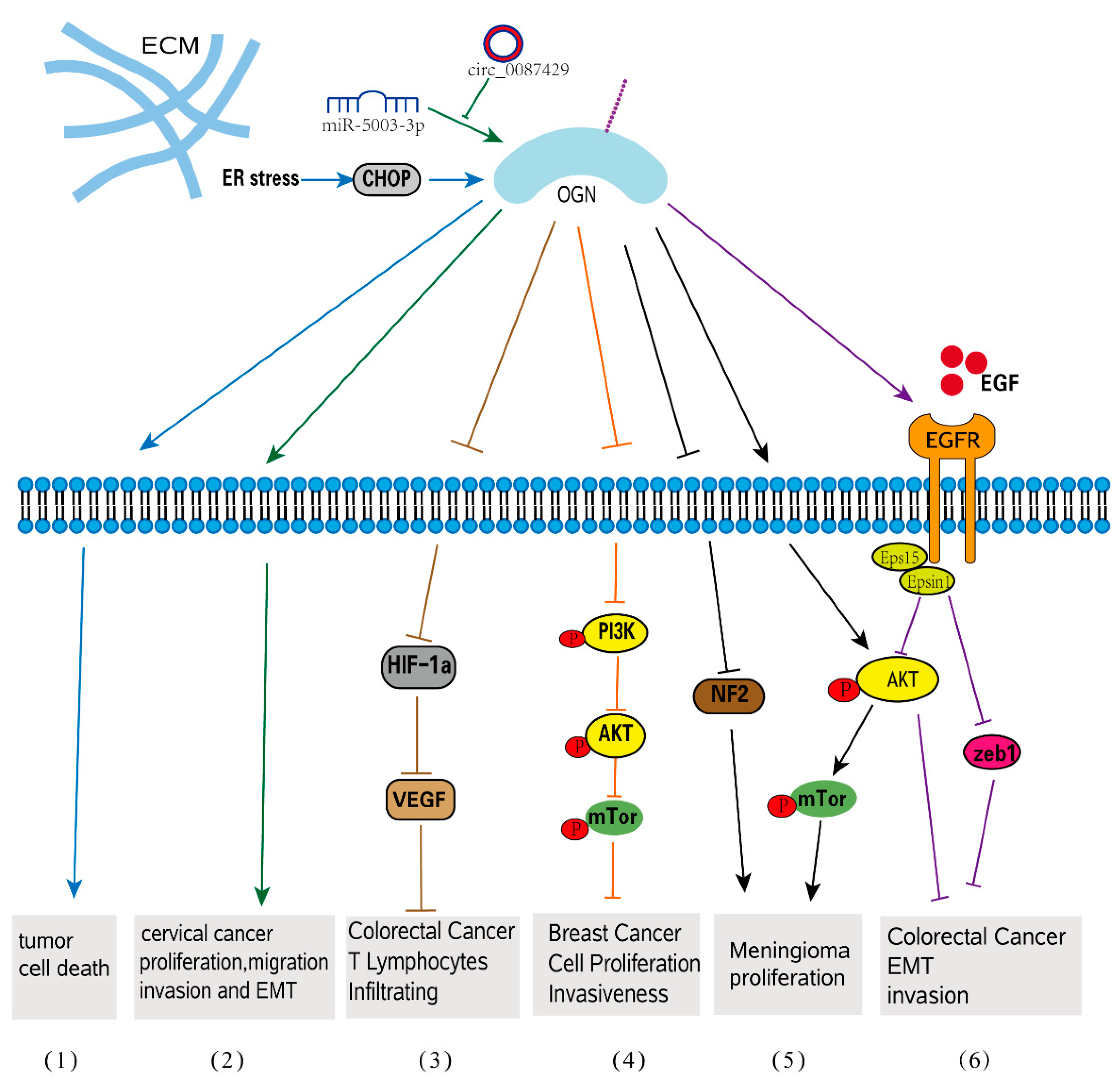
4. OGN Function in Tumorigenesis
4.1. OGN in Tumor Initiation
4.2. OGN in Celluar Progression
4.3. OGN in EMT-Related Tumorigenesis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef] [PubMed]
- Muncie, J.M.; Weaver, V.M. The Physical and Biochemical Properties of the Extracellular Matrix Regulate. Cell Fate. Curr. Top. Dev. Biol. 2018, 130, 1–37. [Google Scholar] [PubMed]
- Schaefer, L.; Dikic, I. Autophagy: Instructions from the extracellular matrix. Matrix Biol. 2021, 100–101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hocking, A.M.; Shinomura, T.; McQuillan, D.J. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998, 17, 1–19. [Google Scholar] [CrossRef]
- Kalamajski, S.; Oldberg, A. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010, 29, 248–253. [Google Scholar] [CrossRef]
- Jensen, M.M.; Karring, H. The origins and developments of sulfation-prone tyrosine-rich and acidic N- and C-terminal extensions of class ll and lll small leucine-rich repeat proteins shed light on connective tissue evolution in vertebrates. BMC Evol. Biol. 2020, 20, 73. [Google Scholar] [CrossRef]
- Pang, X.; Dong, N.; Zheng, Z. Small Leucine-Rich Proteoglycans in Skin Wound Healing. Front. Pharmacol. 2019, 10, 1649. [Google Scholar] [CrossRef]
- Colineau, L.; Laabei, M.; Liu, G.; Ermert, D.; Lambris, J.D.; Riesbeck, K.; Blom, A.M. Interaction of Streptococcus pyogenes with extracellular matrix components resulting in immunomodulation and bacterial eradication. Matrix Biol. Plus. 2020, 6–7, 100020. [Google Scholar] [CrossRef]
- Morrione, A.; Neill, T.; Iozzo, R.V. Dichotomy of decorin activity on the insulin-like growth factor-I system. FEBS J. 2013, 280, 2138–2149. [Google Scholar] [CrossRef]
- Santra, M.; Reed, C.C.; Iozzo, R.V. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J. Biol. Chem. 2002, 277, 35671–35681. [Google Scholar] [CrossRef]
- Hildebrand, A.; Romaris, M.; Rasmussen, L.M.; Heinegard, D.; Twardzik, D.R.; Border, W.A.; Ruoslahti, E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem. J. 1994, 302, 527–534. [Google Scholar] [CrossRef]
- Zou, W.; Wan, J.; Li, M.; Xing, J.; Chen, Q.; Zhang, Z.; Gong, Y. Small leucine rich proteoglycans in host immunity and renal diseases. J. Cell. Commun. Signal. 2019, 13, 463–471. [Google Scholar] [CrossRef]
- Zeng-Brouwers, J.; Pandey, S.; Trebicka, J.; Wygrecka, M.; Schaefer, L. Communications via the Small Leucine-rich Proteoglycans: Molecular Specificity in Inflammation and Autoimmune Diseases. J. Histochem. Cytochem. 2020, 68, 887–906. [Google Scholar] [CrossRef]
- Singla, S.; Hu, C.; Mizeracki, A.; Mehta, J.L. Decorin in atherosclerosis. Ther. Adv. Cardiovasc. Dis. 2011, 5, 305–314. [Google Scholar] [CrossRef]
- Appunni, S.; Anand, V.; Khandelwal, M.; Gupta, N.; Rubens, M.; Sharma, A. Small Leucine Rich Proteoglycans (decorin, biglycan and lumican) in cancer. Clin. Chim. Acta 2019, 491, 1–7. [Google Scholar] [CrossRef]
- Madisen, L.; Neubauer, M.; Plowman, G.; Rosen, D.; Segarini, P.; Dasch, J.; Thompson, A.; Ziman, J.; Bentz, H.; Purchio, A.F. Molecular cloning of a novel bone-forming compound: Osteoinductive factor. DNA Cell Biol. 1990, 9, 303–309. [Google Scholar] [CrossRef]
- Chen, S.; Birk, D.E. The regulatory roles of small leucine-rich proteoglycans in extracellular matrix assembly. FEBS J. 2013, 280, 2120–2137. [Google Scholar] [CrossRef]
- Tanaka, K.; Matsumoto, E.; Higashimaki, Y.; Katagiri, T.; Sugimoto, T.; Seino, S.; Kaji, H. Role of osteoglycin in the linkage between muscle and bone. J. Biol. Chem. 2012, 287, 11616–11628. [Google Scholar] [CrossRef]
- Tasheva, E.S.; Koester, A.; Paulsen, A.Q.; Garrett, A.S.; Boyle, D.L.; Davidson, H.J.; Song, M.; Fox, N.; Conrad, G.W. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol. Vis. 2002, 8, 407–415. [Google Scholar]
- Hu, X.; Li, Y.Q.; Li, Q.G.; Ma, Y.L.; Peng, J.J.; Cai, S.J. Osteoglycin-induced VEGF Inhibition Enhances T Lymphocytes Infiltrating in Colorectal Cancer. EBioMedicine 2018, 34, 35–45. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Cary, N.R.; Osbourn, J.K.; Weissberg, P.L. Identification of osteoglycin as a component of the vascular matrix. Differential expression by vascular smooth muscle cells during neointima formation and in atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Juchtmans, N.; Dhollander, A.A.; Coudenys, J.; Audenaert, E.A.; Pattyn, C.; Lambrecht, S.; Elewaut, D. Distinct dysregulation of the small leucine-rich repeat protein family in osteoarthritic acetabular labrum compared to articular cartilage. Arthritis. Rheumatol. 2015, 67, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D. Structure of the extracellular matrix in normal and fibrotic liver: Collagens and glycoproteins. Semin. Liver. Dis. 1990, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nastase, M.V.; Iozzo, R.V.; Schaefer, L. Key roles for the small leucine-rich proteoglycans in renal and pulmonary pathophysiology. Biochim. Biophys. Acta 2014, 1840, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, H. Overexpressed microRNA-140 inhibits pulmonary fibrosis in interstitial lung disease via the Wnt signaling pathway by downregulating osteoglycin. Am. J. Physiol. Cell Physiol. 2020, 319, C895–C905. [Google Scholar] [CrossRef]
- Beecher, N.; Carlson, C.; Allen, B.R.; Kipchumba, R.; Conrad, G.W.; Meek, K.M.; Quantock, A.J. An X-ray diffraction study of corneal structure in mimecan-deficient mice. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4046–4049. [Google Scholar] [CrossRef][Green Version]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef]
- Mohan, V.; Das, A.; Sagi, I. Emerging roles of ECM remodeling processes in cancer. Semin. Cancer Biol. 2020, 62, 192–200. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef]
- Aggelidakis, J.; Berdiaki, A.; Nikitovic, D.; Papoutsidakis, A.; Papachristou, D.J.; Tsatsakis, A.M.; Tzanakakis, G.N. Biglycan Regulates MG63 Osteosarcoma Cell Growth Through a LPR6/beta-Catenin/IGFR-IR Signaling Axis. Front. Oncol. 2018, 8, 470. [Google Scholar] [CrossRef]
- Papoutsidakis, A.; Giatagana, E.M.; Berdiaki, A.; Spyridaki, I.; Spandidos, D.A.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Lumican mediates HTB94 chondrosarcoma cell growth via an IGFIR/Erk1/2 axis. Int. J. Oncol. 2020, 57, 791–803. [Google Scholar] [CrossRef]
- Tasheva, E.S. Analysis of the promoter region of human mimecan gene. Biochim. Biophys. Acta 2002, 1575, 123–129. [Google Scholar] [CrossRef]
- Deckx, S.; Heymans, S.; Papageorgiou, A.P. The diverse functions of osteoglycin: A deceitful dwarf, or a master regulator of disease? FASEB J. 2016, 30, 2651–2661. [Google Scholar] [CrossRef]
- Schaefer, L.; Iozzo, R.V. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J. Biol. Chem. 2008, 283, 21305–21309. [Google Scholar] [CrossRef]
- Nastase, M.V.; Janicova, A.; Wygrecka, M.; Schaefer, L. Signaling at the Crossroads: Matrix-Derived Proteoglycan and Reactive Oxygen Species Signaling. Antioxid. Redox Signal. 2017, 27, 855–873. [Google Scholar] [CrossRef]
- Rienks, M.; Papageorgiou, A.P.; Frangogiannis, N.G.; Heymans, S. Myocardial extracellular matrix: An ever-changing and diverse entity. Circ. Res. 2014, 114, 872–888. [Google Scholar] [CrossRef]
- Iozzo, R.V. The family of the small leucine-rich proteoglycans: Key regulators of matrix assembly and cellular growth. Crit. Rev. Biochem. Mol. Biol. 1997, 32, 141–174. [Google Scholar] [CrossRef]
- Prydz, K.; Dalen, K.T. Synthesis and sorting of proteoglycans. J. Cell Sci. 2000, 113 Pt 2, 193–205. [Google Scholar] [CrossRef]
- Funderburgh, J.L.; Corpuz, L.M.; Roth, M.R.; Funderburgh, M.L.; Tasheva, E.S.; Conrad, G.W. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J. Biol. Chem. 1997, 272, 28089–28095. [Google Scholar] [CrossRef]
- Tasheva, E.S.; Corpuz, L.M.; Funderburgh, J.L.; Conrad, G.W. Differential splicing and alternative polyadenylation generate multiple mimecan mRNA transcripts. J. Biol. Chem. 1997, 272, 32551–32556. [Google Scholar] [CrossRef]
- Tasheva, E.S.; Maki, C.G.; Conrad, A.H.; Conrad, G.W. Transcriptional activation of bovine mimecan by p53 through an intronic DNA-binding site. Biochim. Biophys. Acta 2001, 1517, 333–338. [Google Scholar] [CrossRef]
- Tasheva, E.S.; Funderburgh, M.L.; McReynolds, J.; Funderburgh, J.L.; Conrad, G.W. The bovine mimecan gene. Molecular cloning and characterization of two major RNA transcripts generated by alternative use of two splice acceptor sites in the third exon. J. Biol. Chem. 1999, 274, 18693–18701. [Google Scholar] [CrossRef]
- Tasheva, E.S.; Pettenati, M.; Von Kap-Her, C.; Conrad, G.W. Assignment of mimecan gene (OGN) to human chromosome band 9q22 by in situ hybridization. Cytogenet. Cell Genet. 2000, 88, 326–327. [Google Scholar] [CrossRef]
- Hu, R.M.; Han, Z.G.; Song, H.D.; Peng, Y.D.; Huang, Q.H.; Ren, S.X.; Gu, Y.J.; Huang, C.H.; Li, Y.B.; Jiang, C.L.; et al. Gene expression profiling in the human hypothalamus-pituitary-adrenal axis and full-length cDNA cloning. Proc. Natl. Acad. Sci. USA 2000, 97, 9543–9548. [Google Scholar] [CrossRef]
- Tasheva, E.S.; Conrad, G.W. The UV responsive elements in the human mimecan promoter: A functional characterization. Mol. Vis. 2003, 9, 1–9. [Google Scholar]
- Sahar, T.; Nigam, A.; Anjum, S.; Waziri, F.; Jain, S.K.; Wajid, S. Differential expression of Lumican, Mimecan, Annexin A5 and Serotransferrin in ectopic and matched eutopic endometrium in ovarian endometriosis: A case-control study. Gynecol. Endocrinol. 2021, 37, 56–60. [Google Scholar] [CrossRef]
- Nikitovic, D.; Aggelidakis, J.; Young, M.F.; Iozzo, R.V.; Karamanos, N.K.; Tzanakakis, G.N. The biology of small leucine-rich proteoglycans in bone pathophysiology. J. Biol. Chem. 2012, 287, 33926–33933. [Google Scholar] [CrossRef]
- Fang, Y.; Chang, Z.; Xu, Z.; Hu, J.; Zhou, H.; Yu, S.; Wan, X. Osteoglycin silencing exerts inhibitory effects on myocardial fibrosis and epithelial/endothelial-mesenchymal transformation in a mouse model of myocarditis. Biofactors 2020, 46, 1018–1030. [Google Scholar] [CrossRef]
- Yang, M.; Hu, H.; Wu, S.; Ding, J.; Yin, B.; Huang, B.; Li, F.; Guo, X.; Han, L. EIF4A3-regulated circ_0087429 can reverse EMT and inhibit the progression of cervical cancer via miR-5003-3p-dependent upregulation of OGN expression. J. Exp. Clin. Cancer Res. 2022, 41, 165. [Google Scholar] [CrossRef]
- Seher, A.; Nickel, J.; Mueller, T.D.; Kneitz, S.; Gebhardt, S.; ter Vehn, T.M.; Schlunck, G.; Sebald, W. Gene expression profiling of connective tissue growth factor (CTGF) stimulated primary human tenon fibroblasts reveals an inflammatory and wound healing response in vitro. Mol. Vis. 2011, 17, 53–62. [Google Scholar]
- Li, X.; Massa, P.E.; Hanidu, A.; Peet, G.W.; Aro, P.; Savitt, A.; Mische, S.; Li, J.; Marcu, K.B. IKKalpha, IKKbeta, and NEMO/IKKgamma are each required for the NF-kappa B-mediated inflammatory response program. J. Biol. Chem. 2002, 277, 45129–45140. [Google Scholar] [CrossRef] [PubMed]
- Kurpakus Wheater, M.; Kernacki, K.A.; Hazlett, L.D. Corneal cell proteins and ocular surface pathology. Biotech. Histochem. 1999, 74, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.M.; Li, F.; Yu, H.M.; Li, R.Y.; Ma, Q.Y.; Ye, T.J.; Lu, Z.Y.; Chen, J.L.; Song, H.D. The mimecan gene expressed in human pituitary and regulated by pituitary transcription factor-1 as a marker for diagnosing pituitary tumors. J. Clin. Endocrinol. Metab. 2005, 90, 6657–6664. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Zuo, C.L.; Ma, J.H.; Zhang, X.N.; Ru, Y.; Li, P.; Pan, C.M.; Liu, Z.; Cao, H.M.; Chen, M.D.; et al. Glucocorticoid up-regulates mimecan expression in corticotroph cells. Mol. Cell. Endocrinol. 2010, 321, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.Y.; Zhang, X.N.; Jiang, H.; Wang, Z.Q.; Zhang, H.J.; Xue, L.Q.; Chen, M.D.; Song, H.D. Mimecan in pituitary corticotroph cells may regulate ACTH secretion and the HPAA. Mol. Cell Endocrinol. 2011, 341, 71–77. [Google Scholar] [CrossRef]
- Cao, H.M.; Ye, X.P.; Ma, J.H.; Jiang, H.; Li, S.X.; Li, R.Y.; Li, X.S.; Guo, C.C.; Wang, Z.Q.; Zhan, M.; et al. Mimecan, a Hormone Abundantly Expressed in Adipose Tissue, Reduced Food Intake Independently of Leptin Signaling. EBioMedicine 2015, 2, 1718–1724. [Google Scholar] [CrossRef]
- Rienks, M.; Papageorgiou, A.; Wouters, K.; Verhesen, W.; Leeuwen, R.V.; Carai, P.; Summer, G.; Westermann, D.; Heymans, S. A novel 72-kDa leukocyte-derived osteoglycin enhances the activation of toll-like receptor 4 and exacerbates cardiac inflammation during viral myocarditis. Cell. Mol. Life Sci. 2017, 74, 1511–1525. [Google Scholar] [CrossRef]
- Kampmann, A.; Fernandez, B.; Deindl, E.; Kubin, T.; Pipp, F.; Eitenmuller, I.; Hoefer, I.E.; Schaper, W.; Zimmermann, R. The proteoglycan osteoglycin/mimecan is correlated with arteriogenesis. Mol. Cell Biochem. 2009, 322, 15–23. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, J.; Liu, H.F.; Zhang, X.N.; Zhan, M.; Chen, F.L. Overexpression of mimecan in human aortic smooth muscle cells inhibits cell proliferation and enhances apoptosis and migration. Exp. Ther. Med. 2015, 10, 187–192. [Google Scholar] [CrossRef][Green Version]
- Ma, Z.G.; Yuan, Y.P.; Wu, H.M.; Zhang, X.; Tang, Q.Z. Cardiac fibrosis: New insights into the pathogenesis. Int. J. Biol. Sci. 2018, 14, 1645–1657. [Google Scholar] [CrossRef]
- Jellis, C.; Martin, J.; Narula, J.; Marwick, T.H. Assessment of nonischemic myocardial fibrosis. J. Am. Coll. Cardiol. 2010, 56, 89–97. [Google Scholar] [CrossRef]
- Zuo, Z.; Li, M.H.; Zheng, X.H.; Yao, W.M.; Wang, H.; Li, X.L. Elevated plasma levels of osteoglycin in cardiovascular patients: A systematic review and meta-analysis. Ann. Palliat. Med. 2022, 11, 498–505. [Google Scholar] [CrossRef]
- Petretto, E.; Sarwar, R.; Grieve, I.; Lu, H.; Kumaran, M.K.; Muckett, P.J.; Mangion, J.; Schroen, B.; Benson, M.; Punjabi, P.P.; et al. Integrated genomic approaches implicate osteoglycin (Ogn) in the regulation of left ventricular mass. Nat. Genet. 2008, 40, 546–552. [Google Scholar] [CrossRef]
- Van Aelst, L.N.; Voss, S.; Carai, P.; Van Leeuwen, R.; Vanhoutte, D.; Sanders-van Wijk, S.; Eurlings, L.; Swinnen, M.; Verheyen, F.K.; Verbeken, E.; et al. Osteoglycin prevents cardiac dilatation and dysfunction after myocardial infarction through infarct collagen strengthening. Circ. Res. 2015, 116, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Jazbutyte, V.; Fiedler, J.; Kneitz, S.; Galuppo, P.; Just, A.; Holzmann, A.; Bauersachs, J.; Thum, T. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age 2013, 35, 747–762. [Google Scholar] [CrossRef]
- Leask, A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. Res. 2010, 106, 1675–1680. [Google Scholar] [CrossRef]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Zuo, C.; Li, X.; Huang, J.; Chen, D.; Ji, K.; Yang, Y.; Xu, T.; Zhu, D.; Yan, C.; Gao, P. Osteoglycin attenuates cardiac fibrosis by suppressing cardiac myofibroblast proliferation and migration through antagonizing lysophosphatidic acid 3/matrix metalloproteinase 2/epidermal growth factor receptor signalling. Cardiovasc. Res. 2018, 114, 703–712. [Google Scholar] [CrossRef]
- Blyszczuk, P.; Muller-Edenborn, B.; Valenta, T.; Osto, E.; Stellato, M.; Behnke, S.; Glatz, K.; Basler, K.; Luscher, T.F.; Distler, O.; et al. Transforming growth factor-beta-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur. Heart J. 2017, 38, 1413–1425. [Google Scholar]
- Shin, D.H.; Park, H.M.; Jung, K.A.; Choi, H.G.; Kim, J.A.; Kim, D.D.; Kim, S.G.; Kang, K.W.; Ku, S.K.; Kensler, T.W.; et al. The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic. Biol. Med. 2010, 48, 1051–1063. [Google Scholar] [CrossRef]
- Linke, F.; Zaunig, S.; Nietert, M.M.; von Bonin, F.; Lutz, S.; Dullin, C.; Janovska, P.; Beissbarth, T.; Alves, F.; Klapper, W.; et al. WNT5A: A motility-promoting factor in Hodgkin lymphoma. Oncogene 2017, 36, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bianco, P.; Fisher, L.W.; Young, M.F.; Termine, J.D.; Robey, P.G. Expression and localization of the two small proteoglycans biglycan and decorin in developing human skeletal and non-skeletal tissues. J. Histochem. Cytochem. 1990, 38, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Hausser, H.; Altenburger, M.; Ugorcakova, J.; August, C.; Fisher, L.W.; Schaefer, R.M.; Kresse, H. Decorin, biglycan and their endocytosis receptor in rat renal cortex. Kidney Int. 1998, 54, 1529–1541. [Google Scholar] [CrossRef]
- Stokes, M.B.; Hudkins, K.L.; Zaharia, V.; Taneda, S.; Alpers, C.E. Up-regulation of extracellular matrix proteoglycans and collagen type I in human crescentic glomerulonephritis. Kidney Int. 2001, 59, 532–542. [Google Scholar] [CrossRef]
- Mogyorosi, A.; Ziyadeh, F.N. What is the role of decorin in diabetic kidney disease? Nephrol. Dial. Transplant. 1999, 14, 1078–1081. [Google Scholar] [CrossRef]
- Suzuki, K.; Wang, R.; Kubota, H.; Shibuya, H.; Saegusa, J.; Sato, T. Kinetics of biglycan, decorin and thrombospondin-1 in mercuric chloride-induced renal tubulointerstitial fibrosis. Exp. Mol. Pathol. 2005, 79, 68–73. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, B.Y.; Li, X.L.; Wang, Y.J.; Zhang, Z.; Pei, F.; Wang, Q.Z.; Zhang, J.; Cai, Y.W.; Cheng, M.; et al. Restoration of Mimecan Expression by Grape Seed Procyanidin B2 Through Regulation of Nuclear Factor-kappaB in Mice with Diabetic Nephropathy. Iran. J. Kidney Dis. 2016, 10, 325–331. [Google Scholar]
- Afratis, N.A.; Bouris, P.; Skandalis, S.S.; Multhaupt, H.A.; Couchman, J.R.; Theocharis, A.D.; Karamanos, N.K. IGF-IR cooperates with ERalpha to inhibit breast cancer cell aggressiveness by regulating the expression and localisation of ECM molecules. Sci. Rep. 2017, 7, 40138. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The Concise Guide to Pharmacology 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 2019, 176 (Suppl. S1), S21–S141. [Google Scholar] [CrossRef]
- Lomnytska, M.I.; Becker, S.; Hellman, K.; Hellstrom, A.C.; Souchelnytskyi, S.; Mints, M.; Hellman, U.; Andersson, S.; Auer, G. Diagnostic protein marker patterns in squamous cervical cancer. Proteomics. Clin. Appl. 2010, 4, 17–31. [Google Scholar] [CrossRef]
- Lee, J.Y.; Eom, E.M.; Kim, D.S.; Ha-Lee, Y.M.; Lee, D.H. Analysis of gene expression profiles of gastric normal and cancer tissues by SAGE. Genomics 2003, 82, 78–85. [Google Scholar] [CrossRef]
- Rower, C.; Ziems, B.; Radtke, A.; Schmitt, O.; Reimer, T.; Koy, C.; Thiesen, H.J.; Gerber, B.; Glocker, M.O. Toponostics of invasive ductal breast carcinoma: Combination of spatial protein expression imaging and quantitative proteome signature analysis. Int. J. Clin. Exp. Pathol. 2011, 4, 454–467. [Google Scholar]
- Li, L.; Zhang, Z.; Wang, C.; Miao, L.; Zhang, J.; Wang, J.; Jiao, B.; Zhao, S. Quantitative proteomics approach to screening of potential diagnostic and therapeutic targets for laryngeal carcinoma. PLoS ONE 2014, 9, e90181. [Google Scholar] [CrossRef]
- Sponziello, M.; Lavarone, E.; Pegolo, E.; Di Loreto, C.; Puppin, C.; Russo, M.A.; Bruno, R.; Filetti, S.; Durante, C.; Russo, D.; et al. Molecular differences between human thyroid follicular adenoma and carcinoma revealed by analysis of a murine model of thyroid cancer. Endocrinology 2013, 154, 3043–3053. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Yang, L.; Sun, W. Elevated OGN expression correlates with the EMT signature and poor prognosis in ovarian carcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 584–589. [Google Scholar]
- Hu, X.; Li, Y.Q.; Li, Q.G.; Ma, Y.L.; Peng, J.J.; Cai, S.J. Osteoglycin (OGN) reverses epithelial to mesenchymal transition and invasiveness in colorectal cancer via EGFR/Akt pathway. J. Exp. Clin. Cancer Res. 2018, 37, 41. [Google Scholar] [CrossRef]
- Mei, Y.; Du, Z.; Hu, C.; Greenwald, N.F.; Abedalthagafi, M.; Agar, N.Y.R.; Dunn, P.G.; Bi, W.L.; Santagata, S.; Dunn, I.F. Osteoglycin promotes meningioma development through downregulation of NF2 and activation of mTOR signaling. Cell Commun. Signal. 2017, 15, 34. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Lu, B.; Xu, E.; Huang, Q.; Lai, M. Differential expression of mimecan and thioredoxin domain-containing protein 5 in colorectal adenoma and cancer: A proteomic study. Exp. Biol. Med. 2007, 232, 1152–1159. [Google Scholar] [CrossRef]
- Wassermann-Dozorets, R.; Rubinstein, M. C/EBPbeta LIP augments cell death by inducing osteoglycin. Cell Death Dis. 2017, 8, e2733. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, R.; Dong, M.; Zhang, Z.; Li, H.; Zhan, C.; Li, X. Osteoglycin (OGN) Inhibits Cell Proliferation and Invasiveness in Breast Cancer via PI3K/Akt/mTOR Signaling Pathway. Onco. Targets Ther. 2019, 12, 10639–10650. [Google Scholar] [CrossRef]
- Lim, W.; An, Y.; Yang, C.; Bazer, F.W.; Song, G. Chrysophanol induces cell death and inhibits invasiveness via mitochondrial calcium overload in ovarian cancer cells. J. Cell Biochem. 2018, 119, 10216–10227. [Google Scholar] [CrossRef]
- Ren, L.; Li, Z.; Dai, C.; Zhao, D.; Wang, Y.; Ma, C.; Liu, C. Chrysophanol inhibits proliferation and induces apoptosis through NF-kappaB/cyclin D1 and NF-kappaB/Bcl-2 signaling cascade in breast cancer cell lines. Mol. Med. Rep. 2018, 17, 4376–4382. [Google Scholar]
- Yao, Y.; Yan, Z.; Lian, S.; Wei, L.; Zhou, C.; Feng, D.; Zhang, Y.; Yang, J.; Li, M.; Chen, Y. Prognostic value of novel immune-related genomic biomarkers identified in head and neck squamous cell carcinoma. J. Immunother. Cancer 2020, 8, e000444. [Google Scholar] [CrossRef]
- Ren, Q.; Khoo, W.H.; Corr, A.P.; Phan, T.G.; Croucher, P.I.; Stewart, S.A. Gene expression predicts dormant metastatic breast cancer cell phenotype. Breast. Cancer Res. 2022, 24, 10. [Google Scholar] [CrossRef]
- Yu, X.; Yu, B.; Fang, W.; Xiong, J.; Ma, M. Identification hub genes of consensus molecular subtype correlation with immune infiltration and predict prognosis in gastric cancer. Discov. Oncol. 2021, 12, 41. [Google Scholar] [CrossRef]
- Ren, H.; Liu, X.; Li, F.; He, X.; Zhao, N. Identification of a Six Gene Prognosis Signature for Papillary Thyroid Cancer Using Multi-Omics Methods and Bioinformatics Analysis. Front. Oncol. 2021, 11, 624421. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T. To differentiate or not—Routes towards metastasis. Nat. Rev. Cancer 2012, 12, 425–436. [Google Scholar] [CrossRef]
- Grunwald, V.; Hidalgo, M. Developing inhibitors of the epidermal growth factor receptor for cancer treatment. J. Natl. Cancer Inst. 2003, 95, 851–867. [Google Scholar] [CrossRef]
- Wu, D.M.; Zhang, T.; Liu, Y.B.; Deng, S.H.; Han, R.; Liu, T.; Li, J.; Xu, Y. The PAX6-ZEB2 axis promotes metastasis and cisplatin resistance in non-small cell lung cancer through PI3K/AKT signaling. Cell Death Dis. 2019, 10, 349. [Google Scholar] [CrossRef]
- Yan, S.P.; Chu, D.X.; Qiu, H.F.; Xie, Y.; Wang, C.F.; Zhang, J.Y.; Li, W.C.; Guo, R.X. LncRNA LINC01305 silencing inhibits cell epithelial-mesenchymal transition in cervical cancer by inhibiting TNXB-mediated PI3K/Akt signalling pathway. J. Cell Mol. Med. 2019, 23, 2656–2666. [Google Scholar] [CrossRef]
- Pearlman, R.L.; Montes de Oca, M.K.; Pal, H.C.; Afaq, F. Potential therapeutic targets of epithelial-mesenchymal transition in melanoma. Cancer Lett. 2017, 391, 125–140. [Google Scholar] [CrossRef]
- Cui, X.; Song, B.; Hou, L.; Wei, Z.; Tang, J. High expression of osteoglycin decreases the metastatic capability of mouse hepatocarcinoma Hca-F cells to lymph nodes. Acta Biochim. Biophys Sin. 2008, 40, 349–355. [Google Scholar] [CrossRef][Green Version]
- Deckx, S.; Heggermont, W.; Carai, P.; Rienks, M.; Dresselaers, T.; Himmelreich, U.; van Leeuwen, R.; Lommen, W.; van der Velden, J.; Gonzalez, A.; et al. Osteoglycin prevents the development of age-related diastolic dysfunction during pressure overload by reducing cardiac fibrosis and inflammation. Matrix Biol. 2018, 66, 110–124. [Google Scholar] [CrossRef]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef]

| Cancer Type | Expression (Protein/mRNA) | Level of Expression | Role | Function in Cancer | Ref. |
|---|---|---|---|---|---|
| Colon cancer | Protein | Downregulated | Tumor suppressor | Suppressing invasion, migration, and EMT and prolonging OS | [86] |
| Colon cancer | Protein | Downregulated | Tumor suppressor | Enhancing T iymphocytes infiltrating | [20] |
| Breast Cancer | mRNA/Protein | Downregulated | Tumor suppressor | Inhibiting cell proliferation, invasiveness, and EMT and prolonging OS | [90] |
| Liver cancer | Protein | Downregulated | Tumor suppressor | Decreasing metastatic potential to peripheral lymph nodes and prolonging OS | [90] |
| Cervical cancer | mRNA/Protein | Downregulated | Tumor suppressor | Suppressing proliferation, migration, and invasion and reversing EMT | [49] |
| Ovarian cancer | mRNA/Protein | Upregulated | Oncogene | Being associated with EMT and poor prognosis | [85] |
| Meningioma | mRNA/Protein | Upregulated | Oncogene | Accelerating proliferation | [87] |
| Melanoma | mRNA/Protein | Upregulated | Oncogene | Increasing cell death | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nulali, J.; Zhan, M.; Zhang, K.; Tu, P.; Liu, Y.; Song, H. Osteoglycin: An ECM Factor Regulating Fibrosis and Tumorigenesis. Biomolecules 2022, 12, 1674. https://doi.org/10.3390/biom12111674
Nulali J, Zhan M, Zhang K, Tu P, Liu Y, Song H. Osteoglycin: An ECM Factor Regulating Fibrosis and Tumorigenesis. Biomolecules. 2022; 12(11):1674. https://doi.org/10.3390/biom12111674
Chicago/Turabian StyleNulali, Jiayida, Ming Zhan, Kaiwen Zhang, Pinghui Tu, Yu Liu, and Huaidong Song. 2022. "Osteoglycin: An ECM Factor Regulating Fibrosis and Tumorigenesis" Biomolecules 12, no. 11: 1674. https://doi.org/10.3390/biom12111674
APA StyleNulali, J., Zhan, M., Zhang, K., Tu, P., Liu, Y., & Song, H. (2022). Osteoglycin: An ECM Factor Regulating Fibrosis and Tumorigenesis. Biomolecules, 12(11), 1674. https://doi.org/10.3390/biom12111674






