Sex Hormone-Specific Neuroanatomy of Takotsubo Syndrome: Is the Insular Cortex a Moderator?
Abstract
1. Introduction
2. The Brain–Heart Connection
2.1. Cortical Regulation in the Circulatory System in Response to Emotional Stress
The Central Autonomic Network (CAN)
2.2. The Insular Cortex and the Processing of Emotion
2.3. The Insular Cortex and the Regulation of the Cardiovascular System
- A.
- Animal Studies
- B.
- Human Studies
- B.1.
- The Role of Hemispheric Laterality in Sympathovagal Modulation
- B.2.
- The Insular Cortex and Heart Rate, Arrhythmia, and Sympathovagal Balance
- B.2.1.
- Epilepsy Studies
- B.2.2.
- Neuroimaging Studies
3. Stroke Involving the Insular Cortex and the Disrupted Cardiovascular System
3.1. Stroke Involving the Insular Cortex and Cerebrogenic Cardiac Arrhythmias
3.2. Stroke Involving the Insular Cortex and Asystole/Disrupted Diurnal BP Variation/Reduced Baroreflex Sensitivity/Acute Kidney Injury
3.3. Stroke Involving the Insular Cortex and Cardiac Overload/Injury
4. Takotsubo Syndrome
4.1. Pathophysiology of Takotsubo Syndrome
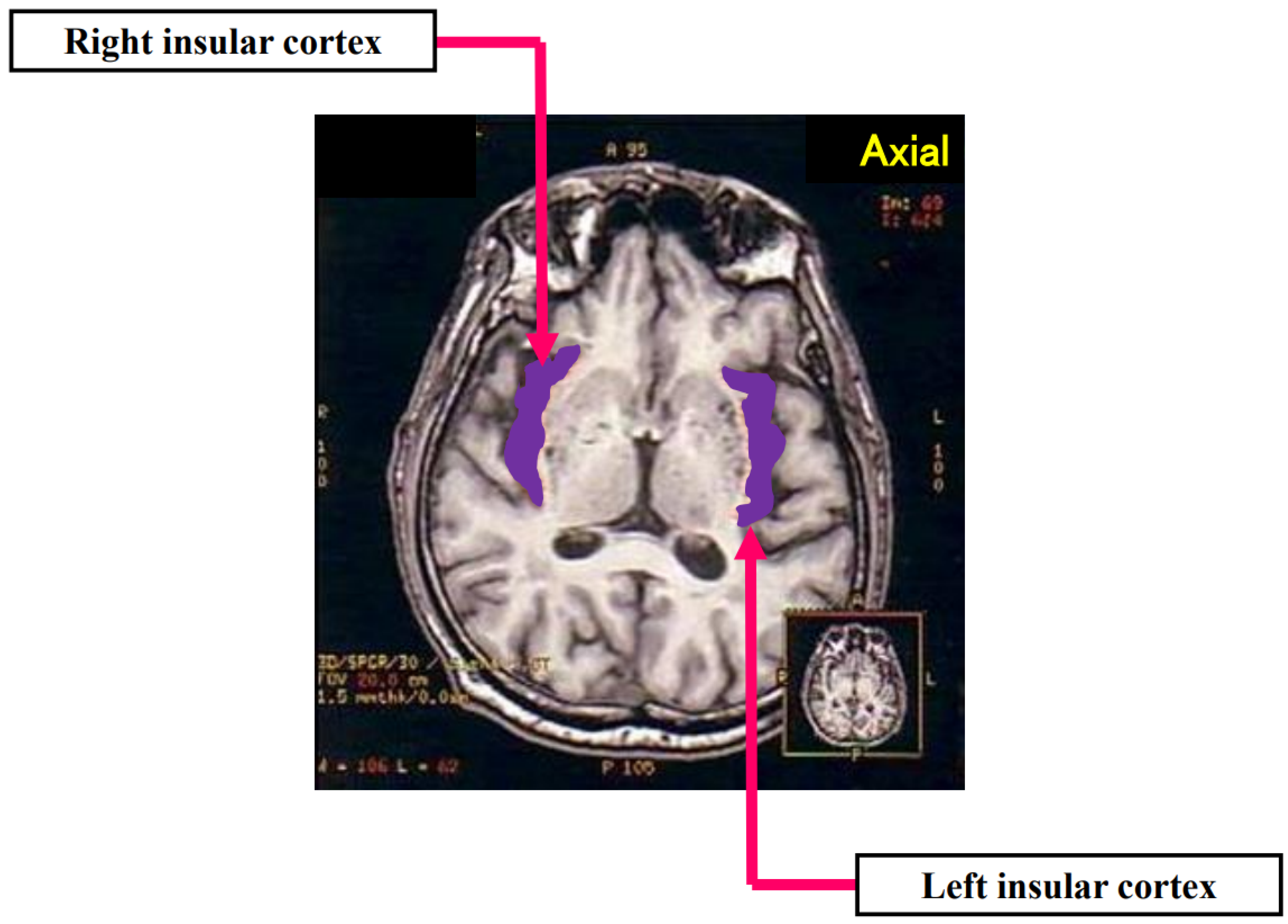
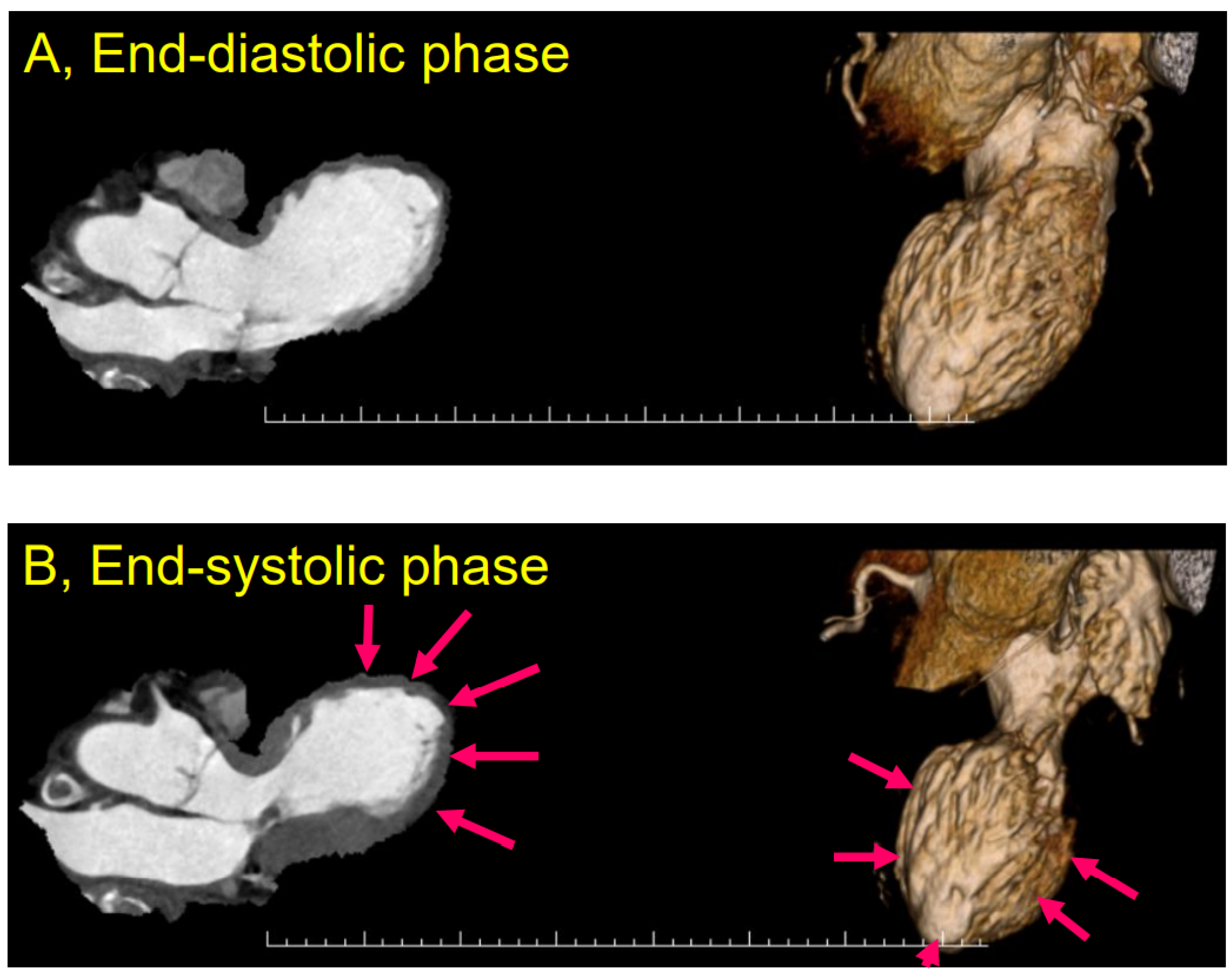
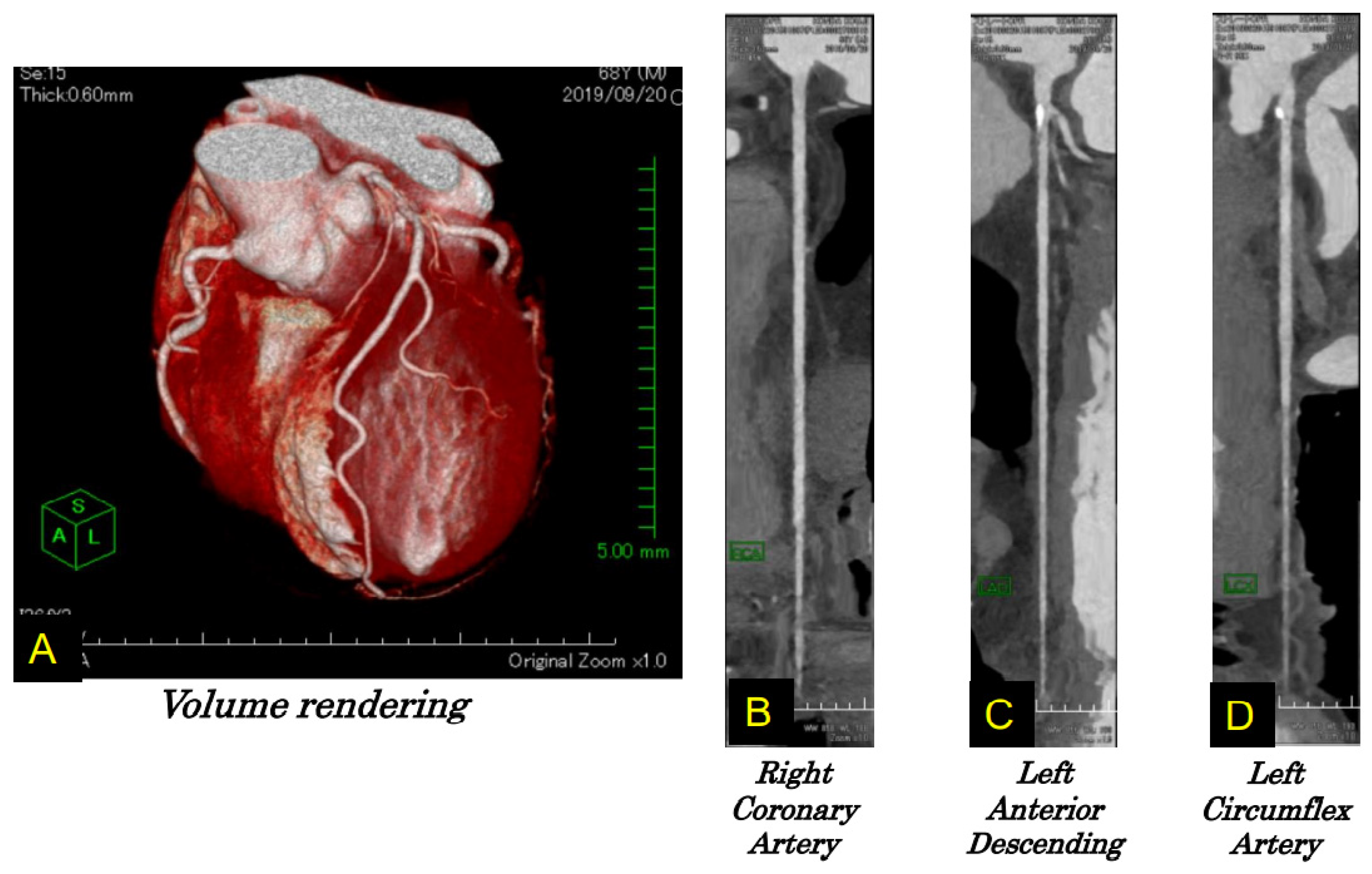
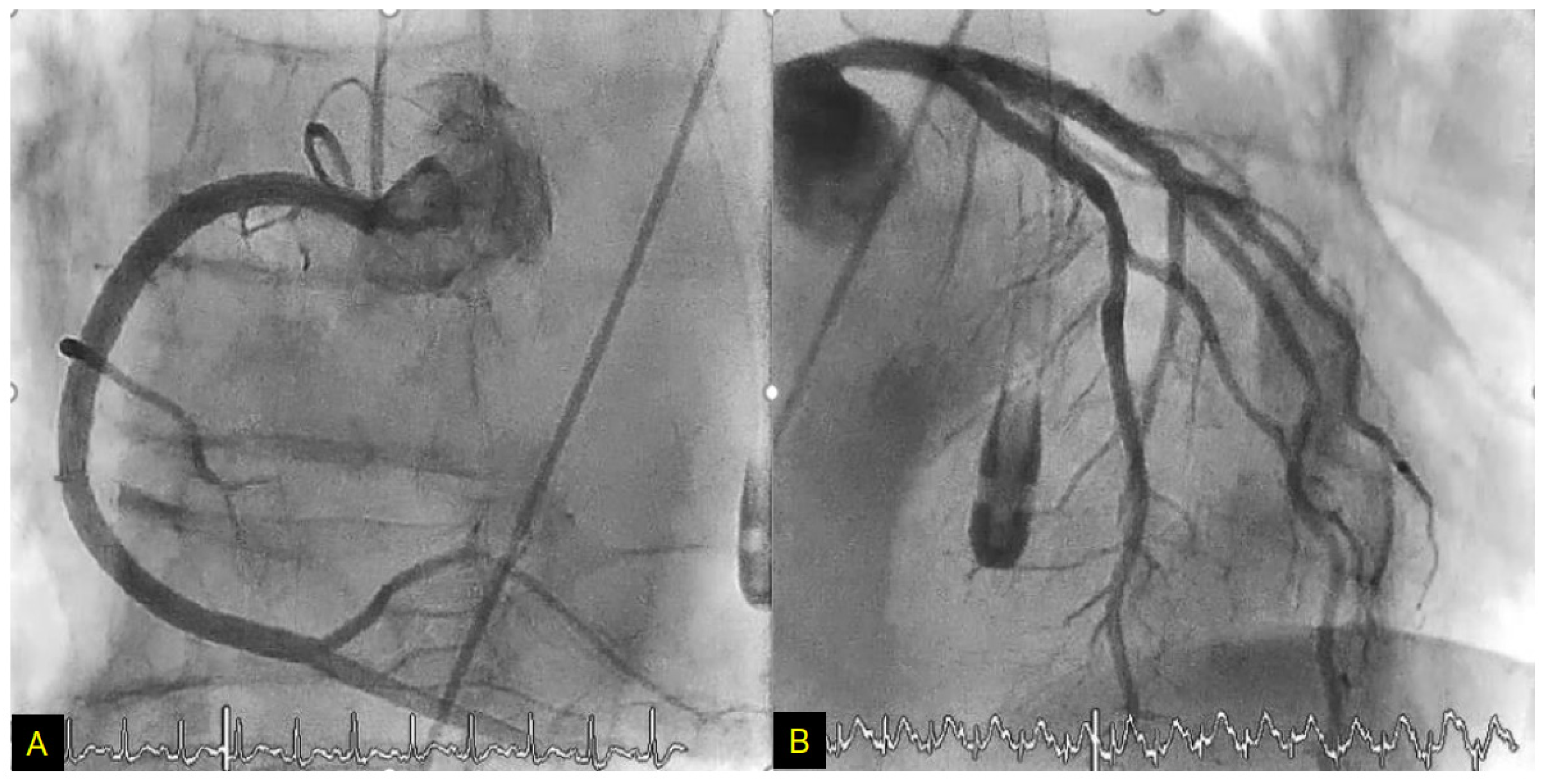
4.2. Case Report
4.3. Ic Stroke and Takotsubo Syndrome
5. Possible Role of Estrogen Deficiency in Takotsubo Syndrome
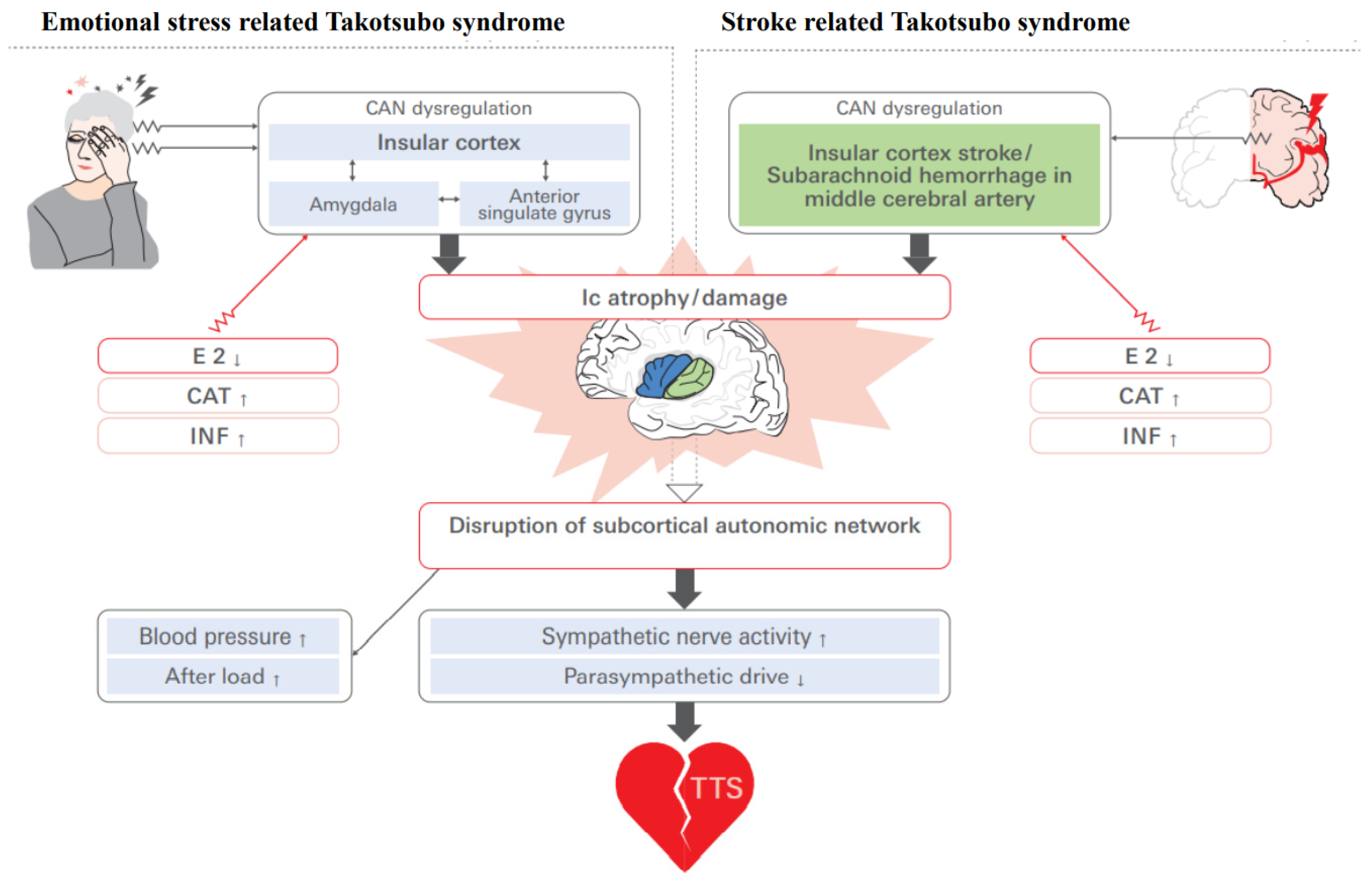
6. Sex-Specific Neuroanatomy of Takotsubo Syndrome: How Does the Ic Interact?
6.1. Estrogen Deficiency and Functional Cerebral Asymmetry in TTS
6.2. Circuitry and Functional Aspects of the Ic in Takotsubo Syndrome
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Remark
Abbreviations
References
- Dote, K.; Sato, H.; Uchinda, A.T.; Ishihara, M. Myocardial stunning due to simultaneous multivessel spasms: A review of 5 cases. J. Cardiol. 1991, 21, 203–214. [Google Scholar] [PubMed]
- Samuels, M.A. The brain-heart connection. Circulation 2007, 116, 77–84. [Google Scholar] [CrossRef]
- Nagai, M.; Hoshide, S.; Kario, K. The insular cortex and cardiovascular system: A new insight into the brain-heart axis. J. Am. Soc. Hypertens. 2010, 4, 174–182. [Google Scholar] [CrossRef]
- Nagai, M.; Dote, K.; Kato, M.; Sasaki, S.; Oda, N.; Kagawa, E.; Nakano, Y.; Yamane, A.; Higashihara, T.; Miyauchi, S.; et al. The insular cortex and Takotsubo cardiomyopathy. Curr. Pharm. Des. 2017, 23, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Drača, S. A possible relationship between Takotsubo cardiomyopathy and female sex steroid-related modulation of functional cerebral asymmetry. Med. Hypotheses 2015, 84, 238–240. [Google Scholar] [CrossRef]
- Benarroch, E.E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Nagai, M.; Kishi, K.; Kato, S. Insular cortex and neuropsychiatric disorders: A review of recent literature. Eur. Psychiatry 2007, 22, 387–394. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Ketter, T.A.; Parekh, P.I.; Horwitz, B.; Herscovitch, P.; Post, R.M. Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatry 1995, 152, 341–351. [Google Scholar]
- Reiman, E.M.; Lane, R.D.; Ahern, G.L.; Schwartz, G.E.; Davidson, R.J.; Friston, K.J.; Yun, L.S.; Chen, K. Neuroanatomical correlates of externally and internally generated human emotion. Am. J. Psychiatry 1997, 154, 918–925. [Google Scholar]
- Damasio, A.R.; Grabowski, T.J.; Bechara, A.; Damasio, H.; Ponto, L.L.; Parvizi, J.; Hichwa, R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat. Neurosci. 2000, 3, 1049–1056. [Google Scholar] [CrossRef]
- Lane, R.D.; Reiman, E.M.; Ahern, G.L.; Schwartz, G.E.; Davidson, R.J. Neuroanatomical correlates of happiness, sadness, and disgust. Am. J. Psychiatry 1997, 154, 926–933. [Google Scholar] [PubMed]
- Gu, X.; Hof, P.R.; Friston, K.J.; Fan, J. Anterior insular cortex and emotional awareness. J. Comp. Neurol. 2013, 521, 3371–3388. [Google Scholar] [CrossRef]
- Oppenheimer, S.M.; Cechetto, D.F. Cardiac chronotropic organization of the rat insular cortex. Brain Res. 1990, 533, 66–72. [Google Scholar] [CrossRef]
- Oppenheimer, S.M.; Saleh, T.M.; Wilson, J.X.; Cechetto, D.F. Plasma and organ catecholamine levels following stimulation of the rat insular cortex. Brain Res. 1992, 569, 221–228. [Google Scholar] [CrossRef]
- Oppenheimer, S.M.; Wilson, J.X.; Guiraudon, C.; Cechetto, D.F. Insular cortex stimulation produces lethal cardiac arrhythmias: A mechanism of sudden death? Brain Res. 1991, 550, 115–121. [Google Scholar] [CrossRef]
- Ming, Q.; Ma, H.; Li, J.; Yang, F.; Li, J.; Liang, J.; Li, D.; Lin, W. Changes in autonomic nervous function and influencing factors in a rat insular cortex electrical kindling model. Neurosci. Lett. 2020, 721, 134782. [Google Scholar] [CrossRef] [PubMed]
- Balint, B.; Jaremek, V.; Thorburn, V.; Whitehead, S.N.; Sposato, L.A. Left atrial microvascular endothelial dysfunction, myocardial inflammation and fibrosis after selective insular cortex ischemic stroke. Int. J. Cardiol. 2019, 292, 148–155. [Google Scholar] [CrossRef]
- Min, J.; Farooq, M.U.; Greenberg, E.; Aloka, F.; Bhatt, A.; Kassab, M.; Morgan, J.P.; Majid, A. Cardiac dysfunction after left permanent cerebral focal ischemia. The Brain and Heart Connection. Stroke 2009, 40, 2560–2563. [Google Scholar] [CrossRef]
- Cai, Y.; Lu, X.; Cheng, X.; Lv, Q.; Xu, G.; Liu, X. Increased renal dysfunction, apoptosis, and fibrogenesis through sympathetic hyperactivity after focal cerebral infarction. Transl. Stroke Res. 2021, in press. [CrossRef]
- Marins, F.R.; Limborço-Filho, M.; D’Abreu, B.F.; Machado de Almeida, P.W.; Gavioli, M.; Xavier, C.H.; Oppenheimer, S.M.; Guatimosim, S.; Fontes, M.A.P. Autonomic and cardiovascular consequences resulting from experimental hemorrhagic stroke in the left or right intermediate insular cortex in rats. Auton. Neurosci. 2020, 227, 102695. [Google Scholar] [CrossRef]
- Zamrini, E.Y.; Meador, K.J.; Loring, D.W.; Nichols, F.T.; Lee, G.P.; Figueroa, R.E.; Thompson, W.O. Unilateral cerebral inactivation produces differential left/right heart rate responses. Neurology 1990, 40, 1408–1411. [Google Scholar] [CrossRef]
- Yoon, B.W.; Morillo, C.A.; Cechetto, D.F.; Hachinski, V. Cerebral hemispheric lateralization in cardiac autonomic control. Arch. Neurol. 1997, 54, 741–744. [Google Scholar] [CrossRef]
- Hilz, M.J.; Dutsch, M.; Perrine, K.; Nelson, P.K.; Rauhut, U.; Devinsky, O. Hemispheric influence on autonomic modulation and baroreflex sensitivity. Ann. Neurol. 2001, 49, 575–584. [Google Scholar] [CrossRef]
- Costagliola, G.; Orsini, A.; Coll, M.; Brugada, R.; Parisi, P.; Striano, P. The brain-heart interaction in epilepsy: Implications for diagnosis, therapy, and SUDEP prevention. Ann. Clin. Transl. Neurol. 2021, 8, 1557–1568. [Google Scholar] [CrossRef]
- Lanz, M.; Oehl, B.; Brandt, A.; Schulze-Bonhage, A. Seizure induced cardiac asystole in epilepsy patients undergoing long term video-EEG monitoring. Seizure 2011, 20, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Marynissen, T.; Govers, N.; Vydt, T. Ictal asystole: Case report with review of literature. Acta Cardiol. 2012, 67, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Tayah, T.; Savard, M.; Desbiens, R.; Nguyen, D.K. Ictal bradycardia and asystole in an adult with a focal left insular lesion. Clin. Neurol. Neurosurg. 2013, 115, 1885–1887. [Google Scholar] [CrossRef] [PubMed]
- Al-Otaibi, F.; Wong, S.W.; Shoemaker, J.K.; Parrent, A.G.; Mirsattari, S.M. The cardioinhibitory responses of the right posterior insular cortex in an epileptic patient. Stereotact. Funct. Neurosurg. 2010, 88, 390–397. [Google Scholar] [CrossRef]
- Wali, A.; Siddiqui, F. Focal cortical dysplasia with prolonged ictal asystole, a case report. Clin. Neurophysiol. Pract. 2020, 6, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Okadome, T.; Mukaino, T.; Uehara, T.; Tanaka, H.; Kamada, T.; Miyoshi, A.; Akamatsu, N.; Ohara, S.; Shigeto, H. Ictal asystole as a manifestation of pure insular epilepsy. Seizure 2021, 91, 192–195. [Google Scholar] [CrossRef]
- Lagarde, S.; Singh, R.; Bartolomei, F.; Guedj, E. Insular interictal positron emission tomography hypometabolism in patients with ictal asystole. Epilepsia 2021, 62, e117–e122. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, S.M.; Gelb, A.; Girvin, J.P.; Hachinski, V.C. Cardiovascular effects of human insular cortex stimulation. Neurology 1992, 42, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Larsen, A.; Principe, A.; Ley, M.; Navarro-Cuartero, J.; Rocamora, R. Characterization of the insular role in cardiac function through intracranial electrical stimulation of the human insula. Ann. Neurol. 2021, 89, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Chouchou, F.; Mauguière, F.; Vallayer, O.; Catenoix, H.; Isnard, J.; Montavont, A.; Jung, J.; Pichot, V.; Rheims, S.; Mazzola, L. How the insula speaks to the heart: Cardiac responses to insular stimulation in humans. Hum. Brain Mapp. 2019, 40, 2611–2622. [Google Scholar] [CrossRef]
- Catenoix, H.; Mauguiere, F.; Guenot, M.; Isnard, J.; Ryvlin, P. Recording the insula during ictal asystole. Int. J. Cardiol. 2013, 169, e28–e30. [Google Scholar] [CrossRef] [PubMed]
- Obaid, S.; Zerouali, Y.; Nguyen, D.K. Insular epilepsy: Semiology and noninvasive investigations. J. Clin. Neurophysiol. 2017, 34, 315–323. [Google Scholar] [CrossRef]
- Zhang, Z.; Oppenheimer, S.M. Electrophysiological evidence for reciprocal insulo-insular connectivity of baroreceptor-related neurons. Brain Res. 2000, 863, 25–41. [Google Scholar] [CrossRef]
- Critchley, H.D.; Rotshtein, P.; Nagai, Y.; O’Doherty, J.; Mathias, C.J.; Dolan, R.J. Activity in the human brain predicting different heart rate responses to emotional facial expression. Neuroimage 2005, 24, 751–762. [Google Scholar] [CrossRef]
- Napadow, V.; Dhond, R.; Conti, G.; Makris, N. Brain correlates of autonomic modulation: Combining heart rate variability with fMRI. Neuroimage 2008, 42, 169–177. [Google Scholar] [CrossRef]
- Lane, R.; Reiman, E.; Ahern, G.; Thayer, J. Activity in medial prefrontal cortex correlates with vagal component of heart rate variability during emotion. Brain Cogn. 2001, 47, 97–100. [Google Scholar]
- Lane, R.D.; McRae, K.; Reiman, E.M.; Chen, K.; Ahern, G.L.; Thayer, J.F. Neural correlates of heart rate variability. Neurimage 2009, 44, 213–222. [Google Scholar] [CrossRef]
- Gianaros, P.J.; Van Der Veen, F.M.; Jennings, J.R. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology 2004, 41, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Garibotto, V.; Corpataux, T.; Dupuis-Lozeron, E.; Haller, S.; Fontolliet, T.; Picard, F. Higher nicotinic receptor availability in the cingulo-insular network is associated with lower cardiac parasympathetic tone. J. Comp. Neurol. 2019, 527, 3014–3022. [Google Scholar] [CrossRef]
- Winder, K.; Linker, R.A.; Seifert, F.; Wang, R.; Lee, D.H.; Engelhorn, T.; Dörfler, A.; Fröhlich, K.; Hilz, M. Cerebral lesion correlates of sympathetic cardiovascular activation in multiple sclerosis. Hum. Brain Mapp. 2019, 40, 5083–5093. [Google Scholar] [CrossRef]
- Kimmerly, D.S.; Morris, B.L.; Floras, J.S. Apnea-induced cortical BOLD-fMRI and peripheral sympathoneural firing response patterns of awake healthy humans. PLoS ONE 2013, 8, e82525. [Google Scholar] [CrossRef]
- Gianaros, P.J.; Sheu, L.K.; Uyar, F.; Koushik, J.; Jennings, J.R.; Wager, T.D.; Singh, A.; Verstynen, T.D. A brain phenotype for stressor-evoked blood pressure reactivity. J. Am. Heart Assoc. 2017, 6, e006053. [Google Scholar] [CrossRef]
- Song, X.; Roy, B.; Fonarow, G.C.; Woo, M.A.; Kumar, R. Brain structural changes associated with aberrant functional responses to the Valsalva maneuver in heart failure. J. Neurosci. Res. 2018, 96, 1610–1622. [Google Scholar] [CrossRef]
- Mithani, K.; Mikhail, M.; Morgan, B.R.; Wong, S.; Weil, A.G.; Deschenes, S.; Wang, S.; Bernal, B.; Guillen, M.R.; Ochi, A.; et al. Connectomic profiling identifies responders to vagus nerve stimulation. Ann. Neurol. 2019, 86, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Fentz, V.; Gormsen, J. Electrocardiographic patterns in patients with cerebrovascular accidents. Circulation 1962, 25, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, J.M.; Neil-Dwyer, G.; Brice, J. Electrocardiographic changes and their prognostic significance in subarachnoid hemorrhage. J. Neurol. Neurosurg. Psychiatry 1974, 37, 755–759. [Google Scholar] [CrossRef]
- Dimant, J.; Grob, D. Electrocardiographic changes and myocardial damage in patients with acute cerebrovascular accidents. Stroke 1977, 8, 448–455. [Google Scholar] [CrossRef]
- Mandrioli, J.; Zini, A.; Cavazzuti, M.; Panzetti, P. Neurogenic T wave inversion in pure left insular stroke associated with hyperhomocysteinaemia. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1788–1789. [Google Scholar] [CrossRef][Green Version]
- Lindberg, D.M.; Jauch, E.C. Neurogenic T waves preceding acute ischemic stroke. Circulation 2006, 114, e369–e370. [Google Scholar] [CrossRef] [PubMed]
- van Bree, M.D.; Roos, Y.B.; van der Bilt, I.A.; Wilde, A.A.; Sprengers, M.E.; de Gans, K.; Vergouwen, M.D. Prevalence and characterization of ECG abnormalities after intracerebral hemorrhage. Neurocrit. Care 2010, 12, 50–55. [Google Scholar] [CrossRef]
- Grad, A.; Kiauta, T.; Osredkar, J. Effect of elevated plasma norepinephrine on electrocardiographic changes in subarachnoid hemorrhage. Stroke 1991, 22, 746–749. [Google Scholar] [CrossRef]
- Tatschl, C.; Stöllberger, C.; Matz, K.; Yilmaz, N.; Eckhardt, R.; Nowotny, M.; Dachenhausen, A.; Brainin, M. Insular involvement is associated with QT prolongation: ECG abnormalities in patients with acute stroke. Cerebrovasc. Dis. 2006, 21, 47–53. [Google Scholar] [CrossRef]
- Abboud, H.; Berroir, S.; Labreuche, J.; Orjuela, K.; Amarenco, P. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann. Neurol. 2006, 59, 691–699. [Google Scholar] [CrossRef]
- Blech, B.; O’Carroll, C. Acute right middle cerebral artery occlusion resulting in acute systolic heart failure, cerebral T-waves, and QTc prolongation: A case report. Neurologist 2018, 23, 135–137. [Google Scholar] [CrossRef]
- Christensen, H.; Boysen, G.; Christensen, A.F.; Johannesen, H.H. Insular lesions, ECG abnormalities, and outcome in acute stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Vingerhoets, F.; Bogousslavsky, J.; Regli, F.; Van Melle, G. Atrial fibrillation after acute stroke. Stroke 1993, 24, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Ruiz, A.; Vargas-Gonzalez, J.C.; Fridman, S.; Sposato, L.A. Phenotypes of atrial fibrillation diagnosed before-versus-after ischaemic stroke and TIA: Study protocol for a systematic review and meta-analysis. BMJ Open 2021, 11, e044288. [Google Scholar] [CrossRef] [PubMed]
- Fridman, S.; Jimenez-Ruiz, A.; Vargas-Gonzalez, J.C.; Sposato, L.A. Differences between atrial fibrillation detected before and after stroke and TIA: A systematic review and meta-analysis. Cerebrovasc. Dis. 2021. [CrossRef] [PubMed]
- Belvis, R.; Marti-Fàbregas, J.; Franquet, E.; Cocho, D.; Valencia, C.; Martí-Vilalta, J.L. Asystolias in the acute phase of brain stroke. Report of a case. Neurologia 2003, 18, 170–174. [Google Scholar]
- Sykora, M.; Diedler, J.; Steiner, T. Repetitive asystole in right insular haemorrhage. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1282–1283. [Google Scholar] [CrossRef]
- Sander, D.; Klingelhofer, J. Changes of circadian blood pressure patterns after hemodynamic and thromboembolic brain infarction. Stroke 1994, 25, 1730–1737. [Google Scholar] [CrossRef]
- Sander, D.; Winbeck, K.; Klingelhofer, J.; Etgen, T.; Conrad, B. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology 2001, 57, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Strittmatter, M.; Fischer, C.; Georg, T.; Schmitz, B. Lateralization in autonomic dysfunction in ischemic stroke involving the insular cortex. Neuroreport 2004, 15, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Sykora, M.; Diedler, J.; Rupp, A.; Turcani, P.; Steiner, T. Impaired baroreceptor reflex sensitivity in acute stroke is associated with insular involvement, but not with carotid atherosclerosis. Stroke 2009, 40, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Hoshide, S.; Ishikawa, J.; Shimada, K.; Kario, K. Insular cortex atrophy as an independent determinant of disrupted diurnal rhythm of ambulatory blood pressure in elderly hypertension. Am. J. Hypertens. 2009, 22, 723–729. [Google Scholar] [CrossRef]
- Iltumur, K.; Yavavli, A.; Apak, I.; Ariturk, Z.; Toprak, N. Elevated plasma N-terminal pro-brain natriuretic peptide levels in acute ischemic stroke. Am. Heart J. 2006, 151, 1115–1122. [Google Scholar] [CrossRef]
- Laowattana, S.; Zeger, S.L.; Lima, J.A.; Goodman, S.N.; Wittstein, I.S.; Oppenheimer, S.M. Left insular stroke is associated with adverse cardiac outcome. Neurology 2006, 66, 477–483. [Google Scholar] [CrossRef]
- Ay, H.; Koroshetz, W.J.; Benner, T.; Vangel, M.G.; Melinosky, C.; Arsava, E.M.; Ayata, C.; Zhu, M.; Schwamm, L.H.; Sorensen, A.G. Neuroanatomical correlates of stroke-related myocardial injury. Neurology 2006, 66, 1325–1329. [Google Scholar] [CrossRef]
- Krause, T.; Werner, K.; Fiebach, J.B.; Villringer, K.; Piper, S.K.; Haeusler, K.G.; Endres, M.; Scheitz, J.F.; Nolte, C.H. Stroke in right dorsal anterior insular cortex is related to myocardial injury. Ann. Neurol. 2017, 81, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Scheitz, J.F.; Erdur, H.; Haeusler, K.G.; Audebert, H.J.; Roser, M.; Laufs, U.; Endres, M.; Nolte, C.H. Insular cortex lesions, cardiac troponin, and detection of previously unknown atrial fibrillation in acute ischemic stroke: Iinsights from the Troponin Elevation in Acute Ischemic Stroke Study. Stroke 2015, 46, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Ruiz, A.; Fridman, S.; Sposato, L.A. Study protocol for a systematic review and meta-analysis of comorbidities and stroke characteristics associated with troponin elevation after acute stroke. BMJ Open 2021, 11, e043613. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef]
- Tsuchihashi, K.; Ueshima, K.; Uchida, T.; Oh-mura, N.; Kimura, K.; Owa, M.; Yoshiyama, M.; Miyazaki, S.; Haze, K.; Ogawa, H.; et al. Transient left ventricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 38, 11–18. [Google Scholar] [CrossRef]
- Akashi, Y.J.; Goldstein, D.S.; Barbaro, G.; Ueyama, T. Takotsubo cardiomyopathy: A new form of acute, reversible heart failure. Circulation 2008, 118, 2754–2762. [Google Scholar] [CrossRef]
- Bybee, K.A.; Prasad, A. Stress-related cardiomyopathy syndromes. Circulation 2008, 118, 397–409. [Google Scholar] [CrossRef]
- Allen, D.; Parmar, G.; Ravandi, A.; Hussain, F.; Kass, M. Happiness can break your heart: A rare case of Takotsubo cardiomyopathy after good news. Can. J. Cardiol. 2015, 31, 228.e1–228.e2. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Kobayashi, Y.; Kobatake, H.; Dote, K.; Kato, M.; Oda, N.; Kunita, E.; Kagawa, E.; Yamane, A.; Osawa, A.; et al. Happy heart syndrome: A case of Takotsubo syndrome with left internal carotid artery occlusion. Clin. Auton. Res. 2020, 30, 347–350. [Google Scholar] [CrossRef]
- Patel, S.M.; Chokka, R.G.; Prasad, K.; Prasad, A. Distinctive clinical characteristics according to age and gender in apical ballooning syndrome (takotsubo/stress cardiomyopathy): An analysis focusing on men and young women. J. Card. Fail. 2013, 19, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Yoshikawa, T.; Maekawa, Y.; Ueda, T.; Isogai, T.; Konishi, Y.; Sakata, K.; Nagao, K.; Yamamoto, T.; Takayama, M. Characterization of predictors of in-hospital cardiac complications of takotsubo cardiomyopathy: Multi-center registry from Tokyo CCU network. J. Cardiol. 2014, 63, 269–273. [Google Scholar] [CrossRef]
- Myers, M.G.; Norris, J.W.; Hachniski, V.C.; Sole, M.J. Plasma norepinephrine in stroke. Stroke 1981, 12, 200–204. [Google Scholar] [CrossRef]
- Cruickshank, J.M.; Neil-Dwyer, G.; Stott, A. The possible role of catechoramines, corticosteroids and potassium in the production of ECG changes associated with subarachnoid hemorrhages. Br. Heart J. 1974, 36, 697–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kolin, A.; Kvasnicka, J. Pseudoinfarction pattern of the QRS complex in experimental cardiac hypoxia induced by noradrenaline. Cardiologia 1963, 43, 362–370. [Google Scholar] [CrossRef]
- Melville, K.I.; Shister, H.E. Cardiac responses to epinephrine and norepinephrine during prolonged cholesterol and high fat feeding in rabbits. Am. J. Cardiol. 1959, 4, 391–400. [Google Scholar] [CrossRef]
- Barger, A.C.; Liebowitz, M.R.; Herd, J.A. Chronic catheterization of the coronary artery: Infusion of autonomic drugs in the unanesthetized dog. Fed. Proc. 1961, 20, 107. [Google Scholar]
- Kariyanna, P.T.; Chandrakumar, H.P.; Jayarangaiah, A.; Khan, A.; Vulkanov, V.; Ashamalla, M.; Salifu, M.O.; McFarlane, S.I. Apical Takotsubo cardiomyopathy in a COVID-19 patient presenting with stroke: A case report and pathophysiologic insights. Am. J. Med. Case Rep. 2020, 8, 350–357. [Google Scholar] [CrossRef]
- Gogolla, N. The brain remembers where and how inflammation struck. Cell 2021, 184, 5851–5853. [Google Scholar] [CrossRef] [PubMed]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915. [Google Scholar] [CrossRef] [PubMed]
- Förster, C.; Silwedel, C.; Golenhofen, N.; Burek, M.; Kietz, S.; Mankertz, J.; Drenckhahn, D. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J. Physiol. 2005, 565, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Blecharz, K.; Kahles, T.; Schwarz, T.; Kraft, P.; Göbel, K.; Meuth, S.G.; Burek, M.; Thum, T.; Stoll, G.; et al. Glucocorticoid insensitivity at the hypoxic blood-brain barrier can be reversed by inhibition of the proteasome. Stroke 2011, 42, 1081–1089. [Google Scholar] [CrossRef]
- Ittner, C.; Burek, M.; Störk, S.; Nagai, M.; Förster, C.Y. Increased catecholamine levels and inflammatory mediators alter barrier properties of brain microvascular endothelial cells in vitro. Front. Cardiovasc. Med. 2020, 7, 73. [Google Scholar] [CrossRef]
- Osawa, A.; Nagai, M.; Dote, K.; Kato, M.; Oda, N.; Kunita, E.; Kagawa, E.; Yamane, A.; Kobatake, H.; Shiota, H.; et al. A mid-ventricular variant of Takotsubo syndrome: Was it triggered by insular cortex damage? ESC Heart Fail. 2021, 8, 3408–3412. [Google Scholar] [CrossRef]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef]
- Akashi, Y.J.; Nef, H.M.; Lyon, A.R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Heubach, J.F.; Blaschke, M.; Harding, S.E.; Ravens, U.; Kaumann, A.J. Cardiostimulant and cardiodepressant effects through overexpressed human beta2-adrenoceptors in murine heart: Regional differences and functional role of beta1-adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 367, 380–390. [Google Scholar] [CrossRef]
- Yoshimura, S.; Toyoda, K.; Ohara, T.; Nagasawa, H.; Ohtani, N.; Kuwashiro, T.; Naritomi, H.; Minematsu, K. Takotsubo cardiomyopathy in acute ischemic stroke. Ann. Neurol. 2008, 64, 547–554. [Google Scholar] [CrossRef]
- Jung, J.M.; Kim, J.G.; Kim, J.B.; Cho, K.H.; Yu, S.; Oh, K.; Kim, Y.H.; Choi, J.Y.; Seo, W.K. Takotsubo-like myocardial dysfunction in ischemic stroke: A hospital-based registry and systematic literature review. Stroke 2016, 47, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.B.; Shah, S.; Venkatasubba Rao, C.P.; Suarez, J.I.; Bershad, E.M. Clinical characteristics of myocardial stunning in acute stroke. J. Clin. Neurosci. 2014, 21, 1279–1282. [Google Scholar] [CrossRef]
- Porto, I.; Della Bona, R.; Leo, A.; Proietti, R.; Pieroni, M.; Caltagirone, C.; Spalletta, G.; Bolognese, L.; Cravello, L. Stress cardiomyopathy (tako-tsubo) triggered by nervous system diseases: A systematic review of the reported cases. Int. J. Cardiol. 2013, 167, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.M.; Grossardt, B.R.; Rhodes, D.J.; Brown, R.D., Jr.; Roger, V.L.; Melton, L.J., 3rd; Rocca, W.A. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G.; et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef]
- Barros, R.P.; Machado, U.F.; Gustafsson, J.A. Estrogen receptors: New players in diabetes mellitus. Trends Mol. Med. 2006, 12, 425–431. [Google Scholar] [CrossRef]
- Burek, M.; Arias-Loza, P.A.; Roewer, N.; Förster, C.Y. Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 298–304. [Google Scholar] [CrossRef]
- Zhu, Y.; Bian, Z.; Lu, P.; Karas, R.H.; Bao, L.; Cox, D.; Hodgin, J.; Shaul, P.W.; Thoren, P.; Smithies, O.; et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 2002, 295, 505–508. [Google Scholar] [CrossRef]
- Förster, C.; Kietz, S.; Hultenby, K.; Warner, M.; Gustafsson, J.A. Characterization of the ERbeta−/− mouse heart. Proc. Natl. Acad. Sci. USA 2004, 101, 14234–14239. [Google Scholar] [CrossRef]
- Lizotte, E.; Grandy, S.A.; Tremblay, A.; Allen, B.G.; Fiset, C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol. Biochem. 2009, 23, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Dimberg, U.; Petterson, M. Facial reactions to happy and angry facial expressions: Evidence for right hemisphere dominance. Psychophysiology 2000, 37, 693–696. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Sarcon, A.; Diekmann, J.; Bataiosu, D.R.; Cammann, V.L.; Jurisic, S.; Napp, L.C.; Jaguszewski, M.; Scherff, F.; Brugger, P.; et al. Happy heart syndrome: Role of positive emotional stress in Takotsubo syndrome. Eur. Heart J. 2016, 37, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.R. The insular lobe in primates including humans. Neurol. Res. 1985, 7, 2–10. [Google Scholar] [CrossRef]
- Augustine, J.R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res. Rev. 1996, 22, 229–244. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Mufson, E.J. The Insula of Reil in man and monkey: Architectonics, connectivity, and function. In Cerebral Cortex; Peters, A., Jones, E.G., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 179–226. [Google Scholar]
- Ruggiero, D.A.; Mraovitch, S.; Granata, M.; Anwar, M.; Reis, D.J. A role of insular cortex in cardiovascular function. J. Comp. Neurol. 1987, 257, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Xiao, X.; Yang, T.; Ritola, K.; Hantman, A.; Li, Y.; Huang, Z.J.; Li, B. A genetically defined insula-brainstem circuit selectively controls motivational vigor. Cell 2021, 184, 6344–6360.e18. [Google Scholar] [CrossRef]
- Shipley, M.T.; Sanders, M.S. Special senses are really special: Evidence for a reciprocal, bilateral pathway between insular cortex and nucleus parabrachialis. Brain Res. Bull. 1982, 8, 493–501. [Google Scholar] [CrossRef]
- Butcher, K.S.; Cechetto, D.F. Receptors in lateral hypothalamic area involved in insular cortex sympathetic responses. Am. J. Physiol. 1998, 275, 689–696. [Google Scholar] [CrossRef]
- Templin, C.; Hänggi, J.; Klein, C.; Topka, M.S.; Hiestand, T.; Levinson, R.A.; Jurisic, S.; Lüscher, T.F.; Ghadri, J.R.; Jäncke, L. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur. Heart J. 2019, 40, 1183–1187. [Google Scholar] [CrossRef]
- Dichtl, W.; Tuovinen, N.; Barbieri, F.; Adukauskaite, A.; Senoner, T.; Rubatscher, A.; Hintringer, F.; Siedentopf, C.; Bauer, A.; Gizewski, E.R.; et al. Functional neuroimaging in the acute phase of Takotsubo syndrome: Volumetric and functional changes of the right insular cortex. Clin. Res. Cardiol. 2020, 109, 1107–1113. [Google Scholar] [CrossRef]
- Cechetto, D.F.; Hachinski, V. Cardiovascular consequences of experimental stroke. In Bailliere’s Clinical Neurology; Cechetto, D.F., Hachinski, V., Eds.; WB Saunders: London, UK, 1997; Volume 6, pp. 297–308. [Google Scholar]
- Sturm, V.E.; Brown, J.A.; Hua, A.Y.; Lwi, S.J.; Zhou, J.; Kurth, F.; Eickhoff, S.B.; Rosen, H.J.; Kramer, J.H.; Miller, B.L.; et al. Network architecture underlying basal autonomic outflow: Evidence from frontotemporal dementia. J. Neurosci. 2018, 38, 8943–8955. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.P. The head and the heart of fear. Science 2021, 374, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.S.; Dolensek, N.; Weiand, C.; Gogolla, N. Fear balance is maintained by bodily feedback to the insular cortex in mice. Science 2021, 374, 1010–1015. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, M.; Förster, C.Y.; Dote, K. Sex Hormone-Specific Neuroanatomy of Takotsubo Syndrome: Is the Insular Cortex a Moderator? Biomolecules 2022, 12, 110. https://doi.org/10.3390/biom12010110
Nagai M, Förster CY, Dote K. Sex Hormone-Specific Neuroanatomy of Takotsubo Syndrome: Is the Insular Cortex a Moderator? Biomolecules. 2022; 12(1):110. https://doi.org/10.3390/biom12010110
Chicago/Turabian StyleNagai, Michiaki, Carola Yvette Förster, and Keigo Dote. 2022. "Sex Hormone-Specific Neuroanatomy of Takotsubo Syndrome: Is the Insular Cortex a Moderator?" Biomolecules 12, no. 1: 110. https://doi.org/10.3390/biom12010110
APA StyleNagai, M., Förster, C. Y., & Dote, K. (2022). Sex Hormone-Specific Neuroanatomy of Takotsubo Syndrome: Is the Insular Cortex a Moderator? Biomolecules, 12(1), 110. https://doi.org/10.3390/biom12010110






