Abstract
1α,25-Dihydroxyvitamin D3 [1α,25(OH)2D3, 1] is an active form of vitamin D3 and regulates various biological phenomena, including calcium and phosphate homeostasis, bone metabolism, and immune response via binding to and activation of vitamin D receptor (VDR). Lithocholic acid (LCA, 2) was identified as a second endogenous agonist of VDR, though its potency is very low. However, the lithocholic acid derivative 3 (Dcha-20) is a more potent agonist than 1α,25(OH)2D3, (1), and its carboxyl group has similar interactions to the 1,3-dihydroxyl groups of 1 with amino acid residues in the VDR ligand-binding pocket. Here, we designed and synthesized amide derivatives of 3 in order to clarify the role of the carboxyl group. The synthesized amide derivatives showed HL-60 cell differentiation-inducing activity with potency that depended upon the substituent on the amide nitrogen atom. Among them, the N-cyanoamide 6 is more active than either 1 or 3.
1. Introduction
Vitamin D receptor (VDR) is a ligand-dependent transcriptional factor belonging to the nuclear receptor superfamily [1,2], and mediates most of the biological functions of vitamin D3, including calcium and phosphate homeostasis, bone metabolism, and immune regulation. The endogenous agonist of VDR is 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3, 1], an active metabolite of vitamin D3 (Figure 1), which induces increased expression of target genes. Various vitamin D derivatives have been synthesized as candidate drugs for skin and bone diseases [3,4,5], though most of them have the same secosteroid structure as 1α,25(OH)2D3 (1).
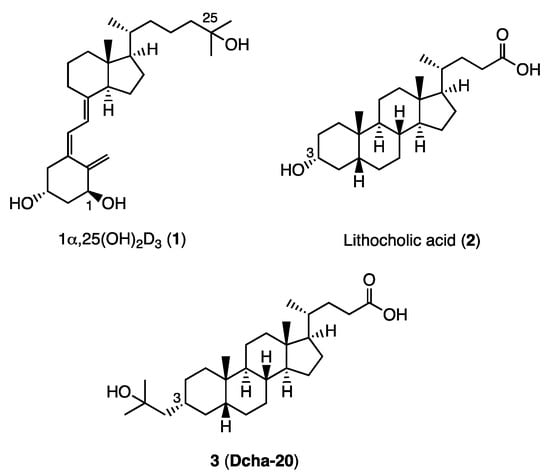
Figure 1.
Structures of 1α,25(OH)2D3 (1), lithocholic acid (LCA, (2), and lithocholic acid derivative (3) (Dcha-20).
Lithocholic acid (LCA, 2, Figure 1) is a secondary bile acid formed from chenodeoxycholate, and was identified as a second endogenous agonist of VDR [6,7,8,9]. However, its VDR binding affinity and potency are very low, compared with those of 1. We recently developed a potent lichocholic acid derivative 3 (Dcha-20) that has a 2-hydroxy-2-methylprop-1-yl moiety instead of the 3-hydroxyl group at the 3 α position of 2 (Figure 1) [10]. In HL-60 cell differentiation-inducing assay, 3 was more potent than 1. Gaikwad, S. et al. recently reported that 3 shows potent vitamin D activity with a lower calcemic activity than 1 [11].
Analysis of the crystal structure of VDR ligand-binding domain (LBD) bound to 3 (Dcha-20) showed that the 3-substituent forms direct hydrogen bonds with the two histidine residues His301 and His393, like the 25-hydroxyl group of 1α,25(OH)2D3 (1), but different from the indirect interaction, via a water molecule, of the 3-hydroxyl group of LCA (2) with the same amino acid residues of the VDR [8,11]. The carboxyl group of 3 forms hydrogen bonds with Tyr143 and Ser274, as in the case of 1α,25(OH)2D3 (1) or LCA (2). The carboxyl group of LCA (2) also formed indirect hydrogen bonds with Arg270 and Ser233 via a water molecule, while the corresponding interaction was not observed in the case of 3. The 3-hydroxyl group of 1α,25(OH)2D3 (1) forms direct hydrogen bonds with these amino acid residues in the crystal. Further, our preliminary results on the pharmacokinetics of 3 indicated that this compound is eliminated very quickly from the serum in mice (data not shown), and the carboxyl group is one of the target functional groups for improvement of this undesirable feature. Therefore, in this study, we designed and synthesized several lithocholic acid amide derivatives 4–8 with various N-substituents in order to clarify the role of the carboxyl group in the VDR binding and vitamin D activity of 3 (Figure 2).
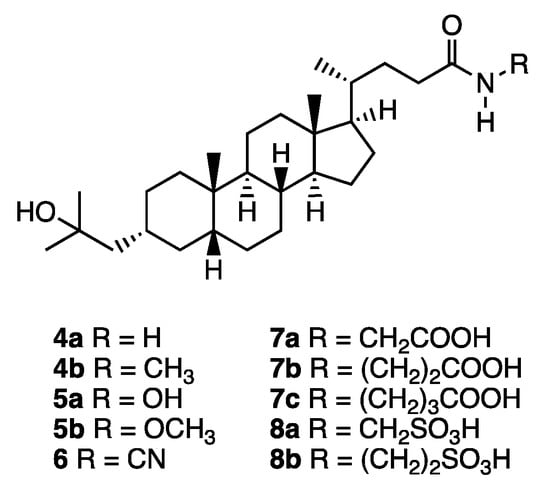
Figure 2.
Structures of lithocholic acid amide derivatives 4–8.
2. Results and Discussion
2.1. Synthesis
The lithocholic acid amide derivatives 4–8 were synthesized from LCA (2) via compound 3 (Dcha-20), which was prepared by modification of our previous method [10] (Scheme 1). Acetylation of the 3-hydroxyl group of LCA (2), followed by reduction of the carboxyl group and benzylation, afforded compound 11. After hydrolysis of the acetate and oxidation, the 3-keto compound 13 was converted to a mixture of 3-formyl derivatives (15a:15b = 8:2) via Wittig reaction. Treatment of this mixture with potassium carbonate increased the ratio of the desired 3 α-isomer 15a (15a:15b = 10:1).
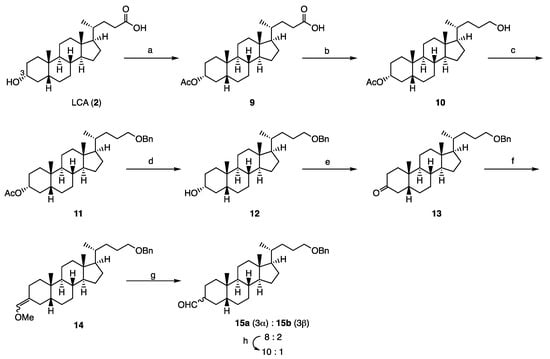
Scheme 1.
Synthesis of key compound 15a. Reagents and conditions: (a) Ac2O, DMAP, pyridine (quant); (b) NaBH4, Et3N, ClCOOEt, THF (quant); (c) CCl3C(=NH)OBn, TfOH, 1,4-dioxane (81%); (d) K2CO3, MeOH, THF (97%); (e) H2SO4, CrO3, acetone (98%); (f) t-BuOK, MeOCH2PPh3Cl, THF (quant); (g) HCl, THF (98%); (h) K2CO3, MeOH, THF (66%).
Compound 3 was synthesized from aldehyde 15a in 7 steps according to our reported method (Scheme 2). Briefly, 15a was reduced to the 3-hydroxymethyl compound 16, followed by tosylation and reaction with sodium cyanide, to afford the nitrile 18. Two-step methylation of 18 gave compound 20 with a 2-hydroxy-2-methylprop-1-yl moiety at the 3 position. The terminal polar group in the side chain of 20 was converted to a carboxyl group in 2 steps to afford 3 (Dcha-20). The amide derivatives 4–8 were synthesized from 3 by the method shown in Scheme 3.
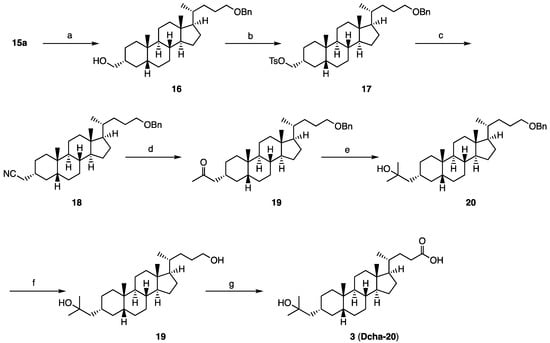
Scheme 2.
Synthesis of compound 3 (Dcha-20). Reagents and conditions: (a) NaBH4, MeOH (quant); (b) TsCl, pyridine (98%); (c) NaCN, DMSO (94%); (d) (1) MeLi, (2) HCl (82%); (e) (1) MeLi, (2) NH4Cl aq (85%); (f) H2, Pd(OH)2-C, MeOH (95%); (g) H2SO4, CrO3, acetone (83%).
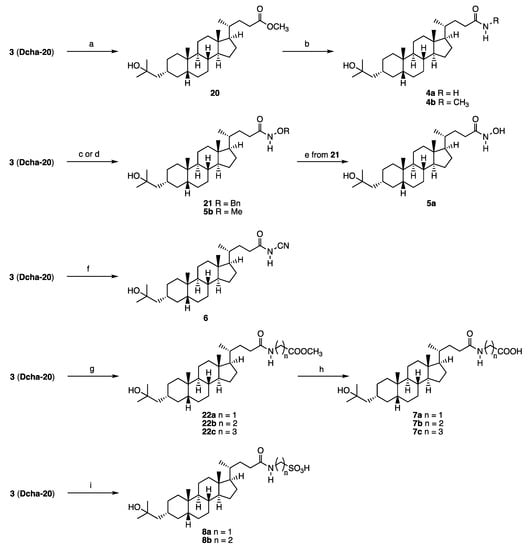
Scheme 3.
Synthesis of amide derivatives 4–8. Reagents and conditions: (a) AcCl, MeOH (quant); (b) NH3 or CH3NH2, MeOH 4a: 51%, 4b: 30%); (c) BnONH2·HCl, DIEA, HOBt, DCC, CH2Cl2 (21: quant); (d) (1) ClCO2Et, Et3N, (2) CH3ONH2·HCl (5b: 73%); (e) H2, Pd-C, MeOH (55%); (f) H2NCN, DMAP, EDCI, DIPEA, CH2Cl2 (89%); (g) H2N(CH2)2COOCH3·HCl, NMM, EDCI, CH2Cl2 (22a: 83%, 22b: 96%, 22c: 82%); (h) NaOH, EtOH (7a: 77%, 7b: quant, 7c: 85%); (i) H2N(CH2)2SO3H, DMT-MM, Et3N, DMF (8a: 74%, 8b: 93%).
2.2. Biological Evaluation
The vitamin D activity of the synthesized lithocholic acid amide derivatives was evaluated in terms of cell differentiation-inducing activity toward human acute promyelocytic leukemia cell line HL-60 [12]. HL-60 cell differentiation was evaluated in terms of the ratio of nitroblue tetrazolium (NBT)-positive cells (Figure 3 and Table 1). All the amide derivatives examined induced dose-dependent differentiation of HL-60 cells. In this assay, the unsubstituted amide 4a exhibited more potent activity (EC50: 0.44 nM), compared with that of the carboxylic acid derivative 3 (Dcha-20, EC50: 1.01 nM) or 1α,25(OH)2D3 (1, EC50: 0.74 nM). Interestingly, N-monomethylation of compound 4a, yielding compound 4b, diminished the activity. N-Methyl group would disturb the hydrogen bond formation of amide group with the amino acid residues of VDR. The introduction of an N-hydroxyl (compound 5a, EC50: 1.18 nM) or N-methoxyl group (compound 5b, EC50: 1.45 nM) slightly decreased the activity, though these compounds still showed activity comparable to that of 3. Interestingly, compound 6 bearing an N-cyano group (EC50: 0.32 nM) was the most active among the synthesized amide derivatives, being more potent than 3 or 1.
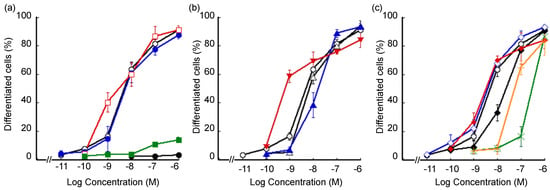
Figure 3.
HL-60 cell differentiation-inducing activity of lithocholic acid amide derivatives. Differentiated cells were determined by measuring the ratio of nitroblue tetrazolium (NBT)-positive cells. (a) ○: 1α,25(OH)2D3 (1), ●: LCA (2), ●: 3 (Dcha-20), □: 4a, ■: 4b, (b) ○: 1α,25(OH)2D3 (1), △: 5a, ▲: 5b, ▼: 6, (c) ○: 1α,25(OH)2D3 (1),◆: 7a, ◇: 7b, ◆: 7c, ×: 8a, +: 8b.

Table 1.
EC50 vales of novel lithocholic acid amide derivatives.
Among the three derivatives bearing an N-carboxyalkyl group, compound 7a with one methylene group between the amide and carboxyl groups (EC50: 2.03 nM) showed lower activity than the parent amide compound 4a (EC50: 0.44 nM), while compounds 7b (EC50: 0.45 nM) and 7c (EC50: 0.64 nM) with longer alkyl chains were more active than 7a. A similar tendency was observed for the compounds bearing an N-sulfoalkyl group. Thus, compound 8b (EC50: 6.56 nM) was more active than compound 8a (EC50: 18.5 nM). Terminal polar groups (carboxyl for 7 and sulfo for 8) adjacent to the amide group appear to have a negative effect, possibly blocking hydrogen bond formation of the amide with amino acid residues of VDR, whereas groups more distant from the amide group might have positive effects such as formation of additional hydrogen bond(s).
Next, we examined the VDR trasactivation ability for selected compounds (Figure 4 and Table 1), according to the method reported in our previous study [13]. 1α,25(OH)2D3 (1, EC50: 0.058 nM) and compound 3 (Dcha-20, EC50: 0.083 nM) showed potent transactivation activity at the concentrations above 0.1 nM, while LCA (2) did not show the activity at the concentration below 1 μ M. All the amide derivatives examined showed dose-dependent transactivation activity, which was well correlated with the activity in HL-60 cell assay. Among them compounds 6 (EC50: 0.10 nM) and 7c (EC50: 0.081 nM) were as potent as 1α,25(OH)2D3 (1) and compound 3 (Dcha-20). The results indicated that the differentiation-inducing activity of the lithocholic acid amide derivatives would be mediated by VDR.
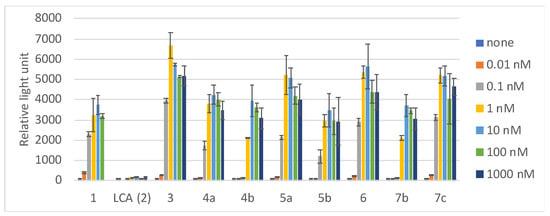
Figure 4.
VDR transactivation ability of lithocholic acid amide derivatives in HEK293 cells.
2.3. X-ray Crystallographic Analysis
We next attempted X-ray crystallographic analysis of the complex of rat VDR LBD (residues 116–423, Δ165–211) with several of the lithocholic acid amide derivatives. According to the method reported in our previous study [10,14], a synthetic peptide containing the target sequence of the coactivator MED1 (mediator of RNA polymerase II transcription subunit 1, also known as ARC205 or DRIP205) was included in the crystallization solution of VDR LBD and the test compound. However, the analysis was successful only for the complex of compound 7b (Table 2). The electron density map clearly shows the VDR LBD, the coactivator peptide, the ligand and a relatively low number of water molecules. Figure 5a shows the overall structure of the VDR LBD complex with 7b; it is similar to those previously reported for VDR LBD complexes with other lithocholic acid derivatives [10,14,15]. The interactions of compound 7b with amino acid residues of the VDR LBD (Figure 5b) are compared with those of 3 (Dcha-20) in Figure 5c. The hydroxyl group in the 3-substituent of 7b forms direct hydrogen bonds with two histidine residues, His301 (O···N distance: 2.79 Å) and His393 (O···N distance: 2.68 Å). This is the same as in the case of 3 (Dcha-20), in which the O···N distances were 2.80 Å for His301 and 2.66 Å for His393, whereas LCA (2) forms indirect hydrogen bonds with these amino acid residues via a water molecule. The direct interactions of the hydroxy group in the 3-substituent with two histidines may contribute to the potent activity of 7b and 3. The carboxyl group of compound 3 (Dcha-20) formed hydrogen bonds with the phenolic hydroxyl group of Tyr143 (O··O distance: 2.81 Å) in helix 1 and the hydroxymethyl group of Ser274 (O··O distance: 3.13 Å) in helices 4/5, whereas the amide group of 7b did not form a hydrogen bond with any amino acid residue. Instead, the terminal carboxyl group of 7b formed hydrogen bonds with Arg270 (O···N distance: 2.82 Å) and the backbone amide bond of Tyr143 (O···N distance: 2.91 Å). Similar hydrogen bond formation with these amino acid residues of the VDR LBD was observed in secosteroid derivatives bearing a hydroxylated substituent at the 2-position of the cyclohexane ring.

Table 2.
Data collection and refinement statistics for the crystal structure of VDR LBD bound to 7b and coactivator peptide. Values in parentheses are for the highest-resolution shell.
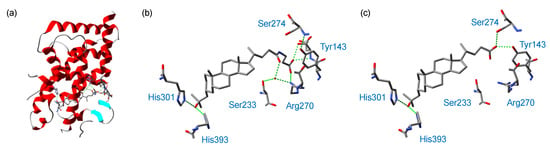
Figure 5.
Crystal structure of the complex of VDR LBD with 7b. (a) Overall structure, and (b) binding to the VDR, compared with (c) that of 3 (Dcha-20) (PDB: 7C7V) [10].
3. Conclusions
We designed and synthesized several lithocholic acid amide derivatives of 3 (Dcha-20) as a lead compound. The carboxyl group of compound 3 (Dcha-20) can be replaced with various amide bonds without decreasing the activity. Among the synthesized amide derivatives, compounds 4a, 6 with an N-cyano group and 7b with an N-2-carboxyethyl group showed the most potent activity. Crystallographic analysis of the complex of the VDR LBD with 7b showed that the terminal carboxyl group, but not the amide group, forms hydrogen bonds with amino acid residues of the VDR LBD. The hydroxyl group in the 3-substituent also forms direct hydrogen bonds with two histidine residues, His301 and His393. Recently, compound 3 (Dcha-20) was reported to have lower calcemic activity than 1α,25(OH)2D3 (1) [11], which would be favorable for clinical application, but preliminary studies on the pharmacokinetics of 3 (Dcha-20) indicated that it is eliminated very quickly in mice. The carboxyl group of 3 (Dcha-20) appears to be important for both the potent vitamin D activity and the pharmacokinetic properties. The novel amide derivatives of compound 3 also showed potent vitamin D activities, and studies on their pharamacokinetic properties are now on going. Our results suggest that it may be possible to develop lithocholic acid derivatives having potent activity and drug-like pharmacokinetic properties by chemical modification at the terminal polar group in the side chain.
4. Experimental
4.1. General
1H and 13C NMR spectra were recorded on JNM-ECS 400, JNM-ECS 500, and Bruker Avance 600 spectrometers. The 1H NMR chemical shifts are reported in parts per million (ppm) relative to the centerline of the singlet signal of the solvent molecule (7.26 ppm for chloroform); coupling constants are given in hertz (Hz). The 13C NMR chemical shifts are reported in ppm relative to the centerline of the triplet at 77.16 ppm for CDCl3. Mass spectral data were obtained on a Bruker Daltonics micro TOF-2focus in the positive and negative ion detection modes.
4.2. Synthesis
Synthesis of compound 9: Acetic anhydride (6.174 g, 60.48 mmol) and 4-dimethylaminopyridine (97 mg, 0.80 mmol) were added to a solution of lithocholic acid (1.522 g, 4.04 mmol) in dry pyridine (40 mL). The mixture was stirred for 20 h at room temperature, then quenched with water and extracted with a mixture of ethyl acetate and n-hexane (1:1). The organic layer was washed with 2 M hydrochloric acid and brine, dried over sodium sulfate, and filtered. The filtrate was concentrated to give 9 (1.757 g, quant.) as a yellow solid. 1H NMR (600 MHz, CDCl3) δ 4.74-4.70 (m, 1 H), 2.40 (ddd, J = 15.6, 10.2, 5.4 Hz, 1 H), 2.26 (ddd, J = 15.6, 9.6, 6.6 Hz, 1 H), 2.03 (s, 3 H), 1.98-1.95 (m, 1 H), 1.85-1.80 (m, 5 H), 1.68-1.00 (m, 20 H), 0.92 (d, J = 6.6 Hz, 3 H), 0.92 (s, 3 H), 0.64 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 178.33, 170.73, 74.39, 56.43, 55.90, 42.70, 41.82, 40.33, 40.08, 35.72, 35.28, 34.98, 34.54, 32.18, 30.71, 30.60, 28.16, 26.96, 26.57, 26.27, 24.14, 23.30, 21.50, 20.78, 18.20, 12.01; HRMS calcd for C26H42NaO4 (M + Na)+ 441.2975, found 441,2970.
Synthesis of compound 10: Triethylamine (533 mg, 5.27 mmol) and ethyl chloroformate (616 mg, 5.680 mmol) were added to a solution of 9 (1.757 g, 4.04 mmol) in distilled THF (40 mL). The mixture was stirred for 2 h at room temperature, then cooled to 0 °C, and sodium borohydride (737 mg, 19.5 mmol) and dry methanol (20 mL) were added to it. The reaction mixture was stirred for 2 h 15 min at 0 °C and then quenched with water. After removal of the solvent in vacuo, the residue was extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate, filtered, and concentrated. The residue was purified by silica gel column chromatography (ethyl acetate/n-hexane = 1:3) to give 10 (1.672 g, quant.) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 4.74-4.69 (m, 1 H), 3.65-3.58 (m, 2 H), 2.03 (s, 3 H), 1.99-1.96 (m, 1 H), 1.97-1.80 (m, 4 H), 1.70-1.02 (m, 23H), 0.93 (s, 3 H), 0.92 (d, J = 6.6 Hz, 3 H), 0.65 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 170.68, 74.37, 63.58, 56.43, 56.10, 42.62, 41.81, 40.32, 40.08, 35.70, 35.51, 34.95, 34.51, 32.16, 31.74, 29.31, 28.25, 26.95, 26.55, 26.26, 24.13, 23.28, 21.47, 20.76, 18.57, 11.98; HRMS calcd for C26H44NaO3 (M + Na)+ 427.3183, found 427.3174.
Synthesis of compound 11: Trifluoromethanesulfonic acid (0.25 mL, 2.85 mmol) was added to a solution of 10 (1.309 g, 3.24 mmol) in dry 1,4-dioxane (40.0 ml) and benzyl 2,2,2-trichloroacetimidate (2.206 g, 8.74 mmol) at 0 °C under an argon atmosphere. The reaction mixture was stirred for 3 h at room temperature, then cooled to 0 °C, quenched with saturated sodium hydrogen carbonate, and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate, filtered, and concentrated. The residue was purified by silica gel column chromatography (ethyl acetate/n-hexane = 1:14) to give compound 11 (1.297 g, 81%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.35-7.32 (m, 4 H), 7.30-7.27 (m, 1 H), 4.74-4.70 (m, 1 H), 4.51 (d, J = 12.0 Hz, 1 H), 4.49 (d, J = 12.0, 1 H), 3.46-3.40 (m, 2 H), 2.03 (s, 3 H), 1.98-1.96 (m, 1 H), 1.84-1.79 (m, 4 H), 1.68-1.66 (m, 2 H), 1.56-1.02 (m, 21 H), 0.92 (s, 3 H), 0.91 (d, J = 6.6 Hz, 3 H), 0.63 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 170.71, 138.61, 128.30, 127.60, 127.43, 74.41, 72.29, 71.01, 56.44, 56.14, 42.63, 41.83, 40.33, 40.10, 35.73, 35.53, 34.97, 34.53, 32.18, 32.12, 28.24, 26.98, 26.56, 26.28, 26.25, 24.16, 23.30, 21.48, 20.77, 18.55, 11.99; HRMS calcd for C33H50NaO3 (M + Na)+ 517.3652, found 517.3638.
Synthesis of compound 12: Potassium carbonate (1.464 g, 10.6 mmol) was added to a solution of 11 (1.344 g, 2.72 mmol) in dry methanol (30 mL) and distilled THF (5 mL). The mixture was stirred for 6 h 20 min at room temperature under an argon atmosphere and then quenched with acetic acid. After removal of the solvent in vacuo, the residue was extracted with ethyl acetate. The organic layer was washed with water and brine, dried over sodium sulfate, filtered, and concentrated. The residue was purified by silica gel column chromatography (ethyl acetate/n-hexane = 1:3) to give 12 (1.190 g, 97%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.36-7.33 (m, 4 H), 7.30-7.27 (m, 1 H), 4.51 (d, J = 13.2 Hz, 1 H), 4.49 (d, J = 12.0 Hz, 1 H), 3.64-3.60 (m, 1 H), 3.46-3.40 (m, 2 H), 1.96 (dt, J = 12.0, 3.0 Hz, 1 H), 1.86-1.66 (m, 7 H), 1.59-0.94 (m, 20 H), 0.91 (s, 3 H), 0.91 (d, J = 6.6 Hz, 3 H), 0.63 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 138.65, 128.32, 127.61, 172.43, 72.80, 71.88, 71.02, 56.46, 56.13, 42.64, 42.04, 40.37, 40.13, 36.39, 35.79, 35.57, 35.29, 34.53, 32.13, 30.50, 28.27, 27.16, 26.39, 26.28, 24.20, 23.35, 20.78, 18.56, 12.00; HRMS calcd for C31H48NaO2 (M + Na)+ 475.3547, found 475.3541.
Synthesis of compound 13: Sulfuric acid (0.23 mL) was added to a cooled solution of chromium (VI) oxide (267 mg) in water (0.77 mL) just prior to use. An aliquot (0.7 mL) of this Jones reagent was added to a solution of 12 (1.173 g, 2.59 mmol) in dry acetone (30 mL). The mixture was stirred for 30 min at room temperature, then quenched with 2-propanol, and the solvent was removed in vacuo. The extract was extracted with diethyl ether. The organic layer was washed with brine, dried over sodium sulfate, filtered, and concentrated. The residue was purified by silica gel column chromatography (ethyl acetate/n-hexane = 1:4) to give 13 (1.148 g, 98%) as a colorless oil. 1H NMR (600 MHz, CDCl3) δ 7.36-7.33 (m, 4 H), 7.29-7.27 (m, 1 H), 4.51 (d, J = 12.6 Hz, 1 H), 4.49 (d, J = 12.6 Hz, 1 H), 3.47-3.40 (m, 2 H), 2.70 (t, J = 14.4 Hz, 1 H), 2.33 (td, J = 14.4, 5.4 Hz, 1 H), 2.17-2.14 (m, 1 H), 2.05-2.00 (m, 3 H), 1.88-1.79 (m, 3 H), 1.70-1.08 (m, 19 H), 1.01 (s, 3 H), 0.92 (d, J = 6.6 Hz, 3 H), 0.67 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 213.64, 138.62, 128.31, 127.60, 127.44, 72.81, 70.99, 56.40, 56.12, 44.32, 42.68, 42.35, 40.65, 40.01, 37.21, 36.99, 35.54, 35.48, 34.85, 32.12, 28.22, 26.58, 26.17, 25.74, 24.14, 22.63, 21.15, 18.57, 12.03; HRMS calcd for C31H46NaO2 (M + Na)+ 473.3390, found 473.3379.
Synthesis of compound 14: A mixture of (methoxylmethyl)triphenylphosphonium chloride (18.111 g, 52.8 mmol) and potassium tert-butoxide (6.188 g, 46.7 mmol) in distilled THF (112 mL) was stirred for 45 min at 0 °C under an argon atmosphere. A solution of 13 (6.812 g, 15.1 mmol) in distilled THF (18 mL) was added to it. The resulting mixture was allowed to warm to room temperature, stirred for 2 h, quenched with water, and extracted with ethyl acetate. The organic layer was washed with water and brine, dried over sodium sulfate, filtered, and concentrated. The residue was purified by silica gel column chromatography (chloroform/n-hexane = 3:2) to give 14 (7.280 g, quant.) as a mixture of geometrical isomers. Each isomer was isolated in a small amount to determine the structure. (E)-14: 1H NMR (600 MHz, CDCl3) δ 7.35-7.33 (m, 4 H), 7.29-7.27 (m, 1 H), 5.76 (s, 1 H), 4.51 (d, J = 12.0 Hz, 1 H), 4.49 (d, J = 12.0 Hz, 1 H), 3.52 (s, 3 H), 3.46-3.40 (m, 2 H), 2.31 (dd, J = 13.8, 3.6 Hz, 1 H), 2.12 (t, J = 13.8 Hz, 1 H), 1.98-1.95 (m, 1 H), 1.86-1.79 (m, 3 H), 1.71-1.66 (m, 2 H), 1.57-0.92 (m, 20 H), 0.91 (s, 3 H), 0.91 (d, J = 5.4 Hz, 3 H), 0.64 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 138.75, 138.63, 128.32, 127.61, 127.43, 118.80, 72.82, 71.07, 59.28, 56.60, 56.23, 43.67, 42.72, 40.24, 40.15, 38.68, 35.76, 35.59, 32.22, 28.29, 26.99, 26.34, 26.28, 25.71, 25.12, 24.24, 23.73, 20.97, 18.61, 12.06; HRMS calcd for C33H50NaO2 (M + Na)+ 501.3707, found 501.3698. (Z)-14: 1H NMR (600 MHz, CDCl3) δ 7.35-7.33 (m, 4 H), 7.29-7.27 (m, 1 H), 5.72 (s, 1 H), 4.51 (d, J = 12.0 Hz, 1 H), 4.49 (d, J = 12.0 Hz, 1 H), 3.52 (s, 3 H), 3.46-3.40 (m, 2 H), 2.46 (d, J = 13.8 Hz, 1 H), 2.39 (t, J = 13.6 Hz, 1 H), 1.98-1.95 (m, 1 H), 1.86-1.79 (m, 3 H), 1.71-1.66 (m, 2 H), 1.57-0.92 (m, 20 H), 0.91 (s, 3 H), 0.91 (d, J = 5.4 Hz, 3 H), 0.64 (s, 3 H). 13C; HRMS calcd for C33H50NaO2 (M + Na)+ 501.3707, found 501.3698.
Synthesis of compound 15: 6 M hydrochloric acid (30 mL) was added to a solution of 14 (6.975 g, 14.6 mmol) in distilled THF (80 mL), and the mixture was stirred for 5 h at room temperature. The reaction mixture was quenched with water, and extracted with ethyl acetate. The organic layer was washed with water and brine, dried over sodium sulfate, filtered, and concentrated. The residue was purified by silica gel column flash chromatography (dichloromethane/n-hexane = 1:1) to give a mixture of 15a and 15b (6.664 g, 98%, 15a:15b = 8:2) as a colorless oil. 15a: 1H NMR (600 MHz, CDCl3) δ 9.64 (d, J =1.8 Hz, 1 H), 7.36-7.33 (m, 4 H), 7.29-7.27 (m, 1 H), 4.51 (d, J = 13.2 Hz, 1 H), 4.49 (d, J = 12.0 Hz, 1 H), 3.46-3.40 (m, 2 H), 2.27 (dtt, J = 12.6, 3.6, 1.8 Hz, 1 H), 1.97-0.97 (m, 28 H), 0.96 (s, 3 H), 0.91 (d, J = 6.6 Hz, 3 H), 0.63 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 205.13, 138.64, 128.31, 127.61, 127.43, 72.80, 71.01, 56.36, 56.11, 51.32, 42.62, 42.56, 40.32, 40.03, 36.06, 35.74, 35.56, 35.10, 32.12, 28.24, 27.16, 26.28, 24.16, 23.81, 20.78, 18.55, 12.00; HRMS calcd for C32H48NaO2 (M + Na)+ 487.3547, found 487.3535. 15b: 1H NMR (600 MHz, CDCl3) δ 9.71 (s, 1 H), 7.36-7.33 (m, 4 H), 7.23-7.27 (m, 1 H), 4.51 (d, J = 12.6 Hz, 1 H), 4.49 (d, J = 12.6 Hz, 1 H), 3.46-3.39 (m, 2 H), 2.45 (br, 1 H), 2.04-0.85 (m, 28 H), 0.91 (d, J = 6.6 Hz, 3 H), 0.87 (s, 3 H), 0.63 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 206.55, 138.64, 128.33, 127.63, 127.46, 72.82, 71.03, 56.56, 56.16, 47.08, 42.68, 40.15, 39.98, 39.60, 35.58, 35.54, 34.90, 33.48, 32.14, 28.26, 26.91, 26.26, 26.07, 25.00, 24.18, 23.85, 20.85, 19.60, 18.58, 12.02; HRMS calcd for C32H48NaO2 (M + Na)+ 487.3547, found 487.3531.
The ratio of 15a in the mixture of the epimers was increased by treatment of the mixture with K2CO3, MeOH, THF (66%), and the isolated 15a was converted to compound 3 (Dcha-20), according to our reported method.
Synthesis of compound 20: Acetyl chloride (0.01 mL, 0.140 mmol) was added to a cooled solution of 3 (64 mg, 0.15 mmol) in methanol (7 mL) at 0 °C under an argon atmosphere. The mixture was stirred for 4 h at room temperature, then quenched with water at 0 °C, and the precipitate was collected to obtain 20 (72 mg, quant.) as a colorless solid. 1H NMR (400 MHz, CDCl3) δ 3.66 (s, 3H), 2.39-2.31 (m, 1H), 2.25-2.17 (m, 1H), 1.94 (d, J = 11.4 Hz, 1H), 1.89-1.73 (m, 4H), 1.59-0.90 (m, 25H), 1.22 (s, 6H), 0.91 (d, J = 6.4 Hz, 3H), 0.91 (s, 3H), 0.64 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 174.84, 71.65, 60.39, 56.50, 55.89, 51.50, 51.23, 43.55, 42.70, 40.46, 40.15, 37.43, 35.89, 35.80, 35.34, 34.97, 34.68, 31.01, 30.97, 30.02, 29.97, 28.18, 27.40, 26.45, 24.17, 23.97, 21.07, 20.78, 18.24, 14.17, 12.01; HRMS calcd for C29H50NaO3 (M + Na)+ 469.3652, found 484.3.655.
Synthesis of compound 4: A methanolic solution of conc. ammonia (4 mL) was added to 20 (37 mg, 0.084 mmol). The mixture was stirred for 7 d at room temperature, and then for 4 d at 30 °C. The solvent was removed in vacuo, and the residue was purified by GPC (chloroform) to give 4a (18 mg, 51%) as a colorless solid. 1H NMR (400 MHz, CDCl3) δ 5.36 (brs, 1H), 5.22 (brs, 1H), 2.32-2.25 (m, 1H), 2.15-2.07 (m, 1H), 1.95 (d, J = 11.4 Hz, 1H), 1.87-1.73 (m, 4H), 1.59-0.91 (m, 25H), 1.22 (s, 6H), 0.93 (d, J = 6.4 Hz, 3H), 0.91 (s, 3H), 0.64 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 175.82, 71.65, 56.50, 55.93, 51.22, 43.54, 42.72, 40.46, 40.17, 37.42, 35.89, 35.80, 35.45, 34.97, 34.67, 32.75, 31.59, 30.03, 29.96, 29.76, 28.25, 27.39, 26.45, 24.17, 23.96, 20.78, 18.33, 12.02; HRMS calcd for C28H49NaO2 (M+Na)+ 454.3656, found 454.3649.
Compound 4b was synthesized similarly. 4b: 1H NMR (400 MHz, CDCl3) δ 5.38 (brs, 1H), 2.80 (d, J = 5.0 Hz, 3H), 2.23-2.19 (m, 1H), 2.08-2.02 (m, 1H), 1.94 (d, J = 11.4 Hz, 1H), 1.85-1.73 (m, 4H), 1.49-0.90 (m, 25H), 1.22 (s, 6H), 0.91 (d, J = 6.4 Hz, 3H), 0.91 (s, 3H), 0.63 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 174.19, 71.66, 56.51, 55.97, 51.23, 43.55, 42.70, 40.47, 40.17, 37.43, 35.89, 35.80, 35.52, 34.97, 34.68, 33.55, 31.82, 30.03, 29.96, 29.77, 28.24, 27.40, 26.45, 26.30, 24.17, 23.97, 20.78, 18.35, 12.02; HRMS calcd for C29H51NaO2 (M + Na)+ 468.3796, found 468.3805.
Synthesis of compound 21: O-Benzylhydroxylamine hydrochloride (24 mg, 0.15 mmol), N,N-diisopropylethylamine (20 mg, 0.16 mmol) and 1-hydroxybenzotriazole (17 mg, 0.13 mmol) were successively added to a solution of 3 (49 mg, 0.11 mmol) in dry dichloromethane (10 mL) at room temperature. After 10 min, N,N’-dicyclohexylcarbodiimide (29 mg, 0.14 mmol) was added to it. The mixture was stirred for 23 h at room temperature, and then filtered. The filtrate was washed with 5% hydrochloric acid, and brine, dried over sodium sulfate, filtered, and concentrated. The residue was purified by silica gel column chromatography (ethyl acetate/n-hexane = 1:2) to give 21 (68 mg, quant.) as a colorless solid. 1H NMR (600 MHz, CDCl3) δ 7.85 (br, 1 H), 7.41-7.39 (m, 5 H), 4.92 (br, 2 H), 1.94-0.92 (m, 31 H), 1.22 (s, 6 H), 0.91 (s, 3 H), 0.88 (d, J = 4.8 Hz, 3 H), 0.62 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 171.44, 137.83, 129.24, 128.59, 128.18, 78.03, 71.66, 56.48, 55.88, 51.21, 43.53, 42.69, 40.45, 40.15, 37.41, 35.88, 35.78, 35.41, 34.95, 34.66, 31.43, 30.02, 29.93, 29.75, 29.66, 28.19, 27.38, 26.43, 24.15, 23.95, 20.76, 18.26, 12.02; HRMS calcd for C35H56NO3 (M + H)+ 538.4255, found 538.4241.
Synthesis of compound 5b: Compound 3 (20 mg, 0.047 mmol) in DMF (0.5 mL) was added to a solution of triethylamine (12 mg, 0.12 mmol) in DMF (0.5 mL). The mixture was stirred at 0 °C for 10 min, and then ethyl chloroformate (6 mg, 0.055 mmol) in DMF (0.5 mL) was added to it. The resulting mixture was stirred at 0 °C for 45 min, and a mixture of methoxyamine hydrochloride (4 mg, 0.052 mmol) and triethylamine (12 mg, 0.12 mmol) in DMF (1.0 mL) was added to it. Stirring was continued at room temperature for 4 h, then the solvent was removed in vacuo, and the residue was extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate, filtered, and concentrated. The residue was purified by GPC (chloroform) to give 5b (16 mg, 73%) as a colorless solid. 1H NMR (400 MHz, CDCl3) δ 7.98 (brs, 1H), 3.76 (s, 3H), 2.17 (brs, 1H), 1.94 (d, J = 10.5, 1H), 1.87-1.73 (m, 4H), 1.59-0.91 (m, H), 1.22 (s, 6H), 0.92 (d, J = 8.2 Hz, 3H), 0.91 (s, 3H), 0.64 (s, 3H) ; 13C NMR (150MHz, CDCl3) δ 171.64, 71.73, 64.56, 56.56, 55.95, 51.28, 43.60, 42.78, 40.52, 40.22, 37.48, 35.95, 35.85, 35.51, 35.03, 34.73, 31.45, 30.25, 30.09, 30.01, 29.83, 28.28, 27.45, 26.50, 24.22, 24.02, 20.84, 18.38, 12.08; HRMS calcd for C29H51NaO3 (M + Na)+ 484.3761, found 484.3762.
Synthesis of compound 5a: Palladium hydroxide (13 mg) was added to a solution of 21 (68 mg, 0.11 mmol) in dry methanol (15 mL). The mixture was stirred for 24 h at room temperature under a hydrogen atmosphere, then filtered, and the filtrate was concentrated. The residue was purified by silica gel column chromatography (ethyl acetate/n-hexane = 2:1, ethyl acetate, then, ethyl acetate/methanol = 20:1) to give 5a (31 mg, 55%) as a colorless solid. 1H NMR (600 MHz, CDCl3) δ 5.40 (br, 1 H), 5.33 (br, 1 H), 2.28 (ddd, J = 15.6, 10.8, 4.2 Hz 1 H), 2.11 (ddd, J = 16.8, 10.8, 6.0 Hz 1 H), 1.95-1.92 (m, 1 H), 1.85-0.94 (m, 28 H), 1.22 (s, 6 H), 0.92 (d, J = 6.6 Hz, 3 H), 0.90 (s, 3 H), 0.63 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 176.04, 71.69, 56.52, 55.94, 51.23, 43.56, 42.73, 40.47, 40.18, 37.44, 35.90, 35.81, 35.46, 34.98, 34.69, 32.77, 31.61, 30.04, 29.97, 29.78, 28.27, 27.41, 26.46, 24.18, 23.98, 20.80, 18.35, 12.04; Anal. calcd for C28H49NO3: C, 75.12; H, 11.03; N, 3.15, found: C, 76.35; H, 11.04; N, 3.20.
Synthesis of compound 6: 4-Dimethylaminopyridine (23 mg, 0.19 mmol), cyanamide (19 mg, 0.45 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (42 mg, 0.22 mmol) and N,N-diisopropylethylamine (39 mg, 0.30 mmol) were successively added to a solution of 3 (59 mg, 0.14 mmol) in dry dichloromethane (5 mL). The mixture was stirred for 18 h at room temperature under an argon atmosphere, then diluted with dichloromethane, washed with 2 M hydrochloric acid and brine, dried over sodium sulfate, filtered, and concentrated. The residue was purified by silica gel flash column chromatography (chloroform/methanol = 10:1) to give 6 (5 mg, 89%) as a colorless oil. 1H NMR (600 MHz, pyridine-d5) δ 2.65 (br, 1 H), 2.52 (br, 1 H), 2.01-1.95 (m, 1 H), 1.86-1.72 (m, 6 H), 1.65-0.85 (m, 22 H), 1.44 (s, 6 H), 0.92 (s, 3 H), 0.88 (d, J = 6.0 Hz, 3 H), 0.56 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 174.15, 108.26, 71.57, 56.39, 55.76, 51.03, 43.46, 42.64, 40.36, 40.05, 37.34, 35.82, 35.70, 35.21, 34.85, 34.57, 32.21, 30.49, 29.68, 29.62, 29.51, 28.08, 27.30, 26.35, 24.07, 23.85, 20.68, 18.09, 11.90; HRMS calcd for C29H47N2O2 (M-H)- 455.3643, found 455.3627.
Synthesis of compound 22: L-Glycine methyl ester hydrochloride (8 mg, 0.06 mmol) and N-methylmorpholine (13 mg, 0.12 mmol) were added to a solution of 3 (20 mg, 0.047 mmol) in dry dichloromethane (8 mL). 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (12 mg, 0.06 mmol) was added to the mixture under an argon atmosphere. The resulting mixture was stirred for 24 h at room temperature, then diluted with dichloromethane, washed with 2 M hydrochloric acid, and brine, dried over sodium sulfate, filtered, and concentrated. The residue was purified by silica gel column chromatography (dichloromethane/methanol = 19:1) to give 22a (19 mg, 83%) as a colorless solid. 1H NMR (600 MHz, CDCl3) δ 5.91 (br, 1 H), 4.05 (d, J = 5.4 Hz, 2 H), 3.77 (s, 3 H), 2.30 (ddd, J = 15.6, 10.8, 5.4 Hz 1 H), 2.13 (ddd, J = 15.0, 10.2, 6.0 Hz 1 H), 1.96-1.94 (m, 1 H), 1.87-1.73 (m, 5 H), 1.56-0.96 (m, 23 H), 1.22 (s, 6 H), 0.92 (d, J = 6.6 Hz, 3 H), 0.91 (s, 3 H), 0.63 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 173.58, 170.52, 71.55, 56.40, 55.85, 52.19, 51.13, 43.45, 42.61, 41.07, 40.36, 40.07, 37.33, 35.79, 35.70, 35.36, 34.87, 34.58, 33.14, 31.46, 29.93, 29.86, 29.67, 28.13, 27.30, 26.35, 24.07, 23.87, 20.68, 18.23, 11.92; HRMS calcd for C31H53NNaO4 (M + Na)+ 526.3867, found 526.3860.
Compounds 22b and 22c were synthesized similarly. 22b: 1H NMR (600 MHz, CDCl3) δ 6.01 (t, J = 6.6 Hz, 1 H), 3.70 (s, 3 H), 3.51 (q, J = 6.0 Hz, 2 H), 2.54 (t, J = 6.0 Hz, 2 H), 2.24-2.18 (m, 1 H), 2.07-2.01 (m, 1 H), 1.95-1.92 (m, 1 H), 1.87-1.73 (m, 4 H), 1.56-0.96 (m, 24 H), 1.22 (s, 6 H), 0.91 (s, 3 H), 0.90 (d, J = 6.0 Hz, 3 H), 0.63 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 173.64, 173.32, 71.67, 56.50, 55.96, 51.83, 51.22, 43.55, 42.70, 40.46, 40.17, 37.43, 35.89, 35.80, 35.47, 34.97, 34.67, 33.81, 33.62, 31.70, 30.02, 29.95, 29.77, 28.24, 27.40, 26.45, 24.17, 23.96, 20.78, 18.33, 12.01; HRMS calcd for C32H55NNaO4 (M + Na)+ 540.4023, found 540.4033. 22c: 1H NMR (600 MHz, CDCl3) δ 5.63 (br, 1 H), 3.68 (s, 3 H), 3.29 (q, J = 7.2 Hz, 2 H), 2.37 (t, J = 7.2 Hz, 2 H), 2.22 (ddd, J = 15.6, 10.2, 4.2 Hz, 1 H), 2.04 (ddd, J = 15.6, 10.2, 6.0 Hz, 1 H), 1.94-1.02 (m, 1 H), 1.84 (q, J = 7.2 Hz, 2 H), 1.77-1.73 (m, 3 H), 1.57-0.93 (m, 25 H), 1.22 (s, 6 H), 0.91 (d, J = 6.6 Hz, 3 H), 0.91 (s, 3 H), 0.63 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 173.98, 173.80, 71.66, 56.49, 55.95, 51.75, 51.21, 43.54, 42.69, 40.45, 40.16, 38.92, 37.42, 35.88, 35.79, 35.48, 34.96, 34.66, 33.61, 31.77, 31.46, 30.01, 29.94, 29.76, 28.24, 27.39, 26.44, 24.54, 24.16, 23.95, 20.77, 18.34, 12.00; HRMS calcd for C33H57NNaO4 (M + Na)+ 554. 4180, found 554.4179.
Synthesis of compound 7: 15% w/v aqueous sodium hydroxide (1 mL) was added to a solution of 22a (8 mg, 0.035 mmol) in ethanol (5 mL), and the mixture was stirred for 3 h at room temperature. Ethanol was removed in vacuo, and the solution was acidified with conc. hydrochloric acid until a precipitate was formed. This was collected and washed with water to give 7a (13 mg, 77%) as a colorless solid. 1H NMR (600 MHz, CD3OD) δ 3.86 (s, 2 H), 2.30 (ddd, J = 13.8, 10.2, 5.4 Hz, 1 H), 2.15 (ddd, J = 13.8, 9.6, 6.6 Hz, 1 H), 2.00-1.98 (m, 1 H), 1.91-1.85 (m, 2 H), 1.82-1.76 (m, 2 H), 1.60-0.98 (m, 24 H), 1.17 (s, 6 H), 0.96 (d, J = 6.0 Hz, 3 H), 0.93 (s, 3 H), 0.68 (s, 3 H); 13C NMR (150 MHz, CD3OD) δ 177.10, 173.50, 72.01, 57.95, 57.46, 52.08, 49.56, 45.15, 43.91, 42.03, 41.90, 41.57, 38.69, 37.27, 36.83, 36.22, 35.82, 33.81, 33.08, 31.00, 29.93, 29.91, 29.25, 28.63, 27.75, 25.30, 24.54, 21.94, 18.83, 12.47; HRMS calcd for C30H51NNaO4 (M + Na)+ 512.3710, found 512.3707.
Compounds 7b and 7c were synthesized similarly. 7b: 1H NMR (600 MHz, CD3OD) δ 3.39 (t, J = 6.6 Hz, 2 H), 2.48 (t, J = 6.6 Hz, 2 H), 2.21-2.19 (m, 1 H), 2.10-2.05 (m, 1 H), 2.00-1.98 (m, 1 H), 1.91-1.87 (m, 2 H), 1.78-1.73 (m, 2 H), 1.59-1.49 (m, 4 H), 1.40-0.96 (m, 26 H), 0.94 (d, J = 7.2 Hz, 3 H), 0.93 (s, 3 H), 0.67 (s, 3 H); 13C NMR (150 MHz, CD3OD) δ 176.87, 175.48, 72.00, 57.94, 57.43, 52.08, 45.14, 43.90, 41.89, 41.55, 38.68, 37.26, 36.85, 36.42, 36.21, 35.81, 34.77, 34.00, 33.28, 30.99, 29.92, 29.90, 29.28, 28.62, 27.74, 25.28, 24.53, 21.93, 18.81, 12.45; HRMS calcd for C31H53NNaO4 (M + Na)+ 526.3867, found 526.3862. 7c: 1H NMR (600 MHz, CDCl3) δ 5.63 (br, 1 H), 3.68 (s, 3 H), 3.29 (q, J = 7.2 Hz, 2 H), 2.37 (t, J = 7.2 Hz, 2 H), 2.22 (ddd, J = 15.6, 10.2, 4.2 Hz, 1 H), 2.04 (ddd, J = 15.6, 10.2, 6.0 Hz, 1 H), 1.94-1.02 (m, 1 H), 1.84 (q, J = 7.2 Hz, 2 H), 1.77-1.73 (m, 3 H), 1.57-0.93 (m, 25 H), 1.22 (s, 6 H), 0.91 (d, J = 6.6 Hz, 3 H), 0.91 (s, 3 H), 0.63 (s, 3 H); 13C NMR (150 MHz, CDCl3) δ 173.98, 173.80, 71.66, 56.49, 55.95, 51.75, 51.21, 43.54, 42.69, 40.45, 40.16, 38.92, 37.42, 35.88, 35.79, 35.48, 34.96, 34.66, 33.61, 31.77, 31.46, 30.01, 29.94, 29.76, 28.24, 27.39, 26.44, 24.54, 24.16, 23.95, 20.77, 18.34, 12.00; HRMS calcd for C33H57NNaO4 (M + Na)+ 554. 4180, found 554.4179.
Synthesis of compound 8: 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (123 mg, 0.44 mmol) and triethylamine (437 mg, 4.32 mmol) were added to a solution of 3 (75 mg, 0.17 mmol) in dry N,N-dimethylformamide (8 mL). The mixture was stirred for 10 min at room temperature under an argon atmosphere, and aminomethanesulfuric acid (132.2 mg, 1.190 mmol) was added to it. The mixture was stirred for 20 h at room temperature, then filtered, and the solvent was removed in vacuo. The residue was extracted with ethyl acetate and water. The water layer was cooled to 0 °C, and conc. hydrochloric acid (5.0 mL) was added. The resulting precipitate was collected, and washed with water to give 8a (67 mg, 74%) as a pale yellow solid. 1H NMR (600 MHz, CD3OD) δ 4.16 (q, J = 13.2 Hz, 2 H), 2.18 (br, 1 H), 2.02 (br, 1 H), 1.85 (d, J = 11.4 Hz, 1 H), 1.75 (m, 2 H), 1.63 (d, J = 13.8 Hz, 2 H), 1.44-0.84 (m, 24 H), 1.03 (s, 6 H), 0.81 (d, J = 6.0 Hz, 3 H), 0.79 (s, 3 H), 0.53 (s, 3 H); HRMS calcd for C29H50NO5S (M-H)- 524.3415, found 524.3404.
Compound 8b was synthesized similarly. 8b: 1H NMR (600 MHz, Pyrdine-d5) δ 4.23 (t, J = 5.4 Hz, 2 H), 3.48 (t, J = 5.4 Hz, 2 H), 2.40 (ddd, J = 14.4, 10.2, 4.2 Hz, 1 H), 2.25 (ddd, J = 16.2, 10.2, 6.0 Hz, 1 H), 2.00-1.98 (m, 1 H), 1.82-0.96 (m, 28 H), 1.42 (s, 6 H), 0.90 (s, 3 H), 0.85 (d, J = 6.0 Hz, 3 H), 0.53 (s, 3 H); 13C NMR (150 MHz, Pyrdine-d5) δ 174.07, 70.15, 56.46, 56.19, 52.01, 51.70, 44.00, 42.79, 40.71, 40.33, 37.92, 36.52, 36.41, 35.96, 35.73, 35.45, 34.90, 33.78, 32.26, 30.78, 30.74, 30.33, 28.35, 27.80, 26.75, 24.36, 24.24, 21.08, 18.55, 12.19; HRMS calcd for C30H52NO5S (M-H)- 538.3572, found 528.3576.
4.3. HL-60 Cell Differentiation Assay
HL-60 cells were cultured in RPMI-1640 medium supplemented with 5% FBS and penicillin G and streptomycin at 37 °C under 5% CO2 in air [14]. The cells were diluted to 8.0 × 104 cells/mL with RPMI-1640 (5% FBS), and an ethanol solution of a test compound was added to give 10−9 to 10−6 M final concentration. Control cells were treated with the same volume of ethanol alone. 1α,25(OH)2D3 was always assayed at the same time as a positive control. The cells were incubated at 37 °C under 5% CO2 in air for 4 days. The percentage of differentiated cells was determined by nitro-blue tetrazolium (NBT) reduction assay. Cells were incubated at 37 °C for 20 min in RPMI-1640 (5% FBS) and an equal volume of phosphate-buffered saline (PBS) containing NBT (0.2%) and 12-O-tetradecanoylphorbol 13-acetate (TPA; 200 ng/mL). The percentage of cells containing blue-black formazan was determined in a minimum of 200 cells. All experiments were done in triplicate.
4.4. Transactivation Assay
Human embryonic kidney HEK293 cells (RIKEN Cell Bank, Tsukuba, Japan) were cultured in Dulbecco’s modified Eagle’s medium containing 5% FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Nacalai Tesque, Kyoto, Japan). Transfections used 15 ng of pCMX-hVDR, 50 ng of TK-Spp × 3-LUC reporter plasmid, and 10 ng of pCMX-β-galactosidase for each well of a 96-well plate, and were performed by the calcium phosphate coprecipitation assay as described previously [13]. Eight hours after transfection, test compounds were added. Cells were harvested after 16–24 h and were assayed for luciferase and β-galactosidase activity using a luminometer and a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Luciferase data were normalized to the internal β-galactosidase control. All experiments were done in triplicate.
4.5. X-ray Crystallographic Analysis
Crystals of VDR complexes were prepared according to the method of Vanhooke et al. [16] with some modifications. The rat VDR LBD (residues 116–423, Δ165–211) was cloned as an N-terminal His6-tagged fusion protein into the pET14b expression vector and overproduced in Escherichia coli C41. The cells were grown at 37 °C in LB medium (including ampicillin 100 mg/L) and subsequently induced for 6 h with 15 µM iso-propyl-β-d-thiogalactopyranoside (IPTG) at 23 °C. The purification procedure included affinity chromatography on a Ni-NTA column, followed by dialysis and cation-exchange chromatography (SP-Sepharose). After tag removal by thrombin digestion, the protease was removed by filtration through a HiTrap benzamidine column and the protein was further purified by gel filtration on a Super-dex200 column. The purity and homogeneity of the rVDR LBD were assessed by SDS-PAGE.
Purified rVDR LBD solution was concentrated to about 0.75 mg/mL by ultrafiltration. To an aliquot (800 µL) of the protein solution a ligand was added (approx. 10 equiv). Then the solution was further concentrated to about 1/8, and a solution (25 mM Tris-HCl, pH 8.0; 50 mM NaCl; 10 mM DTT; 0.02% NaN3) of coactivator peptide (H2N-KNHPMLMNLLKDN-CONH2) derived from DRIP205 was added. This solution of VDR/ligand/peptide was allowed to crystallize by the vapor diffusion method using a series of precipitant solutions containing 0.2 M potassium citrate tribasic monohydrate, 20% (w/v) PEG3350. Droplets for crystallization were prepared by mixing 2 μL of complex solution and 1 μL precipitant solution, and equilibrated against 500 μL of precipitant solution at 20 °C.
Prior to diffraction data collection, crystals were soaked in a cryoprotectant solution containing 0.2 M potassium citrate tribasic monohydrate, 20% (w/v) PEG3350, and 17–20% ethylene glycol. Diffraction data sets were collected at 100 K in a stream of nitrogen gas at beamline BL-17A of KEK-PF (Tsukuba, Japan). Reflections were recorded with an oscillation range per image of 1.0°. Diffraction data were indexed, integrated, and scaled using the program HKL2000 (HKL Research Inc., Charlottesville, VA, USA). The structures were solved by molecular replacement with the program Phaser in Phenix [17], using the rat VDR LBD coordinates (PDB code: 2ZLC), and finalized sets of atomic coordinates were obtained after iterative rounds of model modification with the program COOT [18] and refinement with REFMAC [19]. The coordinates and structure factors have been deposited in the Protein Data Bank (Entry ID: 7VQP).
Author Contributions
A.Y., H.K. (Haru Kawasaki) and H.M. synthesized the compounds. K.T., N.N. and N.I. examined the crystal structure. N.H., Y.K., M.I. and M.M. examined the biological activities. H.K. (Hiroyuki Kagechika) and A.T. planed and supervised the scientific works, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by JSPS KAKENHI Grant No. 16K08318, 20K06963 (to AT), JSPS Core-to-Core Program, and Japan Agency for Medical Research and Development (AMED) under Grant Number JP20am0101098 (Platform Project for Supporting Drug Discovery and Life Science Research, BINDS). A part of this research is based on the Cooperative Research Project of the Research Center for Biomedical Engineering. A.T. thanks The Naito Foundation, the Cosmetology Research Foundation, and the Tokyo Biochemical Research Foundation for their support. The X-ray crystal structure analysis was performed with the approval of the Photon Factory Program Advisory Committee (Proposal No. 2019G595).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Feldman, D.; Pike, J.W.; Adams, J.S. (Eds.) Vitamin D, 4th ed.; Elsevier Science: London, UK, 2017. [Google Scholar]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2015, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Okamura, W.H.; Norman, A.W. Structure-Function Relationships in the Vitamin D Endocrine System. Endocr. Rev. 1995, 16, 200–257. [Google Scholar] [PubMed]
- Maestro, M.A.; Molnár, F.; Mouriño, A.; Carlberg, C. Vitamin D Receptor 2016: Novel Ligands and Structural Insights. Expert Opin. Ther. Pat. 2016, 26, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Maestro, M.A.; Molnár, F.; Carlberg, C. Vitamin D and Its Synthetic Analogs. J. Med. Chem. 2019, 62, 6854–6875. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Lu, T.T.; Xie, W.; Whitfield, G.K.; Domoto, H.; Evans, R.M.; Haussler, M.R.; Mangelsdorf, D.J. Vitamin D Receptor As an Intestinal Bile Acid Sensor. Science 2002, 296, 1313–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belorusova, A.Y.; Eberhardt, J.; Potier, N.; Stote, R.H.; Dejaegere, A.; Rochel, N. Structural Insights into the Molecular Mechanism of Vitamin D Receptor Activation by Lithocholic Acid Involving a New Mode of Ligand Recognition. J. Med. Chem. 2014, 57, 4710–4719. [Google Scholar] [CrossRef] [PubMed]
- Marchionatti, A.M.; Pérez, A.; Rivoira, M.A.; Rodríguez, V.A.; Tolosa de Talamoni, N.G. Lithocholic Acid: A New Emergent Protector of Intestinal Calcium Absorption under Oxidant Conditions. Biochem. Cell Biol. 2017, 95, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizawa, M.; Akagi, D.; Makishima, M. Lithocholic Acid Is a Vitamin D Receptor Ligand That Acts Preferentially in the Ileum. Int. J. Mol. Sci. 2018, 19, 1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, H.; Masuno, H.; Kawasaki, H.; Yoshihara, A.; Numoto, N.; Ito, N.; Ishida, H.; Yamamoto, K.; Hirata, N.; Kanda, Y.; et al. Lithocholic Acid Derivatives as Potent Vitamin D Receptor Agonists. J. Med. Chem. 2021, 64, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, S.; González, C.M.; Vilariño, D.; Lasanta, G.; Villaverde, C.; Mouriño, A.; Verlinden, L.; Verstuyf, A.; Peluso-Iltis, C.; Rochel, N.; et al. Lithocholic acid-based design of noncalcemic vitamin D receptor agonists. Bioorg. Chem. 2021, 111, 104878. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Koeffler, H.P.; Donaldson, C.A.; Pike, J.W.; Haussler, M.R. 1,25-Dihydroxyvitamin D3-Induced Differentiation in a Human Promyelocytic Leukemia Cell Line (HL-60): Receptor-Mediated Maturation to Macrophage-like Cells. J. Cell Biol. 1984, 98, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, T.; Ishizawa, M.; Maekawa, K.; Nakabayashi, M.; Watarai, Y.; Uchida, H.; Tokiwa, H.; Ikura, T.; Ito, N.; Makishima, M.; et al. Combination of triple bond and adamantane ring on the vitamin D side chain produced partial agonists for vitamin D receptor. J. Med. Chem. 2014, 57, 4073–4087. [Google Scholar] [CrossRef] [PubMed]
- Masuno, H.; Ikura, T.; Morizono, D.; Orita, I.; Yamada, S.; Shimizu, M.; Ito, N. Crystal Structures of Complexes of Vitamin D Receptor Ligand-Binding Domain with Lithocholic Acid Derivatives. J. Lipid Res. 2013, 54, 2206–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, C.M.; Gaikwad, S.; Lasanta, G.; Loureiro, J.; Nilsson, N.; Peluso-Iltis, C.; Rochel, N.; Mouriñoa, A. Design, synthesis and evaluation of side-chain hydroxylated derivatives of lithocholic acid as potent agonists of the vitamin D receptor (VDR). Bioorg. Chem. 2021, 115, 105202. [Google Scholar] [CrossRef] [PubMed]
- Vanhooke, J.L.; Benning, M.M.; Bauer, C.B.; Pike, J.W.; DeLuca, H.F. Molecular Structure of the Rat Vitamin D Receptor Ligand Binding Domain Complexed with 2-Carbon-Substituted Vitamin D3 Hormone Analogues and a LXXLL-Containing Coactivator Peptide. Biochemistry 2004, 43, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Liebschner, D.; Afonine, P.V.; Baker, M.L.; Bunkóczi, G.; Chen, V.B.; Croll, T.I.; Hintze, B.; Hung, L.W.; Jain, S.; McCoy, A.J.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Cryst. D 2019, 75, 861–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Cryst. Sect. D 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Cryst. Sect. D 2011, 67, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).