Zinc’s Association with the CmPn/CmP Signaling Network in Breast Cancer Tumorigenesis
Abstract
1. Introduction
2. Cellular Zinc Level Is Influenced by Dietary Supplementation
2.1. Cellular Zinc Concentrations Depend on Dietary Consumption
2.2. Zinc Cellular Specific Actions
3. Zinc Plays a Significant Role in Tumorigenesis
3.1. Function of Zinc Contributes to the Progression of Cell Tumorigenesis
3.2. Zinc Transport Protein in Breast Cancer Cells
4. Zinc and the CmPn/CmP Signaling Network
4.1. Zinc Is a Critical Nutrient in Mammalian Female Reproductive System
4.2. Zinc Supplementation for Cancer Prevention
4.3. Zinc Is an Essential Nutrient in PRG Biogenesis and PRG-Mediated Signaling
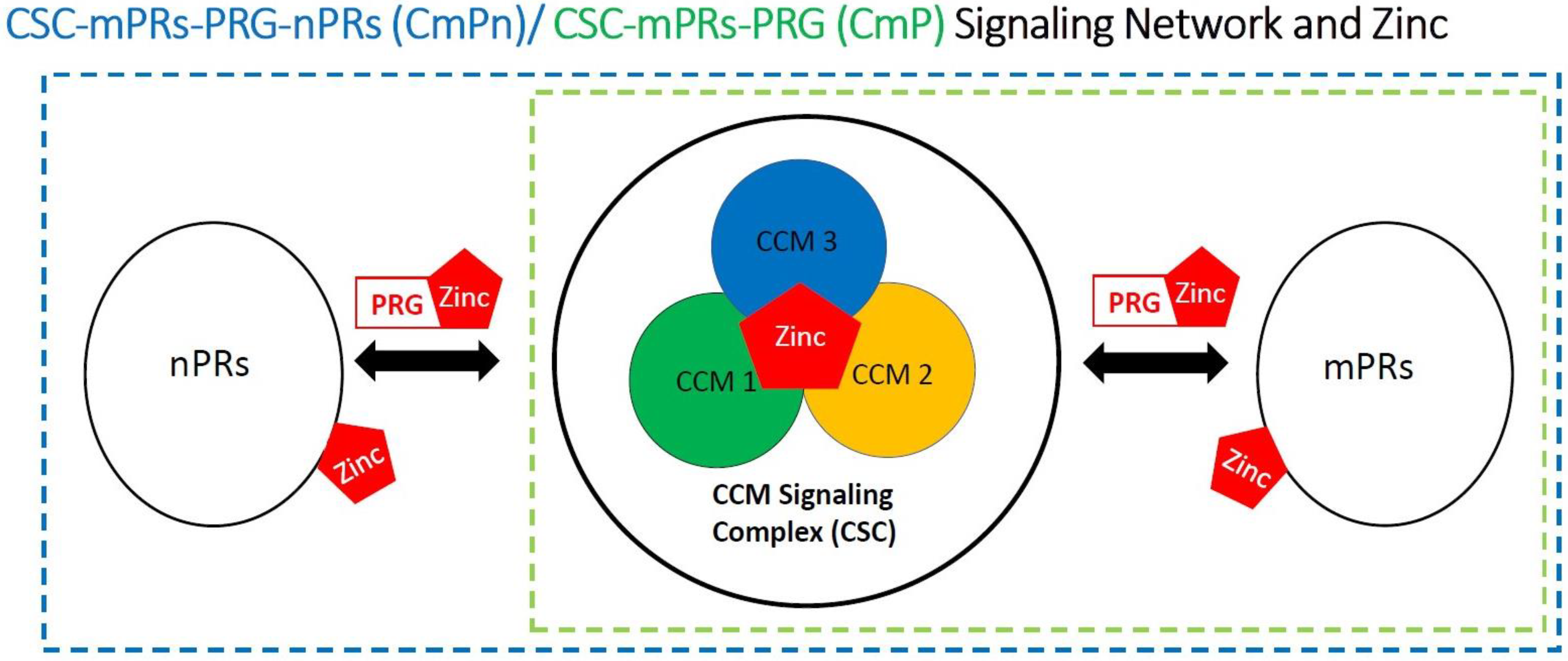
4.4. Zinc Associated with the CSC
4.5. Involvement of Zinc within the CmPn/CmP Signaling Network
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Baquet, C.R.; Mishra, S.I.; Commiskey, P.; Ellison, G.L.; DeShields, M. Breast cancer epidemiology in blacks and whites: Disparities in incidence, mortality, survival rates and histology. J. Natl. Med. Assoc. 2008, 100, 480–488. [Google Scholar] [CrossRef]
- Venturelli, S.; Leischner, C.; Helling, T.; Renner, O.; Burkard, M.; Marongiu, L. Minerals and Cancer: Overview of the Possible Diagnostic Value. Cancers 2022, 14, 1256. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Angulo, A.M.; Morales-Vasquez, F.; Hortobagyi, G.N. Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. 2007, 608, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.M.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J. Natl. Cancer Inst. 2015, 107, djv048. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Vail, G.; Roepke, T.A. Membrane-initiated estrogen signaling via Gq-coupled GPCR in the central nervous system. Steroids 2019, 142, 77–83. [Google Scholar] [CrossRef]
- Azeez, J.M.; Susmi, T.R.; Remadevi, V.; Ravindran, V.; Sasikumar Sujatha, A.; Ayswarya, R.N.S.; Sreeja, S. New insights into the functions of progesterone receptor (PR) isoforms and progesterone signaling. Am. J. Cancer Res. 2021, 11, 5214–5232. [Google Scholar]
- Thomas, P. Membrane Progesterone Receptors (mPRs, PAQRs): Review of Structural and Signaling Characteristics. Cells 2022, 11, 1785. [Google Scholar] [CrossRef]
- Carey, L.A.; Perou, C.M.; Livasy, C.A.; Dressler, L.G.; Cowan, D.; Conway, K.; Karaca, G.; Troester, M.A.; Tse, C.K.; Edmiston, S.; et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006, 295, 2492–2502. [Google Scholar] [CrossRef]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thurlimann, B.; Senn, H.J.; Panel, M. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Vogel-Gonzalez, M.; Musa-Afaneh, D.; Rivera Gil, P.; Vicente, R. Zinc Favors Triple-Negative Breast Cancer’s Microenvironment Modulation and Cell Plasticity. Int. J. Mol. Sci. 2021, 22, 9188. [Google Scholar] [CrossRef]
- Franklin, R.B.; Costello, L.C. The Important Role of the Apoptotic Effects of Zinc in the Development of Cancers. J. Cell Biochem. 2009, 106, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Grattan, B.J.; Freake, H.C. Zinc and Cancer: Implications for LIV-1 in Breast Cancer. Nutrients 2012, 4, 648–675. [Google Scholar] [CrossRef] [PubMed]
- McCall, K.A.; Huang, C.; Fierke, C.A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef]
- Taccioli, C.; Chen, H.; Jiang, Y.; Liu, X.P.; Huang, K.; Smalley, K.J.; Farber, J.L.; Croce, C.M.; Fong, L.Y. Dietary zinc deficiency fuels esophageal cancer development by inducing a distinct inflammatory signature. Oncogene 2012, 31, 4550–4558. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, H. Aberrance of Zinc Metalloenzymes-Induced Human Diseases and Its Potential Mechanisms. Nutrients 2021, 13, 4456. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Xu, Z.; Cheng, X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol. Med. 2020, 17, 612–625. [Google Scholar] [CrossRef]
- Woo, W.; Xu, Z.M. Body zinc distribution profile during N-methyl-N-nitrosourea-induced mammary tumorigenesis in rats at various levels of dietary zinc intake. Biol. Trace Elem Res. 2002, 87, 157–169. [Google Scholar] [PubMed]
- Chakraborty, M.; Hershfinkel, M. Zinc Signaling in the Mammary Gland: For Better and for Worse. Biomedicines 2021, 9, 1204. [Google Scholar] [CrossRef] [PubMed]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef]
- Fong, L.Y.Y.; Nguyen, V.T.; Farber, J.L. Esophageal cancer prevention in zinc-deficient rats: Rapid induction of apoptosis by replenishing zinc. J. Natl. Cancer Inst. 2001, 93, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.Y.Y.; Jiang, Y.; Riley, M.; Liu, X.; Smalley, K.J.; Guttridge, D.C.; Farber, J.L. Prevention of upper aerodigestive tract cancer in zinc-deficient rodents: Inefficacy of genetic or pharmacological disruption of COX-2. Int. J. Cancer 2008, 122, 978–989. [Google Scholar] [CrossRef]
- Alam, S.; Kelleher, S.L. Cellular mechanisms of zinc dysregulation: A perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients 2012, 4, 875–903. [Google Scholar] [CrossRef]
- Yildiz, A.; Kaya, Y.; Tanriverdi, O. Effect of the Interaction Between Selenium and Zinc on DNA Repair in Association With Cancer Prevention. J. Cancer Prev. 2019, 24, 146–154. [Google Scholar] [CrossRef]
- Maret, W. Regulation of Cellular Zinc Ions and Their Signaling Functions. In Zinc Signaling; Fukada, T., Kambe, T., Eds.; Springer: Singapore, 2019; pp. 5–22. [Google Scholar]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef]
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics 2010, 2, 306–317. [Google Scholar] [CrossRef]
- Lonergan, Z.R.; Skaar, E.P. Nutrient Zinc at the Host-Pathogen Interface. Trends Biochem. Sci. 2019, 44, 1041–1056. [Google Scholar] [CrossRef]
- Chandler, P.; Kochupurakkal, B.S.; Alam, S.; Richardson, A.L.; Soybel, D.I.; Kelleher, S.L. Subtype-specific accumulation of intracellular zinc pools is associated with the malignant phenotype in breast cancer. Mol. Cancer 2016, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Margalioth, E.J.; Schenker, J.G.; Chevion, M. Copper and zinc levels in normal and malignant tissues. Cancer 1983, 52, 868–872. [Google Scholar] [CrossRef]
- Feng, Y.; Zeng, J.W.; Ma, Q.; Zhang, S.; Tang, J.; Feng, J.F. Serum copper and zinc levels in breast cancer: A meta-analysis. J. Trace Elem. Med. Biol. 2020, 62, 126629. [Google Scholar] [CrossRef] [PubMed]
- Riesop, D.; Hirner, A.V.; Rusch, P.; Bankfalvi, A. Zinc distribution within breast cancer tissue: A possible marker for histological grading? J. Cancer Res. Clin. Oncol. 2015, 141, 1321–1331. [Google Scholar] [CrossRef]
- Yamasaki, S.; Sakata-Sogawa, K.; Hasegawa, A.; Suzuki, T.; Kabu, K.; Sato, E.; Kurosaki, T.; Yamashita, S.; Tokunaga, M.; Nishida, K.; et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 2007, 177, 637–645. [Google Scholar] [CrossRef]
- Nagamatsu, S.; Nishito, Y.; Yuasa, H.; Yamamoto, N.; Komori, T.; Suzuki, T.; Yasui, H.; Kambe, T. Sophisticated expression responses of ZNT1 and MT in response to changes in the expression of ZIPs. Sci. Rep. 2022, 12, 7334. [Google Scholar] [CrossRef]
- Eide, D. Molecular biology of iron and zinc uptake in eukaryotes. Curr. Opin. Cell Biol. 1997, 9, 573–577. [Google Scholar] [CrossRef]
- Guerinot, M.L. The ZIP family of metal transporters. Biochim. Biophys. Acta 2000, 1465, 190–198. [Google Scholar] [CrossRef]
- Cuajungco, M.P.; Ramirez, M.S.; Tolmasky, M.E. Zinc: Multidimensional Effects on Living Organisms. Biomedicines 2021, 9, 208. [Google Scholar] [CrossRef]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-function analysis of HKE4, a member of the new LIV-1 subfamily of zinc transporters. Biochem. J. 2004, 377, 131–139. [Google Scholar] [CrossRef]
- Taylor, K.M.; Morgan, H.E.; Smart, K.; Zahari, N.M.; Pumford, S.; Ellis, I.O.; Robertson, J.F.; Nicholson, R.I. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol. Med. 2007, 13, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M. A distinct role in breast cancer for two LIV-1 family zinc transporters. Biochem. Soc. Trans 2008, 36, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Matsui, C.; Takatani-Nakase, T.; Hatano, Y.; Kawahara, S.; Nakase, I.; Takahashi, K. Zinc and its transporter ZIP6 are key mediators of breast cancer cell survival under high glucose conditions. FEBS Lett. 2017, 591, 3348–3359. [Google Scholar] [CrossRef] [PubMed]
- Hogstrand, C.; Kille, P.; Nicholson, R.I.; Taylor, K.M. Zinc transporters and cancer: A potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med. 2009, 15, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kasper, G.; Weiser, A.A.; Rump, A.; Sparbier, K.; Dahl, E.; Hartmann, A.; Wild, P.; Schwidetzky, U.; Castanos-Velez, E.; Lehmann, K. Expression levels of the putative zinc transporter LIV-1 are associated with a better outcome of breast cancer patients. Int. J. Cancer 2005, 117, 961–973. [Google Scholar] [CrossRef]
- Jones, S.; Farr, G.; Nimmanon, T.; Ziliotto, S.; Gee, J.M.W.; Taylor, K.M. The importance of targeting signalling mechanisms of the SLC39A family of zinc transporters to inhibit endocrine resistant breast cancer. Explor. Target Antitumor Ther. 2022, 3, 224–239. [Google Scholar] [CrossRef]
- Lisle, R.S.; Anthony, K.; Randall, M.A.; Diaz, F.J. Oocyte-cumulus cell interactions regulate free intracellular zinc in mouse oocytes. Reproduction 2013, 145, 381–390. [Google Scholar] [CrossRef][Green Version]
- Sharir, H.; Zinger, A.; Nevo, A.; Sekler, I.; Hershfinkel, M. Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J. Biol. Chem. 2010, 285, 26097–26106. [Google Scholar] [CrossRef]
- Hershfinkel, M. The Zinc Sensing Receptor, ZnR/GPR39, in Health and Disease. Int. J. Mol. Sci. 2018, 19, 439. [Google Scholar] [CrossRef]
- Ventura-Bixenshpaner, H.; Asraf, H.; Chakraborty, M.; Elkabets, M.; Sekler, I.; Taylor, K.M.; Hershfinkel, M. Enhanced ZnR/GPR39 Activity in Breast Cancer, an Alternative Trigger of Signaling Leading to Cell Growth. Sci. Rep. 2018, 8, 8119. [Google Scholar] [CrossRef]
- Mero, M.; Asraf, H.; Sekler, I.; Taylor, K.M.; Hershfinkel, M. ZnR/GPR39 upregulation of K(+)/Cl(-)-cotransporter 3 in tamoxifen resistant breast cancer cells. Cell Calcium 2019, 81, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.M.; Vogt, S.; O’Halloran, T.V.; Woodruff, T.K. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 2010, 6, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, M.L.; Kong, B.Y.; Kim, A.M.; O’Halloran, T.V.; Woodruff, T.K. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol. Reprod. 2012, 86, 114. [Google Scholar] [CrossRef] [PubMed]
- Que, E.L.; Duncan, F.E.; Bayer, A.R.; Philips, S.J.; Roth, E.W.; Bleher, R.; Gleber, S.C.; Vogt, S.; Woodruff, T.K.; O’Halloran, T.V. Zinc sparks induce physiochemical changes in the egg zona pellucida that prevent polyspermy. Integr. Biol. 2017, 9, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Diaz, F.J. Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology 2012, 153, 873–886. [Google Scholar] [CrossRef]
- Bedwal, R.S.; Bahuguna, A. Zinc, copper and selenium in reproduction. Experientia 1994, 50, 626–640. [Google Scholar] [CrossRef]
- Sandstead, H.H.; Fosmire, G.J.; McKenzie, J.M.; Halas, E.S. Zinc deficiency and brain development in the rat. Fed. Proc. 1975, 34, 86–88. [Google Scholar]
- Keen, C.L.; Hurley, L.S. Effects of zinc deficiency on prenatal and postnatal development. Neurotoxicology 1987, 8, 379–387. [Google Scholar]
- Uriu-Adams, J.Y.; Keen, C.L. Zinc and reproduction: Effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Res. B Dev. Reprod. Toxicol. 2010, 89, 313–325. [Google Scholar] [CrossRef]
- Aliarabi, H.; Fadayifar, A.; Alimohamady, R.; Dezfoulian, A.H. The Effect of Maternal Supplementation of Zinc, Selenium, and Cobalt as Slow-Release Ruminal Bolus in Late Pregnancy on Some Blood Metabolites and Performance of Ewes and Their Lambs. Biol. Trace Elem. Res. 2019, 187, 403–410. [Google Scholar] [CrossRef]
- Tamura, T.; Goldenberg, R.L.; Johnston, K.E.; DuBard, M. Maternal plasma zinc concentrations and pregnancy outcome. Am. J. Clin. Nutr. 2000, 71, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Grober, U.; Holzhauer, P.; Kisters, K.; Holick, M.F.; Adamietz, I.A. Micronutrients in Oncological Intervention. Nutrients 2016, 8, 163. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, K.; Cymbaluk-Ploska, A. The Role of Zinc and Copper in Gynecological Malignancies. Nutrients 2020, 12, 3732. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, G.; Shi, H.; Zhao, X.; Wang, B.; Dong, P.; Watari, H.; Pfeffer, L.M.; Yue, J. Zinc regulates primary ovarian tumor growth and metastasis through the epithelial to mesenchymal transition. Free Radic. Biol. Med. 2020, 160, 775–783. [Google Scholar] [CrossRef]
- Huang, H.Y.; Caballero, B.; Chang, S.; Alberg, A.J.; Semba, R.D.; Schneyer, C.R.; Wilson, R.F.; Cheng, T.Y.; Vassy, J.; Prokopowicz, G.; et al. The efficacy and safety of multivitamin and mineral supplement use to prevent cancer and chronic disease in adults: A systematic review for a National Institutes of Health state-of-the-science conference. Ann. Intern. Med. 2006, 145, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M. Nutritional supplements and cancer: Potential benefits and proven harms. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, e478–e486. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Ullah, H.; Santarcangelo, C.; Di Minno, A.; Khan, H.; Daglia, M.; Arciola, C.R. Micronutrient Food Supplements in Patients with Gastro-Intestinal and Hepatic Cancers. Int. J. Mol. Sci. 2021, 22, 8014. [Google Scholar] [CrossRef]
- Grungreiff, K.; Gottstein, T.; Reinhold, D.; Blindauer, C.A. Albumin Substitution in Decompensated Liver Cirrhosis: Don’t Forget Zinc. Nutrients 2021, 13, 4011. [Google Scholar] [CrossRef]
- Lewandowska, A.; Religioni, U.; Czerw, A.; Deptala, A.; Karakiewicz, B.; Partyka, O.; Pajewska, M.; Sygit, K.; Cipora, E.; Kmiec, K.; et al. Nutritional Treatment of Patients with Colorectal Cancer. Int. J. Environ. Res. Public Health 2022, 19, 6881. [Google Scholar] [CrossRef]
- Kasprzyk, A.; Bilmin, K.; Chmielewska-Ignatowicz, T.; Pawlikowski, J.; Religioni, U.; Merks, P. The Role of Nutritional Support in Malnourished Patients With Lung Cancer. Vivo 2021, 35, 53–60. [Google Scholar] [CrossRef]
- Nett, H.; Steegmann, J.; Tollkuhn-Prott, B.; Holzle, F.; Modabber, A. A prospective randomized comparative trial evaluating postoperative nutritional intervention in patients with oral cancer. Sci. Rep. 2022, 12, 14213. [Google Scholar] [CrossRef] [PubMed]
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 159, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Boeing, H.; Stelmach-Mardas, M.; Gottschald, M.; Dietrich, S.; Hoffmann, G.; Chaimani, A. Dietary Supplements and Risk of Cause-Specific Death, Cardiovascular Disease, and Cancer: A Systematic Review and Meta-Analysis of Primary Prevention Trials. Adv. Nutr. 2017, 8, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Nasiadek, M.; Stragierowicz, J.; Klimczak, M.; Kilanowicz, A. The Role of Zinc in Selected Female Reproductive System Disorders. Nutrients 2020, 12, 2464. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.S.; Shrader, R.E. Abnormal development of preimplantation rat eggs after three days of maternal dietary zinc deficiency. Nature 1975, 254, 427–429. [Google Scholar] [CrossRef]
- Peters, J.M.; Wiley, L.M.; Zidenberg-Cherr, S.; Keen, C.L. Influence of short-term maternal zinc deficiency on the in vitro development of preimplantation mouse embryos. Proc. Soc. Exp. Biol. Med. 1991, 198, 561–568. [Google Scholar] [CrossRef]
- Garner, T.B.; Hester, J.M.; Carothers, A.; Diaz, F.J. Role of zinc in female reproduction. Biol. Reprod. 2021, 104, 976–994. [Google Scholar] [CrossRef]
- Derar, D.; Ali, A.; Almundarij, T.; Abd-Elmoniem, E.; Alhassun, T.; Zeitoun, M. Association between Serum Trace Elements Levels, Steroid Concentrations, and Reproductive Disorders in Ewes and Does. Vet. Med. Int. 2022, 2022, 8525089. [Google Scholar] [CrossRef]
- Sunar, F.; Gormus, Z.I.; Baltaci, A.K.; Mogulkoc, R. The effect of low dose zinc supplementation to serum estrogen and progesterone levels in post-menopausal women. Biol. Trace Elem. Res. 2008, 126 (Suppl. S1), S11–S14. [Google Scholar] [CrossRef]
- Miro, F.; Smyth, C.D.; Hillier, S.G. Development-related effects of recombinant activin on steroid synthesis in rat granulosa cells. Endocrinology 1991, 129, 3388–3394. [Google Scholar] [CrossRef]
- Chang, H.M.; Cheng, J.C.; Klausen, C.; Leung, P.C. BMP15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells. Mol. Endocrinol. 2013, 27, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Chang, H.M.; Cheng, J.C.; Leung, P.C.; Sun, Y.P. TGF-beta1 downregulates StAR expression and decreases progesterone production through Smad3 and ERK1/2 signaling pathways in human granulosa cells. J. Clin. Endocrinol. Metab. 2014, 99, E2234–E2243. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kelleher, S.L. Molecular regulation of lactation: The complex and requisite roles for zinc. Arch. Biochem. Biophys. 2016, 611, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C.; Park, S.; Vass, T.; Lydon, J.P.; O’Malley, B.W.; Weinberg, R.A. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. USA 1998, 95, 5076–5081. [Google Scholar] [CrossRef] [PubMed]
- Obr, A.E.; Edwards, D.P. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol. Cell Endocrinol. 2012, 357, 4–17. [Google Scholar] [CrossRef]
- Habib, F.K.; Maddy, S.Q.; Stitch, S.R. Zinc induced changes in the progesterone binding properties of the human endometrium. Eur. J. Endocrinol. 1980, 94, 99–106. [Google Scholar] [CrossRef]
- Vasiliauskaite-Brooks, I.; Sounier, R.; Rochaix, P.; Bellot, G.; Fortier, M.; Hoh, F.; De Colibus, L.; Bechara, C.; Saied, E.M.; Arenz, C.; et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 2017, 544, 120–123. [Google Scholar] [CrossRef]
- Kelder, J.; Pang, Y.; Dong, J.; Schaftenaar, G.; Thomas, P. Molecular modeling, mutational analysis and steroid specificity of the ligand binding pocket of mPRalpha (PAQR7): Shared ligand binding with AdipoR1 and its structural basis. J. Steroid. Biochem. Mol. Biol. 2022, 219, 106082. [Google Scholar] [CrossRef]
- Padarti, A.; Zhang, J. Recent advances in cerebral cavernous malformation research. Vessel Plus 2018, 2, 21. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Vasquez, M.; Grajeda, B.; Ellis, C.; Zhang, J. Systems-wide analysis unravels the new roles of CCM signal complex (CSC). Heliyon 2019, 5, e02899. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Smith, M.; Falahati, K.; Zhang, J. Comparative omics of CCM signaling complex (CSC). Chin. Neurosurg. J. 2020, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Abou-Fadel, J.; Zhang, J. Systems Wide Analysis of CCM Signaling Complex Alterations in CCM-Deficient Models Using Omics Approaches. Methods Mol. Biol. 2020, 2152, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Abou-Fadel, J.; Bhalli, M.; Grajeda, B.; Zhang, J. CmP Signaling Network Leads to Identification of Prognostic Biomarkers for Triple-Negative Breast Cancer in Caucasian Women. Genet Test Mol. Biomark. 2022, 26, 198–219. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Grajeda, B.; Jiang, X.; Cailing-De La, O.A.; Flores, E.; Padarti, A.; Bhalli, M.; Le, A.; Zhang, J. CmP signaling network unveils novel biomarkers for triple negative breast cancer in African American women. Cancer Biomark 2022, Preprint. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Jiang, X.; Grajeda, B.; Padarti, A.; Ellis, C.C.; Flores, E.; Cailing-De La, O.A.; Zhang, J. CCM signaling complex (CSC) couples both classic and non-classic Progesterone receptor signaling. Cell Commun. Signal 2022, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Sesterhenn, A.M.; Iwinska-Zelder, J.; Dalchow, C.V.; Bien, S.; Werner, J.A. Acute haemorrhage in patients with advanced head and neck cancer: Value of endovascular therapy as palliative treatment option. J. Laryngol. Otol. 2006, 120, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.B.; Shi, H.B.; Park, S.; Lee, D.G.; Shim, J.H.; Lee, D.H.; Suh, D.C. Acute bleeding in the head and neck: Angiographic findings and endovascular management. Am. J. Neuroradiol. 2014, 35, 360–366. [Google Scholar] [CrossRef]
- Franklin, R.B.; Feng, P.; Milon, B.; Desouki, M.M.; Singh, K.K.; Kajdacsy-Balla, A.; Bagasra, O.; Costello, L.C. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol. Cancer 2005, 4, 32–45. [Google Scholar] [CrossRef]
- Meerarani, P.; Ramadass, P.; Toborek, M.; Bauer, H.-C.; Bauer, H.; Hennig, B. Zinc protects against apoptosis of endothelial cells induced by linoleic acid and tumor necrosis factor α. Am. J. Clin. Nutr. 2000, 71, 81–87. [Google Scholar] [CrossRef]
- Karadas, S.; Sayin, R.; Aslan, M.; Gonullu, H.; Kati, C.; Dursun, R.; Duran, L.; Gonullu, E.; Demir, H. Serum Levels of Trace Elements and Heavy Metals in Patients with Acute Hemorrhagic Stroke. J. Membrane Biol. 2014, 247, 175–180. [Google Scholar] [CrossRef]
- Mammadova-Bach, E.; Braun, A. Zinc Homeostasis in Platelet-Related Diseases. Int. J. Mol. Sci. 2019, 20, 5258. [Google Scholar] [CrossRef] [PubMed]
- Grungreiff, K.; Gottstein, T.; Reinhold, D. Zinc Deficiency-An Independent Risk Factor in the Pathogenesis of Haemorrhagic Stroke? Nutrients 2020, 12, 3548. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Rigamonti, D.; Badr, A.; Zhang, J. Ccm1 regulates microvascular morphogenesis during angiogenesis. J. Vasc. Res. 2011, 48, 130–140. [Google Scholar] [CrossRef]
- Chapman, E.M.; Lant, B.; Ohashi, Y.; Yu, B.; Schertzberg, M.; Go, C.; Dogra, D.; Koskimaki, J.; Girard, R.; Li, Y.; et al. A conserved CCM complex promotes apoptosis non-autonomously by regulating zinc homeostasis. Nat. Commun. 2019, 10, 1791. [Google Scholar] [CrossRef]
- Ito, S.; Greiss, S.; Gartner, A.; Derry, W.B. Cell-nonautonomous regulation of C. elegans germ cell death by kri-1. Curr. Biol. 2010, 20, 333–338. [Google Scholar] [CrossRef]
- Maddaluno, L.; Rudini, N.; Cuttano, R.; Bravi, L.; Giampietro, C.; Corada, M.; Ferrarini, L.; Orsenigo, F.; Papa, E.; Boulday, G.; et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 2013, 498, 492–496. [Google Scholar] [CrossRef]
- Zhou, Z.; Rawnsley, D.R.; Goddard, L.M.; Pan, W.; Cao, X.-J.; Jakus, Z.; Zheng, H.; Yang, J.; Arthur, J.; Simon, C.; et al. The Cerebral Cavernous Malformation Pathway Controls Cardiac Development via Regulation of Endocardial MEKK3 Signaling and KLF Expression. Developmental. Cell 2015, 32, 168–180. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, A.T.; Wong, W.-Y.; Bamezai, S.; Goddard, L.M.; Shenkar, R.; Zhou, S.; Yang, J.; Wright, A.C.; Foley, M.; et al. Cerebral cavernous malformations arise from endothelial gain of MEKK3–KLF2/4 signalling. Nature 2016, 532, 122–126. [Google Scholar] [CrossRef]
- .Cunha, S.I.; Magnusson, P.U.; Dejana, E.; Lampugnani, M.G. Deregulated TGF-β/BMP Signaling in Vascular Malformations. Circ. Res. 2017, 121, 981–999. [Google Scholar] [CrossRef]
- Lopez-Ramirez, M.A.; Fonseca, G.; Zeineddine, H.A.; Girard, R.; Moore, T.; Pham, A.; Cao, Y.; Shenkar, R.; de Kreuk, B.-J.; Lagarrigue, F.; et al. Thrombospondin1 (TSP1) replacement prevents cerebral cavernous malformations. J. Exp. Med. 2017, 214, 3331–3346. [Google Scholar] [CrossRef]
- Choi, J.P.; Wang, R.; Yang, X.; Wang, X.; Wang, L.; Ting, K.K.; Foley, M.; Cogger, V.; Yang, Z.; Liu, F.; et al. Ponatinib (AP24534) inhibits MEKK3-KLF signaling and prevents formation and progression of cerebral cavernous malformations. Sci. Adv. 2018, 4, eaau0731. [Google Scholar] [CrossRef]
- Lopez-Ramirez, M.A.; Pham, A.; Girard, R.; Wyseure, T.; Hale, P.; Yamashita, A.; Koskimaki, J.; Polster, S.; Saadat, L.; Romero, I.A.; et al. Cerebral cavernous malformations form an anticoagulant vascular domain in humans and mice. Blood 2019, 133, 193–204. [Google Scholar] [CrossRef]
- Su, V.L.; Calderwood, D.A. Signalling through cerebral cavernous malformation protein networks. Open Biol. 2020, 10, 200263. [Google Scholar] [CrossRef]
- Wei, S.; Li, Y.; Polster, S.P.; Weber, C.R.; Awad, I.A.; Shen, L. Cerebral Cavernous Malformation Proteins in Barrier Maintenance and Regulation. Int. J. Mol. Sci. 2020, 21, 675. [Google Scholar] [CrossRef]
- Lopez-Ramirez, M.A.; Lai, C.C.; Soliman, S.I.; Hale, P.; Pham, A.; Estrada, E.J.; McCurdy, S.; Girard, R.; Verma, R.; Moore, T.; et al. Astrocytes propel neurovascular dysfunction during cerebral cavernous malformation lesion formation. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Jiang, X.; Padarti, A.; Qu, Y.; Sheng, S.; Abou-Fadel, J.; Badr, A.; Zhang, J. Alternatively spliced isoforms reveal a novel type of PTB domain in CCM2 protein. Sci. Rep. 2019, 9, 15808–15819. [Google Scholar] [CrossRef]
- Antwi-Adjei, E.; Burguete, A.S.; Ghabrial, A.S. Furry is a component of the CCM3-GCKIII signaling pathway. Vessel Plus 2021, 5, 35. [Google Scholar] [CrossRef]
- Zhang, J. Learn from the past, review the present, and look towards the future. Vessel Plus 2022, 6, 20. [Google Scholar] [CrossRef]
- Berman, J.R.; Kenyon, C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 2006, 124, 1055–1068. [Google Scholar] [CrossRef]
- Yamawaki, T.M.; Arantes-Oliveira, N.; Berman, J.R.; Zhang, P.; Kenyon, C. Distinct activities of the germline and somatic reproductive tissues in the regulation of Caenorhabditis elegans’ longevity. Genetics 2008, 178, 513–526. [Google Scholar] [CrossRef]
- Baumbach, G.L.; Didion, S.P.; Faraci, F.M. Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for CuZn superoxide dismutase. Stroke 2006, 37, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P.; Kinzenbaw, D.A.; Schrader, L.I.; Faraci, F.M. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension 2006, 48, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Didion, S.P.; Kinzenbaw, D.A.; Faraci, F.M. Critical role for CuZn-superoxide dismutase in preventing angiotensin II-induced endothelial dysfunction. Hypertension 2005, 46, 1147–1153. [Google Scholar] [CrossRef]
- Didion, S.P.; Ryan, M.J.; Didion, L.A.; Fegan, P.E.; Sigmund, C.D.; Faraci, F.M. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ. Res. 2002, 91, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Morita-Fujimura, Y.; Fujimura, M.; Gasche, Y.; Copin, J.C.; Chan, P.H. Overexpression of copper and zinc superoxide dismutase in transgenic mice prevents the induction and activation of matrix metalloproteinases after cold injury-induced brain trauma. J Cerebr. Blood Flow Metab. 2000, 20, 130–138. [Google Scholar] [CrossRef]
- Goitre, L.; Balzac, F.; Degani, S.; Degan, P.; Marchi, S.; Pinton, P.; Retta, S.F. KRIT1 regulates the homeostasis of intracellular reactive oxygen species. PLoS ONE 2010, 5, e11786. [Google Scholar] [CrossRef]
- Goitre, L.; De Luca, E.; Braggion, S.; Trapani, E.; Guglielmotto, M.; Biasi, F.; Forni, M.; Moglia, A.; Trabalzini, L.; Retta, S.F. KRIT1 loss of function causes a ROS-dependent upregulation of c-Jun. Free Radic. Biol. Med. 2014, 68, 134–147. [Google Scholar] [CrossRef]
- Jung, K.H.; Han, D.M.; Jeong, S.G.; Choi, M.R.; Chai, Y.G.; Cho, G.W. Proteomic analysis reveals KRIT1 as a modulator for the antioxidant effects of valproic acid in human bone-marrow mesenchymal stromal cells. Drug Chem. Toxicol. 2015, 38, 286–292. [Google Scholar] [CrossRef]
- Trapani, E.; Retta, S.F. Cerebral cavernous malformation (CCM) disease: From monogenic forms to genetic susceptibility factors. J. Neurosurg Sci. 2015, 59, 201–209. [Google Scholar]
- Retta, S.F.; Glading, A.J. Oxidative stress and inflammation in cerebral cavernous malformation disease pathogenesis: Two sides of the same coin. Int. J. Biochem. Cell Biol. 2016, 81, 254–270. [Google Scholar] [CrossRef]
- Fontes-Dantas, F.L.; da Fontoura Galvao, G.; Veloso da Silva, E.; Alves-Leon, S.; Cecilia da Silva Rego, C.; Garcia, D.G.; Marques, S.A.; Blanco Martinez, A.M.; Reis da Silva, M.; Marcondes de Souza, J. Novel CCM1 (KRIT1) Mutation Detection in Brazilian Familial Cerebral Cavernous Malformation: Different Genetic Variants in Inflammation, Oxidative Stress, and Drug Metabolism Genes Affect Disease Aggressiveness. World Neurosurg. 2020, 138, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Perrelli, A.; Retta, S.F. Fluorescence Analysis of Reactive Oxygen Species (ROS) in Cellular Models of Cerebral Cavernous Malformation Disease. Methods Mol. Biol. 2020, 2152, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Swamy, H.; Glading, A.J. Contribution of protein-protein interactions to the endothelial-barrier-stabilizing function of KRIT1. J. Cell Sci. 2022, 135, jcs258816. [Google Scholar] [CrossRef] [PubMed]
- Abou-Fadel, J.; Jiang, X.; Padarti, A.; Goswami, D.; Smith, M.; Grajeda, B.; Walker, W.; Zhang, J. CCM signaling complex (CSC) is a master regulator governing homeostasis of progestins and their mediated signaling cascades. bioRxiv 2020. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Qu, Y.; Gonzalez, E.; Smith, M.; Zhang, J. Emerging roles of CCM genes during tumorigenesis with potential application as novel biomarkers across major types of cancers. Oncol. Rep. 2020, 43, 1945–1963. [Google Scholar] [CrossRef]
- Abou-Fadel, J.; Jiang, X.; Padarti, A.; Goswami, D.G.; Smith, M.; Grajeda, B.; Bhalli, M.; Le, A.; Walker, W.E.; Zhang, J. mPR-Specific Actions Influence Maintenance of the Blood–Brain Barrier (BBB). Int. J. Mol. Sci. 2022, 23, 9684. [Google Scholar]
- Renteria, M.; Belkin, O.; Jang, D.; Aickareth, J.; Bhalli, M.; Zhang, J. CmPn signaling networks in the tumorigenesis of breast cancer. Front. Endocrinol. 2022, 13, 1013892. [Google Scholar] [CrossRef]
| Main Point | Key Findings | Pubmed | References |
|---|---|---|---|
| The CSC is linked to tumorigenesis with mPRs in various cancers. | Differential expression of CCM and mPR correlated with various types and grades of major human cancers, especially breast and liver cancers. | PMID: 32186778 | [136,138] |
| Establishing the CmPn signaling network in nPR(+) breast cancers | The CSC role in coupling classic, non-classic, or combined PRG signaling pathways via the effects of an intricate homeostatic concentration of progesterone to form the CmPn signaling network | PMID: 35971177 | [96,138] |
| Establishing the CmP signaling network in nPR(-) breast cancers | Through establishingCmP signaling network in nPR(-) breast cancers, we discovered novel biomarker signature panels for Triple-Negative Breast Cancers (TNBCs) between African and Caucasian Women | PMID: 35431232; 35481969 | [94,95,138] |
| Establishing the CmP signaling network in nPR(-) vasular ECs | Deficiency of any CCM genes, in combination with mPR-specific PRG actions, leads to perturbed CmP signaling network in nPR(-) ECs both in vitro and in vivo, result in compromising blood brain barrier integrity and increases the risk of hemorrhage | PMID: 36077089; 35098046 | [135,137,138] |
| Exploring molecuular signaling within the CmPn/CmP signaling networks with multiomics | molecuular signaling within the CmPn/CmP signaling networks were investigated with multiomics, such as RNAseq and proteomics. Major molecular pathways were presented with pathway analysis and visualization | PMID: 36077089; 35098046 | [91,94,95,96,135,136] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renteria, M.; Belkin, O.; Aickareth, J.; Jang, D.; Hawwar, M.; Zhang, J. Zinc’s Association with the CmPn/CmP Signaling Network in Breast Cancer Tumorigenesis. Biomolecules 2022, 12, 1672. https://doi.org/10.3390/biom12111672
Renteria M, Belkin O, Aickareth J, Jang D, Hawwar M, Zhang J. Zinc’s Association with the CmPn/CmP Signaling Network in Breast Cancer Tumorigenesis. Biomolecules. 2022; 12(11):1672. https://doi.org/10.3390/biom12111672
Chicago/Turabian StyleRenteria, Mellisa, Ofek Belkin, Justin Aickareth, David Jang, Majd Hawwar, and Jun Zhang. 2022. "Zinc’s Association with the CmPn/CmP Signaling Network in Breast Cancer Tumorigenesis" Biomolecules 12, no. 11: 1672. https://doi.org/10.3390/biom12111672
APA StyleRenteria, M., Belkin, O., Aickareth, J., Jang, D., Hawwar, M., & Zhang, J. (2022). Zinc’s Association with the CmPn/CmP Signaling Network in Breast Cancer Tumorigenesis. Biomolecules, 12(11), 1672. https://doi.org/10.3390/biom12111672







