Chitosan Augments Tetramycin against Soft Rot in Kiwifruit and Enhances Its Improvement for Kiwifruit Growth, Quality and Aroma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vitro Toxicity Tests
2.3. Field Experiments
2.3.1. Study Site
2.3.2. Field Control Experiment of Soft Rot Disease in Kiwifruit
2.4. Determination of Control Effect, Development and Quality
2.5. Analytical Methods
2.6. Statistical Analyses
3. Results
3.1. Toxicity Effects of Chitosan and Tetramycin against Soft Rot Pathogens
3.2. Field Control Effect of Chitosan and Tetramycin on Soft Rot Disease of Kiwifruit
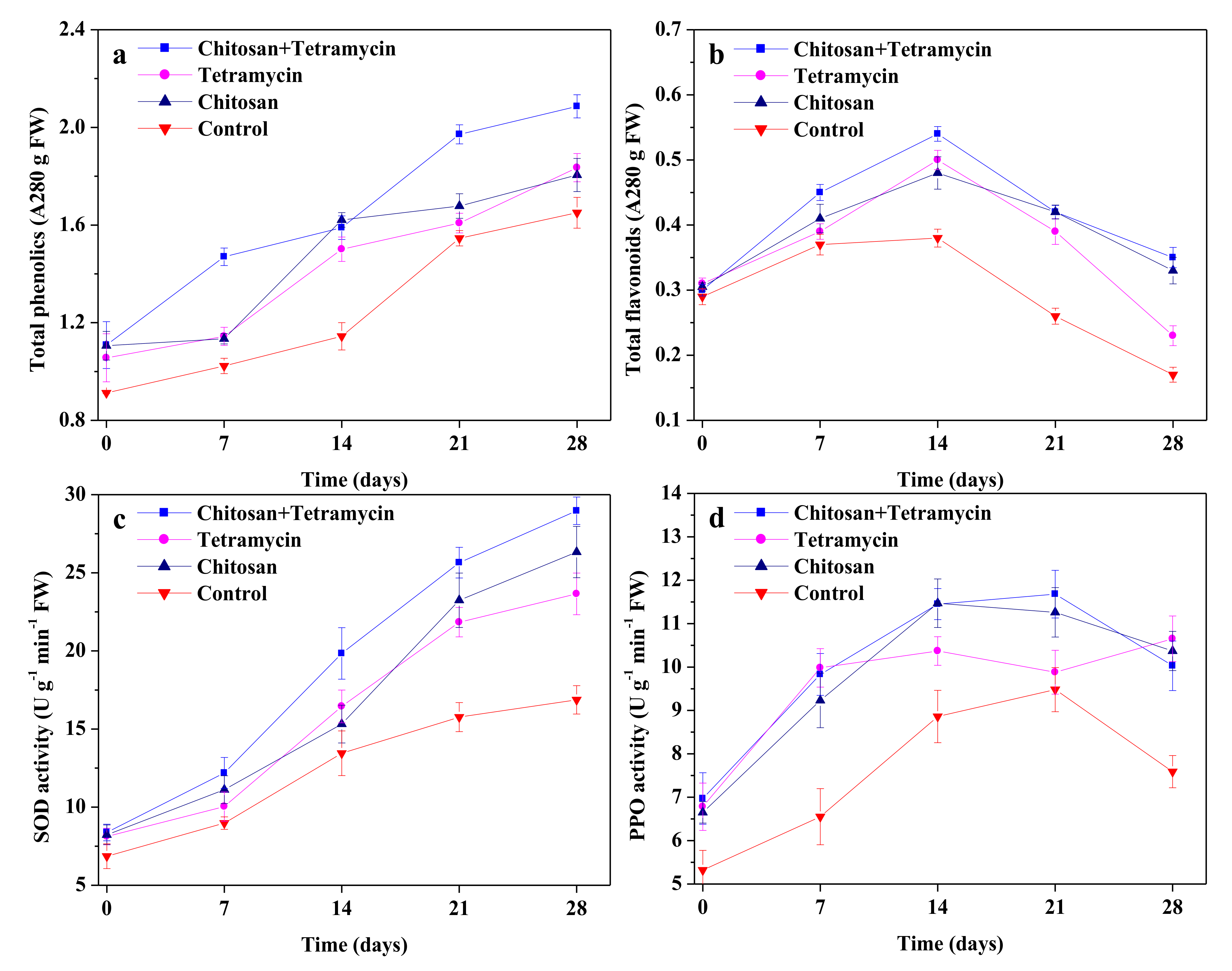
3.3. The Effects of Chitosan and Tetramycin on Total Phenolics, Total Flavonoids, SOD Activity and PPO Activity in Kiwifruit
3.4. The Effects of Chitosan and Tetramycin on Growth and Quality of Kiwifruit
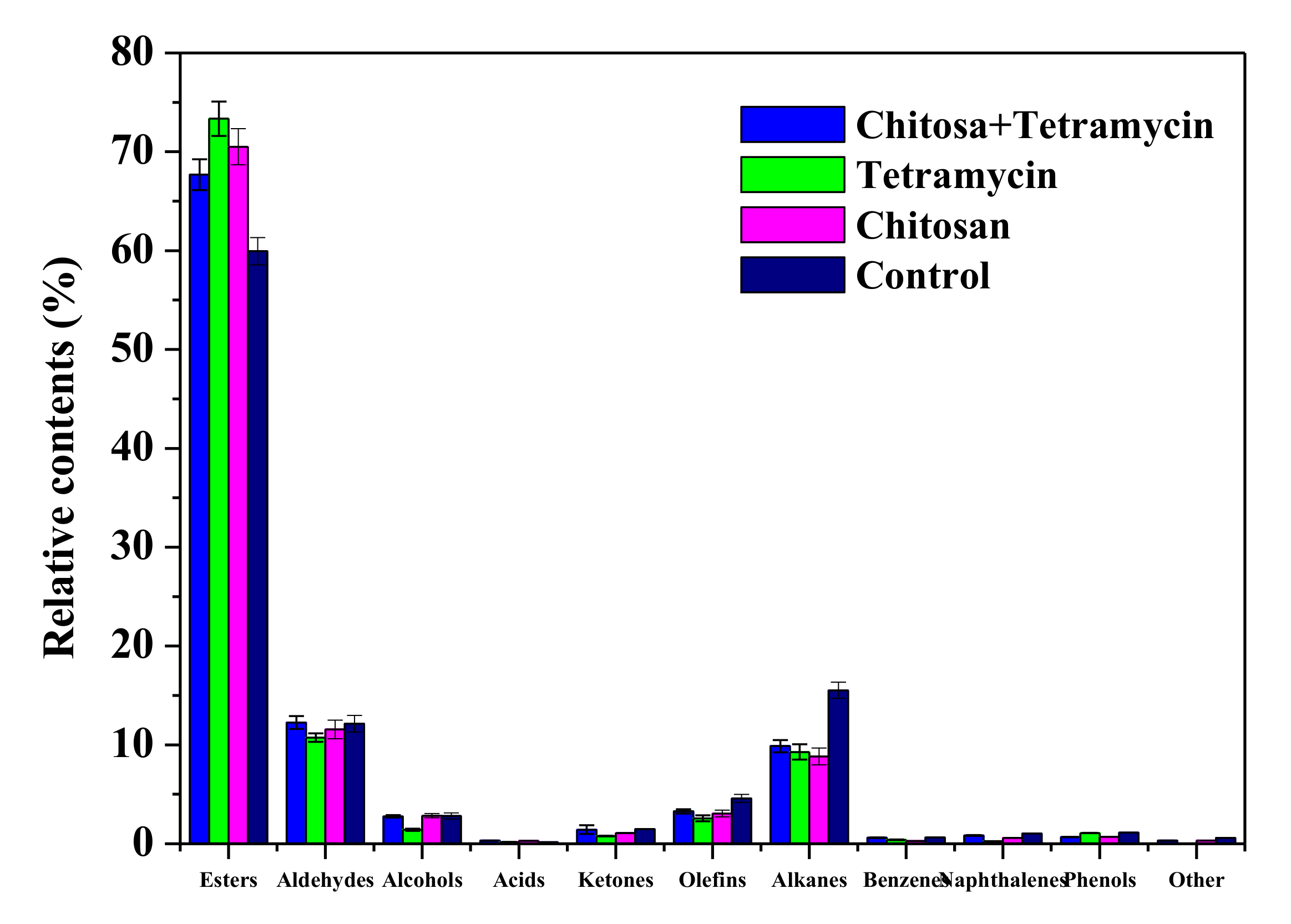
3.5. The Effects of Chitosan and Tetramycin on Aroma Compounds of Kiwifruit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, H.L.; Zhou, H.S.; Li, P.X. Lacquer wax coating improves the sensory and quality attributes of kiwifruit during ambient storage. Sci. Hortic. 2019, 244, 31–41. [Google Scholar] [CrossRef]
- Zhang, M.L.; Xu, L.Y.; Zhang, L.Y.; Guo, Y.H.; Qi, X.; He, L. Effects of quercetin on postharvest blue mold control in kiwifruit. Sci. Hortic. 2018, 228, 18–25. [Google Scholar] [CrossRef]
- Fatemi, H.; Mohammadi, S.; Aminifard, M.H. Effect of postharvest salicylic acid treatment on fungal decay and some postharvest quality factors of kiwi fruit. Arch. Phytopathol. Plant Protect. 2013, 46, 1338–1345. [Google Scholar] [CrossRef]
- Hur, J.S.; Oh, S.O.; Lim, K.M.; Jung, J.S.; Kim, J.W.; Koh, Y.J. Novel effects of TiO2 photocatalytic ozonation on control of postharvest fungal spoilage of kiwifruit. Postharvest Biol. Technol. 2005, 35, 109–113. [Google Scholar] [CrossRef]
- Hawthorne, B.T.; Rees–George, J.; Samuels, G.J. Fungi associated with leaf spots and post–harvest fruit rots of kiwifruit (Actinidia chinensis) in New Zealand. New Zealand J. Bot. 1982, 20, 143–150. [Google Scholar] [CrossRef]
- Manning, M.A.; Meier, X.; Olsen, T.L.; Johnston, P.R. Fungi associated with fruit rots of Actinidia chinensis ‘Hort16A’ in New Zealand. New Zeal. J. Crop Hort. Sci. 2003, 31, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.J.; Hur, J.S.; Jung, J.S. Postharvest fruit rots of kiwifruit (Actinidia deliciosa) in Korea. New Zealand J. Crop Hort. Sci. 2005, 22, 303–310. [Google Scholar] [CrossRef]
- Prodi, A.; Sandalo, S.; Tonti, S.; Nipoti, P.; Pisi, A. Phiaphora–like fungi associated with kiwifruit elephantiasis. Plant Physiol. 2008, 90, 487–494. [Google Scholar] [CrossRef]
- Luongo, L.; Santori, A.; Riccioni, L.; Belisario, A. Phomopsis sp. associated with post–harvest fruit rot of kiwifruit in Italy. J. Plant Pathol. 2011, 93, 205–209. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, X.M.; Long, Y.H.; Li, M. Control of soft rot in kiwifruit by pre–harvest application of chitosan composite coating and its effect on preserving and improving kiwifruit quality. Food Sci. 2016, 37, 274–281. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.H.; Wang, Q.P.; Li, J.H.; An, H.M.; Wu, X.M.; Li, M. The effect of preharvest 28.6% chitosan composite film sprays for controlling soft rot on kiwifruit and its defense responses. Hortic. Sci. 2019, 46, 180–194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Long, Y.H.; Li, J.H.; Li, M.; Xing, D.K.; An, H.M.; Wu, X.M.; Wu, Y.Y. A chitosan composite film sprayed before pathogen infection effectively controls postharvest soft rot in kiwifruit. Agronomy 2020, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Cameron, A.; Sarojini, V. Pseudomonas syringae pv. actinidiae: Chemical control, resistance mechanisms and possible alternatives. Plant. Pathol. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Jones, E.E.; Casonato, S.; Monk, J.; Ridgway, H.J. Biological control of Pseudomonas syringae pv. actinidiae (Psa), the causal agent of bacterial canker of kiwifruit, using endophytic bacteria recovered from a medicinal plant. Biol. Control. 2018, 116, 103–112. [Google Scholar] [CrossRef]
- Bo, C.; Fen, Y.; Zheng, X.; Cui, D.; Shao, Y.; Zhu, C. Genome mining of the biosynthetic gene cluster of the polyene macrolide antibiotic tetramycin and characterization of a P450 monooxygenase involved in the hydroxylation of the tetramycin B polyol segment. Chem. Biochem. 2012, 13, 34–42. [Google Scholar] [CrossRef]
- Ren, J.; Cui, Y.; Zhang, F.; Cui, H.; Ni, X.; Chen, F.; Li, L.; Xia, H. Enhancement of nystatin production by redirecting precursor fluxes after disruption of the tetramycin gene from Streptomyces ahygroscopicus. Microbiol. Res. 2014, 169, 602–608. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, L.; Zhang, Q.; Xu, C.; Zhu, H.; Lu, Z.; Shen, L.; Wang, G.; Jie, D. Effect of tetramycin on mycelia growth and spore germination of rice blast pathogen. J. Microbiol. 2010, 2, 43–45. [Google Scholar]
- Song, Y.; He, L.; Chen, L.; Ren, Y.; Lu, H.; Geng, S.; Mu, W.; Liu, F. Baseline sensitivity and control efficacy of antibiosis fungicide tetramycin against Botrytis cinerea. Eur. J. Plant Pathol. 2016, 146, 337–347. [Google Scholar] [CrossRef]
- Chen, L.L.; Guo, B.B.; Li, B.X.; Mu, W.; Liu, F. Toxicity and control efficacy of tetramycin against Passalora fulva. Chin. J. Pestic. Sci. 2017, 19, 324–330. [Google Scholar]
- Ma, D.C.; Zhu, J.M.; He, L.; Cui, K.; Mu, W.; Liu, F. Baseline sensitivity and control efficacy of tetramycin against Phytophthora capsici isolates in china. Plant Dis. 2017, 102, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; He, L.; Li, X.; Lin, J.; Mu, W.; Liu, F. Toxicity and biochemical action of the antibiotic fungicide tetramycin on Colletotrichum scovillei. Pestic. Biochem. Physiol. 2018, 147, 51–58. [Google Scholar] [CrossRef]
- Ma, D.C.; Zhu, J.M.; Jiang, J.G.; Zhao, Y.H.; Li, B.X.; Mu, W.; Liu, F. Evaluation of bioactivity and control efficacy of tetramycin against Corynespora cassiicola. Pestic. Biochem. Physiol. 2018, 152, 106–113. [Google Scholar] [CrossRef]
- Wang, L.P.; Chang, G.B.; Meng, S.; Sun, C.H. Study on the poplar canker disease controlled using four hygromycin in field. J. Microbiol. 2014, 34, 68–70. [Google Scholar]
- Li, H.; Liu, J.B.; Wang, T.J.; Jiang, H.; Zhang, R.B.; Guan, W.J. Research progress of ATP–binding cassette transporters in Polyene antibiotic biosynthesis Gene Cluster. Microbiol. China 2014, 41, 950–958. [Google Scholar]
- Wang, Q.; Zhang, C.; Long, Y.; Wu, X.; Su, Y.; Lei, Y.; Ai, Q. Bioactivity and control efficacy of the novel antibiotic tetramycin against various kiwifruit diseases. Antibiotics 2021, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zahid, N.; Manickam, S.; Siddiqui, Y.; Alderson, P.G.; Maqbool, M. Induction of lignin and pathogenesis related proteins in dragon fruit plants in response to submicron chitosan dispersions. Crop Prot. 2014, 63, 83–88. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohyd. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Reglinski, T.; Elmer, P.A.G.; Taylor, J.T.; Wood, P.N.; Hoyte, S.M. Inhibition of Botrytis cinerea growth and suppression of botrytis bunch rot in grapes using chitosan. Plant Pathology 2010, 59, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Boonlertnirun, S.; Suvannasara, P.; Promsomboon, P.; Boonlertnirun, K. Chitosan in combination with chemical fertilizer on agronomic traits and some physiological responses relating to yield potential of rice (Oryza sativa L.). Braz. J. Med. Biol. Res. 2012, 7, 64–68. [Google Scholar] [CrossRef]
- Lopes, U.P.; Zambolim, L.; Costa, H.; Pereira, O.L.; Finger, F.L. Potassium silicate and chitosan application for gray mold management in strawberry during storage. Crop Prot. 2014, 63, 103–106. [Google Scholar] [CrossRef]
- Ma, Z.X.; Yang, L.Y.; Yan, H.X.; Kennedy, J.F.; Meng, X.H. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohyd. Polym. 2013, 94, 272–277. [Google Scholar] [CrossRef]
- Yan, J.Q.; Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Effects of preharvest oligochitosan sprays on postharvest fungal diseases, storage quality, and defense responses in jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit. Sci. Hortic. 2012, 142, 196–204. [Google Scholar] [CrossRef]
- Mondal, M.M.A.; Puteh, A.B.; Dafader, N.C.; Rafii, M.Y.; Malek, M.A. Foliar application of chitosan improves growth and yield in maize. J. Food Agric. Environ. 2013, 11, 520–523. [Google Scholar]
- Liang, W.L.; Yu, A.X.; Wang, G.D.; Zheng, F.; Hu, P.T.; Jia, J.L.; Xu, H.H. A novel water–based chitosan–La pesticide nanocarrier enhancing defense responses in rice (Oryza sativa L) growth. Carbohyd. Polym. 2018, 199, 437–444. [Google Scholar] [CrossRef]
- Liu, H.; Tian, W.X.; Li, B.; Wu, G.X.; Ibrahim, M.; Tao, Z.Y.; Wang, Y.L.; Xie, G.L.; Li, H.Y.; Sun, G.C. Antifungal effect and mechanism of chitosan against the rice sheath blight pathogen, Rhizoctonia solani. Biotechnol. Lett. 2012, 34, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.H.; Yang, L.Y.; Kennedy, J.F.; Tian, S.P. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohyd. Polym. 2010, 81, 70–75. [Google Scholar] [CrossRef]
- Chen, J.P.; Zou, X.; Liu, Q.; Wang, F.; Feng, W.; Wan, N. Combination effect of chitosan and methyl jasmonate on controlling Alternaria alternata and enhancing activity of cherry tomato fruit defense mechanisms. Crop Prot. 2014, 56, 31–36. [Google Scholar] [CrossRef]
- Shi, Z.J.; Wang, F.; Lu, Y.Y.; Deng, J. Combination of chitosan and salicylic acid to control postharvest green mold caused by Penicillium digitatum in grapefruit fruit. Sci. Hortic. 2018, 223, 54–60. [Google Scholar] [CrossRef]
- Zhong, L.J. Studies on the rice resistance to rice blast induced by tetramycin. J. Anhui Agric. Sci. 2010, 38, 6263–6264. (In Chinese) [Google Scholar]
- Frank, D.; Riordan, P.; Varelis, P.; Zabaras, D.; Watkins, P.; Ceccato, C.; Wijesundera, C. Deconstruction and recreation of ‘Hayward’ volatile flavour using a trained sensory panel, olfactometry and a kiwifruit model matrix. Acta Hortic. 2007, 753, 107–119. [Google Scholar] [CrossRef]
- Friel, E.N.; Wang, M.; Taylor, A.J.; Macrae, E.A. In vitro and in vivo release of aroma compounds from yellow–fleshed kiwifruit. J. Agric. Food Chem. 2007, 55, 6664–6673. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Macrae, E.; Wohlers, M.; Marsh, K. Changes in volatile production and sensory quality of kiwifruit during fruit maturation in Actinidia deliciosa ‘Hayward’ and A. chinensis ‘Hort16A’. Postharvest Biol. Technol. 2011, 59, 16–24. [Google Scholar] [CrossRef]
- Günther, C.S.; Marsh, K.B.; Winz, R.A.; Harker, R.F.; Wohlers, M.W.; White, A.; Goddard, M.R. The impact of cold storage and ethylene on volatile ester production and aroma perception in ‘Hort16A’ Kiwifruit. Food Chem. 2015, 169, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Q.; Wu, X.; Long, Y.; Wu, Y.; Huang, Y.; Tang, J. Effects of forchlorfenuron on amino acids and aroma components of Guichang kiwifruit postharvests. J. Nucl. Agric. Sci. 2019, 33, 2186–2194. (In Chinese) [Google Scholar] [CrossRef]
| Pathogens | Antibiotic Bactericides | Regression Equation | Determination Coefficient (R2) | EC50 (mg kg−1) |
|---|---|---|---|---|
| Chitosan > B. dothidea | 0.3% Tetramycin AS | y = 6.0759 + 1.2511 x | 0.9963 | 0.14 |
| Chitosan | y = 5.1568 + 0.7069 x | 0.9941 | 600.11 | |
| Phomopsis sp. | 0.3% Tetramycin AS | y = 1.1510 + 9.3601 x | 0.9968 | 0.09 |
| Chitosan | y = 5.2904 + 0.9250 x | 0.9865 | 485.41 |
| Treatments | Incidence Rate of Disease Fruit (%) | Control Effect (%) |
|---|---|---|
| Chitosan + tetramycin | 8.00 ± 2.00 cC | 85.33 ± 2.12 aA |
| Tetramycin | 10.00 ± 2.65 cC | 80.99 ± 6.86 aA |
| Chitosan | 32.00 ± 3.61 bB | 40.66 ± 3.29 bB |
| Control | 58.00 ± 3.61 aA |
| Treatments | Longitudinal Diameter (mm) | Transverse Diameter (mm) | Lateral Diameter (mm) | Fruit Shape Index | Single Fruit Volume (cm3) | Single Fruit Weight (g) |
|---|---|---|---|---|---|---|
| Chitosan + Tetramycin | 76.93 ± 0.47 a | 52.97 ± 1.42 a | 42.35 ± 0.72 a | 1.61 ± 0.03 a | 72.29 ± 3.52 a | 82.70 ± 0.56 a |
| Tetramycin | 76.12 ± 0.14 a | 52.02 ± 0.61 a | 42.06 ± 0.24 a | 1.62 ± 0.02 a | 69.73 ± 1.08 ab | 80.05 ± 0.82 b |
| Chitosan | 76.47 ± 0.22 a | 52.27 ± 0.53 a | 42.13 ± 0.81 a | 1.62 ± 0.00 a | 70.49 ± 1.12 ab | 80.08 ± 0.74 b |
| Control | 75.51 ± 0.33 ab | 51.71 ± 0.50 a | 41.26 ± 0.36 a | 1.62 ± 0.02 a | 67.45 ± 1.17 b | 76.79 ± 1.59 c |
| Treatments | Vitamin C (g kg−1) | Total Soluble Sugar (%) | Soluble Solid (%) | Dry Matter (%) | Soluble Protein (%) | Titratable Acidity (%) |
|---|---|---|---|---|---|---|
| Chitosan + tetramycin | 1.91 ± 0.02 a | 12.61 ± 0.24 a | 15.60 ± 0.10 a | 19.56 ± 0.09 a | 1.78 ± 0.04 a | 1.04 ± 0.03 b |
| Tetramycin | 1.88 ± 0.01 a | 12.40 ± 0.07 a | 15.20 ± 0.10 a | 19.14 ± 0.10 ab | 1.75 ± 0.02 a | 1.09 ± 0.02 b |
| Chitosan | 1.89 ± 0.01 a | 12.56 ± 0.11 a | 15.40 ± 0.10 a | 19.37 ± 0.16 a | 1.77 ± 0.02 a | 1.05 ± 0.01 b |
| Control | 1.81 ± 0.01 b | 12.08 ± 0.01 b | 14.37 ± 0.06 b | 18.34 ± 0.03 c | 1.72 ± 0.03 b | 1.16 ± 0.04 a |
| Aroma Components | Relative Contents/% | |||
|---|---|---|---|---|
| Chitosan + Tetramycin | Tetramycin | Chitosan | Control | |
| Ethyl acetate | 0.30 ± 0.01 | 0.29 ± 0.01 | 0.34 ± 0.01 | – |
| Butanoic acid methyl ester | 16.07 ± 1.08 | 15.7 ± 1.03 | 16.11 ± 1.21 | 14.52 ± 1.75 |
| Butanoic acid ethyl ester | 22.09 ± 1.21 | 21.7 ± 1.25 | 22.21 ± 1.35 | 20.41 ± 1.88 |
| Hexanoic acid methyl ester | 1.35 ± 0.01 | 1.31 ± 0.01 | 1.04 ± 0.02 | 1.78 ± 0.12 |
| Hexanoic acid ethyl ester | 3.06 ± 0.13 | 4.62 ± 0.42 | 2.88 ± 0.13 | – |
| Butanoic acid 2–methyl propyl ester | 0.51 ± 0.01 | 0.44 ± 0.01 | 0.47 ± 0.02 | – |
| 2–Furancarboxylic acid ethyl ester | 0.33 ± 0.01 | 0.32 ± 0.02 | 0.33 ± 0.01 | 0.28 ± 0.05 |
| Benzoic acid methyl ester | 15.64 ± 1.03 | 15.54 ± 1.39 | 16.77 ± 1.25 | 15.36 ± 1.55 |
| Octanoic acid methyl ester | 1.13 ± 0.04 | 1.20 ± 0.01 | 1.22 ± 0.03 | 1.10 ± 0.68 |
| Benzoic acid ethyl ester | 6.03 ± 0.87 | 10.97 ± 0.33 | 7.88 ± 0.45 | 5.40 ± 1.01 |
| Octanoic acid ethyl ester | 0.75 ± 0.04 | 0.35 ± 0.03 | 0.67 ± 0.32 | 0.72 ± 0.35 |
| Benzoic acid hexyl ester | – | 0.05 ± 0.00 | – | – |
| Butyl benzoate | 0.18 ± 0.11 | 0.69 ± 0.04 | 0.33 ± 0.23 | 0.18 ± 0.11 |
| Decanoic acid ethyl ester | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | – |
| Octadecyl 2–amyl sulfate | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | – |
| 2– methyl propyl phthalate | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | – |
| Dibutyl phthalate | 0.11 ± 0.01 | 0.04 ± 0.00 | 0.15 ± 0.01 | 0.19 ± 0.04 |
| 2–Butenedioic acid (E)–, bis(2–ethylhexyl) ester | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.03 ± 0.00 | – |
| (E) –2–hexenal | 9.07 ± 0.34 | 8.78 ± 0.20 | 8.94 ± 0.26 | 7.97 ± 1.20 |
| Benzeneacetaldehyde | – | 0.08 ± 0.01 | 0.07 ± 0.00 | – |
| Nonanal | 2.55 ± 0.85 | 1.52 ± 0.05 | 1.88 ± 0.07 | 3.55 ± 0.83 |
| Decanal | 0.62 ± 0.11 | 0.28 ± 0.03 | 0.58 ± 0.04 | 0.62 ± 0.11 |
| 2–Undecenal | – | 0.05 ± 0.00 | 0.07 ± 0.00 | – |
| Fifteen aldehydes | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | – |
| 1–Deoxy–d–arabitol | 0.81 ± 0.04 | – | 0.80 ± 0.02 | 0.77 ± 0.11 |
| Eucalyptol | 1.13 ± 0.22 | 1.05 ± 0.07 | 1.19 ± 0.06 | 1.22 ± 0.24 |
| à–Terpineol | 0.31 ± 0.01 | 0.29 ± 0.01 | 0.27 ± 0.01 | 0.23 ± 0.01 |
| (E)–4, 6–dimethyl–1–methyl sulfide –1, 5–heptadiene –4–alcohol | – | 0.08 ± 0.01 | – | – |
| Trans –2, 6–dimethyl–6 – (p–methyl–phenyl) –heptenol | 0.11 ± 0.01 | – | 0.13 ± 0.01 | 0.13 ± 0.02 |
| (Z)–13– docosahlenol | 0.41 ± 0.01 | – | 0.45 ± 0.01 | 0.46 ± 0.05 |
| E–2–Hexenyl benzoate | 0.16 ± 0.02 | – | 0.17 ± 0.02 | 0.15 ± 0.04 |
| Hexanoic acid anhydride | – | 0.04 ± 0.00 | – | – |
| 2– methyl pentahydride | 0.15 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | – |
| 7– isopropyl – dicyclic [0,3,3] octane–2–ketone | 0.21 ± 0.01 | – | – | 0.21 ± 0.01 |
| 3–tert–Butyl–2–pyrazolin–5–one | – | 0.04 ± 0.00 | – | – |
| (S)– 6– (1– methyl vinyl)– cyclohexenone | 0.13 ± 0.01 | – | 0.15 ± 0.01 | 0.11 ± 0.02 |
| Beta–Malaysia | 0.10 ± 0.01 | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.11 ± 0.01 |
| Trans geranyl acetone | 0.31 ± 0.02 | 0.29 ± 0.03 | 0.34 ± 0.01 | 0.33 ± 0.02 |
| trans–á–Ionone | 0.15 ± 0.01 | 0.06 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.02 |
| 3–(2–pentene)–1,2,4–cyclopentaerone | 0.52 ± 0.01 | 0.24 ± 0.03 | 0.38 ± 0.01 | 0.56 ± 0.03 |
| Benzophenone | – | 0.08 ± 0.01 | – | – |
| 1,7, 7–trimethyl–hept–2–ene | 0.38 ± 0.02 | 0.27 ± 0.02 | 0.33 ± 0.02 | 1.27 ± 0.13 |
| à–Cubebene | 0.98 ± 0.05 | 1.15 ± 0.05 | 1.23 ± 0.02 | 0.76 ± 0.11 |
| (E)–á–Famesene | 0.17 ± 0.04 | – | – | 0.16 ± 0.04 |
| isoledene | 0.18 ± 0.03 | – | 0.18 ± 0.03 | 0.15 ± 0.02 |
| cis–Calamenene | 1.07 ± 0.14 | 1.12 ± 0.01 | 1.16 ± 0.01 | 1.66 ± 0.14 |
| à–Calacorene | 0.32 ± 0.02 | – | – | 0.46 ± 0.07 |
| isoaromadendrene epoxide | 0.14 ± 0.01 | – | 0.12 ± 0.01 | 0.12 ± 0.02 |
| Neophytadiene | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | – |
| Octamethyl– cyclotetrasiloxane | 1.48 ± 0.06 | 0.04 ± 0.00 | 0.03 ± 0.00 | 5.15 ± 1.53 |
| Decamethyl– cyclopentasiloxane | 4.52 ± 0.18 | 5.27 ± 0.64 | 4.89 ± 0.64 | 6.08 ± 1.30 |
| 1,1–Dichloro–2–methyl–3–(4,4–diformyl–1,3–butadien–1–yl) cyclopropane | – | 0.03 ± 0.00 | – | – |
| Dodecamethyl– cyclohexasiloxane | 2.05 ± 0.12 | 2.46 ± 0.19 | 2.33 ± 0.13 | 2.55 ± 0.33 |
| Tetradecane | 0.22 ± 0.01 | – | 0.25 ± 0.01 | 0.27 ± 0.03 |
| Tetradecamethyl– cycloheptasiloxane | 1.23 ± 0.13 | 1.00 ± 0.18 | 1.08 ± 0.11 | 1.06 ± 0.13 |
| Hexadecamethyl– cyclooctasiloxane | 0.21 ± 0.01 | 0.32 ± 0.04 | 0.12 ± 0.01 | 0.29 ± 0.01 |
| Octadecamethyl– cyclononasiloxane | 0.16 ± 0.01 | 0.12 ± 0.02 | 0.12 ± 0.02 | 0.12 ± 0.01 |
| Eicosamethyl– cyclodecasiloxane | – | 0.04 ± 0.01 | – | – |
| 1, 4–bis – (1–methylethyl) benzene | 0.13 ± 0.01 | – | 0.04 ± 0.01 | 0.11 ± 0.01 |
| 1, 1–propane diphenyl | – | 0.04 ± 0.01 | – | – |
| 1, 1–2–butene –1, 4–2–benzene | 0.21 ± 0.01 | 0.26 ± 0.06 | 0.22 ± 0.02 | 0.23 ± 0.02 |
| 2 – methyl naphthalene | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.03 ± 0.01 | – |
| 1, 7–dimethyl naphthalene | 0.22 ± 0.01 | 0.05 ± 0.01 | 0.28 ± 0.01 | 0.29 ± 0.02 |
| 1–Isopropyl–4,7–dimethyl–1,2,3,4,5,6–hexahydronaphthalene | 0.29 ± 0.03 | – | – | 0.28 ± 0.04 |
| 1,6–dimethyl–4 – (1–methylethyl) naphthalene | 0.37 ± 0.03 | 0.24 ± 0.09 | 0.27 ± 0.01 | 0.57 ± 0.09 |
| 1,2,3,4–tetramethene | 0.17 ± 0.02 | – | – | 0.18 ± 0.01 |
| Butylated Hydroxytoluene | 0.38 ± 0.02 | 0.05 ± 0.01 | 0.44 ± 0.01 | 0.32 ± 0.02 |
| (S)– (S) –2–methyl–5 – (1,2, 2–tricyclopentyl) phenol | 0.29 ± 0.10 | 1.03 ± 0.04 | 0.25 ± 0.01 | 0.81 ± 0.10 |
| Dibenzyl ketoxime | 0.31 ± 0.01 | – | 0.32 ± 0.01 | 0.34 ± 0.03 |
| (Z) –oleate amide | – | – | – | 0.24 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, C.; Wu, X.; Long, Y.; Su, Y. Chitosan Augments Tetramycin against Soft Rot in Kiwifruit and Enhances Its Improvement for Kiwifruit Growth, Quality and Aroma. Biomolecules 2021, 11, 1257. https://doi.org/10.3390/biom11091257
Wang Q, Zhang C, Wu X, Long Y, Su Y. Chitosan Augments Tetramycin against Soft Rot in Kiwifruit and Enhances Its Improvement for Kiwifruit Growth, Quality and Aroma. Biomolecules. 2021; 11(9):1257. https://doi.org/10.3390/biom11091257
Chicago/Turabian StyleWang, Qiuping, Cheng Zhang, Xiaomao Wu, Youhua Long, and Yue Su. 2021. "Chitosan Augments Tetramycin against Soft Rot in Kiwifruit and Enhances Its Improvement for Kiwifruit Growth, Quality and Aroma" Biomolecules 11, no. 9: 1257. https://doi.org/10.3390/biom11091257
APA StyleWang, Q., Zhang, C., Wu, X., Long, Y., & Su, Y. (2021). Chitosan Augments Tetramycin against Soft Rot in Kiwifruit and Enhances Its Improvement for Kiwifruit Growth, Quality and Aroma. Biomolecules, 11(9), 1257. https://doi.org/10.3390/biom11091257





