Do Neurotrophins Connect Neurological Disorders and Heart Diseases?
Abstract
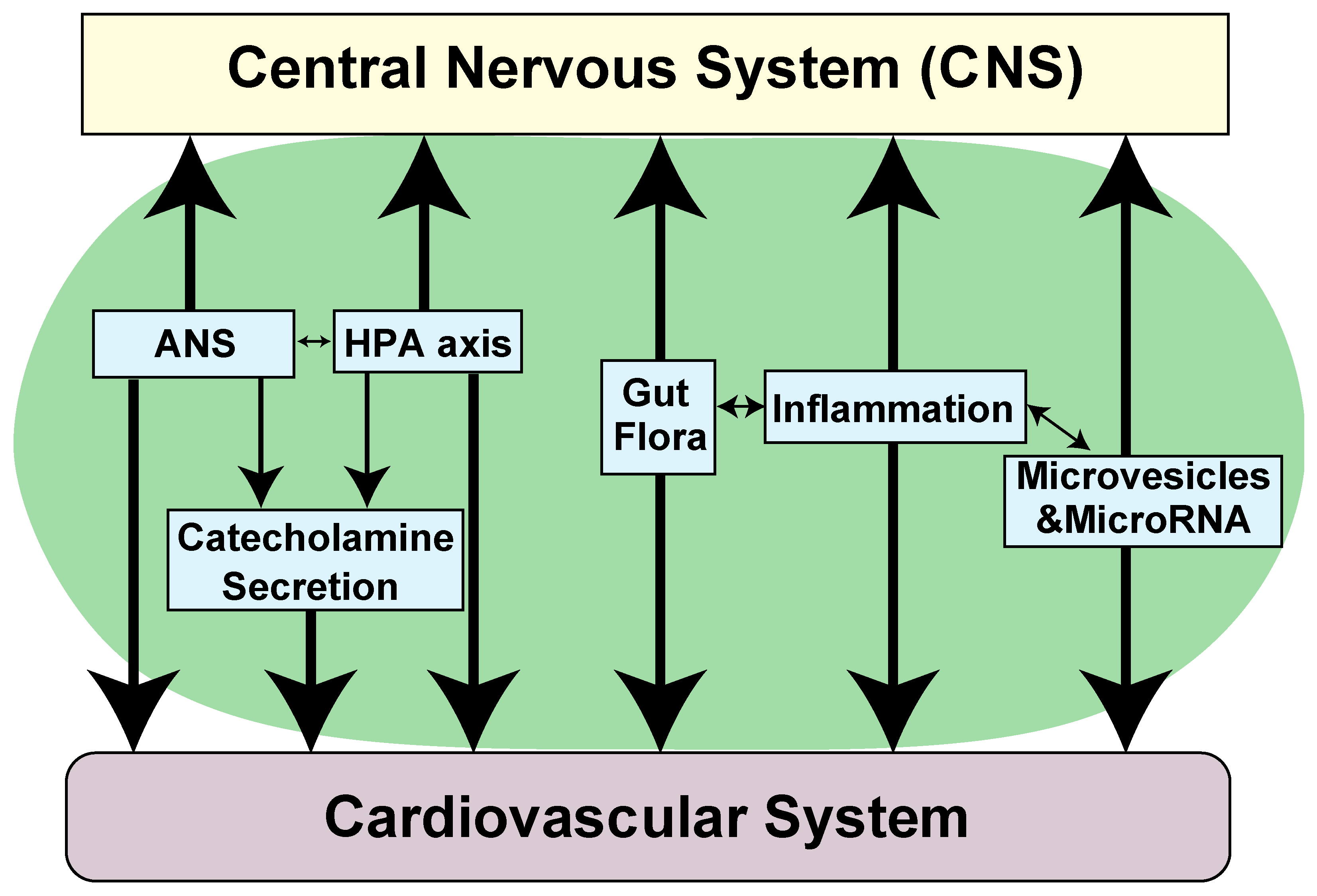
:1. Introduction
2. NTs Regulate Cardiovascular Function, Development, and Angiogenesis
2.1. The Physiological Role of NTs in the Cardiovascular System or Autonomic Nervous System
2.2. NTs Regulate Heart Morphogenesis Directly and Indirectly
2.3. NTs Regulate Angiogenesis
3. Pathophysiological Role of NTs in Primary Cardiovascular Diseases
3.1. Ischemic Injury
3.2. Heart Failure (HF)
3.3. Doxorubicin-Induces Cardiotoxicity
4. Pathophysiological Role of NTs in Cardiovascular Abnormalities Associated with Neurological Diseases
4.1. Pathophysiological Role of NTs in Neurological Diseases and How to Modulate BDNF Expression
4.2. Pathophysiological Role of NTs in Cardiovascular Abnormalities Associated with Neurodegenerative Diseases
4.3. Pathophysiological Role of NTs in Cardiovascular Abnormalities Associated with Stroke and Brain Injury
4.4. Pathophysiological Role of NTs in Cardiovascular Abnormalities Associated with Psychiatric Disorders
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Bothwell, M. Recent advances in understanding neurotrophin signaling. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, C.F.; Simi, A. p75 neurotrophin receptor signaling in nervous system injury and degeneration: Paradox and opportunity. Trends Neurosci. 2012, 35, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suter, U.; Heymach, J.V.; Shooter, E.M. 2 Conserved Domains in the Ngf Propeptide Are Necessary and Sufficient for the Biosynthesis of Correctly Processed and Biologically-Active Ngf. EMBO J. 1991, 10, 2395–2400. [Google Scholar] [CrossRef]
- Bruno, M.A.; Cuello, A.C. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc. Natl. Acad. Sci. USA 2006, 103, 6735–6740. [Google Scholar] [CrossRef] [Green Version]
- Hempstead, B.L. Deciphering proneurotrophin actions. Handb. Exp. Pharm. 2014, 220, 17–32. [Google Scholar] [CrossRef]
- Nykjaer, A.; Willnow, T.E.; Petersen, C.M. p75NTR--live or let die. Curr. Opin. Neurobiol. 2005, 15, 49–57. [Google Scholar] [CrossRef]
- Howard, L.; Wyatt, S.; Nagappan, G.; Davies, A.M. ProNGF promotes neurite growth from a subset of NGF-dependent neurons by a p75NTR-dependent mechanism. Development 2013, 140, 2108–2117. [Google Scholar] [CrossRef] [Green Version]
- Kermani, P.; Hempstead, B. BDNF Actions in the Cardiovascular System: Roles in Development, Adulthood and Response to Injury. Front. Physiol. 2019, 10, 455. [Google Scholar] [CrossRef]
- Massara Martinelli, P.; Ribeiro da Silva Camargos, E. Neurotrophic Factors and Heart Diseases. J. Cardiol. Ther. 2016, 3, 483–491. [Google Scholar] [CrossRef]
- Caporali, A.; Emanueli, C. Cardiovascular actions of neurotrophins. Physiol. Rev. 2009, 89, 279–308. [Google Scholar] [CrossRef] [Green Version]
- Pius-Sadowska, E.; Machalinski, B. Pleiotropic activity of nerve growth factor in regulating cardiac functions and counteracting pathogenesis. ESC Heart Fail. 2021, 8, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.J.; Lin, M.I.; Wiegn, P.; Ringstedt, T.; Kraemer, R.; Hahn, R.; Wang, S.; Ibanez, C.F.; Rafii, S.; Hempstead, B.L. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 2000, 127, 4531–4540. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.D.; Theres, H.; Englert, C.; Schedl, A.; Scholz, H. Coronary vessel development requires activation of the TrkB neurotrophin receptor by the Wilms’ tumor transcription factor Wt1. Genes Dev. 2005, 19, 2631–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, M.J.; Hahn, R.; Tessarollo, L.; Hempstead, B.L. Identification of an essential nonneuronal function of neurotrophin 3 in mammalian cardiac development. Nat. Genet. 1996, 14, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Hang, P.Z.; Zhu, H.; Li, P.F.; Liu, J.; Ge, F.Q.; Zhao, J.; Du, Z.M. The Emerging Role of BDNF/TrkB Signaling in Cardiovascular Diseases. Life 2021, 11, 70. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J. Biomed. Biotechnol. 2011, 2011, 201696. [Google Scholar] [CrossRef] [Green Version]
- Tahsili-Fahadan, P.; Geocadin, R.G. Heart-Brain Axis: Effects of Neurologic Injury on Cardiovascular Function. Circ. Res. 2017, 120, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Venkat, P.; Seyfried, D.; Chopp, M.; Yan, T.; Chen, J. Brain-Heart Interaction: Cardiac Complications After Stroke. Circ. Res. 2017, 121, 451–468. [Google Scholar] [CrossRef]
- Baroni, C.; Lionetti, V. The impact of sex and gender on heart-brain axis dysfunction: Current concepts and novel perspectives. Can. J. Physiol. Pharm. 2021, 99, 151–160. [Google Scholar] [CrossRef]
- Shityakov, S.; Hayashi, K.; Stork, S.; Scheper, V.; Lenarz, T.; Forster, C.Y. The Conspicuous Link between Ear, Brain and Heart-Could Neurotrophin-Treatment of Age-Related Hearing Loss Help Prevent Alzheimer’s Disease and Associated Amyloid Cardiomyopathy? Biomolecules 2021, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ieda, M.; Fukuda, K. Development, maturation, and transdifferentiation of cardiac sympathetic nerves. Circ. Res. 2012, 110, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Govoni, S.; Pascale, A.; Amadio, M.; Calvillo, L.; D’Elia, E.; Cereda, C.; Fantucci, P.; Ceroni, M.; Vanoli, E. NGF and heart: Is there a role in heart disease? Pharm. Res. 2011, 63, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Yokoyama, M.; Toko, H.; Tateno, K.; Moriya, J.; Shimizu, I.; Nojima, A.; Ito, T.; Yoshida, Y.; Kobayashi, Y.; et al. Brain-derived neurotrophic factor protects against cardiac dysfunction after myocardial infarction via a central nervous system-mediated pathway. Arter. Thromb. Vasc. Biol. 2012, 32, 1902–1909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zhang, Y.; Cohen, S.B.; DiPetrillo, K. TrkB agonist antibody dose-dependently raises blood pressure in mice with diet-induced obesity. Am. J. Hypertens. 2010, 23, 732–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, N.; Huke, S.; Zhu, G.S.; Tocchetti, C.G.; Shi, S.; Aiba, T.; Kaludercic, N.; Hoover, D.B.; Beck, S.E.; Mankowski, J.L.; et al. Constitutive BDNF/TrkB signaling is required for normal cardiac contraction and relaxation. Proc. Natl. Acad. Sci. USA 2015, 112, E1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulgenzi, G.; Tomassoni-Ardori, F.; Babini, L.; Becker, J.; Barrick, C.; Puverel, S.; Tessarollo, L. BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB.T1 receptor activation. J. Cell Biol. 2015, 210, 1003–1012. [Google Scholar] [CrossRef] [Green Version]
- Rothman, S.M.; Griffioen, K.J.; Wan, R.; Mattson, M.P. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann. N. Y. Acad. Sci. 2012, 1264, 49–63. [Google Scholar] [CrossRef]
- Brady, R.; Zaidi, S.I.A.; Mayer, C.; Katz, D.M. BDNF is a target-derived survival factor for arterial baroreceptor and chemoafferent primary sensory neurons. J. Neurosci. 1999, 19, 2131–2142. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.L.; Jenkins, V.K.; Hsieh, H.Y.; Balkowiec, A. Brain-derived neurotrophic factor in arterial baroreceptor pathways: Implications for activity-dependent plasticity at baroafferent synapses. J. Neurochem. 2009, 108, 450–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, C.G.; Hasser, E.M.; Kunze, D.L.; Katz, D.M.; Kline, D.D. Endogenous brain-derived neurotrophic factor in the nucleus tractus solitarius tonically regulates synaptic and autonomic function. J. Neurosci. 2011, 31, 12318–12329. [Google Scholar] [CrossRef] [PubMed]
- Scarisbrick, I.A.; Jones, E.G.; Isackson, P.J. Coexpression of mRNAs for NGF, BDNF, and NT-3 in the cardiovascular system of the pre- and postnatal rat. J. Neurosci. 1993, 13, 875–893. [Google Scholar] [CrossRef]
- Hiltunen, J.O.; Arumae, U.; Moshnyakov, M.; Saarma, M. Expression of mRNAs for neurotrophins and their receptors in developing rat heart. Circ. Res. 1996, 79, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Tessarollo, L.; Tsoulfas, P.; Donovan, M.J.; Palko, M.E.; Blair-Flynn, J.; Hempstead, B.L.; Parada, L.F. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc. Natl. Acad. Sci. USA 1997, 94, 14776–14781. [Google Scholar] [CrossRef] [Green Version]
- Anastasia, A.; Deinhardt, K.; Wang, S.; Martin, L.; Nichol, D.; Irmady, K.; Trinh, J.; Parada, L.; Rafii, S.; Hempstead, B.L.; et al. Trkb signaling in pericytes is required for cardiac microvessel stabilization. PLoS ONE 2014, 9, e87406. [Google Scholar] [CrossRef] [Green Version]
- Vegh, A.M.D.; Duim, S.N.; Smits, A.M.; Poelmann, R.E.; Ten Harkel, A.D.J.; DeRuiter, M.C.; Goumans, M.J.; Jongbloed, M.R.M. Part and Parcel of the Cardiac Autonomic Nerve System: Unravelling Its Cellular Building Blocks during Development. J. Cardiovasc. Dev. Dis. 2016, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Cantarella, G.; Lempereur, L.; Presta, M.; Ribatti, D.; Lombardo, G.; Lazarovici, P.; Zappala, G.; Pafumi, C.; Bernardini, R. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. Faseb. J. 2002, 16, 1307–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, K.M.; Falcone, D.J.; Kraemer, R. Nerve growth factor activation of Erk-1 and Erk-2 induces matrix metalloproteinase-9 expression in vascular smooth muscle cells. J. Biol. Chem. 2002, 277, 2353–2359. [Google Scholar] [CrossRef] [Green Version]
- Caporali, A.; Sala-Newby, G.B.; Meloni, M.; Graiani, G.; Pani, E.; Cristofaro, B.; Newby, A.C.; Madeddu, P.; Emanueli, C. Identification of the prosurvival activity of nerve growth factor on cardiac myocytes. Cell Death Differ. 2008, 15, 299–311. [Google Scholar] [CrossRef]
- Lin, M.I.; Das, I.; Schwartz, G.M.; Tsoulfas, P.; Mikawa, T.; Hempstead, B.L. Trk C receptor signaling regulates cardiac myocyte proliferation during early heart development in vivo. Dev. Biol. 2000, 226, 180–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporali, A.; Pani, E.; Horrevoets, A.J.; Kraenkel, N.; Oikawa, A.; Sala-Newby, G.B.; Meloni, M.; Cristofaro, B.; Graiani, G.; Leroyer, A.S.; et al. Neurotrophin p75 receptor (p75NTR) promotes endothelial cell apoptosis and inhibits angiogenesis: Implications for diabetes-induced impaired neovascularization in ischemic limb muscles. Circ. Res. 2008, 103, e15–e26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Bray, P.; McCaffrey, T.; March, K.; Hempstead, B.L.; Kraemer, R. p75NTR Mediates Neurotrophin-Induced Apoptosis of Vascular Smooth Muscle Cells. Am. J. Pathol. 2000, 157, 1247–1258. [Google Scholar] [CrossRef] [Green Version]
- Siao, C.J.; Lorentz, C.U.; Kermani, P.; Marinic, T.; Carter, J.; McGrath, K.; Padow, V.A.; Mark, W.; Falcone, D.J.; Cohen-Gould, L.; et al. ProNGF, a cytokine induced after myocardial infarction in humans, targets pericytes to promote microvascular damage and activation. J. Exp. Med. 2012, 209, 2291–2305. [Google Scholar] [CrossRef] [Green Version]
- Hassankhani, A.; Steinhelper, M.E.; Soonpaa, M.H.; Katz, E.B.; Taylor, D.A.; Andrade-Rozental, A.; Factor, S.M.; Steinberg, J.J.; Field, L.J.; Federoff, H.J. Overexpression of NGF within the heart of transgenic mice causes hyperinnervation, cardiac enlargement, and hyperplasia of ectopic cells. Dev. Biol. 1995, 169, 309–321. [Google Scholar] [CrossRef]
- Ieda, M.; Kanazawa, H.; Ieda, Y.; Kimura, K.; Matsumura, K.; Tomita, Y.; Yagi, T.; Onizuka, T.; Shimoji, K.; Ogawa, S.; et al. Nerve growth factor is critical for cardiac sensory innervation and rescues neuropathy in diabetic hearts. Circulation 2006, 114, 2351–2363. [Google Scholar] [CrossRef] [Green Version]
- Nico, B.; Mangieri, D.; Benagiano, V.; Crivellato, E.; Ribatti, D. Nerve growth factor as an angiogenic factor. Microvasc. Res. 2008, 75, 135–141. [Google Scholar] [CrossRef]
- Seo, K.; Choi, J.; Park, M.; Rhee, C. Angiogenesis effects of nerve growth factor (NGF) on rat corneas. J. Vet. Sci. 2001, 2, 125–130. [Google Scholar] [CrossRef]
- Lazarovici, P.; Gazit, A.; Staniszewska, I.; Marcinkiewicz, C.; Lelkes, P.I. Nerve growth factor (NGF) promotes angiogenesis in the quail chorioallantoic membrane. Endothelium 2006, 13, 51–59. [Google Scholar] [CrossRef]
- Emanueli, C.; Salis, M.B.; Pinna, A.; Graiani, G.; Manni, L.; Madeddu, P. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 2002, 106, 2257–2262. [Google Scholar] [CrossRef] [Green Version]
- Raychaudhuri, S.K.; Raychaudhuri, S.P.; Weltman, H.; Farber, E.M. Effect of nerve growth factor on endothelial cell biology: Proliferation and adherence molecule expression on human dermal microvascular endothelial cells. Arch. Derm. Res. 2001, 293, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Steinle, J.J.; Granger, H.J. Nerve growth factor regulates human choroidal, but not retinal, endothelial cell migration and proliferation. Auton. Neurosci. 2003, 108, 57–62. [Google Scholar] [CrossRef]
- Moser, K.V.; Reindl, M.; Blasig, I.; Humpel, C. Brain capillary endothelial cells proliferate in response to NGF, express NGF receptors and secrete NGF after inflammation. Brain Res. 2004, 1017, 53–60. [Google Scholar] [CrossRef]
- Tanaka, A.; Wakita, U.; Kambe, N.; Iwasaki, T.; Matsuda, H. An autocrine function of nerve growth factor for cell cycle regulation of vascular endothelial cells. Biochem. Biophys. Res. Commun. 2004, 313, 1009–1014. [Google Scholar] [CrossRef]
- Rahbek, U.L.; Dissing, S.; Thomassen, C.; Hansen, A.J.; Tritsaris, K. Nerve growth factor activates aorta endothelial cells causing PI3K/Akt- and ERK-dependent migration. Pflug. Arch. 2005, 450, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Dolle, J.P.; Rezvan, A.; Allen, F.D.; Lazarovici, P.; Lelkes, P.I. Nerve growth factor-induced migration of endothelial cells. J. Pharm. Exp. 2005, 315, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Hansen-Algenstaedt, N.; Algenstaedt, P.; Schaefer, C.; Hamann, A.; Wolfram, L.; Cingoz, G.; Kilic, N.; Schwarzloh, B.; Schroeder, M.; Joscheck, C.; et al. Neural driven angiogenesis by overexpression of nerve growth factor. Histochem. Cell Biol. 2006, 125, 637–649. [Google Scholar] [CrossRef]
- Kermani, P.; Rafii, D.; Jin, D.K.; Whitlock, P.; Schaffer, W.; Chiang, A.; Vincent, L.; Friedrich, M.; Shido, K.; Hackett, N.R.; et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J. Clin. Investig. 2005, 115, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Zhang, L.; Chen, S.; Yuan, Z.; Liu, S.; Shen, X.; Zheng, X.; Qi, X.; Lee, K.K.; Chan, J.Y.; et al. BDNF-mediated migration of cardiac microvascular endothelial cells is impaired during ageing. J. Cell Mol. Med. 2012, 16, 3105–3115. [Google Scholar] [CrossRef]
- Cristofaro, B.; Stone, O.A.; Caporali, A.; Dawbarn, D.; Ieronimakis, N.; Reyes, M.; Madeddu, P.; Bates, D.O.; Emanueli, C. Neurotrophin-3 Is a Novel Angiogenic Factor Capable of Therapeutic Neovascularization in a Mouse Model of Limb Ischemia. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Kaess, B.M.; Preis, S.R.; Lieb, W.; Beiser, A.S.; Yang, Q.; Chen, T.C.; Hengstenberg, C.; Erdmann, J.; Schunkert, H.; Seshadri, S.; et al. Circulating brain-derived neurotrophic factor concentrations and the risk of cardiovascular disease in the community. J. Am. Heart Assoc. 2015, 4, e001544. [Google Scholar] [CrossRef] [Green Version]
- Meloni, M.; Caporali, A.; Graiani, G.; Lagrasta, C.; Katare, R.; Van Linthout, S.; Spillmann, F.; Campesi, I.; Madeddu, P.; Quaini, F.; et al. Nerve growth factor promotes cardiac repair following myocardial infarction. Circ. Res. 2010, 106, 1275–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiltunen, J.O.; Laurikainen, A.; Vakeva, A.; Meri, S.; Saarma, M. Nerve growth factor and brain-derived neurotrophic factor mRNAs are regulated in distinct cell populations of rat heart after ischaemia and reperfusion. J. Pathol. 2001, 194, 247–253. [Google Scholar] [CrossRef]
- Ieda, M.; Fukuda, K.; Hisaka, Y.; Kimura, K.; Kawaguchi, H.; Fujita, J.; Shimoda, K.; Takeshita, E.; Okano, H.; Kurihara, Y.; et al. Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression. J. Clin. Investig. 2004, 113, 876–884. [Google Scholar] [CrossRef] [Green Version]
- Hasan, W.; Jama, A.; Donohue, T.; Wernli, G.; Onyszchuk, G.; Al-Hafez, B.; Bilgen, M.; Smith, P.G. Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain Res. 2006, 1124, 142–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejiri, J.; Inoue, N.; Kobayashi, S.; Shiraki, R.; Otsui, K.; Honjo, T.; Takahashi, M.; Ohashi, Y.; Ichikawa, S.; Terashima, M.; et al. Possible role of brain-derived neurotrophic factor in the pathogenesis of coronary artery disease. Circulation 2005, 112, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, P.; Zhao, J.; Cai, B.; Tian, S.; Huang, W.; Guo, J.; Sun, C.; Li, Y.; Du, Z. Brain-derived neurotrophic factor regulates TRPC3/6 channels and protects against myocardial infarction in rodents. Int. J. Biol. Sci. 2015, 11, 536–545. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.; Huan, Y.; Zhao, H.; Deng, J. Application of bFGF and BDNF to improve angiogenesis and cardiac function. J. Surg. Res. 2006, 136, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Ma, Y.; Ramirez, T.A.; Zhang, J.; Dai, Q.; Hensler, J.G.; Lopez, E.F.; Ghasemi, O.; Jin, Y.F.; Lindsey, M.L. Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1830–H1842. [Google Scholar] [CrossRef] [Green Version]
- Kaye, D.M.; Vaddadi, G.; Gruskin, S.L.; Du, X.J.; Esler, M.D. Reduced myocardial nerve growth factor expression in human and experimental heart failure. Circ. Res. 2000, 86, E80–E84. [Google Scholar] [CrossRef] [Green Version]
- Kimura, K.; Kanazawa, H.; Ieda, M.; Kawaguchi-Manabe, H.; Miyake, Y.; Yagi, T.; Arai, T.; Sano, M.; Fukuda, K. Norepinephrine-induced nerve growth factor depletion causes cardiac sympathetic denervation in severe heart failure. Auton. Neurosci. 2010, 156, 27–35. [Google Scholar] [CrossRef]
- Qin, F.; Vulapalli, R.S.; Stevens, S.Y.; Liang, C.S. Loss of cardiac sympathetic neurotransmitters in heart failure and NE infusion is associated with reduced NGF. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H363–H371. [Google Scholar] [CrossRef] [Green Version]
- Lam, N.T.; Currie, P.D.; Lieschke, G.J.; Rosenthal, N.A.; Kaye, D.M. Nerve growth factor stimulates cardiac regeneration via cardiomyocyte proliferation in experimental heart failure. PLoS ONE 2012, 7, e53210. [Google Scholar] [CrossRef]
- Meloni, M.; Descamps, B.; Caporali, A.; Zentilin, L.; Floris, I.; Giacca, M.; Emanueli, C. Nerve growth factor gene therapy using adeno-associated viral vectors prevents cardiomyopathy in type 1 diabetic mice. Diabetes 2012, 61, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Takashio, S.; Sugiyama, S.; Yamamuro, M.; Takahama, H.; Hayashi, T.; Sugano, Y.; Izumiya, Y.; Hokimoto, S.; Minamino, N.; Yasuda, S.; et al. Significance of low plasma levels of brain-derived neurotrophic factor in patients with heart failure. Am. J. Cardiol. 2015, 116, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Barman, H.A.; Sahin, I.; Atici, A.; Durmaz, E.; Yurtseven, E.; Ikitimur, B.; Okuyan, E.; Keles, I. Prognostic significance of brain-derived neurotrophic factor levels in patients with heart failure and reduced left ventricular ejection fraction. Anatol. J. Cardiol. 2019, 22, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sariola, H. The neurotrophic factors in non-neuronal tissues. Cell Mol. Life Sci. 2001, 58, 1061–1066. [Google Scholar] [CrossRef]
- Kerschensteiner, M.; Stadelmann, C.; Dechant, G.; Wekerle, H.; Hohlfeld, R. Neurotrophic cross-talk between the nervous and immune systems: Implications for neurological diseases. Ann. Neurol. 2003, 53, 292–304. [Google Scholar] [CrossRef]
- Nebigil, C.G.; Desaubry, L. Updates in Anthracycline-Mediated Cardiotoxicity. Front Pharmacol 2018, 9, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, D.; Zhang, C.; Liu, N.; Cao, L.; Wang, C.; Feng, Q.; Yao, D.; Long, M.; Jiang, P. Involvement of neurotrophic signaling in doxorubicin-induced cardiotoxicity. Exp. Ther. Med. 2020, 19, 1129–1135. [Google Scholar] [CrossRef]
- Zhao, J.; Du, J.; Pan, Y.; Chen, T.; Zhao, L.; Zhu, Y.; Chen, Y.; Zheng, Y.; Liu, Y.; Sun, L.; et al. Activation of cardiac TrkB receptor by its small molecular agonist 7,8-dihydroxyflavone inhibits doxorubicin-induced cardiotoxicity via enhancing mitochondrial oxidative phosphorylation. Free Radic. Biol. Med. 2019, 130, 557–567. [Google Scholar] [CrossRef]
- Yang, M.; Li, C.; Zhang, Y.; Ren, J. Interrelationship between Alzheimer’s disease and cardiac dysfunction: The brain-heart continuum? Acta Biochim. Biophys. Sin. 2020, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luchsinger, J.A.; Reitz, C.; Honig, L.S.; Tang, M.X.; Shea, S.; Mayeux, R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology 2005, 65, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigin, V.L.; Norrving, B.; Mensah, G.A. Global Burden of Stroke. Circ. Res. 2017, 120, 439–448. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Detraux, J.; Vancampfort, D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin. Neurosci. 2018, 20, 31. [Google Scholar] [PubMed]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Bindocci, E.; Alleva, E. NGF, brain and behavioral plasticity. Neural Plast. 2012, 2012, 784040. [Google Scholar] [CrossRef] [PubMed]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Zuccato, C.; Cattaneo, E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat. Rev. Neurol. 2009, 5, 311–322. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Tuszynski, M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011, 10, 209–219. [Google Scholar] [CrossRef]
- Duman, R.S.; Deyama, S.; Fogaca, M.V. Role of BDNF in the pathophysiology and treatment of depression: Activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 2021, 53, 126–139. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; O’Connor, M.; Wang, G.; Han, F. Brain-Derived Neurotrophic Factor and Its Potential Therapeutic Role in Stroke Comorbidities. Neural Plast. 2020, 2020, 1969482. [Google Scholar] [CrossRef]
- Geral, C.; Angelova, A.; Lesieur, S. From molecular to nanotechnology strategies for delivery of neurotrophins: Emphasis on brain-derived neurotrophic factor (BDNF). Pharmaceutics 2013, 5, 127–167. [Google Scholar] [CrossRef] [Green Version]
- Sambati, L.; Calandra-Buonaura, G.; Doria, A.; Cortelli, P. Diagnosis and management of autonomic failure in neurodegenerative disorders. Eur. Neurol. 2015, 73, 126–133. [Google Scholar] [CrossRef]
- Idiaquez, J.; Roman, G.C. Autonomic dysfunction in neurodegenerative dementias. J. Neurol. Sci. 2011, 305, 22–27. [Google Scholar] [CrossRef]
- Howells, D.W.; Porritt, M.J.; Wong, J.Y.; Batchelor, P.E.; Kalnins, R.; Hughes, A.J.; Donnan, G.A. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp. Neurol. 2000, 166, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Mogi, M.; Togari, A.; Kondo, T.; Mizuno, Y.; Komure, O.; Kuno, S.; Ichinose, H.; Nagatsu, T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci. Lett. 1999, 270, 45–48. [Google Scholar] [CrossRef]
- Rahmani, F.; Saghazadeh, A.; Rahmani, M.; Teixeira, A.L.; Rezaei, N.; Aghamollaii, V.; Ardebili, H.E. Plasma levels of brain-derived neurotrophic factor in patients with Parkinson disease: A systematic review and meta-analysis. Brain Res. 2019, 1704, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Scalzo, P.; Kummer, A.; Bretas, T.L.; Cardoso, F.; Teixeira, A.L. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J. Neurol. 2010, 257, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Alomari, M.A.; Khalil, H.; Khabour, O.F.; Alzoubi, K.H.; Dersieh, E.H. Altered cardiovascular function is related to reduced BDNF in Parkinson’s disease. Exp. Aging Res. 2018, 44, 232–245. [Google Scholar] [CrossRef]
- Fleming, S.M. Cardiovascular autonomic dysfunction in animal models of Parkinson’s disease. J. Parkinsons Dis. 2011, 1, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [Green Version]
- Troncone, L.; Luciani, M.; Coggins, M.; Wilker, E.H.; Ho, C.Y.; Codispoti, K.E.; Frosch, M.P.; Kayed, R.; Del Monte, F. Abeta Amyloid Pathology Affects the Hearts of Patients With Alzheimer’s Disease: Mind the Heart. J. Am. Coll. Cardiol. 2016, 68, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.S.; Bu, X.L.; Wang, Y.R.; Li, L.; Li, W.W.; Liu, Y.H.; Zhu, C.; Yao, X.Q.; Chen, Y.; Gao, C.Y.; et al. Reduced Cardiovascular Functions in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.J. A systemic view of Alzheimer disease—Insights from amyloid-beta metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanila, H. The role of BDNF in Alzheimer’s disease. Neurobiol. Dis. 2017, 97, 114–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turdi, S.; Guo, R.; Huff, A.F.; Wolf, E.M.; Culver, B.; Ren, J. Cardiomyocyte contractile dysfunction in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. PLoS ONE 2009, 4, e6033. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, A.H.; Merrill, D.A.; Coppola, G.; Tsukada, S.; Schroeder, B.E.; Shaked, G.M.; Wang, L.; Blesch, A.; Kim, A.; Conner, J.M.; et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 2009, 15, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, A.H.; Mateling, M.; Kovacs, I.; Wang, L.; Eggert, S.; Rockenstein, E.; Koo, E.H.; Masliah, E.; Tuszynski, M.H. Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice. J. Neurosci. 2013, 33, 15596–15602. [Google Scholar] [CrossRef] [Green Version]
- Critchley, B.J.; Isalan, M.; Mielcarek, M. Neuro-Cardio Mechanisms in Huntington’s Disease and Other Neurodegenerative Disorders. Front. Physiol. 2018, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Stephen, C.; Hersch, S.; Rosas, H. Huntington’s disease and the heart: Electrocardiogram abnormalities suggest cardiac involvement (P5.294). Neurology 2015, 84, P5.294. [Google Scholar]
- Samuels, M.A. The brain-heart connection. Circulation 2007, 116, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Sörös, P.; Hachinski, V. Cardiovascular and neurological causes of sudden death after ischaemic stroke. Lancet Neurol. 2012, 11, 179–188. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, A.; He, T.; Cao, Z. Brain-Heart Axis and Biomarkers of Cardiac Damage and Dysfunction after Stroke: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanne, T.M.; Aberg, N.D.; Nilsson, S.; Jood, K.; Blomstrand, C.; Andreasson, U.; Blennow, K.; Zetterberg, H.; Isgaard, J.; Svensson, J.; et al. Low Circulating Acute Brain-Derived Neurotrophic Factor Levels Are Associated With Poor Long-Term Functional Outcome After Ischemic Stroke. Stroke 2016, 47, 1943–1945. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Yang, Y.L.; Li, Y.Q.; Chang, T.; Man, M.H.; Zhang, X.Y.; Guo, S.C.; Li, L.H. Low Serum Levels of Brain-Derived Neurotrophic Factor Were Associated with Poor Short-Term Functional Outcome and Mortality in Acute Ischemic Stroke. Mol. Neurobiol. 2017, 54, 7335–7342. [Google Scholar] [CrossRef] [PubMed]
- Bejot, Y.; Prigent-Tessier, A.; Cachia, C.; Giroud, M.; Mossiat, C.; Bertrand, N.; Garnier, P.; Marie, C. Time-dependent contribution of non neuronal cells to BDNF production after ischemic stroke in rats. Neurochem. Int. 2011, 58, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taliaz, D.; Loya, A.; Gersner, R.; Haramati, S.; Chen, A.; Zangen, A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J. Neurosci. 2011, 31, 4475–4483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.A.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995, 15, 1768–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef]
- Gregory, T.; Smith, M. Cardiovascular complications of brain injury. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.B.; Smith, M. Systemic complications after head injury: A clinical review. Anaesthesia 2007, 62, 474–482. [Google Scholar] [CrossRef]
- Mrozek, S.; Srairi, M.; Marhar, F.; Delmas, C.; Gaussiat, F.; Abaziou, T.; Larcher, C.; Atthar, V.; Menut, R.; Fourcade, O.; et al. Successful treatment of inverted Takotsubo cardiomyopathy after severe traumatic brain injury with milrinone after dobutamine failure. Heart Lung 2016, 45, 406–408. [Google Scholar] [CrossRef]
- Schneider, H.J.; Kreitschmann-Andermahr, I.; Ghigo, E.; Stalla, G.K.; Agha, A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: A systematic review. JAMA 2007, 298, 1429–1438. [Google Scholar] [CrossRef]
- Huffman, J.C.; Celano, C.M.; Beach, S.R.; Motiwala, S.R.; Januzzi, J.L. Depression and cardiac disease: Epidemiology, mechanisms, and diagnosis. Cardiovasc. Psychiatry Neurol. 2013, 2013, 695925. [Google Scholar] [CrossRef] [Green Version]
- Whooley, M.A.; Wong, J.M. Depression and cardiovascular disorders. Annu. Rev. Clin. Psychol. 2013, 9, 327–354. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Shimizu, E.; Iyo, M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Brain Res. Rev. 2004, 45, 104–114. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Laszlo, A.; Lenart, L.; Illesy, L.; Fekete, A.; Nemcsik, J. The role of neurotrophins in psychopathology and cardiovascular diseases: Psychosomatic connections. J. Neural. Transm. 2019, 126, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Sun, L.H.; Yang, W.; Cui, R.J.; Xu, S.B. The Role of BDNF in the Neuroimmune Axis Regulation of Mood Disorders. Front. Neurol. 2019, 10, 515. [Google Scholar] [CrossRef] [Green Version]
- Castren, E.; Kojima, M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol. Dis. 2017, 97, 119–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef]
- Tschorn, M.; Kuhlmann, S.L.; Rieckmann, N.; Beer, K.; Grosse, L.; Arolt, V.; Waltenberger, J.; Haverkamp, W.; Muller-Nordhorn, J.; Hellweg, R.; et al. Brain-derived neurotrophic factor, depressive symptoms and somatic comorbidity in patients with coronary heart disease. Acta Neuropsychiatr. 2021, 33, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Z.; Duan, J.; Huang, K.; Zhu, B.; Yang, L.; Zheng, L. Serum Levels of FGF21, beta-Klotho, and BDNF in Stable Coronary Artery Disease Patients With Depressive Symptoms: A Cross-Sectional Single-Center Study. Front. Psychiatry 2020, 11, 587492. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. 2020, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, R.; Ibarra, C.; Vicencio, J.M.; Jaimovich, E.; Lavandero, S. New insights into IGF-1 signaling in the heart. Trends Endocrinol. Metab. 2014, 25, 128–137. [Google Scholar] [CrossRef]

| □ | E8 | E9 | E10 | E11 | E12 | E13 | E14 | E15 | E16 | E17 | E18 | P0-3 | P4-Adult | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart | NGF | □ | □ | ± | ± | ± | ± | ± | ± | ± | ± | ± | ± | + |
| BDNF | □ | □ | − | − | ± | ± | ± | ± | ± | − | − | ± | + | |
| NT-3 | □ | □ | + | ± | ± | ± | + | ± | ± | ± | ± | ± | + | |
| NT-4/5 | □ | □ | + | ± | ± | ± | ± | ± | ± | ± | □ | □ | □ | |
| TrkA | □ | □ | − | − | ± | ± | ± | ± | ± | − | − | − | + | |
| TrkB | □ | □ | − | − | ± | ± | ± | ± | ± | ± | ± | ± | ± | |
| TrkC | □ | □ | + | − | ± | ± | + | ± | ± | ± | ± | + | ± | |
| p75NTR | □ | □ | + | ± | ± | ± | + | ± | ± | ± | ± | ± | + | |
| Vascular System | NGF | □ | □ | □ | □ | ± | □ | □ | □ | □ | □ | □ | □ | ± |
| BDNF | □ | □ | □ | □ | □ | □ | ± | □ | □ | □ | □ | □ | ± | |
| NT-3 | □ | □ | □ | □ | □ | □ | ± | □ | □ | □ | □ | □ | ± | |
| NT-4/5 | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | |
| TrkA | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | ± | |
| TrkB | □ | □ | ± | □ | □ | □ | □ | □ | □ | □ | □ | □ | ± | |
| TrkC | □ | □ | ± | + | □ | □ | ± | ± | □ | □ | □ | □ | ± | |
| p75NTR | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | □ | ± | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujitani, M.; Otani, Y.; Miyajima, H. Do Neurotrophins Connect Neurological Disorders and Heart Diseases? Biomolecules 2021, 11, 1730. https://doi.org/10.3390/biom11111730
Fujitani M, Otani Y, Miyajima H. Do Neurotrophins Connect Neurological Disorders and Heart Diseases? Biomolecules. 2021; 11(11):1730. https://doi.org/10.3390/biom11111730
Chicago/Turabian StyleFujitani, Masashi, Yoshinori Otani, and Hisao Miyajima. 2021. "Do Neurotrophins Connect Neurological Disorders and Heart Diseases?" Biomolecules 11, no. 11: 1730. https://doi.org/10.3390/biom11111730
APA StyleFujitani, M., Otani, Y., & Miyajima, H. (2021). Do Neurotrophins Connect Neurological Disorders and Heart Diseases? Biomolecules, 11(11), 1730. https://doi.org/10.3390/biom11111730







