Thromboelastometric Analysis of Anticancer Cerrena unicolor Subfractions Reveal Their Potential as Fibrin Glue Drug Carrier Enhancers
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Culture and Bioactive Fraction Acquisition
2.2. Thromboelastometric Measurements of the Formation Kinetics and Viscoelastic Properties of Fibrin Clots
2.3. Confocal Microscopy of Fibrin Clots
2.4. Thrombin Activity Assay
2.5. Cell Cultures
2.6. Fibrinogen and Fraction Preparation for In Vitro Cell Analyses
2.7. MTT Assay
2.8. NR Assay
2.9. Morphological Identification of Apoptotic and Necrotic Cells
2.10. Evaluation of the MMP Activity—Gelatin Zymography
2.11. Evaluation of the MMP Activity—Immunoblotting Assay
2.12. Statistical Analysis
3. Results
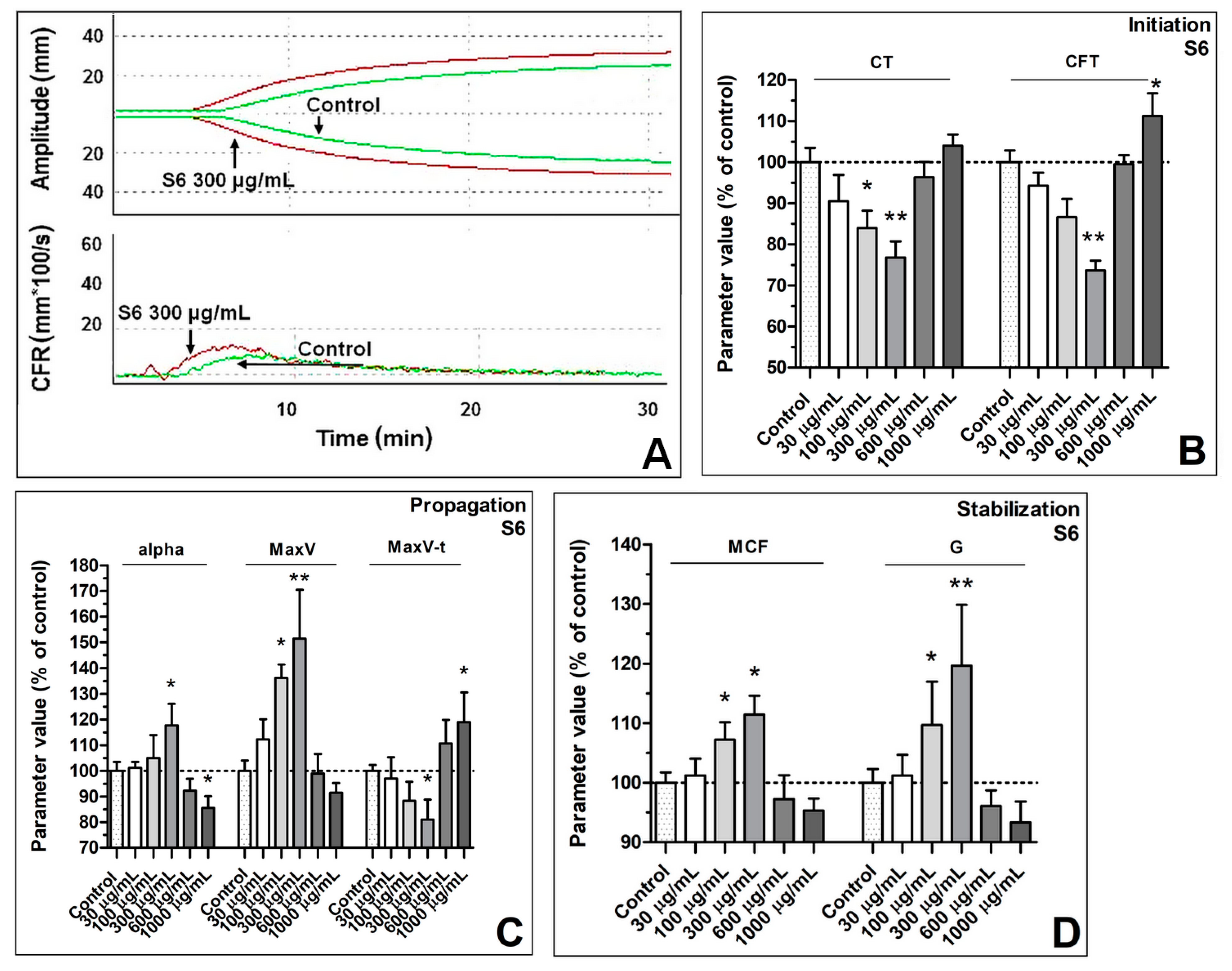
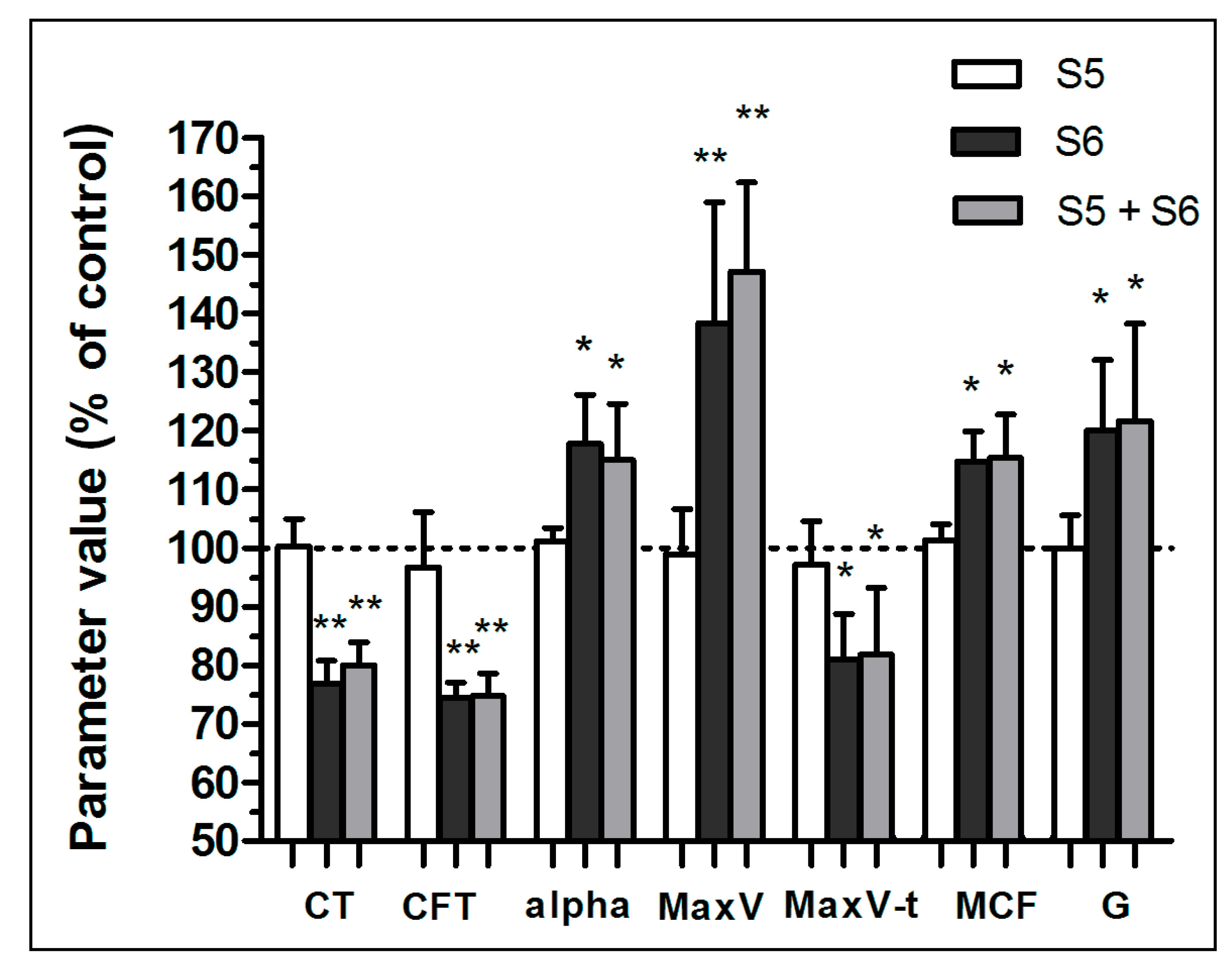
3.1. Enhancement of Formation Dynamics and Viscoelastic Properties of the Fibrin Clot
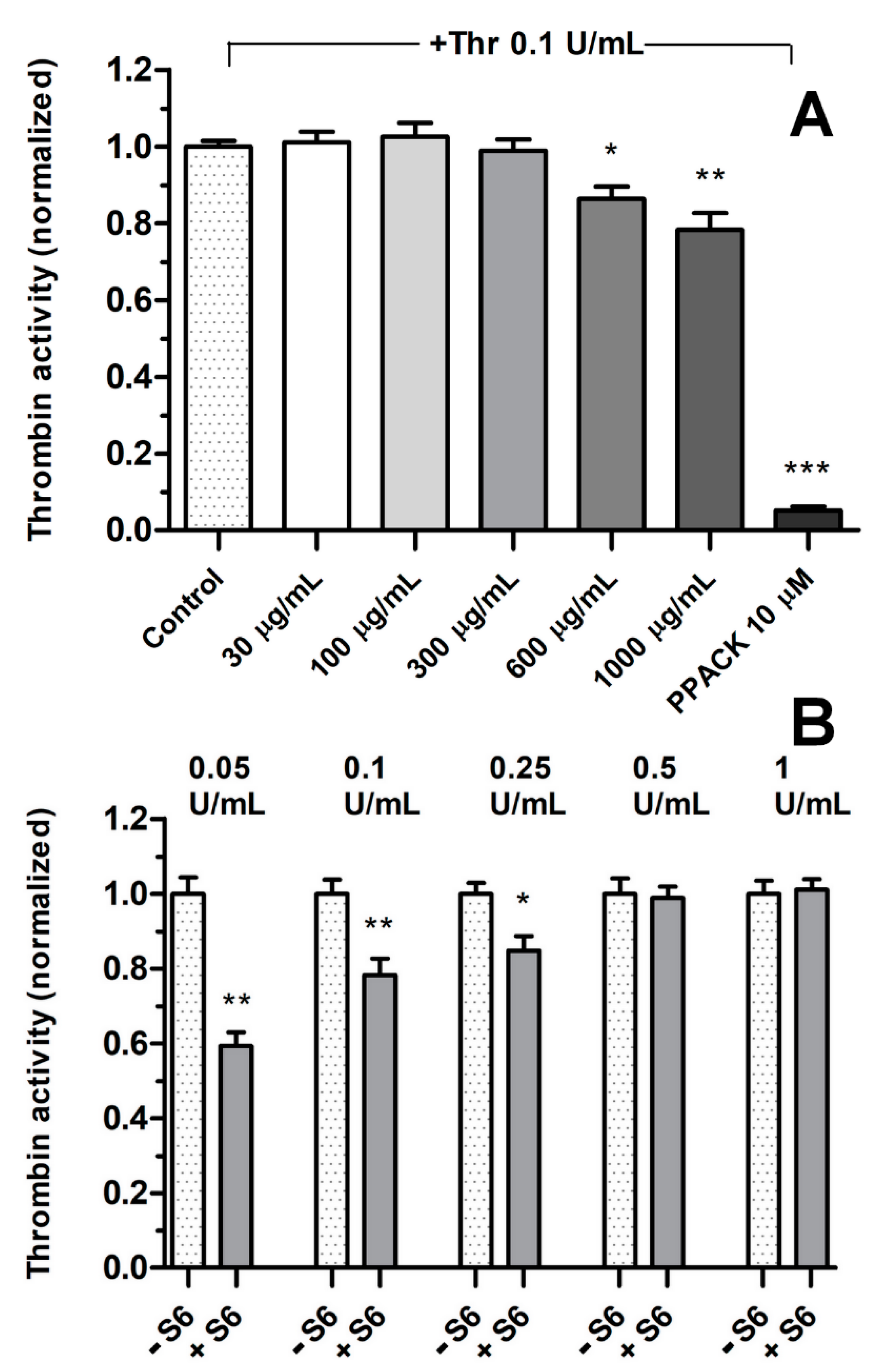
3.2. Effect of Fractions on Thrombin Activity
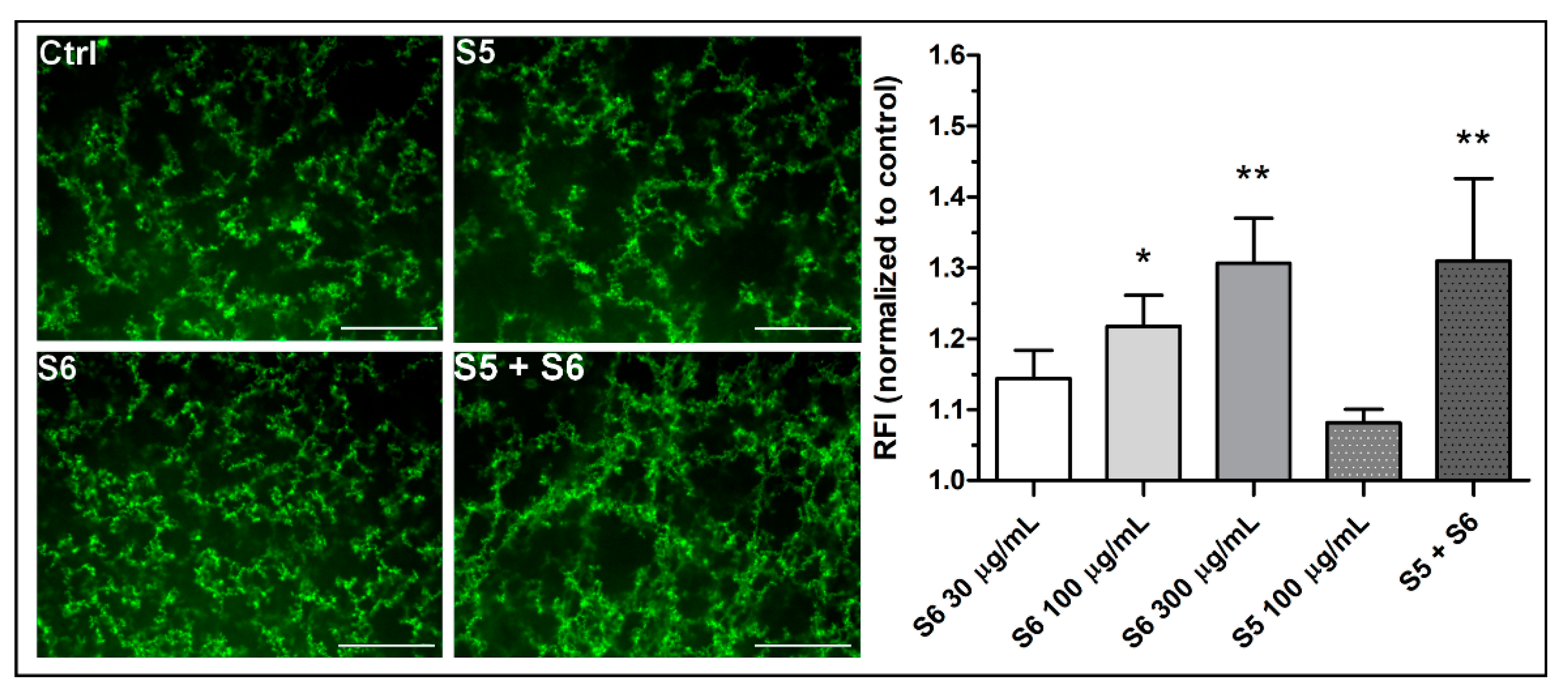
3.3. Confocal Microscopy of the Density of Fibrin Clots
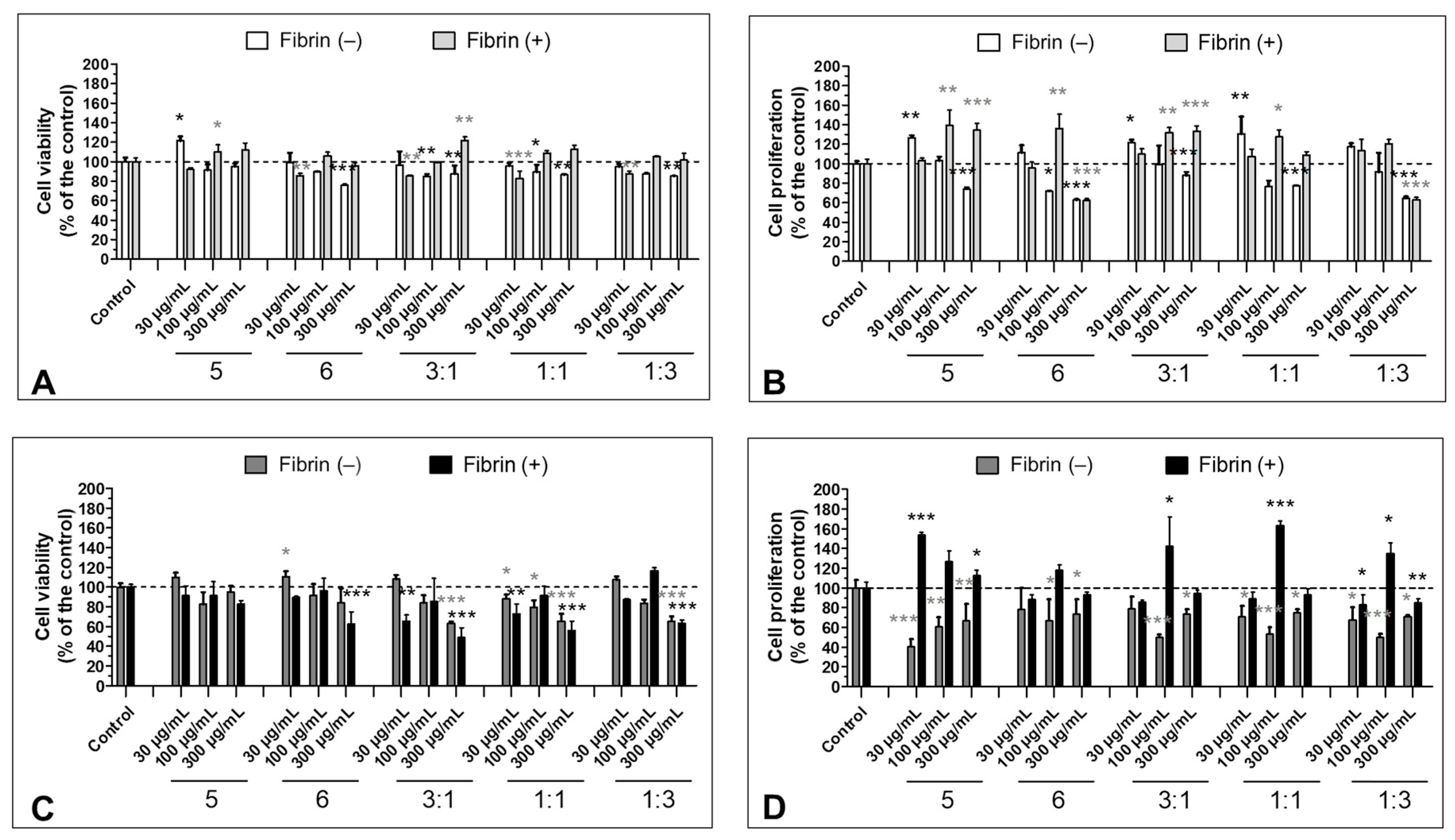
3.4. Effect of Fibrinogen on the Cell Viability
3.5. Effect of the S5 and S6 Fractions in Combination with Fibrin on Cell Viability and Proliferation
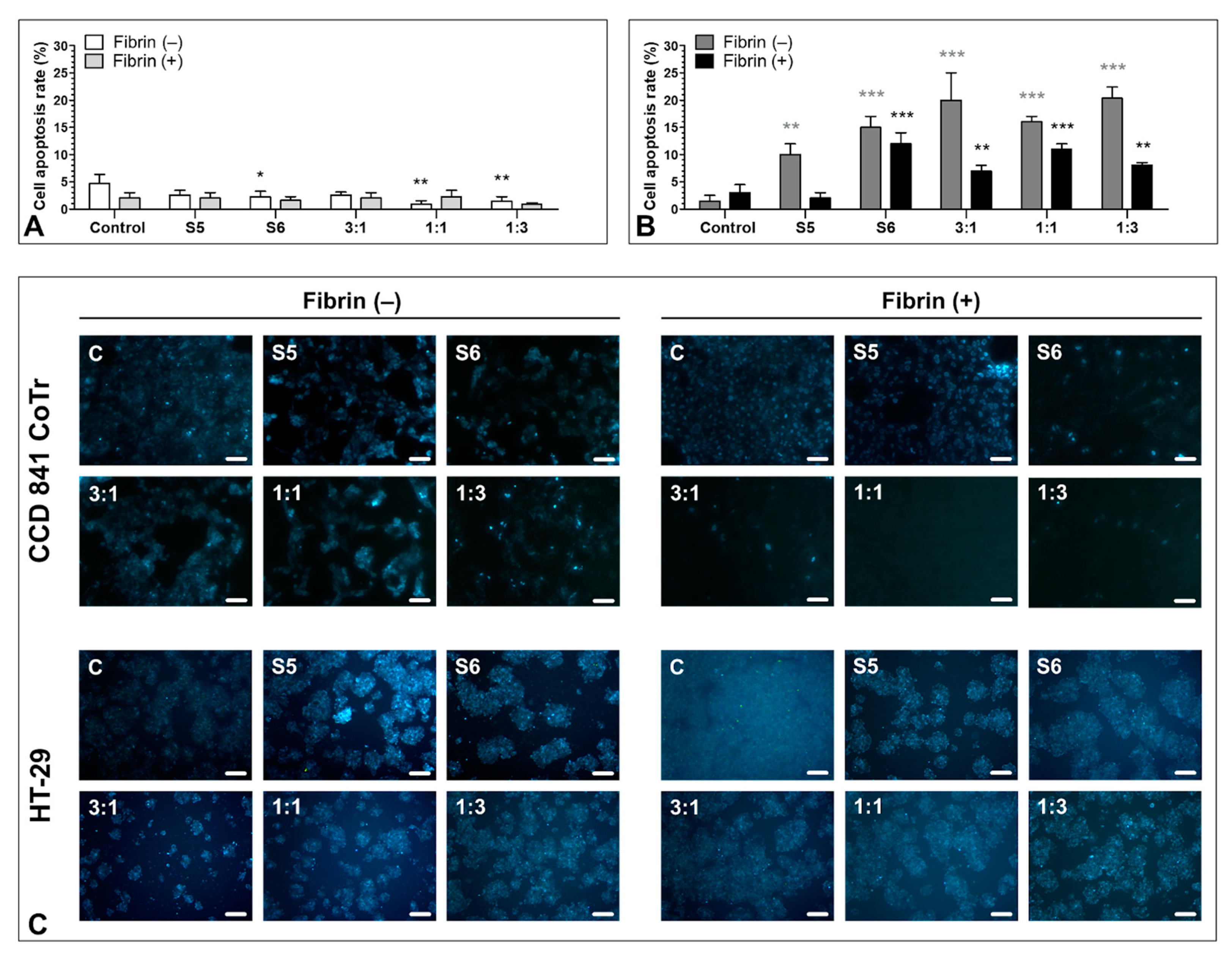
3.6. Pro-Apoptotic Activity of S5 and S6 Fungal Fractions
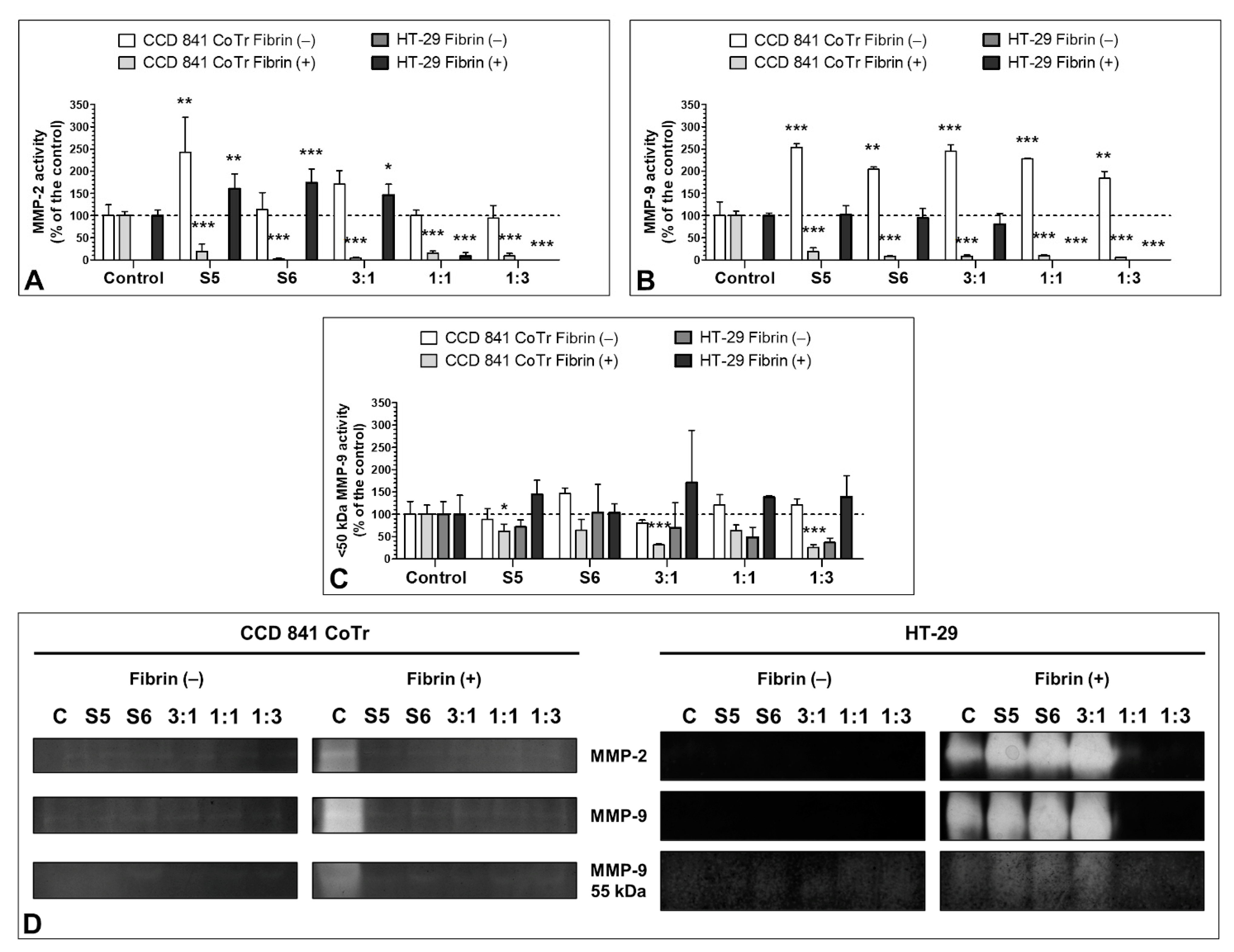
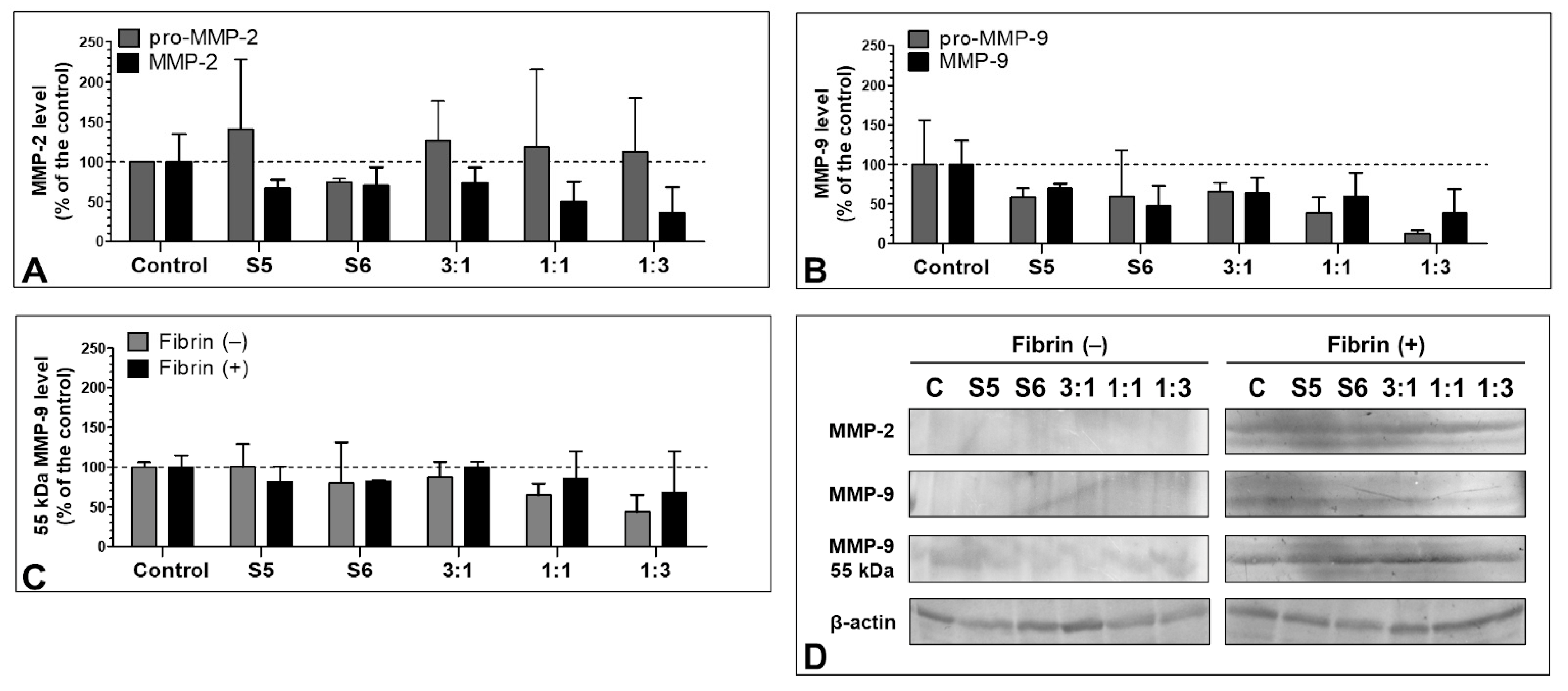
3.7. Effect of the S5 and S6 Fractions with Fibrinogen on MMP-2 and MMP-9
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xu, T.; Beelman, R.B. The Bioactive Com-Pounds in Medicinal Mushrooms Have Potential Protective Effects against Neu-Rodegenerative Diseases. Adv. Food Technol. Nutr. Sci. Open J. 2015, 1, 62–66. [Google Scholar] [CrossRef]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.M.; Aziz, R. Mushrooms: A Potential Natural Source of Anti-Inflammatory Compounds for Medical Applications. Available online: https://www.hindawi.com/journals/mi/2014/805841 (accessed on 21 December 2018).
- Badalyan, S. Medicinal Aspects of Edible Ectomycorrhizal Mushrooms. In Edible Ectomycorrhizal Mushrooms: Current Knowledge and Future Prospects; Zambonelli, A., Bonito, G.M., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 317–334. ISBN 978-3-642-33823-6. [Google Scholar]
- Muszyńska, B.; Kała, K.; Sułkowska-Ziaja, K. Edible Mushrooms and Their In Vitro Culture as a Source of Anticancer Compounds. In Biotechnology and Production of Anti-Cancer Compounds; Springer: Cham, Switzerland, 2017; pp. 231–251. ISBN 978-3-319-53879-2. [Google Scholar]
- Jaszek, M.; Osińska-Jaroszuk, M.; Janusz, G.; Matuszewska, A.; Stefaniuk, D.; Sulej, J.; Polak, J.; Ruminowicz, M.; Grzywnowicz, K.; Jarosz-Wilko\lazka, A. New Bioactive Fungal Molecules with High Antioxidant and Antimicrobial Capacity Isolated from Cerrena unicolor Idiophasic Cultures. BioMed. Res. Int. 2013, 2013, 497492. [Google Scholar] [CrossRef]
- Sánchez, C. Bioactives from Mushroom and Their Application. In Food Bioactives; Springer: Cham, Switzerland, 2017; pp. 23–57. ISBN 978-3-319-51637-0. [Google Scholar]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-Inflammatory Properties of Edible Mushrooms: A Review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef]
- Soares, A.A.; de Sá-Nakanishi, A.B.; Bracht, A.; da Costa, S.M.G.; Koehnlein, E.A.; de Souza, C.G.M.; Peralta, R.M. Hepatoprotective Effects of Mushrooms. Molecules 2013, 18, 7609–7630. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Recent Developments in Mushrooms as Anti-Cancer Therapeutics: A Review. 3 Biotech 2012, 2, 1–15. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Morales, D.; Soler-Rivas, C. Molecular Actions of Hypocholesterolaemic Compounds from Edible Mushrooms. Food Funct. 2018, 9, 53–69. [Google Scholar] [CrossRef]
- Rathee, S.; Rathee, D.; Rathee, D.; Kumar, V.; Rathee, P. Mushrooms as Therapeutic Agents. Rev. Bras. Farmacogn. 2012, 22, 459–474. [Google Scholar] [CrossRef]
- Yoon, S.-J.; Yu, M.-A.; Pyun, Y.-R.; Hwang, J.-K.; Chu, D.-C.; Juneja, L.R.; Mourão, P.A.S. The Nontoxic Mushroom Auricularia auricula Contains a Polysaccharide with Anticoagulant Activity Mediated by Antithrombin. Thromb. Res. 2003, 112, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Telles, C.B.S.; Sabry, D.A.; Almeida-Lima, J.; Costa, M.S.S.P.; Melo-Silveira, R.F.; Trindade, E.S.; Sassaki, G.L.; Wisbeck, E.; Furlan, S.A.; Leite, E.L.; et al. Sulfation of the Extracellular Polysaccharide Produced by the Edible Mushroom Pleurotus sajor-caju Alters Its Antioxidant, Anticoagulant and Antiproliferative Properties in Vitro. Carbohydr. Polym. 2011, 85, 514–521. [Google Scholar] [CrossRef]
- Akahane, K.; Satoh, K.; Ohta, M.; Ozaki, Y. Hot Water Extracts of the Royal Sun Mushroom, Agaricus brasiliensis (Higher Basidiomycetes), Inhibit Platelet Activation via the P2Y1 Receptor. Int. J. Med. Mushrooms 2015, 17, 763–770. [Google Scholar] [CrossRef]
- Khamlue, R.; Naksupan, N.; Ounaroon, A.; Saelim, N. Skin Wound Healing Promoting Effect of Polysaccharides Extracts from Tremella fuciformis and Auricularia auricula on the Ex-Vivo Porcine Skin Wound Healing Model. In Proceedings of the 4th International Conference on Chemical, Biological and Environmental Engineering, Phket, Thailand, 1–2 September 2012; pp. 93–98. [Google Scholar]
- Janusz, G.; Rogalski, J.; Szczodrak, J. Increased Production of Laccase by Cerrena unicolor in Submerged Liquid Cultures. World J. Microbiol. Biotechnol. 2007, 23, 1459–1464. [Google Scholar] [CrossRef]
- Cho, N.S.; Chungbuk, N.U.; Leonowicz, A.; Jarosz Wilkolazka, A.; Ginalska, G.; Cho, H.Y.; Shin, S.J.; Choi, Y.J.; Ohga, S. Degradation of a Non-Phenolic Beta-0-4 Lignin Model Dimer by Cerrena unicolor Laccase and Mediators, Acetovanillone and Acetosyringone. J. Fac. Agr. Kyushu Univ. 2008, 53, 7–12. [Google Scholar]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin Degradation: Microorganisms, Enzymes Involved, Genomes Analysis and Evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, A.; Karp, M.; Jaszek, M.; Janusz, G.; Osińska-Jaroszuk, M.; Sulej, J.; Stefaniuk, D.; Tomczak, W.; Giannopoulos, K. Laccase Purified from Cerrena unicolor Exerts Antitumor Activity against Leukemic Cells. Oncol. Lett. 2016, 11, 2009–2018. [Google Scholar] [CrossRef]
- Mizerska-Dudka, M.; Jaszek, M.; Błachowicz, A.; Rejczak, T.P.; Matuszewska, A.; Osińska-Jaroszuk, M.; Stefaniuk, D.; Janusz, G.; Sulej, J.; Kandefer-Szerszeń, M. Fungus Cerrena unicolor as an Effective Source of New Antiviral, Immunomodulatory, and Anticancer Compounds. Int. J. Biol. Macromol. 2015, 79, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Statkiewicz, M.; Matuszewska, A.; Jaszek, M.; Janusz, G.; Osinska, M.; Sulej, J.; Stefaniuk, D.; Mikula, M.; Ostrowski, J. Antimelanomic Effects of High- and Low-Molecular Weight Bioactive Subfractions Isolated from the Mossy Maze Mushroom, Cerrena unicolor (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, A.; Stefaniuk, D.; Jaszek, M.; Pięt, M.; Zając, A.; Matuszewski, Ł.; Cios, I.; Grąz, M.; Paduch, R.; Bancerz, R. Antitumor Potential of New Low Molecular Weight Antioxidative Preparations from the White Rot Fungus Cerrena unicolor against Human Colon Cancer Cells. Sci. Rep. 2019, 9, 1975. [Google Scholar] [CrossRef]
- Spotnitz, W.D. Fibrin Sealant: The Only Approved Hemostat, Sealant, and Adhesive—A Laboratory and Clinical Perspective. Int. Sch. Res. Not. 2014, 2014, 203943. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Connolly, M.P.; Ramirez, M.G.; Heredia, J.B. de Costs Analysis of Fibrin Sealant for Prevention of Anastomotic Leakage in Lower Colorectal Surgery. Risk Manag. Healthc. Policy 2020, 13, 5–11. [Google Scholar] [CrossRef]
- Angelini, P.; Sciuto, A.; Cuccurullo, D.; Pirozzi, F.; Reggio, S.; Corcione, F. Prevention of Internal Hernias and Pelvic Adhesions Following Laparoscopic Left-Sided Colorectal Resection: The Role of Fibrin Sealant. Surg. Endosc. 2017, 31, 3048–3055. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, T.; Liu, X.; He, Y.; Deng, L.; Guo, J.; Hua, Y.; Luo, T.; Gao, X. Improved Anti-Tumor Efficacy via Combination of Oxaliplatin and Fibrin Glue in Colorectal Cancer. Oncotarget 2017, 9, 2515–2526. [Google Scholar] [CrossRef]
- Kanellos, I.; Mantzoros, I.; Demetriades, H.; Kalfadis, S.; Kelpis, T.; Sakkas, L.; Betsis, D. Healing of Colon Anastomoses Covered with Fibrin Glue after Immediate Postoperative Intraperitoneal Administration of 5-Fluorouracil. Dis. Colon Rectum 2004, 47, 510–515. [Google Scholar] [CrossRef]
- Kanellos, D.; Blouhos, K.; Pramateftakis, M.G.; Kanellos, I.; Demetriades, H.; Sakkas, L.; Betsis, D. Effect of 5-Fluorouracil plus Interferon on the Integrity of Colonic Anastomoses Covering with Fibrin Glue. World J. Surg. 2007, 31, 186–191. [Google Scholar] [CrossRef]
- Luddington, R.J. Thrombelastography/Thromboelastometry. Clin. Lab. Haematol. 2005, 27, 81–90. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Van de Loosdrecht, A.A.; Beelen, R.H.J.; Ossenkoppele, G.J.; Broekhoven, M.G.; Langenhuijsen, M.M.A.C. A Tetrazolium-Based Colorimetric MTT Assay to Quantitate Human Monocyte Mediated Cytotoxicity against Leukemic Cells from Cell Lines and Patients with Acute Myeloid Leukemia. J. Immunol. Methods 1994, 174, 311–320. [Google Scholar] [CrossRef]
- Pięt, M.; Zając, A.; Paduch, R.; Jaszek, M.; Frant, M.; Stefaniuk, D.; Matuszewska, A.; Grzywnowicz, K. Chemopreventive Activity of Bioactive Fungal Fractions Isolated from Milk-Supplemented Cultures of Cerrena unicolor and Pycnoporus sanguineus on Colon Cancer Cells. 3 Biotech. 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Borenfreund, E.; Puerner, J.A. A Simple Quantitative Procedure Using Monolayer Cultures for Cytotoxicity Assays (HTD/NR-90). J. Tissue Cult. Methods 1985, 9, 7–9. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Langner, E.; Bądziul, D.; Wertel, I.; Rzeski, W. Quercetin and Sorafenib as a Novel and Effective Couple in Programmed Cell Death Induction in Human Gliomas. Neurotox. Res. 2014, 26, 64–77. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Fragkostefanakis, S.; Dandachi, F.; Kalaitzis, P. Expression of Arabinogalactan Proteins during Tomato Fruit Ripening and in Response to Mechanical Wounding, Hypoxia and Anoxia. Plant. Physiol. Biochem. 2012, 52, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, A.; Zając, A.; Kurzyna-Szklarek, M.; Cybulska, J.; Zdunek, A. Investigations of Changes in the Arabinogalactan Proteins (AGPs) Structure, Size and Composition during the Fruit Ripening Process. Sci. Rep. 2020, 10, 20621. [Google Scholar] [CrossRef] [PubMed]
- Lorentz, K.M.; Kontos, S.; Frey, P.; Hubbell, J.A. Engineered Aprotinin for Improved Stability of Fibrin Biomaterials. Biomaterials 2011, 32, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Doljak, B.; Stegnar, M.; Urleb, U.; Kreft, S.; Umek, A.; Ciglaric, M.; Strukelj, B.; Popovic, T. Screening for Selective Thrombin Inhibitors in Mushrooms. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2001, 12, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Du, C.; Cheng, H.; Chen, J.; Lin, C. Study on the Anticoagulant or Procoagulant Activities of Type II Phenolic Acid Derivatives. Molecules 2017, 22, 2047. [Google Scholar] [CrossRef] [PubMed]
- Côté, J.; Caillet, S.; Doyon, G.; Sylvain, J.-F.; Lacroix, M. Bioactive Compounds in Cranberries and Their Biological Properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 666–679. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Darmoul, D.; Gratio, V.; Devaud, H.; Lehy, T.; Laburthe, M. Aberrant Expression and Activation of the Thrombin Receptor Protease-Activated Receptor-1 Induces Cell Proliferation and Motility in Human Colon Cancer Cells. Am. J. Pathol. 2003, 162, 1503–1513. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Wang, H.; Peng, Y.; Li, H.; Zhou, Y.; Liu, S.; Wang, F.; Liu, L.; Chang, Y.; et al. Soft Fibrin Matrix Downregulates DAB2IP to Promote Nanog-Dependent Growth of Colon Tumor-Repopulating Cells. Cell Death Dis. 2019, 10, 151. [Google Scholar] [CrossRef]
- Gao, M.; Zhan, Y.-Q.; Yu, M.; Ge, C.-H.; Li, C.-Y.; Zhang, J.-H.; Wang, X.-H.; Ge, Z.-Q.; Yang, X.-M. Hepassocin Activates the EGFR/ERK Cascade and Induces Proliferation of L02 Cells through the Src-Dependent Pathway. Cell. Signal. 2014, 26, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Rabizadeh, E.; Cherny, I.; Lederfein, D.; Sherman, S.; Binkovsky, N.; Rosenblat, Y.; Inbal, A. The Cell-Membrane Prothrombinase, Fibrinogen-like Protein 2, Promotes Angiogenesis and Tumor Development. Thromb. Res. 2015, 136, 118–124. [Google Scholar] [CrossRef]
- Pradhan, S.; Hassani, I.; Seeto, W.J.; Lipke, E.A. PEG-Fibrinogen Hydrogels for Three-Dimensional Breast Cancer Cell Culture. J. Biomed. Mater. Res. A 2017, 105, 236–252. [Google Scholar] [CrossRef]
- Sanoj Rejinold, N.; Muthunarayanan, M.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Curcumin Loaded Fibrinogen Nanoparticles for Cancer Drug Delivery. J. Biomed. Nanotechnol. 2011, 7, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Groblewska, M.; Mroczko, B.; Szmitkowski, M. The Role of Selected Matrix Metalloproteinases and Their Inhibitors in Colorectal Cancer Development. Postepy Hig. Med. Doswiadczalnej Online 2010, 64, 22–30. [Google Scholar]
- Murray, D.; Morrin, M.; Mcdonnell, S. Increased Invasion and Expression of MMP-9 in Human Colorectal Cell Lines by a CD44-Dependent Mechanism. Anticancer Res. 2004, 24, 489–494. [Google Scholar]
- Wu, W.; He, J.T.; Ruan, J.D.; Wang, R.B.; Zhang, Y.D. Expression of MMP-2, MMP-9 and collagen type IV and their relationship in colorectal carcinomas. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2008, 24, 908–909. [Google Scholar]
- Reiss, M.; Han, Y.; Garcia, E.; Goldberg, M.; Hong, Y.; Garner, W. Matrix Metalloproteinase-9 Delays Wound Healing in a Murine Wound Model. Surgery 2010, 147, 295. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Wulsin, D.; Skokos, E.A.; Fleckman, P.; Pirrone, A.; Shipley, J.M.; Senior, R.M.; Bornstein, P. Mice That Lack Matrix Metalloproteinase-9 Display Delayed Wound Healing Associated with Delayed Reepithelization and Disordered Collagen Fibrillogenesis. Matrix Biol. 2009, 28, 65–73. [Google Scholar] [CrossRef]
- Hariono, M.; Yuliani, S.H.; Istyastono, E.P.; Riswanto, F.D.O.; Adhipandito, C.F. Matrix Metalloproteinase 9 (MMP9) in Wound Healing of Diabetic Foot Ulcer: Molecular Target and Structure-Based Drug Design. Wound Med. 2018, 22, 1–13. [Google Scholar] [CrossRef]
- Gill, S.E.; Parks, W.C. Metalloproteinases and Their Inhibitors: Regulators of Wound Healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; den Steen, P.E.V.; Opdenakker, G. Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9): The next Decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Monaco, S.; Gioia, M.; Rodriguez, J.; Fasciglione, G.F.; Di Pierro, D.; Lupidi, G.; Krippahl, L.; Marini, S.; Coletta, M. Modulation of the Proteolytic Activity of Matrix Metalloproteinase-2 (Gelatinase A) on Fibrinogen. Biochem. J. 2007, 402, 503–513. [Google Scholar] [CrossRef]
- Sarker, H.; Hardy, E.; Haimour, A.; Maksymowych, W.P.; Botto, L.D.; Fernandez-Patron, C. Identification of Fibrinogen as a Natural Inhibitor of MMP-2. Sci. Rep. 2019, 9, 4340. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamaoka, Y.; Shinoyama, M.; Kamiya, A. Novel Drug Delivery System Using Autologous Fibrin Glue: Release Properties of Anti-Cancer Drugs. Biol. Pharm. Bull. 2000, 23, 371–374. [Google Scholar] [CrossRef]
- Joo, J.Y.; Park, G.Y.; An, S.S.A. Biocompatible and Biodegradable Fibrinogen Microspheres for Tumor-Targeted Doxorubicin Delivery. Int. J. Nanomed. 2015, 10, 101–111. [Google Scholar] [CrossRef][Green Version]
- Iemma, F.; Spizzirri, U.G.; Puoci, F.; Muzzalupo, R.; Trombino, S.; Picci, N. Radical Crosslinked Albumin Microspheres as Potential Drug Delivery Systems: Preparation and In Vitro Studies. Drug Deliv. 2005, 12, 179–184. [Google Scholar] [CrossRef]
- Rubino, O.P.; Kowalsky, R.; Swarbrick, J. Albumin Microspheres as a Drug Delivery System: Relation Among Turbidity Ratio, Degree of Cross-Linking, and Drug Release. Pharm. Res. 1993, 10, 1059–1065. [Google Scholar] [CrossRef]
- Carter Angela, M.; Cymbalista Charlotte, M.; Spector Tim, D.; Grant Peter, J. Heritability of Clot Formation, Morphology, and Lysis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefaniuk, D.; Misztal, T.; Pięt, M.; Zając, A.; Kopycińska, M.; Matuszewska, A.; Ruminowicz-Stefaniuk, M.; Matuszewski, Ł.; Marcińczyk, N.; Belcarz, A.; et al. Thromboelastometric Analysis of Anticancer Cerrena unicolor Subfractions Reveal Their Potential as Fibrin Glue Drug Carrier Enhancers. Biomolecules 2021, 11, 1263. https://doi.org/10.3390/biom11091263
Stefaniuk D, Misztal T, Pięt M, Zając A, Kopycińska M, Matuszewska A, Ruminowicz-Stefaniuk M, Matuszewski Ł, Marcińczyk N, Belcarz A, et al. Thromboelastometric Analysis of Anticancer Cerrena unicolor Subfractions Reveal Their Potential as Fibrin Glue Drug Carrier Enhancers. Biomolecules. 2021; 11(9):1263. https://doi.org/10.3390/biom11091263
Chicago/Turabian StyleStefaniuk, Dawid, Tomasz Misztal, Mateusz Pięt, Adrian Zając, Magdalena Kopycińska, Anna Matuszewska, Marta Ruminowicz-Stefaniuk, Łukasz Matuszewski, Natalia Marcińczyk, Anna Belcarz, and et al. 2021. "Thromboelastometric Analysis of Anticancer Cerrena unicolor Subfractions Reveal Their Potential as Fibrin Glue Drug Carrier Enhancers" Biomolecules 11, no. 9: 1263. https://doi.org/10.3390/biom11091263
APA StyleStefaniuk, D., Misztal, T., Pięt, M., Zając, A., Kopycińska, M., Matuszewska, A., Ruminowicz-Stefaniuk, M., Matuszewski, Ł., Marcińczyk, N., Belcarz, A., Żuchowski, J., Skrabalak, I., Grąz, M., Ciołek, B., Paduch, R., & Jaszek, M. (2021). Thromboelastometric Analysis of Anticancer Cerrena unicolor Subfractions Reveal Their Potential as Fibrin Glue Drug Carrier Enhancers. Biomolecules, 11(9), 1263. https://doi.org/10.3390/biom11091263







