Decreased Cerebrospinal Fluid Antioxidative Capacity Is Related to Disease Severity and Progression in Early Multiple Sclerosis
Abstract
:1. Introduction
2. Subjects, Materials and Methods
2.1. Patients and Controls
2.2. Clinical Assessments and Follow-Up
2.3. Serum and CSF Sampling and Antioxidative Capacity Analyses
2.4. MRI Protocol
2.5. Statistical Analyses
3. Results
3.1. AOC in Relation to Demographic and Laboratory Data
3.2. CSF AOC in Association with Disease Severity in CIS and RRMS
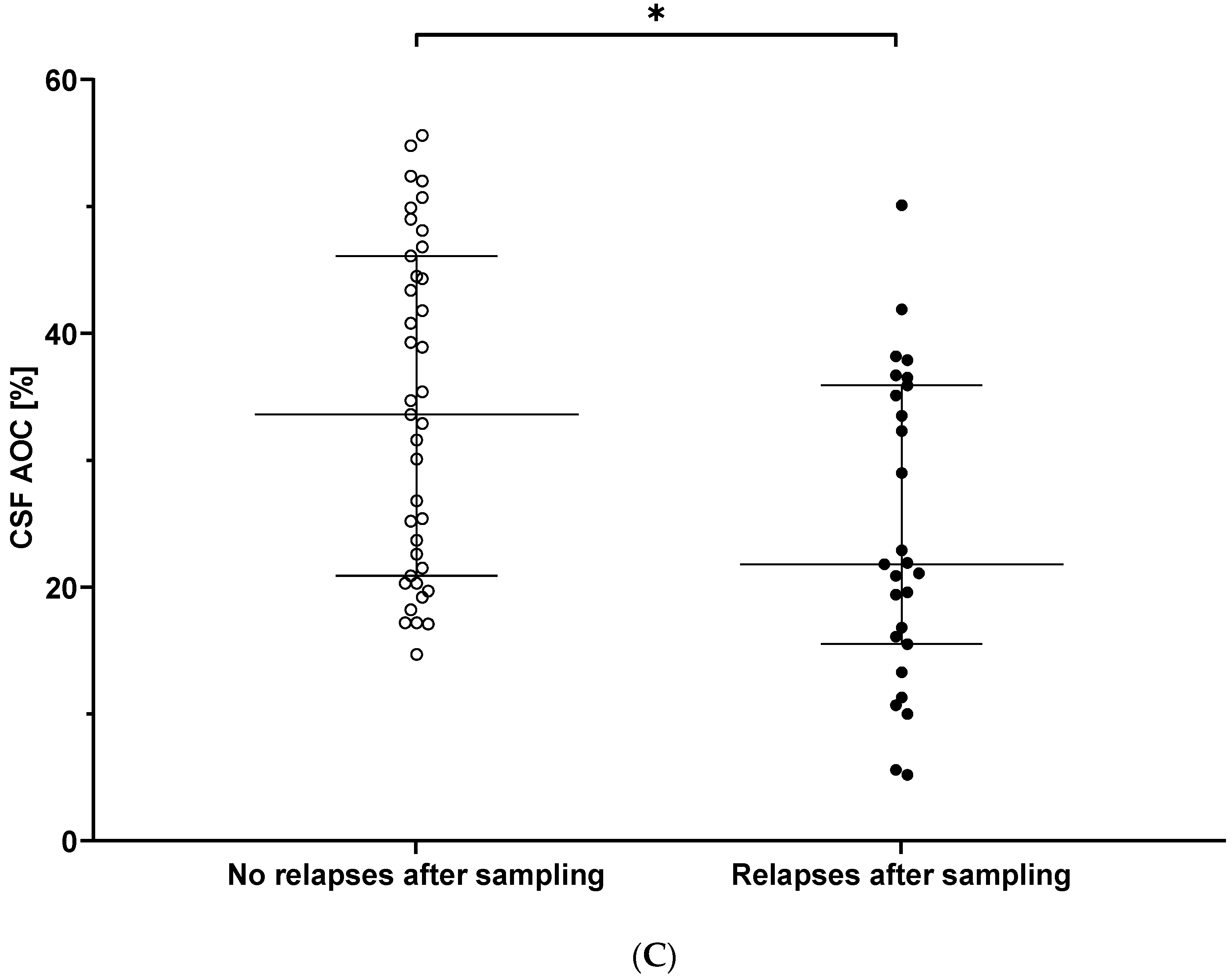
3.3. CSF AOC and Prediction of Clinical Disease Activity over Follow-Up
3.4. Serum AOC and the Association with Clinical Disease Characteristics
3.5. AOC in Relation to MRI Metrics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, N. Multiple sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 43. [Google Scholar] [CrossRef]
- Lassmann, H. Multiple sclerosis pathology. Cold Spring Harb. Perspect. Med. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirshafiey, A.; Mohsenzadegan, M. Antioxidant therapy in multiple sclerosis. Immunopharmacol. Immunotoxicol. 2009, 31, 13–29. [Google Scholar] [CrossRef]
- Stephenson, E.; Nathoo, N.; Mahjoub, Y.; Dunn, J.F.; Yong, V.W. Iron in multiple sclerosis: Roles in neurodegeneration and repair. Nat. Rev. Neurol. 2014, 10, 459–468. [Google Scholar] [CrossRef]
- Ibitoye, R.; Rice, C. Oxidative stress-related biomarkers in multiple sclerosis: A review. Biomark Med. 2016, 10, 889–902. [Google Scholar] [CrossRef] [Green Version]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative damage in multiple sclerosis lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef]
- Koch, M.; Ramsaransing, G.S.M.; Arutjunyan, A.V.; Stepanov, M.; Teelken, A.; Heersema, D.J.; De Keyser, J. Oxidative stress in serum and peripheral blood leukocytes in patients with different disease courses of multiple sclerosis. J. Neurol. 2006, 253, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Reth, M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat. Immunol. 2002, 3, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Besler, H.T.; Çomoğlu, S. Lipoprotein oxidation, plasma total antioxidant capacity and homocysteine level in patients with multiple sclerosis. Nutr. Neurosci. 2003, 6, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Adamczyk-Sowa, M. New insights into the role of oxidative stress mechanisms in the pathophysiology and treatment of multiple sclerosis. Oxidative Med. Cell. Longev. 2016, 2016, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Murat, U.; Md, S.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. WAO J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristori, G.; Brescianini, S.; Pino, A.; Visconti, A.; Vittori, D.; Coarelli, G.; Cotichini, R.; Bocca, B.; Forte, G.; Pozzilli, C.; et al. Serum elements and oxidative status in clinically isolated syndromes: Imbalance and predictivity. Neurology 2011, 76, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Ljubisavljevic, S.; Stojanovic, I.; Vojinovic, S.; Stojanov, D.; Stojanovic, S.; Kocic, G.; Savic, D.; Cvetkovic, T.; Pavlovic, D. Cerebrospinal fluid and plasma oxidative stress biomarkers in different clinical phenotypes of neuroinflammatory acute attacks. Conceptual accession: From fundamental to clinic. Cell. Mol. Neurobiol. 2013, 33, 767–777. [Google Scholar] [CrossRef]
- Ljubisavljevic, S.; Stojanovic, I.; Cvetkovic, T.; Vojinovic, S.; Stojanov, D.; Stojanovic, D.; Stefanovic, N.; Pavlovic, D. Erythrocytes’ antioxidative capacity as a potential marker of oxidative stress intensity in neuroinflammation. J. Neurol. Sci. 2014, 337, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.T.; Sharma, R.; Lim, J.L.; Haider, L.; Frischer, J.M.; Drexhage, J.; Mahad, D.; Bradl, M.; Van Horssen, J.; Lassmann, H. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 2012, 135, 886–899. [Google Scholar] [CrossRef] [Green Version]
- Pasquali, L.; Pecori, C.; Lucchesi, C.; LoGerfo, A.; Iudice, A.; Siciliano, G.; Bonuccelli, U. Plasmatic oxidative stress biomarkers in multiple sclerosis: Relation with clinical and demographic characteristics. Clin. Biochem. 2015, 48, 19–23. [Google Scholar] [CrossRef]
- Karlík, M.; Valkovič, P.; Hančinová, V.; Krížová, L.; Tóthová, Ľ.; Celec, P. Markers of oxidative stress in plasma and saliva in patients with multiple sclerosis. Clin. Biochem. 2015, 48, 24–28. [Google Scholar] [CrossRef]
- Filippi, M.; Preziosa, P.; Rocca, M.A. MRI in multiple sclerosis: What is changing? Curr. Opin. Neurol. 2018, 31, 386–395. [Google Scholar] [CrossRef]
- Gasperini, C.; Prosperini, L.; Tintoré, M.; Sormani, M.P.; Filippi, M.; Rio, J.; Palace, J.; Rocca, M.A.; Ciccarelli, O.; Barkhof, F.; et al. Unraveling treatment response in multiple sclerosis: A clinical and MRI challenge. Neurology 2019, 92, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 Revisions to the “McDonald Criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teunissen, C.; Menge, T.; Altintas, A.; Álvarez-Cermeño, J.C.; Bertolotto, A.; Berven, F.S.; Brundin, L.; Comabella, M.; Degn, M.; Deisenhammer, F.; et al. Consensus definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis. Mult. Scler. J. 2013, 19, 1802–1809. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1453. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.; Alvarez-Cermeño, J.; Bernardi, G.; Cogato, I.; Fredman, P.; Frederiksen, J.; Fredrikson, S.; Gallo, P.; Grimaldi, L.M.; Grønning, M. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensus report. J. Neurol. Neurosurg. Psychiatry 1994, 57, 897–902. [Google Scholar] [CrossRef] [Green Version]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patenaude, B.; Smith, S.M.; Kennedy, D.N.; Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011, 56, 907–922. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.; Jenkinson, M.; Woolrich, M.; Beckmann, C.; Behrens, T.; Johansen-Berg, H.; Bannister, P.; De Luca, M.; Ivana, D.; Flitney, D.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef] [Green Version]
- Plummer, D.L. DispImage: A Display and Analysis Tool for Medical Images. Rev. Neuroradiol. 1992, 5, 489–495. [Google Scholar]
- Aydin, O.; Ellidag, H.Y.; Eren, E.; Kurtulus, F.; Yaman, A.; Yilmaz, N. Ischemia modified albumin is an indicator of oxidative stress in multiple sclerosis. Biochem. Medica 2014, 24, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Miller, E.; Mrowicka, M.; Malinowska, K.; Zolynski, K.; Kedziora, J. Effects of the whole-body cryotherapy on a total anti-oxidative status and activities of some antioxidative enzymes in blood of patinets with multipel sclerosis—Preliminary study. J. Med. Investig. 2010, 57, 168–173. [Google Scholar] [CrossRef] [Green Version]
- Emami Aleagha, M.S.; Siroos, B.; Ahmadi, M.; Balood, M.; Palangi, A.; Haghighi, A.N.; Harirchian, M.H. Decreased concentration of Klotho in the cerebrospinal fluid of patients with relapsing-remitting multiple sclerosis. J. Neuroimmunol. 2015, 281, 5–8. [Google Scholar] [CrossRef]
- Ljubisavljevic, S.; Stojanovic, I.; Vojinovic, S.; Stojanov, D.; Stojanovic, S.; Cvetkovic, T.; Savic, D.; Pavlovic, D. The patients with clinically isolated syndrome and relapsing remitting multiple sclerosis show different levels of advanced protein oxidation products and total thiol content in plasma and CSF. Neurochem. Int. 2013, 62, 988–997. [Google Scholar] [CrossRef]
- Adamczyk, B.; Koziarska, D.; Kasperczyk, S.; Adamczyk-Sowa, M. Are antioxidant parameters in serum altered in patients with relapsing-remitting multiple sclerosis treated with II-line immunomodulatory therapy? Free Radic. Res. 2018, 52, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Keles, M.S.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can. J. Neurol. Sci. 2001, 28, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Seven, A.; Aslan, M.; Incir, S.; Altintaş, A. Evaluation of oxidative and nitrosative stress in relapsing remitting multiple sclerosis: Effect of corticosteroid therapy. Folia Neuropathol. 2013, 51, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xie, K.; Wang, C.; Bi, J. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur. Neurol. 2014, 72, 249–254. [Google Scholar] [CrossRef]
- Adamczyk-Sowa, M.; Pierzchala, K.; Sowa, P.; Mucha, S.; Sadowska-Bartosz, I.; Adamczyk, J.; Hartel, M. Melatonin acts as antioxidant and improves sleep in MS patients. Neurochem. Res. 2014, 39, 1585–1593. [Google Scholar] [CrossRef] [Green Version]
- Tasset, I.; Agüera, E.; Sánchez-López, F.; Feijóo, M.; Giraldo, A.I.; Cruz, A.H.; Gascón, F.; Túnez, I. Peripheral oxidative stress in relapsing-remitting multiple sclerosis. Clin. Biochem. 2012, 45, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Socha, K.; Kochanowicz, J.; Karpińska, E.; Soroczyńska, J.; Jakoniuk, M.; Mariak, Z.; Borawska, M.H. Dietary habits and selenium, glutathione peroxidase and total antioxidant status in the serum of patients with relapsing-remitting multiple sclerosis. Nutr. J. 2014, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Acar, A.; Ugur Cevik, M.; Evliyaoglu, O.; Uzar, E.; Tamam, Y.; Arıkanoglu, A.; Yucel, Y.; Varol, S.; Onder, H.; Taşdemir, N. Evaluation of serum oxidant/antioxidant balance in multiple sclerosis. Acta Neurol. Belg. 2012, 112, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.R.; Kallaur, A.P.; Simão, A.N.C.; Morimoto, H.K.; Lopes, J.; Panis, C.; Petenucci, D.L.; Da Silva, E.; Cecchini, R.; Kaimen-Maciel, D.R.; et al. Oxidative stress in multiple sclerosis patients in clinical remission: Association with the expanded disability status scale. J. Neurol. Sci. 2012, 321, 49–53. [Google Scholar] [CrossRef]
- Oliveira, S.R.; Kallaur, A.P.; Reiche, E.M.V.; Kaimen-Maciel, D.R.; Panis, C.; Lozovoy, M.A.B.; Morimoto, H.K.; Maes, M.; Dichi, I.; Simo, A.N.C. Albumin and protein oxidation are predictors that differentiate relapsing-remitting from progressive clinical forms of multiple sclerosis. Mol. Neurobiol. 2017, 54, 2961–2968. [Google Scholar] [CrossRef]
- Hadžović-Džuvo, A.; Lepara, O.; Valjevac, A.; Avdagić, N.; Hasić, S.; Kiseljaković, E.; Ibragić, S.; Alajbegović, A. Serum total antioxidant capacity in patients with multiple sclerosis. Bosn. J. Basic Med. Sci. 2011, 11, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Siotto, M.; Filippi, M.M.; Simonelli, I.; Landi, D.; Ghazaryan, A.; Vollaro, S.; Ventriglia, M.; Pasqualetti, P.; Rongioletti, M.C.A.; Squitti, R.; et al. Oxidative stress related to iron metabolism in relapsing remitting multiple sclerosis patients with low disability. Front. Neurosci. 2019, 13, 86. [Google Scholar] [CrossRef] [Green Version]
- Alimonti, A.; Ristori, G.; Giubilei, F.; Stazi, M.A.; Pino, A.; Visconti, A.; Brescianini, S.; Monti, M.S.; Forte, G.; Stanzione, P.; et al. Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicology 2007, 28, 450–456. [Google Scholar] [CrossRef]
- Armon-Omer, A.; Waldman, C.; Simaan, N.; Neuman, H.; Tamir, S.; Shahien, R. New insights on the nutrition status and antioxidant capacity in multiple sclerosis patients. Nutrients 2019, 11, 427. [Google Scholar] [CrossRef] [Green Version]
- Sormani, M.P.; Gasperini, C.; Romeo, M.; Rio, J.; Calabrese, M.; Cocco, E.; Enzingher, C.; Fazekas, F.; Filippi, M.; Gallo, A.; et al. Assessing response to interferon-β in a multicenter dataset of patients with MS. Neurology 2016, 87, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Sastre-Garriga, J.; Pareto, D.; Battaglini, M.; Rocca, M.A.; Ciccarelli, O.; Enzinger, C.; Wuerfel, J.; Sormani, M.P.; Barkhof, F.; Yousry, T.A.; et al. MAGNIMS consensus recommendations on the use of brain and spinal cord atrophy measures in clinical practice. Nat. Rev. Neurol. 2020, 16, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güngör, N.; Özyürek, M.; Gülü, K.; Eki, S.D.; Apak, R. Comparative evaluation of antioxidant capacities of thiol-based antioxidants measured by different in vitro methods. Talanta 2011, 83, 1650–1658. [Google Scholar] [CrossRef] [PubMed]



| CIS/RRMS n = 55/n = 11 | Controls n = 67 | |
|---|---|---|
| n female | 44 (66.7) | 45 (67.2) |
| Age (years) | 32.0 (26.4–39.1) | 32.7 (25.2–44.9) |
| Age disease onset (years) | 31.1 (25.2–39.0) | N/A |
| Disease duration (months) | 0.5 (0.3–3.9) | N/A |
| Clinical FU (years) | 4.5 (1.9–7.0) | N/A |
| EDSS | 1.5 (0.0–3.0) | N/A |
| EDSS (in remission) | 1.0 (0.0–2.0) | N/A |
| EDSS last FU (in remission) | 0.0 (0.0–1.5) | N/A |
| n Active disease ≤ 30 days prior to sampling | 45 (68.2) | N/A |
| n Cortisone ≤ 30 days prior to sampling | 19 (28.8) | N/A |
| n DMT | 0 (0) | N/A |
| n DMT at last FU | 33 (50.0) | N/A |
| ARR last FU (n = 21/11) † | 0.5 (0.3–0.9) | N/A |
| CIS/RRMS n = 55/n = 11 | Controls n = 67 | p-Value | |
|---|---|---|---|
| CSF white cell count (nr/µL) (ref. ≤ 4) | 9 (5–17) | 1 (1–2) | <0.001 a |
| n OCB positive | 64 (97.0) | 0 (0) | <0.001 b |
| Qalb (×103) | 4.89 (4.19–6.96) | 4.96 (4.11–5.84) | 0.434 a |
| n increased Qalb (BBB disruption) | 17 (25.8) | 0 (0) | <0.001 b |
| CSF lactate (mmol/L) (normal range < 2.1) | 1.5 (1.4–1.7) | 1.4 (1.4–1.5) | 0.027 a |
| CSF total protein (mg/dL) (normal range < 45) | 35 (27–43) | 30 (27–35) | 0.012 a |
| CSF AOC (%) | 29.5 (19.6–40.8) | 32.8 (23.0–41.4) | 0.180 a |
| Serum AOC (%) | 46.4 (41.1–50.4) | 47.0 (43.0–50.0) | 0.763 a |
| MRI BL n = 47 | MRI FU n = 34 | p-Value n = 22 | |
|---|---|---|---|
| Time from MRI to body fluid sampling a (months), b (years) | 5.06 (1.22–7.52) a | 3.51 (2.53–4.60) b | <0.001 |
| Normalized brain volume (cm3) | 1617.9 (1566.1–1662.7) | 1582.8 (1536.7–1623.2) | 0.001 |
| Normalized grey matter volume (cm3) | 832.6 (786.1–861.4) | 798.4 (772.0–831.8) | 0.004 |
| Normalized white matter volume (cm3) | 779.9 (760.9–818.4) | 787.7 (756.5–805.4) | 0.017 |
| Normalized cortical grey matter volume (cm3) | 674.9 (638.1–708.5) | 641.1 (613.4–682.9) | <0.001 |
| Normalized ventricular volume (cm3) | 28.6 (23.0–42.1) | 35.9 (24.7–44.1) | 0.004 |
| PBVC (%) | N/A | −0.45 (−0.92–−0.02) | N/A |
| Annualized PBVC rate (%/year) | N/A | −0.13 (−0.46–−0.02) | N/A |
| T2LL (cm3) | 5.9 (2.4–10.9) | 5.5 (3.3–7.5) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voortman, M.M.; Damulina, A.; Pirpamer, L.; Pinter, D.; Pichler, A.; Enzinger, C.; Ropele, S.; Bachmaier, G.; Archelos, J.-J.; Marsche, G.; et al. Decreased Cerebrospinal Fluid Antioxidative Capacity Is Related to Disease Severity and Progression in Early Multiple Sclerosis. Biomolecules 2021, 11, 1264. https://doi.org/10.3390/biom11091264
Voortman MM, Damulina A, Pirpamer L, Pinter D, Pichler A, Enzinger C, Ropele S, Bachmaier G, Archelos J-J, Marsche G, et al. Decreased Cerebrospinal Fluid Antioxidative Capacity Is Related to Disease Severity and Progression in Early Multiple Sclerosis. Biomolecules. 2021; 11(9):1264. https://doi.org/10.3390/biom11091264
Chicago/Turabian StyleVoortman, Margarete M., Anna Damulina, Lukas Pirpamer, Daniela Pinter, Alexander Pichler, Christian Enzinger, Stefan Ropele, Gerhard Bachmaier, Juan-Jose Archelos, Gunther Marsche, and et al. 2021. "Decreased Cerebrospinal Fluid Antioxidative Capacity Is Related to Disease Severity and Progression in Early Multiple Sclerosis" Biomolecules 11, no. 9: 1264. https://doi.org/10.3390/biom11091264
APA StyleVoortman, M. M., Damulina, A., Pirpamer, L., Pinter, D., Pichler, A., Enzinger, C., Ropele, S., Bachmaier, G., Archelos, J.-J., Marsche, G., & Khalil, M. (2021). Decreased Cerebrospinal Fluid Antioxidative Capacity Is Related to Disease Severity and Progression in Early Multiple Sclerosis. Biomolecules, 11(9), 1264. https://doi.org/10.3390/biom11091264







